Abstract
The increasing production of iron ore has led to the accumulation of iron ore tailings (IOTs), which pose significant environmental and safety risks when stored in tailings dams. This study investigates the potential of IOTs as a precursor in alkali-activated binder systems, aiming to provide a sustainable solution for mining waste management. Industrial calcium carbide lime and sodium silicate (Na2SiO3) were used as activators in varying concentrations (Na2SiO3: 10%, 15%, 20%, 25%, and 30%; carbide lime: 5%, 7.5%, and 10%), with curing conditions of 23 °C for 7 days. Techniques including unconfined compressive strength tests, X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), and metal leaching tests were employed to evaluate the mechanical performance and environmental safety of the alkali-activated binders. The results reveal that a mixture containing 20% Na2SiO3 and 10% carbide lime achieved the highest compressive strength of 0.33 MPa at 7 days. The binder also showed negligible metal leaching, meeting environmental safety standards. These findings confirm the viability of using IOTs in the development of durable, eco-friendly construction materials, offering a scalable and sustainable solution for the management of mining waste and promoting circular economy principles in the construction sector.
1. Introduction
Mining tailings are a critical issue worldwide, resulting in environmental impacts and challenges for sustainable management. Globally, the mining industry generates millions of tons of tailings, containing heavy metals that can contaminate soil and groundwater, posing severe risks to ecosystem health [1]. In Brazil, according to estimates from the United States Geological Survey [2], approximately 440 million tons of processed iron ore were produced in 2023, making Brazil the second-largest producer in the world. For each ton of ore processed, about 400 kg of tailings are generated [3], potentially containing heavy metals such as lead, chromium, and cadmium.
These mining tailings, with a paste-like consistency, are often deposited in large basins where the slurry slowly consolidates under its own weight [4]. Due to the fine particle size and high-water content, mining tailings exhibit low mechanical stability, making their disposal and storage a critical management concern [5]. Traditional disposal methods lead to various socio-environmental impacts, such as land occupation, environmental pollution, and the risk of tailings dam failures. This risk has been tragically exemplified by the disasters in the cities of Mariana (in 2015) and Brumadinho (in 2019), which resulted in hundreds of deaths and caused irreparable environmental damage [6].
The prediction of geotechnical parameters of mining tailings is complex due to the anthropogenic nature of these materials, which do not behave like conventional soils. The use of conventional cements can aid in stabilization but involves significant environmental and economic issues, such as high CO2 emissions and elevated costs. Additionally, Portland cement has limitations, including low efficiency in stabilizing tailings and a high environmental impact [7].
In this context, alkali activation emerges as a more sustainable and effective technical solution. This technique involves the reaction of a solid aluminosilicate (precursor) in an alkaline environment, induced by an alkaline activator, resulting in an alkali-activated binder or cement with mechanical properties comparable to hydrated Portland cement but with reduced CO2 emissions and lower energy consumption [8]. Examples of precursors include calcined clays, metakaolin, blast furnace slag, fly ash, and agro-industrial residues, such as sugarcane bagasse ash [9]. Activators may include sodium and potassium hydroxides, sodium silicate, and salts of weak acids, such as sodium and potassium carbonates [10].
Carbide lime, a byproduct of the acetylene industry, has emerged as a promising calcium source for alkali-activated systems. This material not only repurposes an industrial waste product but also accelerates alkali activation reactions by supplying the necessary calcium, contributing to circular economy practices and promoting sustainability in tailings management. Carbide lime has shown significant potential in improving the mechanical properties of stabilized materials, offering a more efficient and sustainable approach [11,12,13,14].
Alkali activation offers substantial advantages, including carbon footprint reduction and improved tailings properties, providing a viable alternative for the sustainable management of these materials. Recent studies have explored the use of alkali-activated binders for the stabilization and solidification of mining tailings [12,14,15,16]. However, there is a significant research gap regarding the direct use of iron mining tailings as precursors in alkali-activated systems.
Within the paradigm of the circular economy, the reuse of mining tailings as construction materials represents a key strategy for sustainability. The circular economy aims to maximize the value of materials throughout their lifecycle, minimizing waste and promoting reuse and recycling [17]. Integrating mining tailings into alkali-activated materials not only reduces the need for tailings basin disposal but also creates added value from waste, transforming an environmental liability into a valuable resource for the construction industry [18].
Current research on iron ore tailings (IOTs) primarily focuses on their use as stabilizing agents in cementitious or geopolymeric systems. However, these approaches often face limitations, such as low reactivity, high material variability, and environmental concerns related to cement-based stabilization. Despite advancements in alkali-activated materials, studies exploring IOT as a primary precursor remain scarce, limiting the potential for fully utilizing these industrial byproducts. This study addresses this gap by investigating IOT as the main reactive component in an alkali-activated system, rather than as a mere additive. To this end, unconfined compressive strength tests were conducted to assess mechanical performance, while X-ray diffraction (XRD), Fourier transform infrared (FTIR) spectroscopy, and metal leaching tests were employed to characterize the physicochemical properties of the produced material.
