Influence of Phased Cover Placement on the Acid-Generating Main Waste Stockpile at the Red Dog Mine, Alaska, USA
Abstract
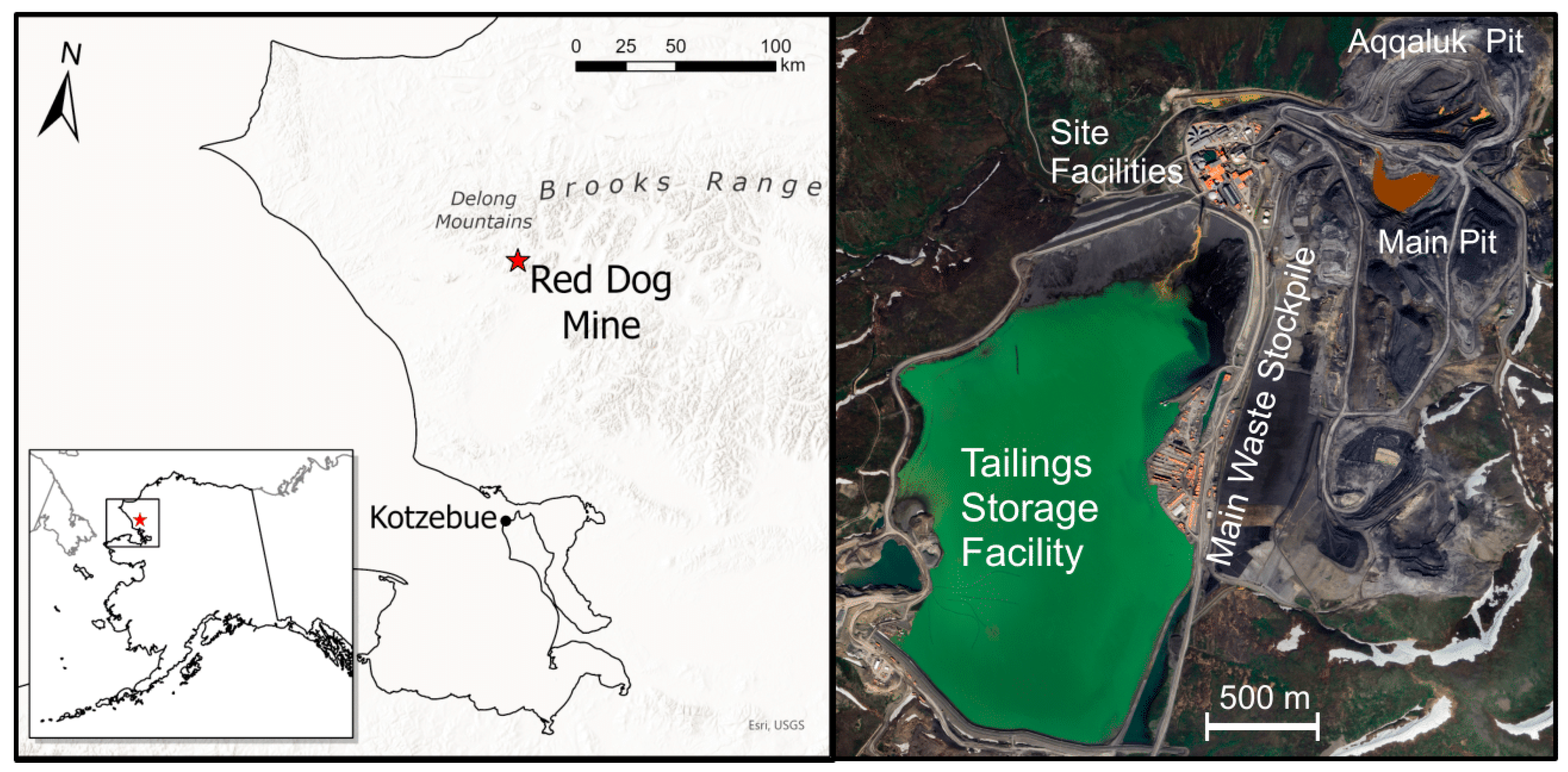
1. Introduction
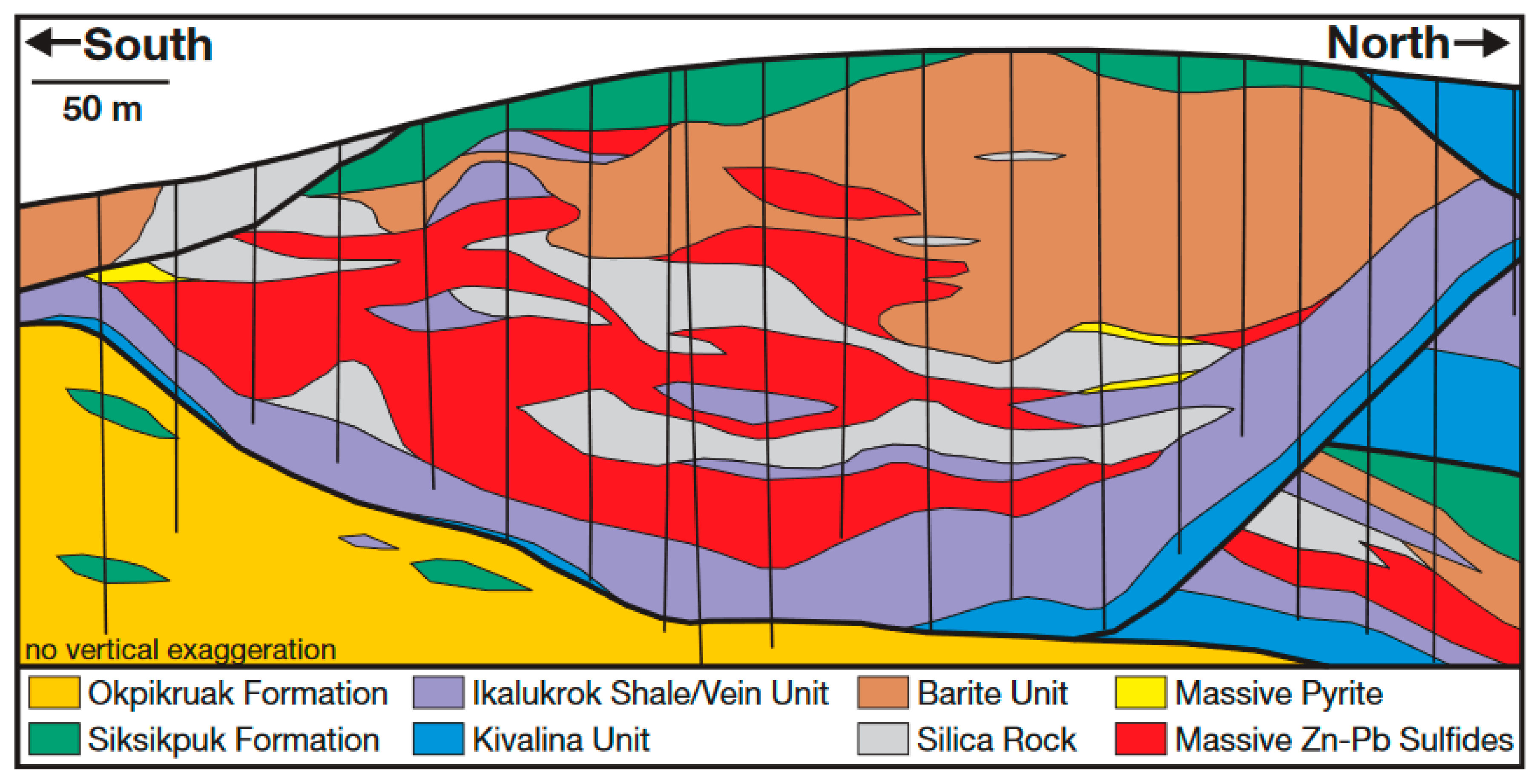
Red Dog Geology and Acidic Drainage
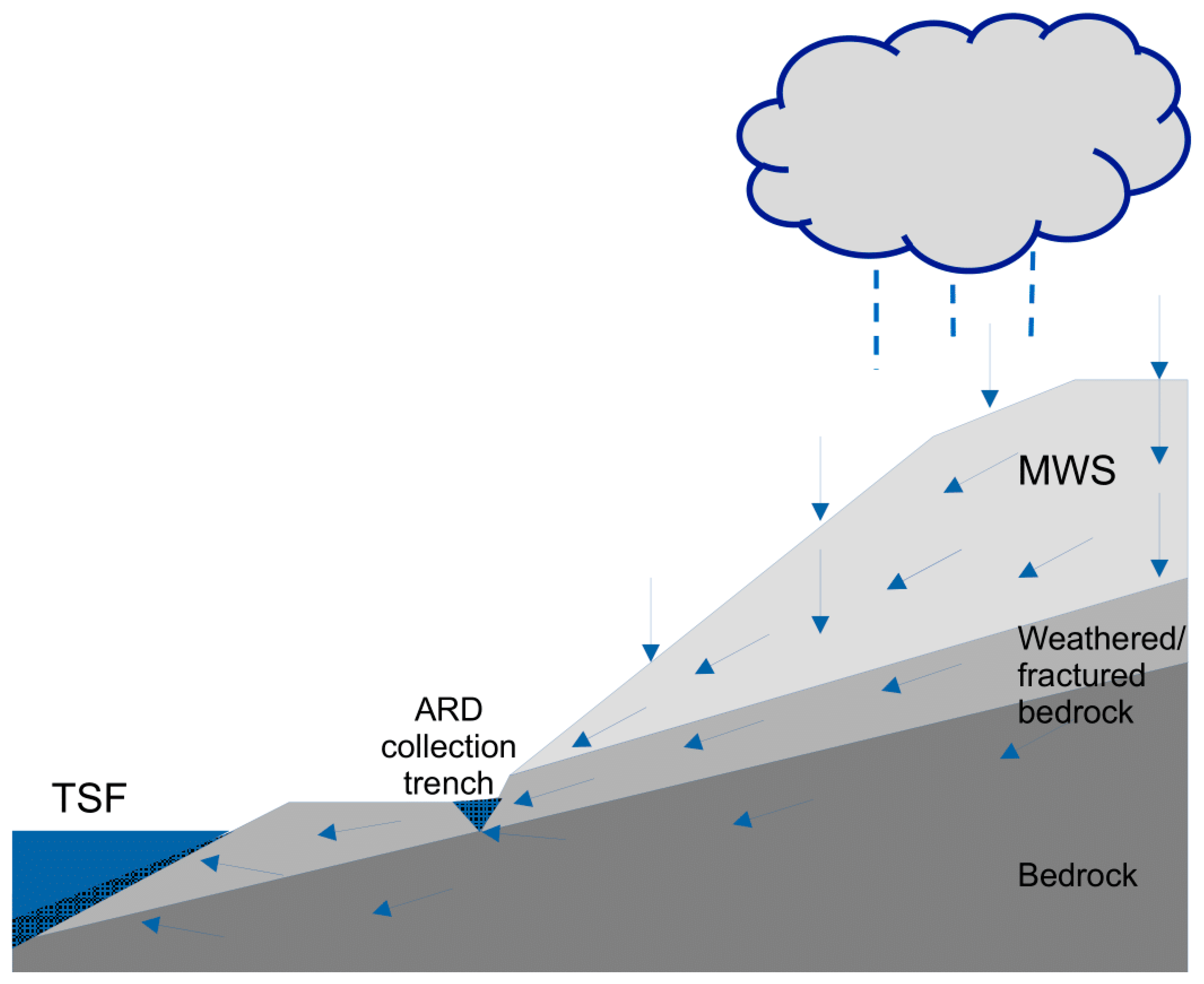
2. Materials and Methods
Statistical Analysis
3. Results
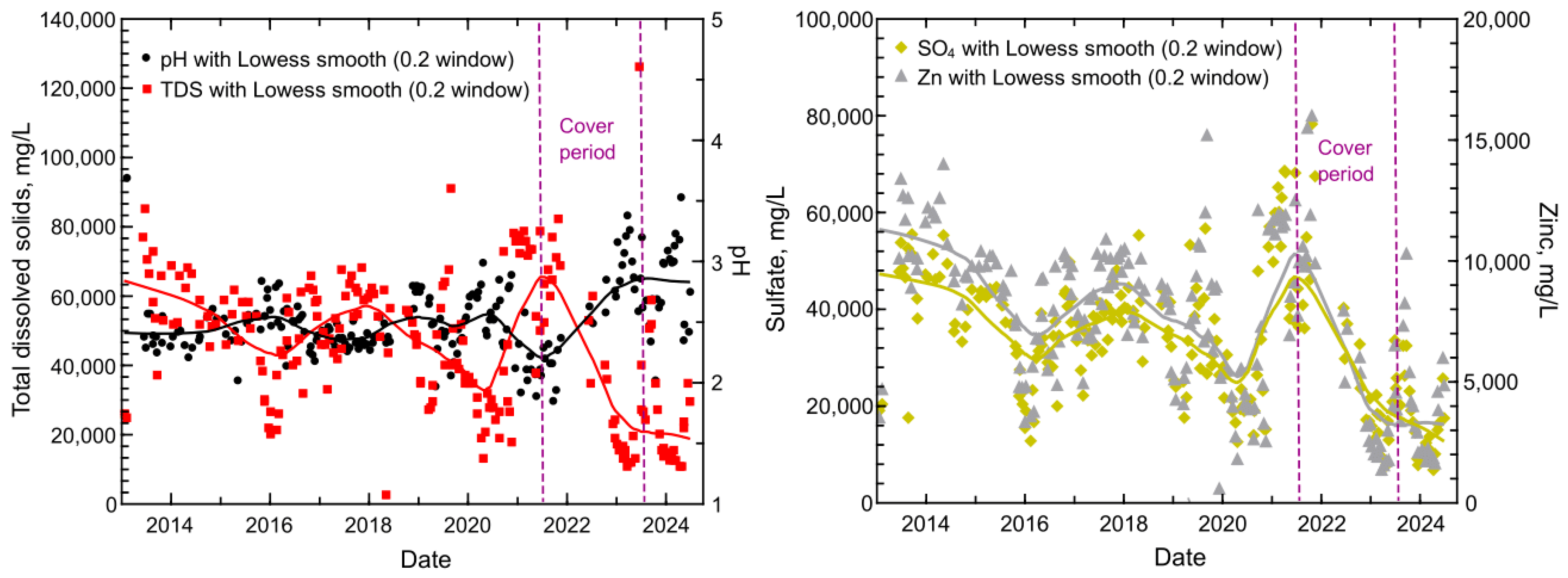
3.1. Summary Statistics and Indications of Decreased Solute Mobilization
3.2. Pre- and Post-Cover ARD Statistical Comparison
3.3. Temporal Trends in Dissolved Solids and pH
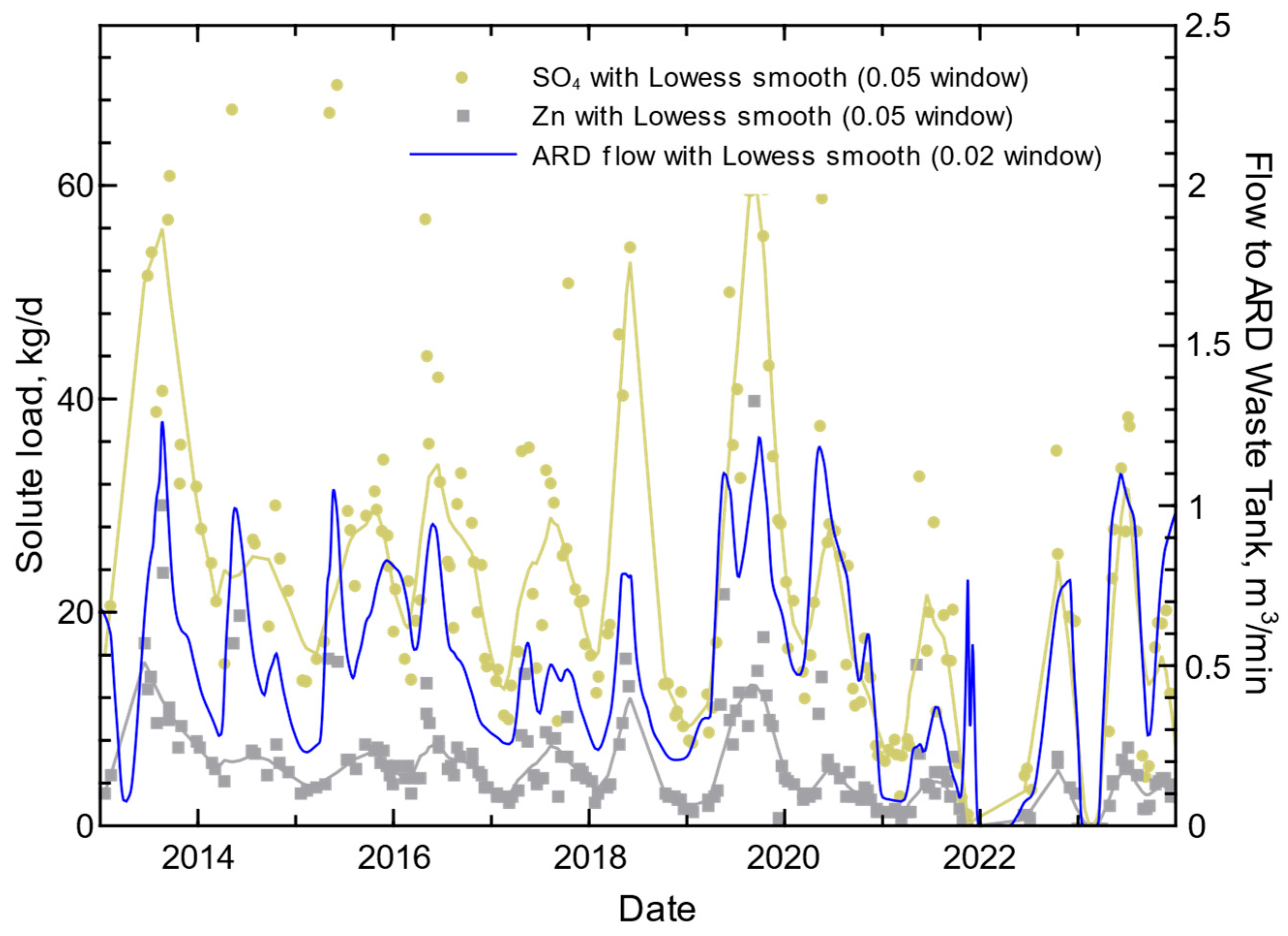
3.4. ARD Tank Chemistry and Inflow
3.5. Estimated Acid Generation and Iron Sulfide Consumption
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clark, J.L.; Christensen, D.; Lum, B. Acid Rock Drainage Source Control Opportunities at the Red Dog Zinc and Lead Mine, USA. In Proceedings of the Mine Closure 2011, Lake Louise, AB, Canada, 18–21 September 2011. [Google Scholar]
- Willowstick Technologies, LLC. Red Dog Mine Waste Rock Dump Drainage Collection Trench; Teck Alaska Incorporated: Anchorage, AK, USA, 2014; p. 70. [Google Scholar]
- Golder Associates, Inc. Summary of 2011 Site Work and Recommendations for 2012 Field Monitoring Program, Main Waste Stockpile Cut-Off Feasibility Study, Red Dog Mine, Alaska; Golder Associates, Inc.: Anchorage, AK, USA, 2012; p. 53. [Google Scholar]
- Golder Associates, Inc. Summary of Main Waste Stockpile 2013 Services, Red Dog Mine, Alaska; 133-95040; Golder Associates, Inc.: Anchorage, AK, USA, 2014; p. 68. [Google Scholar]
- Geosyntec Consultants. Main Waste Stockpile Cover, 2020 Test Plot Monitoring; Geosyntec Consultants: Anchorage, AK, USA, 2020; p. 18. [Google Scholar]
- Moore, D.W.; Young, L.E.; Modene, J.S.; Plahuta, J.T. Geologic Setting and Genesis of the Red Dog Zinc-Lead-Silver Deposit, Western Brooks Range, Alaska. Econ. Geol. 1986, 81, 1696–1727. [Google Scholar] [CrossRef]
- Blevings, S.K.; Kraft, J.L.; Stemler, J.U.; Krolak, T.E. An Overview of the Structure, Stratigraphy, and Zn-Pb-Ag Deposits of the Red Dog District, Northwestern Alaska. In Tectonics, Metallogeny, and Discovery: The North American Cordillera and Similar Accretionary Settings; Colpron, M., Bissig, T., Rusk, B.G., Thompson, J.F.H., Eds.; Society of Economic Geologists: Littleton, CO, USA, 2013; Volume 17, ISBN 978-1-62949-043-4. [Google Scholar]
