1. Introduction
The determination of the elemental composition of minerals and products during their technological processing at mining and refining facilities is important at all stages of material processing (for example, mining and beneficiation). Many analyzing techniques have been developed that allow us to accurately determine the chemical composition of prepared samples [
1,
2,
3]. Such an analysis requires setting up special laboratories that have quite complex and expensive analytical equipment, as well as specially trained and highly qualified personnel. To ensure the operation of such an analytical laboratory, the availability of a large number of chemical reagents is required, which, in addition, has a significant negative impact on the environment. When analyzing materials on such an industrial scale, the main difficulties are related to delivering the representativeness of these samples and the speed of analysis, the results of which often become known only after a few hours.
The operational analysis of materials on the conveyors of mining enterprises in most cases does not require sample preparation, which excludes the influence of a human factor and unpredictable accidents on the results of the analysis [
4]. Such operational analyses can be based on various nuclear physics methods (e.g., radiometric, neutron activation, gamma activation and X-ray fluorescence (XRF)) [
4,
5,
6,
7,
8]. Of course, online analysis methods are usually less sensitive and precise than chemical ones. However, these provide analysis results in real time, which allows us to quickly adjust technologies, automate technological processes and eliminate significant human labor costs. In addition, it is often less important to know the absolute value of a particular element’s concentration on the conveyor than to control its trend and see the course of the concentration change. Each of the nuclear physics methods has its advantages and disadvantages and solves a certain range of applications [
4,
5,
6,
7,
8].
X-ray fluorescence analysis (XRF) is one of the most accurate and simplest analytical methods for studying matter in order to obtain its elemental composition [
6,
8]. With the help of this method, various elements, from aluminum (Al) to uranium (U), can be detected. The XRF method is based on the registration and subsequent analysis of the spectra that arise when the materials under study are irradiated with X-rays. Both X-ray tubes and isotopes of various elements can be used as a radiation source. The industry produces a massive amount of XRF equipment for the analysis of materials in laboratory conditions [
9], and the measurement methods for such equipment are well developed.
At the same time, the development of online XRF equipment for the analysis of materials in industrial environments is not so rapid. There are several industrial XRF analyzers on the market for the elemental composition analysis of liquid solutions and pulp [
10,
11,
12,
13,
14,
15], for the analysis of alloys in coatings [
16,
17] and for the analysis of bulk materials on a conveyor [
18,
19,
20]. The small number of industrial online XRF analyzers on the market is primarily due to non-standard technological problems that need to be solved by each enterprise in its production. In those applications, where the use of non-standard equipment does not require its metrological certification, developers can use their solutions for online X-ray fluorescence analyses of materials [
21,
22,
23,
24,
25,
26,
27,
28,
29]. For monitoring technological processes in the mining industry, measuring equipment with methodological and metrological certification is typically used.
Our previous efforts [
30] were aimed at developing and improving the metrological characteristics of a specialized online XRF analyzer capable of operating under harsh conditions on mining industry conveyors. This paper is devoted to the results of the development and application of an online XRF method for monitoring elements in minerals on the conveyors of mining and processing enterprises. The paper also describes those mining technological applications where we have successfully implemented the online method and presents an analysis of the metrological characteristics achieved in these applications.
2. Online XRF Method
In contrast to laboratory applications, the online use of XRF equipment to analyze the concentration of elements in both pulp and bulk materials implies its integration into the technological line of a processing production [
4]. In such applications, the equipment must operate reliably in a continuous mode for months, without human intervention in the measurement process. For this reason, a reliable industrial online XRF analyzer, capable of continuous operation in harsh industrial conditions, is the key element necessary for the implementation of an online XRF method of controlling elements in minerals on a conveyor. In the mining industry, where analyzers work year-round in unsealed hangars practically in the open air and are exposed to dust, humidity, a wide range of operating temperatures from −40 °C to +50 °C, electromagnetic interference, vibration, etc., the operating conditions are especially harsh.
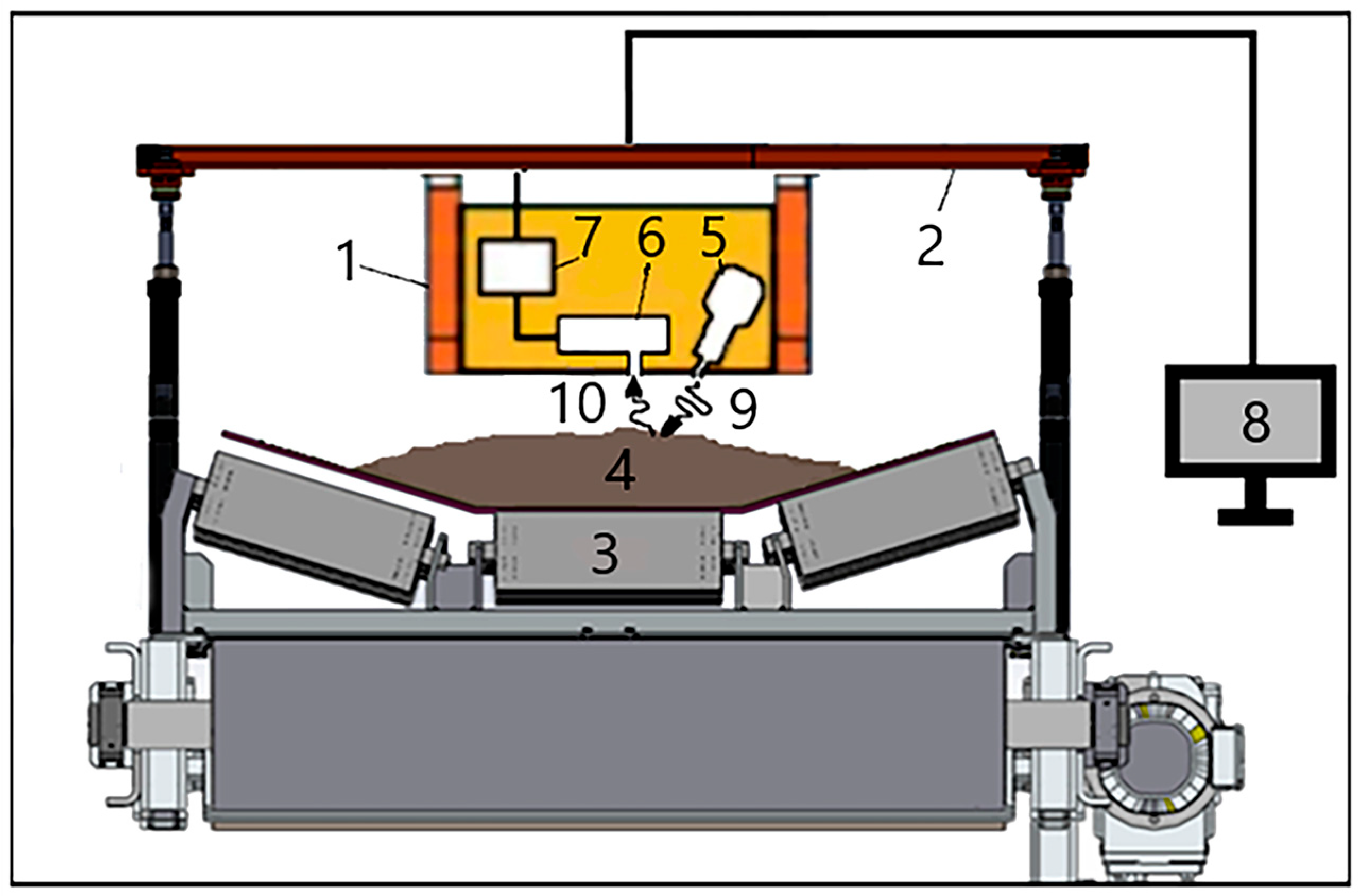
The scheme for the application of the online XRF method for controlling bulk materials on a conveyor is presented in
Figure 1. The industrial XRF analyzer (1) is mounted on a special suspension (2) (see
Section 3) above the conveyor belt (3), with ore (4) usually moving at a speed of 1–2 m/s, and in some enterprises up to 5 m/s. The XRF analyzer contains an X-ray tube (5), an X-ray detector (6), a multi-channel analyzer for the amplification and shaping of signals from the detector, power supplies (7) and a computer (8) equipped with software for the analysis of X-ray spectra and the calculation of the concentration of the required elements.