2. Materials and Methods
2.1. Materials
The materials used in this research were iron ore tailings (IOTs), carbide lime (CL), commercial sodium silicate (SS), and distilled water. The IOTs originated from iron ore production in the state of Minas Gerais, Brazil. The location where mining, extraction, and waste treatment processes occur is situated in the Iron Quadrangle region. CL is a byproduct from an industry located in Sapucaia do Sul, Rio Grande do Sul, Brazil. CL is generated during the production of acetylene gas through the reaction of calcium with water. The sodium silicate solution (SS) was produced in São Paulo, Brazil, with a Na2O/SiO2 ratio of 2.54 and a purity grade of 48%.
2.2. Method
2.2.1. Material Characterization
The IOT samples were dried at 105 °C, while the CL samples were dried at 60 °C and then passed through a 200 mm diameter (0.075 mm) sieve. Table 1 presents the geotechnical properties for consistency limits (Brazilian technical standards—NBR 6459 [19]; NBR 7180 [20]), specific gravity (NBR 6508 [21]), and compaction tests with modified energy (NBR 7182 [22]).

Table 1.
IOT and CL geotechnical properties.
In Figure 1, the particle size distribution curves for IOTs and CL are presented, as obtained through laser particle size analysis in accordance with ISO 13320 [23] and specific gravity testing following NBR 16605 [24], respectively. The IOTs were composed of clay, silt, and fine sand, whereas the CL primarily consisted of particles classified as silt and fine sand.
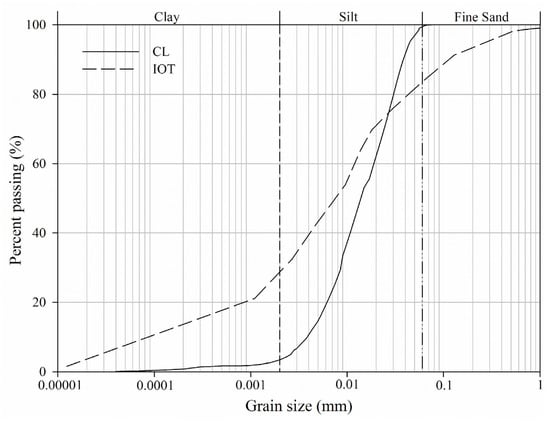
Figure 1.
Particle size distribution of CL and IOT.
The chemical and mineralogical characterization of the materials was performed using X-ray fluorescence (XRF) and X-ray diffraction (XRD). The analyses were conducted only for the IOTs, as the same carbide lime was already characterized by Pelisser et al. [25].
The chemical composition (Table 2) was determined by X-ray fluorescence, using a pressed sample in the STD-1 calibration (Standardless) mode, which involves analysis without standards for chemical elements ranging from fluorine to uranium. The analysis was carried out on a Malvern Panalytical Zetium model equipment (United Kingdom), with loss on ignition (LOI) performed at 1020 °C for 2 h. For the IOTs, it is possible to observe the predominance of iron oxide (Fe2O3)—70.0%; silicon dioxide (SiO2)—14.6%; and aluminum oxide (Al2O3)—7.5%, in addition to other oxides in small proportions. The CL predominantly consisted of calcium oxide (CaO) with 52.5%.

Table 2.
Chemical composition of the materials.
For the mineralogical analysis (Figure 2), a Rigaku diffractometer, model Miniflex® 300, form the United Kingdom, equipped with a fixed Cu anode tube (λ = 1.5406 Å) and operated at 40 kV and 25 mA, was used, with an angular range of 2 to 72° 2θ and a step size of 0.05°/1 s. The mineralogy of the IOTs was analyzed qualitatively and consisted of four crystalline phases: kaolinite (Al2Si2O5(OH)4), quartz (SiO2), hematite (Fe2O3), and goethite (FeO(OH)). The CL was composed of portlandite (Ca(OH)2) and calcite (CaCO3) and predominantly contained calcium oxide (CaO), which made up 52.5% [25].
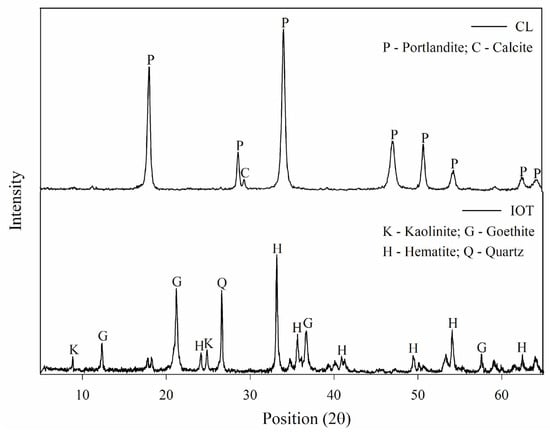
Figure 2.
X-ray diffraction analysis (XRD) of the materials.
The environmental classification of the IOTs and CL was conducted in accordance with the toxicity characteristic leaching procedure (TCLP) [26] and the Brazilian standard NBR 10004 [27], which establishes the criteria for classifying solid waste based on hazardous and non-hazardous characteristics. To determine the potential environmental impact of these materials, leaching and solubilization tests were performed according to NBR 10005 [28] and NBR 10006 [29], respectively.