- Kulas, J.E. Geology of the Red Dog Mine, Western Brooks Range, Alaska; Cominco Alaska Incorporated: Kotzebue, AK, USA, 1992; p. 11. [Google Scholar]
- Gajonera, D. A Study of the Initial Stages of Weathering of Red Dog Mine Waste. Ph.D. Thesis, Queen’s University, Kingston, ON, Canada, 2016. [Google Scholar]
- SRK Consulting. Final Kinetic Test Results, Red Dog Mine; Teck Alaska Incorporated: Kotzebue, AK, USA, 2003. [Google Scholar]
- SRK Consulting. Geochemical Characterization of Aqqaluk and Main Waste Stockpile Waste Rock, Red Dog Mine—Final Report; Teck Alaska Incorporated: Kotzebue, AK, USA, 2020. [Google Scholar]
- Ritchie, A.I.M. Sulfide Oxidation Mechanisms: Controls and Rates of Oxygen Transport. In Environmental Geochemistry of Sulfide Mine Wastes; Jambor, J.L., Blowes, D.W., Eds.; Mineralogical Association of Canada: Ottawa, ON, Canada, 1994; Volume 22, pp. 201–245. [Google Scholar]
- Singer, P.C.; Stumm, W. Acidic Mine Drainage: The Rate-Determining Step. Science 1970, 167, 1121–1123. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.E.; Shumate, K.S.; Singer, P.C.; Stumm, W. Direct Oxidation by Adsorbed Oxygen during Acidic Mine Drainage. Science 1970, 169, 98. [Google Scholar] [CrossRef]
- Holmes, P.R.; Crundwell, F.K. The Kinetics of the Oxidation of Pyrite by Ferric Ions and Dissolved Oxygen: An Electrochemical Study. Geochim. Cosmochim. Acta 2000, 64, 263–274. [Google Scholar] [CrossRef]
- Johnson, A.C.; Romaniello, S.J.; Reinhard, C.T.; Gregory, D.D.; Garcia-Robledo, E.; Revsbech, N.P.; Canfield, D.E.; Lyons, T.W.; Anbar, A.D. Experimental Determination of Pyrite and Molybdenite Oxidation Kinetics at Nanomolar Oxygen Concentrations. Geochim. Cosmochim. Acta 2019, 249, 160–172. [Google Scholar] [CrossRef]
- Moses, C.O.; Kirk Nordstrom, D.; Herman, J.S.; Mills, A.L. Aqueous Pyrite Oxidation by Dissolved Oxygen and by Ferric Iron. Geochim. Cosmochim. Acta 1987, 51, 1561–1571. [Google Scholar] [CrossRef]