While passing under the analyzer, the ore is irradiated by X-ray radiation from the X-ray tube (9) and emits secondary characteristic radiation (10), which is registered by a semiconductor X-ray detector (6). The type of X-ray tube used, its power and anode material are determined by the conditions of the application to be solved.
The height of installing the XRF analyzer above the conveyor depends on the type and size of ore pieces going along the conveyor, the pattern of ore distribution along the width of the conveyor belt and the change in its height on the conveyor over time. In practice, material sizes vary greatly in different conveyor belt applications, ranging from fractions of mm to 25–30 cm. The maximum size of the ore pieces determines the height at which the XRF analyzer should be mounted above the conveyor with the ore, as well as the geometry of the mutual arrangement of the X-ray tube and detector. In order to mount the analyzer at a certain level above the conveyor belt, various suspensions have to be designed and applied, and their design depends on the specific task (see
Section 3). Ideally, the surface of the ore should be completely flat so that the bottom surface of the XRF analyzer is as close as possible to the surface of the material. But, in real applications, this is extremely rare.
In real-world applications, there is always an air gap between the ore surface and the surface of the XRF analyzer, which must be minimized. Since virtually all lines (L-series) of the characteristic X-ray emissions of elements, from aluminum to uranium, are in the 1–30 keV energy range, such soft radiation is easily absorbed in the air gap, leading to a decrease in the sensitivity of the method. No less important is the need not only to minimize but also to ensure the consistency of the width of this air gap. When changing the width of the air gap, the value of radiation absorption in it will change, which will be perceived by the detector as a change in the signal from a controlled element in the ore due to a change in its concentration.
Since the X-ray energy for the excitation of the fluorescence of elements from aluminum to uranium is relatively small (9–45 keV), the layer of the material from which the fluorescent quanta with an acceptable self-absorption value comes out is about 1.0–1.5 mm. This is considered to be the main disadvantage of the XRF material control method (both in a laboratory and online), as it results in a low representativeness (small volume) of the sample tested. However, in the case of the online XRF method, due to a moving conveyor belt, the volume of the samples measured is 3–4 orders of magnitude larger even than that of samples in chemical analysis. Thus, at a 1 m/s speed of the conveyor belt, the track of an irradiated ore material being 10 cm wide and the time of one measurement being 200 s, the sample volume is about 20 × 103 cm3, while in chemical analysis the volume of ore material is approximately 1 cm3.
3. Development and Implementation of the Online XRF Method
Industrial XRF Analyzer. XRF analyzers manufactured for use in laboratory conditions [
8] are not suitable for use in the online monitoring of enterprise conveyors. We continue to develop and implement the online XRF analysis method for mining enterprises based on the industrial CON-X XRF analyzer [
18], which has already undergone several upgrades to meet the increasing level of requirements [
30]. Since the analyzer installation locations in all mining enterprises are practically open hangars all year round, we provided the analyzer with a hermetically sealed housing; the temperature inside the detection system is stabilized, which ensures a reliable measurement of results under all external conditions. This technical solution allowed us to guarantee a temperature instability of the electronic circuit of 0.05% over an operating temperature range of (−20 °C to +45 °C). This in turn ensured a measurement error of no worse than 5% relative to the concentration of most elements, determined using standard samples. At the same time, the specifications of other online XRF analyzers indicate the range of their operating temperatures, but there is no data on the stability of the characteristics and final results in this range [
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20].
However, even then measurement results depend on many factors, such as the distance between a measured sample and the analyzer, the size of ore pieces, the high degree of inhomogeneity of the controlled material, unknown and variable amounts of light elements invisible to the analyzer, the humidity of the measured material, the dustiness of the air, etc. All these factors were considered in the calculation algorithm to ensure the reliable results of online XRF analysis. Furthermore, the analyzer software includes another algorithm that allows for the inclusion and exclusion of “empty belt time” in the analyzer’s analytical calculations, significantly increasing the accuracy of the measurement results. This algorithm is also missing in other online XRF analyzers [
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20].
Software. A software package developed for online XRF analysis provides empirical calibration not only using a reference sample, but also using the fundamental parameter method. This software package provides the ability to conveniently process the obtained spectrum, including deconvolution, smoothing, subtracting the background, the use of the spectral ratio method and the standard-background method [
6,
8]. All the original solutions described allow us to solve a wide range of technological problems in the mining industry.