In the leaching test (NBR 10005 [28]), the materials were first crushed to a particle size of 9.5 mm, ensuring adequate exposure to the leaching solution. The crushed samples were then mixed with an acetic acid solution (pH ~ 2.88) at a solid-to-liquid ratio of 1:20, simulating acidic leaching conditions. The mixture was agitated in a rotary shaker at 30 rpm and maintained at a temperature of 23 ± 2 °C for 18 ± 2 h. After agitation, the leachate was filtered and analyzed for the presence of heavy metals and other contaminants.
The solubilization test (NBR 10006 [29]) followed a similar preparation procedure but used deionized water as the extraction solution, representing a more neutral leaching environment. The same solid-to-liquid ratio of 1:20 was maintained, and the samples were agitated under controlled conditions for the prescribed duration. After extraction, the solubilized solution was filtered and analyzed for soluble elements.
Following the results of these tests, the materials were classified according to NBR 10004 [27], which categorizes waste into Class I (hazardous), Class II-A (non-inert), and Class II-B (inert) based on their potential environmental impact. The classification criteria consider the concentration of toxic elements in the leachate and solubilized extracts, as defined by regulatory thresholds.
The results of the leaching and solubilization tests for the studied materials are presented in Table 3 and Table 4.

Table 3.
Leachate extracts of IOTs and CL.

Table 4.
Solubilized extracts of IOTs and CL.
For the IOTs, the concentrations of metals in the leachate extract were all below the maximum limits allowed by Annex F of NBR 10004 [27]. However, in the solubilized extract, Mn exceeded the limit established by Annex G of NBR 10004 [27]. Therefore, the IOTs were classified as non-hazardous non-inert waste—Class IIA. Regarding the leachate extract of the CL, the concentrations of Hg and Se exceeded the maximum limits specified by Annex F of NBR 10004 [27], resulting in a hazardous waste—Class I [30].
2.2.2. Experimental Program and Statistical Analysis
A complete multi-level factorial design was conducted, based on studies by Falah et al. [31]. The factors investigated were the sodium silicate (SS) solution content and the carbide lime (CL) content, with the levels defined in Table 5, and 3 repetitions for each factorial combination, totaling 45 experiments. The response variable was unconfined compressive strength.

Table 5.
Experimental program.
To define the levels of CL content in the mass ratio (dry weight), an initial lime consumption test (ICL) was performed according to D6276 [32], which indicates the need for 7.5% CL to stabilize the pH at 13.2. Three levels were tested: 5%, 7.5%, and 10%, to evaluate a system with a lower (5%) and higher (10%) calcium content compared to the required stabilization, as defined by Garcia-Lodeiro et al. [33], for the presence of calcium in alkali-activated systems. The proportion of IOTs in the mixture was determined based on the CL content. In other words, a CL content of 5% corresponds to 95% IOTs, 7.5% CL corresponds to 92.5% IOTs, and 10% CL corresponds to 90% IOTs, with the IOT content adjusted accordingly to maintain the total mass balance of the system.
The SS solution contents were defined in five levels: 10%, 15%, 20%, 25%, and 30% [which corresponds to 1.05%, 1.68%, 2.37%, 3.16%, and 4.07% alkali content—Na2O/(IOTs + CL)] and were based on experiments by Falah et al. [31]. The IOT content (%) was adjusted according to the factor levels, and the solid/liquid ratio was set at 0.29 [31].
The results of the experiments were subjected to analysis of variance (ANOVA) with 95% confidence (p-value < 0.05). A generalized model was applied, and the standardized effects of each variable and their interactions on the unconfined compressive strength were compared.
2.2.3. Molding of the Specimens
The sodium silicate solution was prepared using distilled water and maintained at 23 ± 2 °C. The solution was weighed and subsequently added to the dry materials, followed by thorough mixing to ensure homogeneity. The resulting pastes were cast into cylindrical polyvinyl chloride (PVC) molds with a 37 mm diameter and 74 mm height, maintaining a 1:2 ratio, with the bottoms sealed [34]. The pastes were placed in a single layer without compaction, and to minimize the presence of voids and air bubbles, the molds were subjected to successive vibrations on a flat surface until no further bubbles were observed. The samples were then sealed to prevent carbonation and cured at 23 ± 2 °C for 7 days. The selection of a 7-day curing period at 23 °C was based on practical engineering applications where early strength development is a critical factor.
2.2.4. Unconfined Compressive Strength
Following a 7-day curing period, the specimens were extracted from the cylindrical molds, and their dimensions were measured. Subsequently, the specimens underwent unconfined compressive strength testing, conducted using an automatic press (brand: Engetotus, Contagem, Brazil) with a load capacity of 100 tons and a controlled displacement rate of 1.14 mm per minute. The testing procedures adhered to the guidelines specified in ASTM C39/C39M [35].
To stop the ongoing chemical reactions after the 7-day curing period, the samples were immersed in acetone for 48 h. This procedure was implemented to eliminate excess moisture and effectively halt further reactions. Following immersion, the samples were oven-dried at 40 °C for 24 h, in alignment with the methodology established by Padilla-Encinas et al. [36]. This treatment was essential to preserve the mineralogical composition of the samples at the specified curing stage and to enable precise identification of the chemical reactions that had taken place during the initial 7-day curing period.
2.2.5. Mineralogy, Chemical Bond Analysis, and Leaching
The samples that exhibited the best results in unconfined compressive strength for each carbide lime level were subjected to analyses aimed at determining the mineralogy, the formation of chemical bonds, and the capacity to encapsulate metals. Mineralogical characterization and identification of the chemical bonds were performed through X-ray diffraction (XRD) and Fourier transform infrared spectroscopy (FTIR), respectively.