- Rimstidt, J.D.; Vaughan, D.J. Pyrite Oxidation: A State-of-the-Art Assessment of the Reaction Mechanism. Geochim. Cosmochim. Acta 2003, 67, 873–880. [Google Scholar] [CrossRef]
- Eckhardt, B.; Brown, T.; Oester, T. Selection of Climate Change-Informed Design Storm Events for a Tailings Storage Facility at Red Dog Operations, Alaska, USA. In Proceedings of the Tailings and Mine Waste 2023, Vancouver, BC, Canada, 5–8 November 2023; pp. 781–792. [Google Scholar]
- Wendler, G.; Gordon, T.; Stuefer, M. On the Precipitation and Precipitation Change in Alaska. Atmosphere 2017, 8, 253. [Google Scholar] [CrossRef]
- Poujol, B.; Prien, A.F.; Molina, M.J.; Muller, C. Dynamic and Thermodynamic Impacts of Climate Change on Organized Convection in Alaska. Clim. Dyn. 2021, 56, 2569–2593. [Google Scholar] [CrossRef]
- O’Kane Consultants, Inc. Teck Resources—Red Dog Mine, Oxide Stockpile Full-Scale Cover System, 2010–11 Annual Performance Monitoring Report; Teck Resources: Anchorage, AK, USA, 2012; p. 33. [Google Scholar]
- Bigham, J.M.; Nordstrom, D.K. Iron and Aluminum Hydroxysulfates from Acid Sulfate Waters. Rev. Mineral. Geochem. 2000, 40, 351–403. [Google Scholar] [CrossRef]
- Bladh, K.W. The Formation of Goethite, Jarosite, and Alunite during the Weathering of Sulfide-Bearing Felsic Rocks. Econ. Geol. 1982, 77, 176–184. [Google Scholar] [CrossRef]
- Fernández-Remolar, D.C.; Carrizo, D.; Harir, M.; Huang, T.; Amils, R.; Schmitt-Kopplin, P.; Sánchez-García, L.; Gomez-Ortiz, D.; Malmberg, P. Unveiling Microbial Preservation under Hyperacidic and Oxidizing Conditions in the Oligocene Rio Tinto Deposit. Sci. Rep. 2021, 11, 21543. [Google Scholar] [CrossRef]
- Schoepfer, V.A.; Burton, E.D. Schwertmannite: A Review of Its Occurrence, Formation, Structure, Stability, and Interactions with Oxyanions. Earth-Sci. Rev. 2021, 221, 103811. [Google Scholar] [CrossRef]
- Guerra, P.; Valenzuela, J.; Rámila, C.; Cattaneo, G. Settling of Iron and Aluminum Particles in Acid Solutions for Acid Drainage Remediation. Water 2022, 14, 2231. [Google Scholar] [CrossRef]
- Hudson-Edwards, K.A. Uptake and Release of Arsenic and Antimony in Alunite-Jarosite and Beudantite Group Minerals. Am. Mineral. 2019, 104, 633–640. [Google Scholar] [CrossRef]