In addition to calculating the concentrations of elements, the software also calculates an assessment of their statistical accuracy, the detection limit (DL) and the sensitivity of the measurements [
31]. The detection limit (DL) is the minimum concentration which can be detected with certainty in a given measurement time. It is determined from the following expression:
where S
p and S
b are the numbers of photons detected in the spectral line and its background, and C is the concentration of the corresponding element.
The statistical accuracy (σ) shows the achievable repeatability of determination of the element concentration at a fixed sample position. It is determined from the following expression:
The sensitivity (ε) is defined as an increase in the number of photons in the spectral line arriving to the detector in one second, if the concentration is increasing by 1% (in terms of the measured element). It is computed from the following expression:
where t is the measurement time.
Calibration. In order to deliver the required accuracy in measuring the concentration of the measured elements on a conveyor, and to minimize all unaccounted errors (see above), the online analyzer must be calibrated for the specific task it needs to solve. Analyzer calibration, which correlates the X-ray intensity to the actual quantities of the registered elements, is performed by us in laboratory conditions using reference samples [
32,
33]. For such calibration, we use reference samples provided to us by the mining companies themselves. These reference samples are produced by the analytical plant laboratories of mining enterprises and contain the concentrations of monitored elements in the range in which they are present in the ores of these enterprises. The characteristics of the standard reference samples for online XRF analyzer applications (see
Section 4) are presented in
Table 1.
Figure 2 shows an example of a typical calibration curve for measuring Titanium (application 4.5), created using the provided standard samples. The R
2 value for the given graph was 0.9971. For the rest of the elements of interest, all R
2 values are given in
Table 1 without the graphs. As can be seen from the table, all R
2 values are close to the value = 1, thus indicating that the calibration graphs are close to perfect straight lines.
The reference materials ensure accurate and consistent measurements, allowing us to control the metrological characteristics of the XRF analyzer and validate the measuring techniques. All XRF analyzers presented in
Section 4 have been calibrated relative to the reference samples provided by customer enterprises. All these analyzers have methodologies developed for measuring the required elements.
4. Online Analyses Results and Discussions
Below are some typical applications for monitoring the concentration of elements in rocks at mining plants and the solutions that enabled achieving the required measurement accuracy online using the CON-X XRF analyzer on the conveyor belts of these plants. In all of the above solutions, XRF analyzers used X-ray tubes with a Mo anode, although in other applications X-ray tubes with a different anode material were used (Ag, Rh). The power of X-ray tubes in the presented applications varied (HV = 9–45 kV; I = 100–800 μA) depending on the task to be solved. Silicon Drift detectors (SDD) with a sensitive surface area of 10 mm2 were used as X-ray detectors.
4.1. The Chromite Lump (Cr2O3 or FeCr2O4) Content Determination
Control of the chromium content in chromium–iron ore was provided to monitor the quality of the initial products on the conveyor of a mining company. The online XRF analyzer (Baltic Scientifics Instruments, Riga, Latvia) was mounted above a conveyor belt on a standard suspension on four legs with shock absorbers to reduce the vibration of the analyzer (
Figure 3).
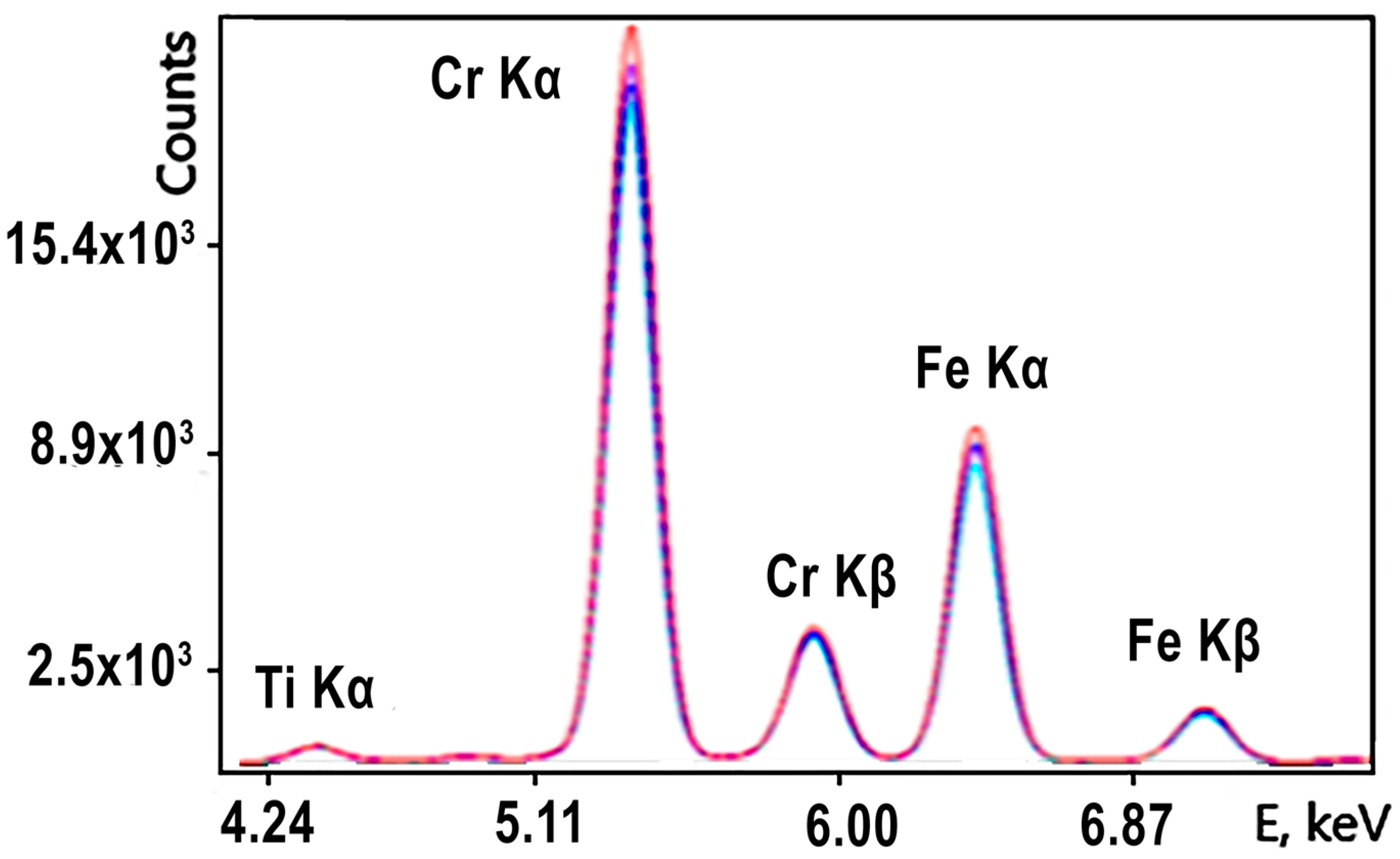
The distance from the bottom of the analyzer (which is also the detector plane) to the surface of the ore pieces was 150 mm. The conveyor belt speed was 1 m/s, and the measurement time in this application was t = 60 s. The typical spectrum of chromium–iron ore flowing through the conveyor recorded online by the XRF analyzer, used to calculate the concentrations of elements in it and the calculation errors, is presented in
Figure 4.