XRD qualitative analyses were conducted using a Rigaku diffractometer, model Miniflex® 300, from the United Kingdom, equipped with a fixed copper anode tube (λ = 1.5484 Å) and operated at 40 kV and 10 mA. The sample was prepared and analyzed by the powder method, being deposited on a glass plate with dimensions of 20 × 20 mm and a depth of 0.5 mm. The angular range utilized was from 5° to 100° 2θ, with a step size of 0.03°, time speed of 0.5 s, and slit widths of SS 1.250° and DS 1.250°. FTIR analyses were carried out using a Perkin Elmer spectrometer, model Spectrum 100S (Shelton, CT, USA), covering a wavenumber range from 600 to 4000 cm−1, and with a resolution of 4 cm−1. The preparation of the samples consisted of the powder method, in which the material passed through a 0.075 mm mesh and was subsequently subjected to the test.
The evaluation of metal leaching from the mixtures was performed according to NBR 10005 [28], and the concentrations were compared to different standards: NBR 10004 [27], CONAMA 460 [37], the Dutch list [38], and the EPA [39]. The metals were quantified using inductively coupled plasma optical emission spectroscopy (ICP-OES), with a Shimadzu spectrometer, model ICPE 9800, from Kyoto, Japan.
3. Results and Discussion
3.1. Mechanical Behavior
The analysis of the results (see Figure 3 and Figure 4) demonstrated that the compressive strength was highly influenced by the interactions among the constituents of the system.
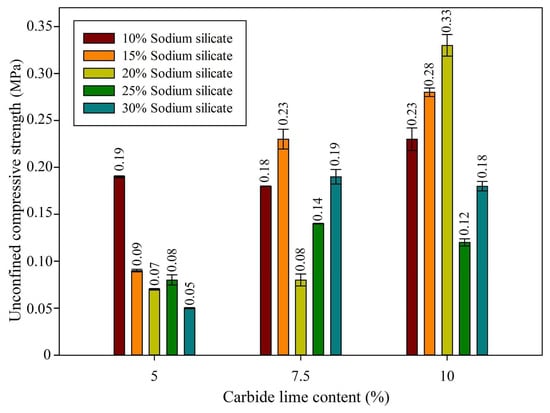
Figure 3.
Unconfined compressive strength of binders produced with 5, 7.5, and 10% lime and IOTs and different concentrations of sodium silicate.
In mixtures with 5% carbide lime, the compressive strength exhibited a decreasing trend with increasing concentrations of sodium silicate, peaking at 0.19 MPa with 10% sodium silicate and progressively declining to 0.05 MPa with 30% sodium silicate. This phenomenon can be explained by the formation of the C-S-H (calcium silicate hydrate) gel, one of the primary products of alkali activation [40]. When the concentration of sodium silicate was increased beyond 10%, the excessive solubilization of silica from the iron ore tailings may have led to the formation of a less dense gel network, resulting in reduced strength. The balance between the calcium provided by the carbide lime and the silica from the tailings and sodium silicate is crucial for proper C-S-H gel formation. An excess of sodium silicate may destabilize this balance, leading to the formation of a less effective gel and, consequently, lower strength [13]. This effect can be observed in the XRD analysis, as shown in Figure 5, where a slight formation can be seen in the C-S-H gel formation region.
When the concentration of carbide lime was increased to 7.5%, the compressive strength increased, with the maximum value of 0.23 MPa observed with 15% sodium silicate. The higher amount of calcium may have promoted the formation of C-S-H gel, leading to a more cohesive and dense matrix, which in turn enhanced the mechanical strength. With 15% sodium silicate, a good balance between the amounts of calcium and silica was achieved, promoting the activation reaction. However, when the sodium silicate concentration was increased to 20%, the strength decreased again, indicating that, while a moderate increase in silica is beneficial for C-S-H gel formation, further increases beyond a certain threshold can undermine the matrix structure, possibly due to excessive silica dissolution, leading to a less cohesive and weaker gel network [12]. With regard to XRD analysis (Figure 5), the formation of the C-S-H gel was more pronounced compared to the 5% CL binder, although it was still not as distinct, indicating a moderate strength gain, as observed.
With 10% carbide lime, the results were more pronounced, with the compressive strength reaching its maximum value of 0.33 MPa with 20% sodium silicate. This increase can be attributed to the higher availability of calcium, which contributed to the formation of C-S-H gel, a material responsible for the cohesion and strength of the system. In addition, the XRD analysis (Figure 5) clearly shows the band in the C-S-H gel region, along with the FTIR analysis (Figure 6), which presents the band in the C-A-S-H gel band, similar to the C-S-H gel, indicating its formation and correlating with the strength values observed. Sodium silicate, in turn, acted as an activator, providing the silica necessary for gel formation. With 20% sodium silicate, the system achieved a good balance, resulting in the highest strength. However, strength began to decrease in mixtures with 25% and 30% sodium silicate, reaching 0.12 MPa and 0.18 MPa, respectively. This decline can be explained by the negative effect of high sodium silicate concentrations. With excessive silica addition, C-S-H gel formation can be compromised due to excessive dissolution of the components of the iron ore tailings, resulting in a more fluid and less cohesive gel network, thereby impairing strength.