- Lecomte, K.L.; Maza, S.N.; Collo, G.; Sarmiento, A.M.; Depetris, P.J. Geochemical Behavior of an Acid Drainage System: The Case of the Amarillo River, Famatina (La Rioja, Argentina). Environ. Sci. Pollut. Res. 2017, 24, 1630–1647. [Google Scholar] [CrossRef] [PubMed]
- Sánchez España, J. Chapter 7—The Behavior of Iron and Aluminum in Acid Mine Drainage: Speciation, Mineralogy, and Environmental Significance. In Thermodynamics, Solubility and Environmental Issues; Letcher, T.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 137–150. ISBN 978-0-444-52707-3. [Google Scholar]
- Nordstrom, D.K. Hydrogeochemical Processes Governing the Origin, Transport and Fate of Major and Trace Elements from Mine Wastes and Mineralized Rock to Surface Waters. Appl. Geochem. 2011, 26, 1777–1791. [Google Scholar] [CrossRef]
- Sánchez-España, J.; Yusta, I.; Burgos, W.D. Geochemistry of Dissolved Aluminum at Low pH: Hydrobasaluminite Formation and Interaction with Trace Metals, Silica and Microbial Cells under Anoxic Conditions. Chem. Geol. 2016, 441, 124–137. [Google Scholar] [CrossRef]
- Sánchez-España, J.; Yusta, I.; Diez-Ercilla, M. Schwertmannite and Hydrobasaluminite: A Re-Evaluation of Their Solubility and Control on the Iron and Aluminium Concentration in Acidic Pit Lakes. Appl. Geochem. 2011, 26, 1752–1774. [Google Scholar] [CrossRef]
- Lee, S.S.; Schmidt, M.; Sturchio, N.C.; Nagy, K.L.; Fenter, P. Effect of pH on the Formation of Gibbsite-Layer Films at the Muscovite (001)–Water Interface. J. Phys. Chem. C 2019, 123, 6560–6571. [Google Scholar] [CrossRef]
- Consani, S.; Carbone, C.; Dinelli, E.; Balić-Žunić, T.; Cutroneo, L.; Capello, M.; Salviulo, G.; Lucchetti, G. Metal Transport and Remobilisation in a Basin Affected by Acid Mine Drainage: The Role of Ochreous Amorphous Precipitates. Environ. Sci. Pollut. Res. 2017, 24, 15735–15747. [Google Scholar] [CrossRef]
- Menshikova, E.; Osovetsky, B.; Blinov, S.; Belkin, P. Mineral Formation under the Influence of Mine Waters (The Kizel Coal Basin, Russia). Minerals 2020, 10, 364. [Google Scholar] [CrossRef]