The spectrum clearly shows the peaks of chromium and iron and the concentrations of oxides which, in the ore, range from 29 to 40% and 25 to 30%, respectively. The detection limits of chromium and iron in the ore were 0.07 and 0.08% abs.
The measurements of reproducibility have been performed by taking different parts of one sample with the purpose of finding out the average value of the Cr Kα spectral line count rate and its standard deviation. These results of the standard deviation and uncertainty in the concentration are shown in
Table 2.
It is possible to determine the resulting concentration determination uncertainty from the empirical calibration curve. In our case it, does not exceed 0.5% (absolute) for Cr2O3, which does not significantly contribute to the total resulting uncertainty of about 2%.
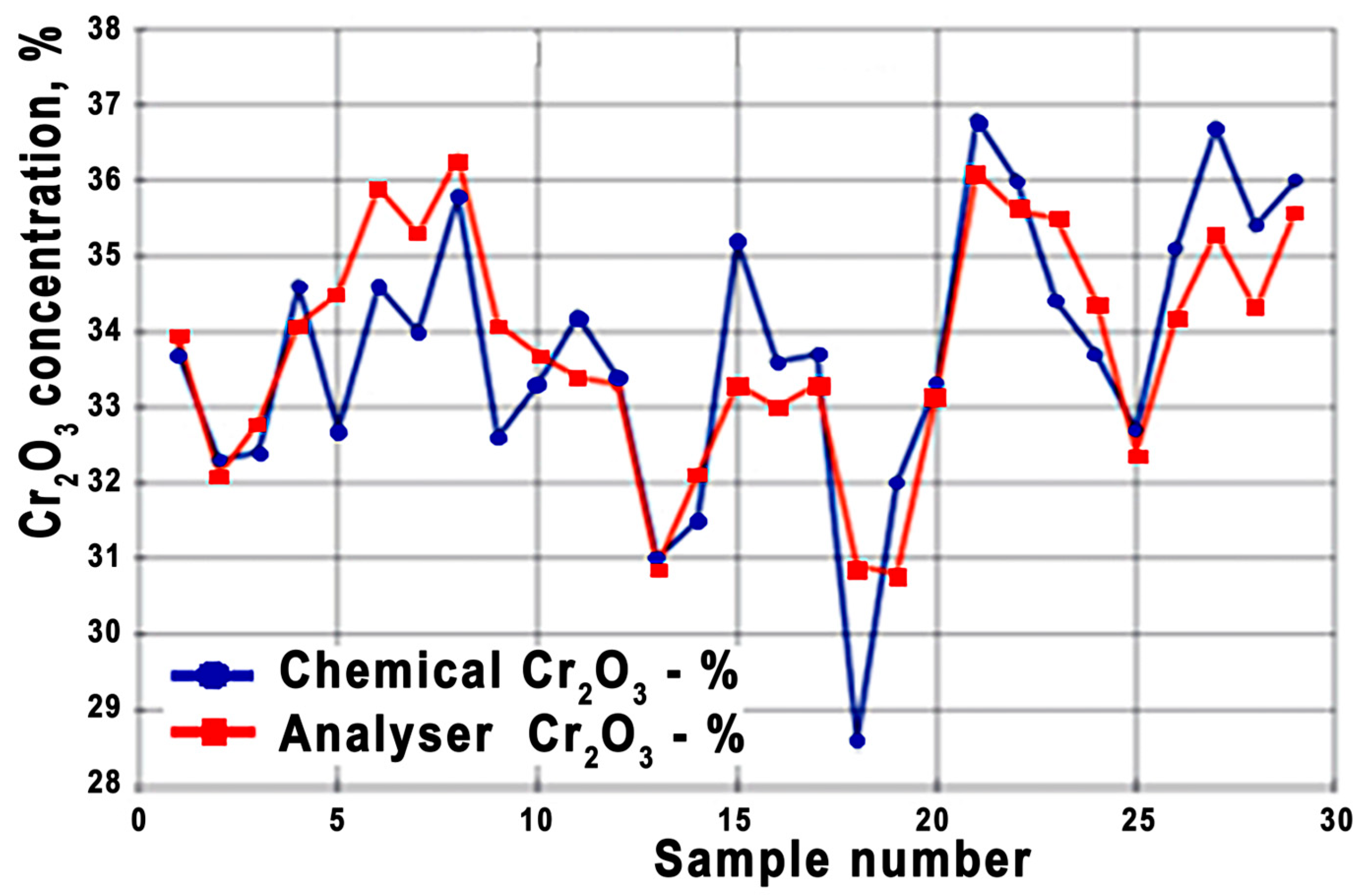
The accuracy of XRF analysis is confirmed by the data from the chemical analyses of more than 300 ore samples, which were taken from the conveyor synchronously with the operation of the spectrometer. Graphs of changes in the concentration of chromium in the ore of various samples are presented in
Figure 5. The standard deviation of the results of the two methods was 1.1% abs for Cr
2O
3 and 0.8% abs for Fe
2O
3.
4.2. Analysis of Copper–Zinc Sulfide Ore
The continuous monitoring of the copper and zinc concentration in copper–zinc sulfide ore lumps is carried out by a company during conveyor transportation. The industrial online analyzer was installed above the conveyor at a height of 80 mm above the ore level on a simple suspension attached to a beam passing over the conveyor (
Figure 6).