The presence of iron ore tailings in the mixtures was crucial for the activation of the system. IOTs contain silica and alumina, which are important precursors for C-S-H gel formation. The interaction between the sodium silicate and the silica present in the IOTs resulted in gel formation, while the carbide lime provided calcium, essential for the activation reaction and the formation of C-S-H gel, as evidenced by the XRD analysis (Figure 5) and the FTIR analysis (Figure 6). Therefore, the iron ore tailings not only acted as a source of silica but also contributed other products, such as iron and aluminum, which may have influenced the properties of the gel. The presence of these elements may have facilitated the formation of additional phases, such as calcium aluminates, which also contributed to the material’s strength [41].
However, the ratios of the components must be carefully controlled to avoid the formation of soluble or weakly cohesive products, which could compromise the mechanical properties of the system. Sodium silicate, when added in excess, can dissolve the silica from the iron ore tailings, damaging the gel structure and resulting in inferior strength. This behavior was observed at higher sodium silicate concentrations, where the strength decreased as the amount of silica available for C-S-H gel formation became excessive, compromising the formation of a cohesive network [42].
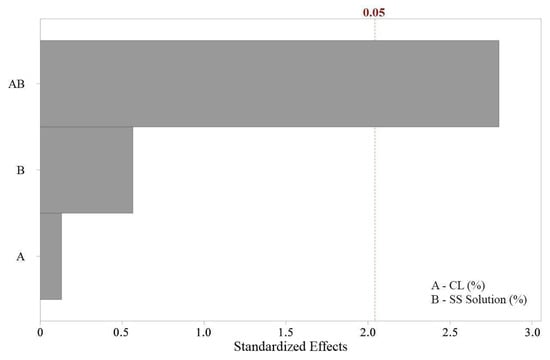
Figure 4.
Standardized effects of factors on unconfined compressive strength.
The results indicate that the best combinations for compressive strength were observed with 10% carbide lime and 20% sodium silicate, achieving a maximum value of 0.33 MPa. This balance between calcium and silica favored the formation of a solid and cohesive network responsible for the high strength observed. The presence of iron ore tailings acted as an essential precursor, but their effectiveness was maximized only when the proportions of sodium silicate and carbide lime were well adjusted. Higher sodium silicate concentrations resulted in a decrease in strength, suggesting that excess silica may compromise the gel structure, weakening cohesion and, consequently, the material’s strength.
When comparing the compressive strength of alkali-activated binders to that of conventional Portland cement-based materials, it is evident that their mechanical performance remains significantly lower. Portland cement-based concretes typically achieve compressive strengths ranging from 20 MPa to 50 MPa, depending on the mix design and curing conditions [8]. In contrast, the maximum compressive strength observed in this study (0.33 MPa) indicates that while these materials exhibited some degree of structural cohesion, their mechanical properties were not yet comparable to traditional binders.
Despite their lower strength values, alkali-activated materials offer significant advantages in terms of sustainability and resource utilization. The incorporation of iron ore tailings, carbide lime, and sodium silicate reduces the need for landfill disposal while also minimizing dependence on clinker production—one of the primary sources of CO2 emissions in conventional cement manufacturing [7,8]. These materials are particularly suitable for applications where high compressive strength is not a critical requirement, such as non-structural building elements (e.g., lightweight panels, partition bricks, and insulation boards) [4,13]. Additionally, the materials may be viable for soil stabilization, embankments, and erosion control structures, where cohesion and environmental benefits outweigh the need for high mechanical performance.
The Pareto chart (Figure 4) was employed to evaluate the relative significance of the individual factors and their interactions on the unconfined compressive strength. The analysis revealed that the first-order interactions (individual effects of carbide lime and sodium silicate concentrations) were not statistically significant, as their standardized effects did not exceed the significance threshold. In contrast, the second-order interactions (combined effects of the carbide lime and sodium silicate concentrations) were found to be statistically significant, with their standardized effects surpassing the threshold. This finding underscores the critical role of the synergistic interaction between the two components in the formation of an appropriate structure, which directly influenced the material’s strength. The results highlight that while neither component alone significantly impacted the compressive strength, their combined interaction was a key determinant of the mechanical performance of the mixtures.
The results indicate that for the carbide lime concentration, increasing the lime proportion did not yield a clear linear effect on the strength. The compressive strength exhibited variability without a continuous upward trend as the CL concentration increased. For instance, at a CL concentration of 5%, combinations with 10% and 15% sodium silicate resulted in strength of 0.19 MPa and 0.09 MPa, respectively, without any evident increasing trend, suggesting that the effect of CL alone was not sufficiently strong to account for variations in strength for the studied curing period.
In a similar manner, while the sodium silicate concentration demonstrated a tendency to increase the strength with higher concentrations, it followed no direct and continuous relationship. At a 5% CL concentration, for example, the strength decreased from 0.19 MPa (with 10% sodium silicate) to 0.05 MPa (with 30% sodium silicate), indicating that the relationship between the sodium silicate and strength was not straightforward and may have been influenced by other factors, such as the interaction between the sodium silicate and the calcium provided by the CL.