- SRK Consulting. Red Dog Geochemical Characterization–Sample Selections for the Main Waste Stockpile; Teck Alaska Incorporated: Kotzebue, AK, USA, 2017. [Google Scholar]
- Jambor, J.L.; Nordstrom, D.K.; Alpers, C.N. Metal-Sulfate Salts from Sulfide Mineral Oxidation. Rev. Mineral. Geochem. 2000, 40, 303–350. [Google Scholar] [CrossRef]
- Elberling, B.; Nicholson, R.V.; Scharer, J.M. A Combined Kinetic and Diffusion Model for Pyrite Oxidation in Tailings: A Change in Controls with Time. J. Hydrol. 1994, 157, 47–60. [Google Scholar] [CrossRef]
- Evangelou, V.P.; Zhang, Y.L. A Review: Pyrite Oxidation Mechanisms and Acid Mine Drainage Prevention. Crit. Rev. Environ. Sci. Technol. 1995, 25, 141–199. [Google Scholar] [CrossRef]
- Fan, R.; Qian, G.; Li, Y.; Short, M.D.; Schumann, R.C.; Chen, M.; Smart, R.S.C.; Gerson, A.R. Evolution of Pyrite Oxidation from a 10-Year Kinetic Leach Study: Implications for Secondary Mineralisation in Acid Mine Drainage Control. Chem. Geol. 2022, 588, 120653. [Google Scholar] [CrossRef]
- Gu, X.; Heaney, P.J.; Reis, F.D.A.A.; Brantley, S.L. Deep Abiotic Weathering of Pyrite. Science 2020, 370, eabb8092. [Google Scholar] [CrossRef]
- Nordstrom, D.K. Aqueous Pyrite Oxidation and the Consequent Formation of Secondary Iron Minerals. In Acid Sulfate Weathering; John Wiley & Sons, Ltd.: New York, NY, USA, 1982; pp. 37–56. ISBN 978-0-89118-905-3. [Google Scholar]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters, 3rd ed.; John Wiley & Sons: New York, NY, USA, 1996; ISBN 978-0-471-67303-3. [Google Scholar]
- Bao, Z.; Bain, J.; Saurette, E.; Zou Finfrock, Y.; Hu, Y.; Ptacek, C.J.; Blowes, D.W. Mineralogy-Dependent Sulfide Oxidation via Polysulfide and Thiosulfate Pathways during Weathering of Mixed-Sulfide Bearing Mine Waste Rock. Geochim. Cosmochim. Acta 2022, 317, 523–537. [Google Scholar] [CrossRef]
- Davis, G.B.; Ritchie, A.I.M. A Model of Oxidation in Pyritic Mine Wastes: Part 1 Equations and Approximate Solution. Appl. Math. Model. 1986, 10, 314–322. [Google Scholar] [CrossRef]
- Dos Santos, E.C.; de Mendonça Silva, J.C.; Duarte, H.A. Pyrite Oxidation Mechanism by Oxygen in Aqueous Medium. J. Phys. Chem. C 2016, 120, 2760–2768. [Google Scholar] [CrossRef]