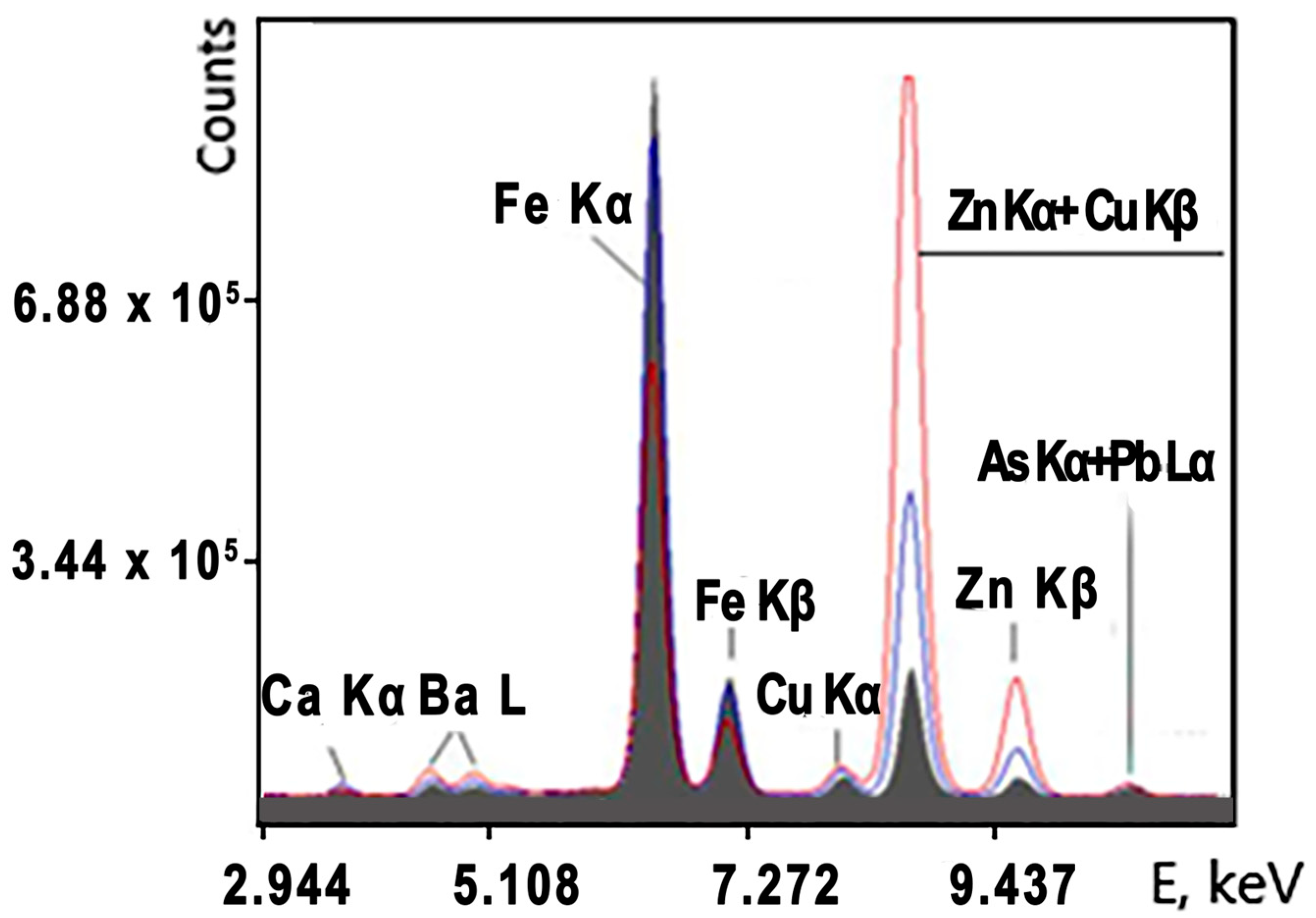
The dimensions of ore lumps were 50–150 mm. The conveyor speed was 1 m/s, and the measurement time of a given application was t = 300 s. A typical copper–zinc sulfide ore spectrum recorded by the analyzer and used to calculate concentrations and measurement errors is shown in
Figure 7.
The spectrum in
Figure 7 indicates that the XRF analyzer is capable of the online measurement of Fe, Cu and Zn in copper–zinc sulfide ore. The spectra clearly show that the copper Ka peaks are very small, and the copper Kb peak is completely covered by the zinc Ka peak, since the zinc concentration is tens of times higher. The concentration ranges for copper in this ore were from 0.05 to 0.8%, and the concentration range for zinc was 2–14%. The statistical parameters of the spectral lines for the sample concentrations of Cu 0.31% and Zn 2.02% are presented in
Table 3.
Sp and Sb are the net peak intensity and the intensity of the background, respectively. This table shows that the statistical accuracy for Cu and Zn is high; however, due to the complexities of online analysis (namely, the constantly changing distance between the surface of the material and the analyzer, the high inhomogeneity of the ore substance, the significant granulometric heterogeneity, etc.), the expected accuracy of determination is usually no better than 2–3% relative. The measurement accuracy (standard deviation) for the samples used in the calibration was about 0.7% relative for Cu (i.e., 0.23% by weight) and 3.6% relative for Zn (0.19% by weight).
A comparison of the results of measuring copper concentrations in the ore online by the XRF method with chemical analysis is shown in
Figure 8.
The standard deviation when compared with the results of chemical analysis for copper was 9.45%. For zinc, the standard deviation (3.6%) is significantly smaller, since the intensity of its peaks is much higher.
4.3. Analysis of Chemical Composition of the Charge Feed
For the production of cast iron from iron ore, an agglomerate is used, the raw materials for which are an iron-ore mixture (IORM) and limestone. The main task of managing the process of the agglomerate charge preparation is to ensure the required chemical composition. The system for the automatic continuous monitoring of the chemical composition of charge components (limestone and IORM) was developed on the basis of the XRF online method [
4].