The statistical significance of the second-order interactions suggests that the impact of the combination of carbide lime and sodium silicate is more intricate than the sum of the individual effects of each component. The maximum compressive strength was obtained with a combination of 10% carbide lime and 20% sodium silicate, yielding a value of 0.33 MPa. This result implies that the synergy between the two components is essential for the optimal performance of the system. However, when the sodium silicate concentration was increased to 30% in the presence of 10% carbide lime, the compressive strength decreased to 0.18 MPa, suggesting that an excessive concentration of sodium silicate may have led to the destabilization of the system.
This behavior can be elucidated by the mechanism of C-S-H gel formation, which is a product of the hydration reaction between the calcium provided by the carbide lime and the silica present in the iron ore tailings and sodium silicate. For the proper formation of C-S-H gel, it is essential to maintain a balance between the quantities of calcium and silica. The interaction between these two components is crucial for forming a robust network that contributes to the strength. Increasing the sodium silicate concentration up to a certain point enhances the formation of the gel, whereas excessive concentrations of sodium silicate can disrupt this balance, thereby compromising the efficacy of the reaction and resulting in reduced strength.
In addition to the primary components, the iron ore tailings also played a significant role in the behavior of the mixture. The tailings contained amorphous silica and crystalline minerals, such as quartz and magnetite, which react with alkalis to form hydration products that contribute to strength. The presence of the tailings may have altered the interactions between the components, possibly explaining the observed variations in strength for different concentrations of carbide lime and sodium silicate.
The mechanism of C-S-H gel formation is central to understanding the observed results. The C-S-H gel formed as a result of the hydration reaction between the calcium from the carbide lime and the silica from the iron ore tailings and sodium silicate. Sodium silicate functions as an alkaline activator, releasing silicate ions, while carbide lime provides calcium ions for gel formation. The balance between calcium and silica is crucial for the proper formation of C-S-H gel. When sodium silicate is present in excess, undesired secondary products may form, competing with the C-S-H gel for available silica, which results in a less dense network and, consequently, a reduction in strength.
The statistical analysis and the experimental results indicate that the combination of carbide lime and sodium silicate must be carefully balanced to ensure efficient formation of C-S-H gel. The optimization of the concentrations of both components is vital for maximizing the compressive strength of the material, and the alkali-activated system with these proportions can serve as a viable alternative for the utilization of iron ore tailings in construction materials.
The results suggest that, while the individual effects of carbide lime and sodium silicate are not sufficiently strong to account for variations in strength, the interaction between these components is fundamental to achieving optimal strength. The appropriate combination of carbide lime and sodium silicate maximizes the compressive strength, whereas excessive concentrations of either or both components may hinder the formation of C-S-H gel and reduce the effectiveness of the alkali-activated system.
3.2. Mineralogy and Chemical Bond Analysis
The X-ray diffraction (XRD) patterns of the samples with 5%, 7.5%, and 10% carbide lime at 7 days are presented in Figure 5, with their phases identified. As mentioned in Section 2.2.5, the samples that exhibited the best results in unconfined compressive strength for each carbide lime level (see Figure 3) were subjected to mineralogy and chemical bond analysis. The XRD patterns for the natural IOT sample are also plotted for comparison. Goethite, quartz, and hematite peaks are primarily identified between 2θ = 17° and 43° in all the samples, including the natural IOTs [43,44,45].
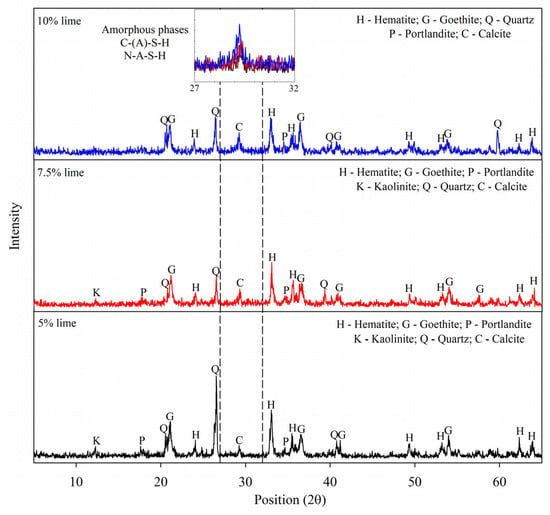
Figure 5.
XRD patterns of binders produced with 5, 7.5, and 10% lime and IOTs.
An indication of C-A-S-H gel formation is identified in the diffraction range 38° to 42°, formed as a result of the reaction with calcite, exhibiting a tendency for increasingly prominent bands over time, and indicating the ongoing hydration process [46]. Additionally, several strong bands, not commonly identified in X-ray diffraction (XRD) patterns, appear between 17° and 28°, primarily in samples hydrated with a significant magnesium content, as observed in this study, where spectra indicative of hydrotalcite were present, related to the reaction products of the mixture [47,48].
Although the high intensity of the quartz bands hinders the identification of amorphous phases in the mineralogical composition of the samples [49], a clear increase in amorphous phases was observed when compared to the diffractogram of the natural IOTs. This suggests that the IOTs acted as a precursor and participated in the formation of cementitious gels [50]. This observation aligns with findings from previous studies, where sodium silicate-activated alkali matrices exhibited a higher prevalence of amorphous phases [47], indicating that crystalline phases are not primarily responsible for strength development, but rather serve to evaluate the gels. Furthermore, this confirms the presence of the N-A-S-H gel, which is detected through the amorphous phases in the angular range of 18–35° [15].