- SRK Consulting. Waste Rock Dump Evaluation of Geochemical, Temperature and Gas Monitoring Data—1999, Project: 1UC009.04; Teck Alaska Incorporated: Kotzebue, AK, USA, 2000. [Google Scholar]
- Alpers, C.N.; Nordstrom, D.K.; Ball, J.W. Solubility of Jarosite Solid Solutions Precipitated from Acid Mine Waters, Iron Mountain, California. Sci. Geol. Bull. 1989, 42, 281–298. [Google Scholar] [CrossRef]
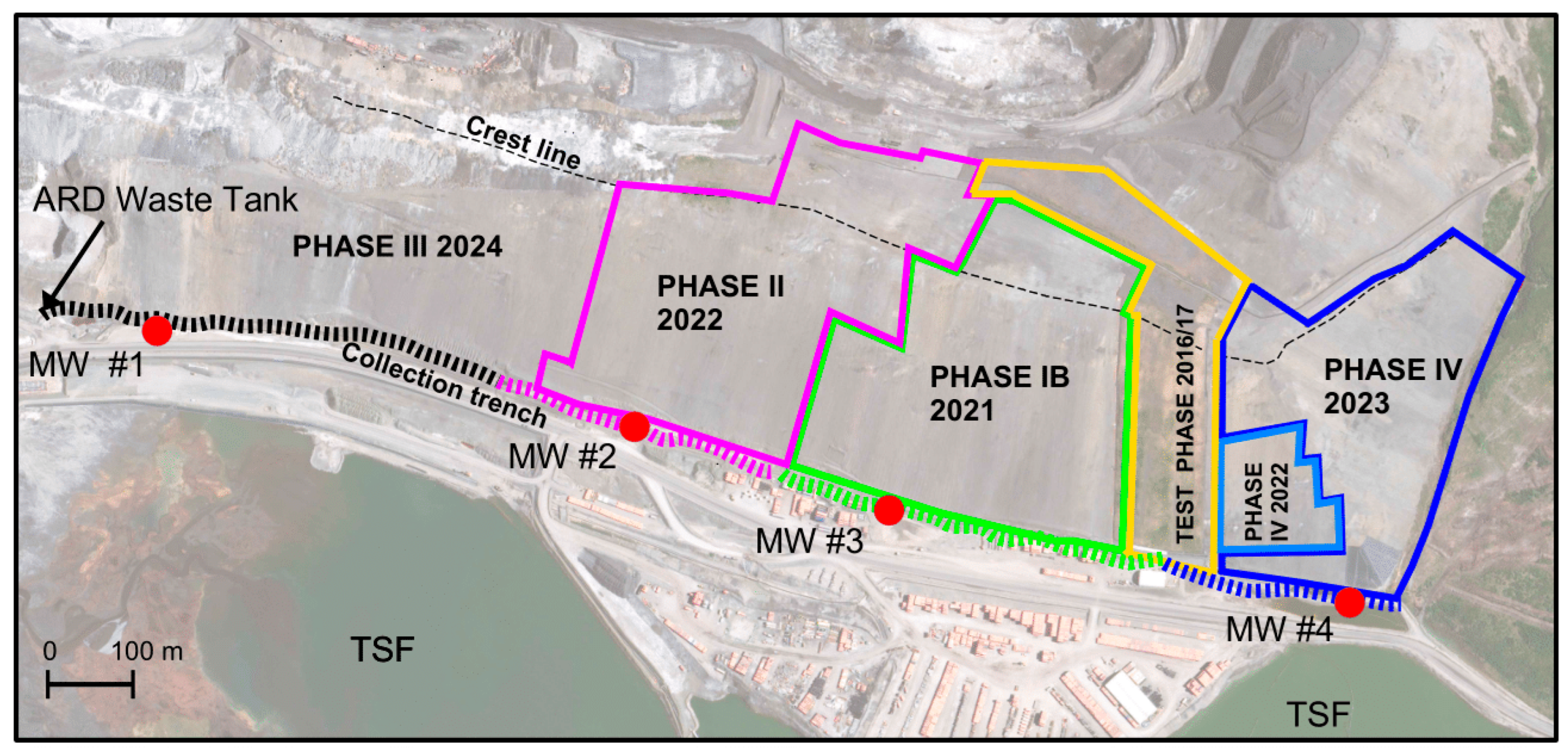




| Cover | Cover Completed | Pre-Cover Data | Area Covered (ha) | Associated Well (Figure 2) |
|---|---|---|---|---|
| Phase IB | 2021 | January 2013 to June 2021 | 12.95 | MW #3 |
| Phase II | 2022 | January 2013 to June 2022 | 8.38 | MW #2 |
| Phase IV | 2023 | January 2013 to June 2023 | 9.87 | MW #4 |
| Phase III 1 | 2024/25 | All data | 14.93 | MW #1 2 |
| Well | pH | Cond. (mS/cm) | ORP (mV) | Temp. (°C) | TDS (g/L) | Fe (g/L) | SO4 (g/L) |
|---|---|---|---|---|---|---|---|
| MW #1 | 2.63 ± 0.39 | 16.00 ± 3.72 | 402 ± 59 | 10.25 ± 2.80 | 54.42 ± 14.65 | 2.29 ± 0.91 | 39.16 ± 12.96 |
| MW #2: | |||||||
| Pre-cover | 2.58 ± 0.30 | 16.31 ± 3.64 | 403 ± 40 | 10.16 ± 3.74 | 49.94 ± 12.12 | 2.48 ± 0.63 | 37.49 ± 9.29 |
| Post-cover | 2.77 ± 0.30 | 13.47 ± 4.17 | 381 ± 30 | 14.21 ± 2.19 | 39.21 ± 12.54 | 1.79 ± 0.87 | 29.71 ± 9.27 |
| MW #3: | |||||||
| Pre-cover | 2.27 ± 0.39 | 29.59 ± 31.84 | 458 ± 51 | 17.55 ± 4.52 | 82.27 ± 25.78 | 2.95 ± 0.81 | 60.69 ± 20.55 |
| Post-cover | 2.29 ± 0.15 | 21.07 ± 5.94 | 470 ± 48 | 17.85 ± 7.40 | 77.13 ± 29.35 | 2.37 ± 0.81 | 51.67 ± 21.21 |
| MW #4: | |||||||
| Pre-cover | 2.86 ± 0.35 | 12.65 ± 6.52 | 365 ± 93 | 14.81 ± 7.23 | 29.10 ± 15.36 | 1.44 ± 0.81 | 21.87 ± 10.79 |
| Post-cover | 3.12 ± 0.61 | 6.27 ± 4.20 | 320 ± 61 | 9.57 ± 4.28 | 16.08 ± 8.40 | 0.68 ± 0.47 | 10.89 ± 5.79 |
| Well | Al (mg/L) | Cd (mg/L) | Cu (mg/L) | Pb (mg/L) | Mn (mg/L) | Se (mg/L) | Zn (g/L) |
|---|---|---|---|---|---|---|---|
| MW #1 | 848 ± 218 | 65 ± 16 | 9.9 ± 3.2 | 0.45 ± 0.80 | 397 ± 105 | 0.03 ± 0.02 | 10.67 ± 2.92 |
| MW #2: | |||||||
| Pre-cover | 786 ± 223 | 32 ± 11 | 2.8 ± 1.6 | 0.15 ± 0.21 | 409 ± 136 | 0.03 ± 0.10 | 7.96 ± 1.78 |