The iron-ore charge feed moves on a conveyor belt at a speed of 1 m/s (
Figure 9) and has the following granulometric composition: the content of the 0–1 mm size class material in IORM is 68–83%; 1–3 mm—12–23%; the remaining part of IORM consists of larger particles with a 3–8 mm size. The IORM level on the conveyor belt is very uneven. To equalize the IORM level and ensure a constant distance between the analyzer detector and the IORM layer on the conveyor (40 mm), a floating suspension with a protective cover was developed. In the case of heavy loads on the conveyor belt, the spectrometer is automatically lifted above the conveyor by a command from the emergency level sensors. The expressiveness of the method for analyzing IORM directly in the process flow depends on the time of the spectrum measurement and is 6 min.
The spectrum of the X-ray fluorescence of IORM is shown in
Figure 10. The spectrum contains the lines of chromium, iron, calcium, argon (in the composition of air) and manganese, the escape peaks of iron, the total peaks of iron superposition and the peaks of the coherent and incoherent scattering of the characteristic radiation of the tube (molybdenum line).
The range of element concentrations in IORM during the industrial test period was 57.5–60.5; 3.58–4.96; and 0.24–2.19% for Fe, CaO and MnO, respectively. The reproducibility of the results of CaO, MnO and Fe determination was 1.8; 12.0; and 0.2% rel., accordingly. For a sample with a CaO content of 2.56 and MnO content of 1.02%, the detection limits were 0.33 and 0.21%, respectively. The resulting detection limit for Fe was 0.16%. The error of the X-ray fluorescence determination of CaO, MnO and Fe in IORM directly in the process flow was 6.4; 19.0; and 0.9% rel., accordingly.
The following
Table 4 shows the calculation data for the detection limits and calculation accuracy for Ca, Cr and Fe using two different excitation modes.
We have tested the reproducibility of our results by repeating the measurement of the same ore sample every 10 min over 4 h. The statistical data for 22 measurements of the online concentration (%) of Ca, Cr and Fe are shown below in
Table 5.
The results of the XRF analysis on the conveyor were controlled by comparing these with the results of the chemical analysis of 500 simultaneously taken samples [
4]. A mechanized sampler was used for sampling IORM on the conveyor. The volume of the sample taken every 6 min was 0.6–0.8 kg. Experimental studies showed that the discrepancy between the results of iron determination in the samples and in the flow is characterized by a standard deviation of 1.6% rel. The XRF in the flow methodology surpasses the technique of IORM analysis through one-time sample selection in terms of accuracy, expressiveness, productivity, efficiency and the possibility of participation in the Automated Process Control System (APCS).
4.4. Cobalt (Co) Content Determination in Iron Cake
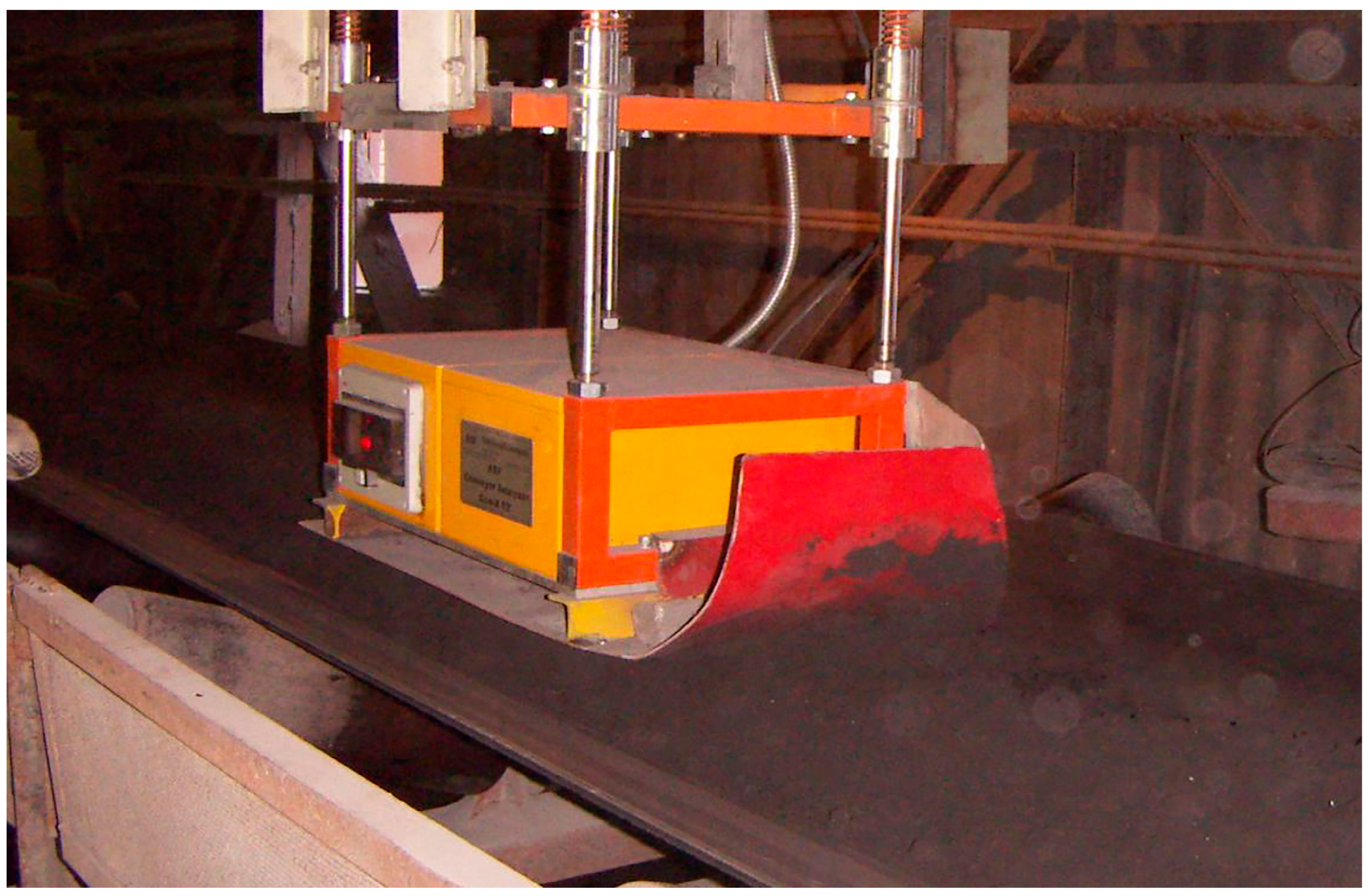
The concentration control of cobalt iron cake is necessary for an enterprise to control the quality in the technological process. Such a control was carried out directly on a belt filter with a cake moving at a speed of 0.3 m/s (
Figure 11). The distance from the lower plane of the analyzer to the surface of the cake was 50 mm, which was provided by the diameter of the wheels supporting the analyzer on the surface of the cake. To prevent the wheels from falling into the cake, a dynamic suspension with a balancer has been developed, which kept the analyzer in a fixed position above the cake.