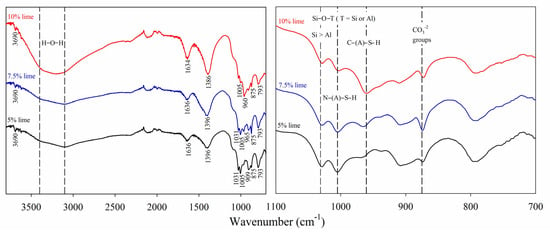
Figure 6.
FTIR spectra of binders produced with 5, 7.5, and 10% lime and IOTs.
Through the qualitative analysis of the FTIR spectra (Figure 6), the primary chemical constituents present in the mixtures were identified. Initially, a shoulder in the band range of approximately 3400 to 3200 cm−1 was observed, which corresponds to the stretching vibration of H-O-H bonds, indicative of water molecules [51]. Additionally, a band at approximately 1636 cm−1 was attributed to the bending vibration of H-O-H bonds, further confirming the presence of water [52].
Among the minerals constituting the IOTs, goethite was prominently identified, associated with the stretching vibration of Fe-O-H bonds at approximately 793 cm−1. Kaolinite was also characterized by Si-O-Al bonds, identified by the band at around 909 cm−1 [53,54]. These observations align with the mineralogical analysis and are further corroborated by the band at approximately 3690 cm−1, which is attributed to the stretching vibrations of O-H bonds [55]. These bonds encompass Al-O-H components in both asymmetric and symmetric axial configurations, as well as in tetrahedral arrangements, characteristic of materials containing IOTs [55,56]. The band at approximately 1031 cm−1 represents a phase enriched in silicon with a low aluminum content, originating from the IOT precursor material [56]. This band corresponds to the asymmetric stretching of Si-O-T (T = Si > Al) bonds, formed by oxides that did not undergo alkali activation, leading to the formation of a silicate network with reduced aluminum substitution when compared to N-A-S-H and C-A-S-H gels [33,55]. Similarly, the band observed in the region of approximately 1005 cm−1, typical of alkali-activated systems, is attributed to the asymmetric stretching of Si-O-T (T = Al or Si), characteristic of N-A-S-H gels [56,57,58,59].
As the lime content in the mixtures increased, a progressive reduction in the intensity of the bands at approximately 909, 1031, and 1005 cm−1 was identified. This phenomenon can be attributed to the increased availability of calcium in the system. Chemically, the addition of lime raises the pH and introduces Ca2+ ions, which compete with Na+ and K+ ions for the stabilization of negative charges in the N-A-S-H gel structure. This alteration in the chemical composition of the alkaline solution favors the formation of C-A-S-H gel over N-A-S-H gel, as calcium has a greater affinity for SiO4 and AlO4 tetrahedra, promoting the incorporation of Ca2+ into the structural networks and leading to the formation of smaller and denser chains [33,60]. In the sample containing 10% lime, a distinct band was observed at approximately 960 cm−1, assuming the possible formation of the C-A-S-H gel [33]. This band, attributed to the asymmetric stretching of the Si-O bonds in the SiO4 tetrahedra, serves as a marker for the reactivity of calcium within the system. However, this spectral region is not exclusive to C-(A)-S-H gel and can also arise from other silicate phases, including various forms of tobermorite and other alkali-activated cement hydration products [33]. Unlike the N-A-S-H gel, which exhibits a more open three-dimensional structure due to the high concentration of Na+ and K+ ions, the C-A-S-H gel forms linear or layered chains, resulting in a more compact and resilient microstructure. The partial substitution of Na+ and K+ ions by Ca2+ in the intertetrahedral bonds reduces ionic mobility within the matrix and enhances chemical stability, which may account for the observed behavior [61,62].
The results clearly demonstrate that the progressive increase in lime content in the mixtures played a crucial role in modifying the chemistry of the cementitious phases, promoting the formation of C-A-S-H gels at the expense of N-A-S-H gels. This transition reflects the enhanced reactivity of calcium, which not only stabilizes silicate and aluminosilicate structures but also leads to the formation of denser and more durable microstructures, as evidenced by the UCS tests. The impact of this evolution is further evident in the FTIR spectra, where the decrease in intensity of bands associated with Si-O-T (T = Al or Si) bonds and N-A-S-H gels, along with the emergence of bands characteristic of C-A-S-H gel, confirms the consolidation of a more compact and chemically stable cementitious matrix.
Interpreting the FTIR spectra of cementitious materials produced from waste presents unique challenges, particularly due to the complex nature of these materials and the overlapping bands in their spectra. Cementitious materials derived from waste often contain a mixture of phases, including unreacted precursors, hydration products, and secondary phases formed during the reaction process. These phases can exhibit similar vibrational modes, leading to overlapping absorption bands in the FTIR spectra. For example, the Si-O stretching and bending vibrations in calcium silicate hydrates (C-S-H) may overlap with those of unreacted silica or other silicate phases. Similarly, carbonate bands from calcite or other carbonation products can interfere with the detection of other phases. To address these challenges, careful deconvolution of the spectra and complementary techniques, such as XRD, are often necessary to accurately identify the phases present. Additionally, the reference spectra of known phases and systematic analysis of band shifts and intensity changes can aid in the interpretation. Despite these difficulties, FTIR remains a valuable tool for characterizing the chemical composition and structural evolution of waste-derived cementitious materials, provided that the limitations and complexities of spectral interpretation are acknowledged.