| Post-cover | 523 ± 258 | 18 ± 18 | 1.3 ± 2.2 | 0.10 ± 0.32 | 295 ± 79 | 0.02 ± 0.01 | 5.82 ± 2.79 |
| MW #3: | |||||||
| Pre-cover | 1201 ± 387 | 67 ± 20 | 7.6 ± 2.9 | 0.03 ± 0.10 | 582 ± 167 | 0.08 ± 0.09 | 14.53 ± 4.34 |
| Post-cover | 964 ± 430 | 73 ± 38 | 7.1 ± 3.1 | 0.01 ± 0.01 | 465 ± 231 | 0.09 ± 0.04 | 12.57 ± 4.25 |
| MW #4: | |||||||
| Pre-cover | 366 ± 236 | 28 ± 17 | 5.1 ± 4.1 | 0.21 ± 0.18 | 444 ± 202 | 0.02 ± 0.05 | 4.47 ± 2.44 |
| Post-cover | 184 ± 147 | 10 ± 4.8 | 1.2 ± 1.1 | 0.10 ± 0.02 | 231 ± 126 | 0.01 ± 0.01 | 2.09 ± 1.04 |
| Well | pH | Conductivity | Temperature | TDS | Fe | SO4 | Zn |
|---|---|---|---|---|---|---|---|
| MW #2: | |||||||
| p value | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| HL est. | −0.25 | 2760 | −4.4 | 13,600 | 810 | 8700 | 2530 |
| MW #3: | |||||||
| p value | <0.01 | <0.01 | 0.02 | 0.12 | <0.01 | 0.21 | 0.01 |
| HL est. | −0.14 | 5802 | 2.6 | 10,650 | 770 | 7200 | 3000 |
| MW #4: | |||||||
| p value | 0.06 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| HL est. | −0.30 | 6107 | 5.2 | 11,800 | 710 | 9765 | 2110 |
| Well | pH | Conductivity | Temperature | TDS | Fe | SO4 | Zn |
|---|---|---|---|---|---|---|---|
| MW #2: | |||||||
| p value | <0.01 | 0.08 | <0.01 | <0.01 | <0.01 | 0.02 | 0.02 |
| HL est. | −0.20 | 1617 | −3.2 | 10,650 | 650 | 8200 | 1395 |
| MW #3: | |||||||
| p value | <0.01 | <0.01 | 0.24 | 0.02 | <0.01 | 0.20 | 0.01 |
| HL est. | −0.20 | 6572 | 1.4 | 17,800 | 820 | 9500 | 3300 |
| MW #4: | |||||||
| p value | 0.76 | 0.76 | 0.98 | 0.72 | 0.9 | 0.96 | 0.72 |
| HL est. | 0.08 | 170 | −0.1 | −1085 | 30.5 | 105 | −201 |
| Well (Period) | pH τ | pH p Value | TDS τ | TDS p Value |
|---|---|---|---|---|
| MW #1 | −0.05 | 0.45 | 0.4 | <0.01 |
| MW #2: | ||||
| Pre-cover | 0.11 | 0.10 | −0.11 | 0.09 |
| Post-cover | 0.21 | 0.15 | −0.61 | <0.01 |
| MW #3: | ||||
| Pre-cover | −0.27 | <0.01 | 0.35 | <0.01 |
| Post-cover | −0.11 | 0.47 | −0.07 | 0.66 |
| MW #4: | ||||
| Pre-cover | 0.13 | 0.06 | −0.42 | <0.01 |
| Post-cover | 0.28 | 0.20 | −0.49 | 0.02 |
| Total ARD: 60% Captured by the Trench | Total ARD: 80% Captured by the Trench | |||||||
|---|---|---|---|---|---|---|---|---|
| FeS2 concentration | 3% | 5% | 7% | 9% | 3% | 5% | 7% | 9% |