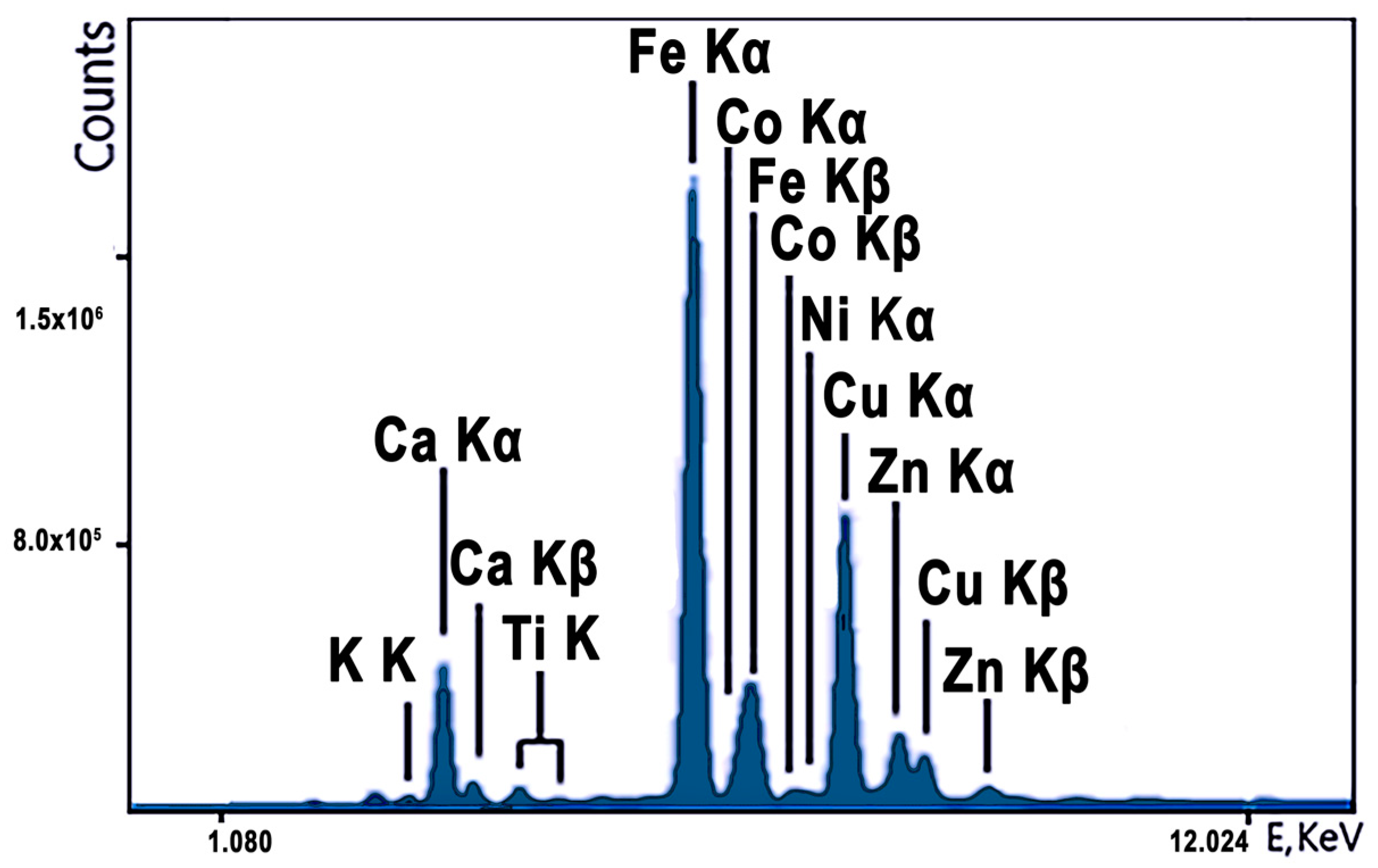
The spectrum of the iron cake for the measurement time t = 300 s is shown in
Figure 12. The spectrum clearly shows the lines of all elements present in the cake. The task of determining the cobalt concentration is complicated by the fact that the intense Fe-Kb line overlaps with the weak Co-Ka line. This creates difficulties in determining the Co concentration, but the analyzer performs the task due to the sufficiently high energy resolution of the detector. To measure the cobalt content in the required concentration range of 0.23–0.36%, the empirical coefficients method was used. This method uses standard samples of different concentrations that are close in composition to the real ore. To determine the cobalt concentration, we used a calculation option in which the cobalt peak area is normalized to the peak area (Fe-Ka), using the fundamental value of the Fe-Ka to Fe-Kb ratio. The best results were obtained when the calibration curve was plotted directly from the ratio of Co/Fe intensities to the incoherent scattering intensity of the radiation.
The results of the repeatability for cobalt contents in online measurements at a constant distance between the analyzer and the sample for three different concentrations (0.27%, 0.23% and 0.24%) have standard deviations of 0.21%, 0.1% and 0.17%.
The statistical parameters (the detection limit, statistical accuracy and sensitivity) for the elements of interest have been calculated using the acquired spectra. All three statistical parameter values for iron (Fe) and copper (Cu) are presented in
Table 6.
In
Figure 13, the chemical and online XRF analyzer measurement results for cobalt content determination are presented. The average result received by the analyzer differs from the average result for the same samples in a chemical laboratory: for Co—0.023% abs. (2.695% rel.); -for Fe—0.642% abs. (6.766% rel.); -for Cu—0.091% abs. (6.058% rel.).
5. Online Analysis of Vanadium Slag
The vanadium content in this slag is to be measured before the recycling of vanadium containing residues. A knowledge of the slag composition will help optimize the metal recovery. The online XRF analyzer is installed on the simplest suspension attached to the structure of the conveyor (
Figure 14). The distance between the slag and the analyzer is 50 mm (variable between 30 and 70 mm). The conveyor speed is 0.4 m/s. The measurement time is 300 s.