| FeS2 (Mt) 1 | 1.8 | 3.0 | 4.2 | 5.4 | 1.8 | 3.0 | 4.2 | 5.4 |
| FeS2 (Mmol) 1 | 15,000 | 25,000 | 35,000 | 45,000 | 15,000 | 25,000 | 35,000 | 45,000 |
| Total flow (103 m3) | 5200 | 5200 | 5200 | 5200 | 3900 | 3900 | 3900 | 3900 |
| ARD H+ (Mmol) 2 | 79,200 | 79,200 | 79,200 | 79,200 | 59,400 | 59,400 | 59,400 | 59,400 |
| Low acid production (Equation (1), 2 × H+ for each mol of FeS2) | ||||||||
| Potential H+ (Mmol) | 30,000 | 50,000 | 70,000 | 90,000 | 30,000 | 50,000 | 70,000 | 90,000 |
| Remaining H+ (%) | −164 | −58 | −13 | 12 | −98 | −19 | 15 | 34 |
| Medium acid production (Equation (3), 4 × H+ for each mol of FeS2) | ||||||||
| Potential H+ (Mmol) | 60,000 | 100,000 | 140,000 | 180,000 | 60,000 | 100,000 | 140,000 | 180,000 |
| Remaining H+ (%) | −32 | 21 | 43 | 56 | 1 | 41 | 58 | 67 |
| High acid production (Equation (2), 16 × H+ for each mol of FeS2) | ||||||||
| Potential H+ (Mmol) | 240,000 | 400,000 | 560,000 | 720,000 | 240,000 | 400,000 | 560,000 | 720,000 |
| Remaining H+ (%) | 67 | 80 | 86 | 89 | 75 | 85 | 89 | 92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Langman, J.B.; Balogh, A.; Aston, D.E.; Link, T.E.; Milan, E.; Eckhardt, B. Influence of Phased Cover Placement on the Acid-Generating Main Waste Stockpile at the Red Dog Mine, Alaska, USA. Mining 2025, 5, 74. https://doi.org/10.3390/mining5040074
Langman JB, Balogh A, Aston DE, Link TE, Milan E, Eckhardt B. Influence of Phased Cover Placement on the Acid-Generating Main Waste Stockpile at the Red Dog Mine, Alaska, USA. Mining. 2025; 5(4):74. https://doi.org/10.3390/mining5040074
Chicago/Turabian StyleLangman, Jeff B., Amanda Balogh, D. Eric Aston, Timothy E. Link, Emile Milan, and Bridget Eckhardt. 2025. "Influence of Phased Cover Placement on the Acid-Generating Main Waste Stockpile at the Red Dog Mine, Alaska, USA" Mining 5, no. 4: 74. https://doi.org/10.3390/mining5040074
APA StyleLangman, J. B., Balogh, A., Aston, D. E., Link, T. E., Milan, E., & Eckhardt, B. (2025). Influence of Phased Cover Placement on the Acid-Generating Main Waste Stockpile at the Red Dog Mine, Alaska, USA. Mining, 5(4), 74. https://doi.org/10.3390/mining5040074