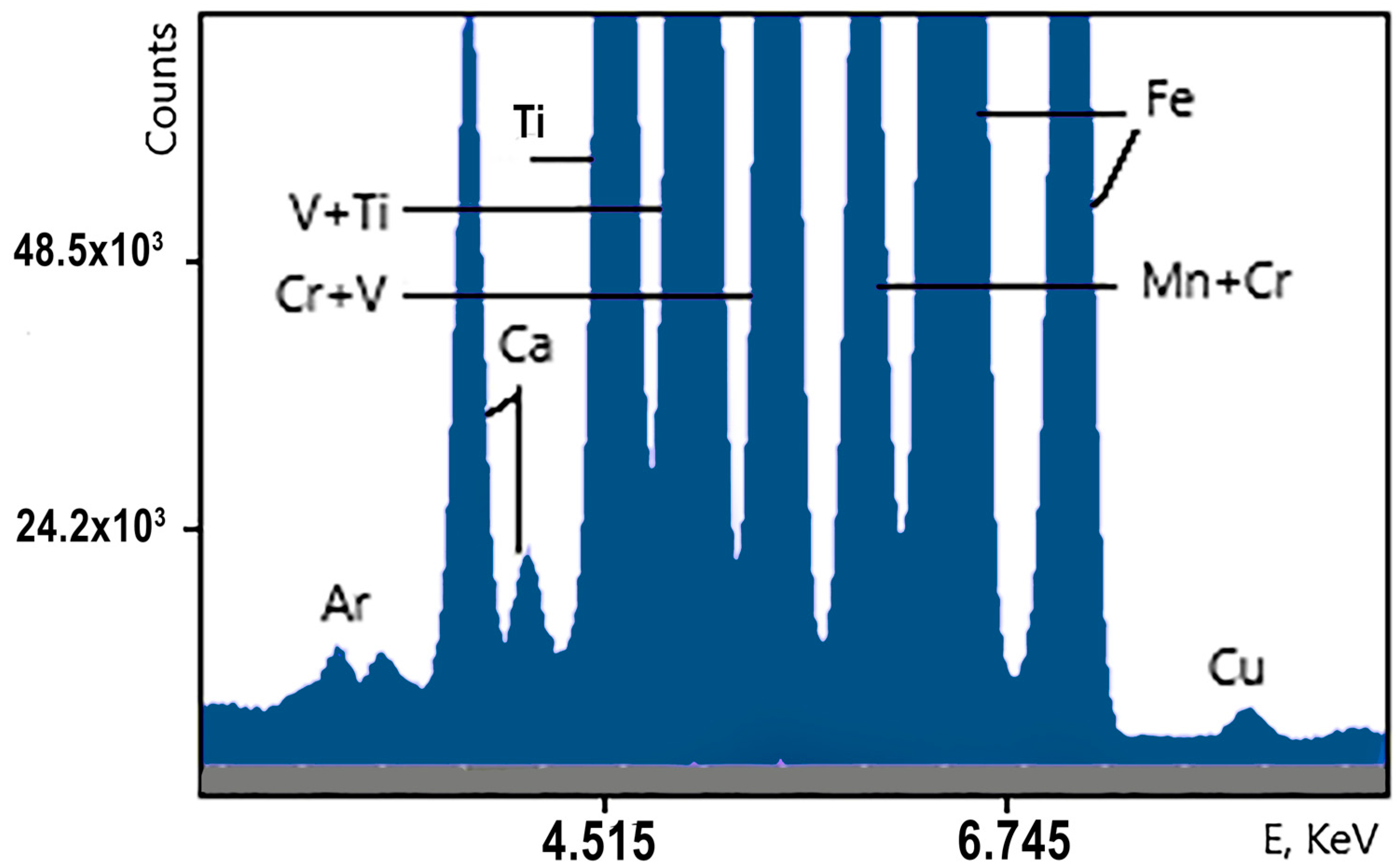
An example of the XRF spectra of the vanadium slag samples is shown in
Figure 15 (overview) and
Figure 16 (zoom into low-intensity lines). Some of the chemical elements giving rise to the spectral lines are indicated. The Ar line originates in the air between the analyzer and the sample.
The statistical analysis of this spectrum is given in
Table 7. These data show that, in five-minute measurements, the online analyzer is able to quantify the amounts of Ca, Ti, V, Cr, Mn and Fe in the vanadium slag samples. We have calculated the following statistical parameters for these elements [
31]: S
p and S
b are the numbers of photons detected in the spectral line and its background; C is the concentration of the corresponding element; DL is the detection limit; ε is the sensitivity of measurements; and σ is the statistical accuracy.
The standard deviation of the data measured over 180 s is 0.09%, with a repeatability of measurements (n = 33), which meets this customer’s requirements. The study indicates that the V content can be determined in the vanadium slag flow directly above the conveyor belt with an accuracy of no worse than 2% relative (respectively, 0.2% V absolute). The amount of some other elements (Ca, Ti, Cr, Mn, Fe) may also be determined online. The relative statistical accuracies of their determination are estimated in
Table 7.
6. Conclusions
The results of this work confirmed the correctness of the strategy we have chosen to develop a specialized industrial online XRF analyzer capable of operating autonomously in a continuous mode in the harsh conditions of the mining industry and measuring the concentrations of elements in mining materials directly on the conveyors of enterprises. The specialized design, which provides the thermal stabilization of the analyzer parameters in a wide range of temperatures and the development and application of a special algorithm for the exclusion of “empty belt time” and other non-standard algorithms in the analyzer’s analytical calculations, significantly increased the accuracy of the measurement results.
The measurement methods developed in this paper using an online XRF analyzer made it possible to directly measure the concentrations of the elements in mining materials of various consistencies: lump, ore, change feed, cake and slag. In all applications, the results obtained for the measurement of concentrations, detection limits of elements and precision measurements met the requirements of enterprises. The calibration of analyzers was carried out in laboratory conditions according to reference samples made in the factory laboratories of these mining enterprises. As the results of this work showed, the discrepancy between the results of measuring the concentrations of elements using XRF online measurements and laboratory measurements of the enterprises themselves does not exceed a few percent in most cases.
We associate the further development of the online XRF methods for measuring and calculating small concentrations of elements against the background of elements with a high concentration, which should make it possible to measure the concentration of rare earth elements in the ore online.