3.3. Leaching Behavior
The concentration of metals in the leachate extracts (Table 6) was assessed for mixtures containing 5% and 10% CL, and these results were compared to the concentrations observed in the IOTs and CL, as well as the leaching limits established by Annex F of NBR 10004 [27]. Furthermore, the results were analyzed in relation to the water quality standards outlined in CONAMA Resolution No. 460 [37], the Dutch List [38], and the EPA [39].

Table 6.
Chemical composition of the leached extracts.
The chemical composition analysis of the leachate extracts from the binders demonstrated a reduction in the concentrations of metals originally present in the IOTs and CL, suggesting effective encapsulation facilitated by the cementitious gels [63]. None of the elements exceeded the limits established by Annex F of NBR 10004 [27], thus indicating that these materials do not exhibit metal toxicity. However, the mixture containing 5% lime displayed copper (Cu) leaching, likely originating from the CL itself, surpassing the permissible limit set by the Dutch List [38]. In contrast, the mixture containing 10% lime successfully encapsulated the copper, which can be attributed to the formation of a more compact cementitious matrix resulting from an improved ratio between the calcium source and the alkaline activator.
The leaching of barium (Ba) in the sample with 10% lime can be explained by the amphoteric nature of Ba, which exhibits increased mobility under highly alkaline conditions, such as those found in alkali-activated binders, leading to the release of higher concentrations of the metal [63,64]. Additionally, the high availability of calcium and sodium in the mixture promoted the formation of more stable structures involving these elements. However, as Ba does not integrate into the cementitious matrix in the same manner, it becomes more readily leached [56].
4. Concluding Remarks
This study evaluated the use of iron ore tailings in alkali-activated systems, combined with carbide lime industrial waste, aiming to explore a sustainable method for reusing these materials. The findings offer important insights that further advance the understanding of utilizing industrial waste in the development of sustainable construction materials.
- (a)
- The study on binder dosage demonstrated the combined influence of carbide lime and sodium silicate solution (Na2SiO3) on compressive strength. The best mechanical performance (0.33 MPa at 7 days of curing) was achieved with the mixture containing 20% sodium silicate and 10% carbide lime. This result not only confirms the feasibility of using iron ore tailings as a precursor for alkali-activated binders but also highlights the potential for optimizing low-cost, high-performance mixtures. This finding contributes to filling the research gap regarding the use of industrial waste as a raw material for sustainable and efficient binder production.
- (b)
- The chemical and mineralogical characterization of the samples, which indicated the formation of N-A-S-H and C-A-S-H gels, is essential for understanding the reaction mechanisms and structure of alkali-activated binders. These discoveries provide valuable insights into the material’s behavior over time, contributing to bridging the knowledge gap on how the properties of alkali-activated binders can be tailored using secondary materials, such as iron ore tailings.
- (c)
- The fact that the binder samples showed no metal toxicity and partially met the leaching quality limits is a significant result from an environmental perspective. This confirms that the reuse of iron ore tailings, in combination with carbide lime and sodium silicate, offers a safe alternative for utilizing these industrial residues. It also contributes to reducing the environmental impact associated with the improper disposal of tailings and industrial waste. This approach not only promotes circular economy practices but also mitigates the negative effects of industrial residues in landfills and water bodies, underscoring a clear environmental advantage by avoiding the extraction of natural raw materials.
Several challenges must be addressed before these binders can be widely adopted. The variability in the composition of industrial byproducts, such as iron ore tailings and carbide lime, may affect reproducibility and consistency in large-scale production. Additionally, the influence of different curing conditions, including temperature and humidity variations, on mechanical performance remains uncertain. The long-term behavior of these materials under environmental exposure, such as freeze–thaw cycles or chemical interactions, also requires further investigation. Another limitation is the relatively low compressive strength, which may restrict potential applications. Future studies should focus on optimizing material formulations and processing conditions to enhance performance and assess their suitability for broader construction applications.
Despite these challenges, this study demonstrates a promising approach to sustainable binder development. In this context, the reuse of these residues can contribute to the reduction of CO2 emissions associated with traditional cement production, which is highly polluting, while also decreasing the volume of waste sent to landfills. This aligns with sustainability strategies and circular economy principles, promoting responsible resource use and reducing the environmental impact of the construction industry. This study fills a significant gap in the development of sustainable cementitious materials and offers a promising alternative for the safe, economical, and environmentally friendly use of industrial residues. Furthermore, the practical and theoretical findings presented here open new possibilities for innovation in the construction materials sector, especially in the context of alkali-activated binders, aligning with the growing demand for sustainability and waste reuse in the construction industry.
Author Contributions
Conceptualization, F.P.d.V., W.M.K.L., G.J.B., M.K. and M.A.P.; methodology, F.P.d.V., W.M.K.L., G.J.B., M.K., M.A.P. and E.P.K.; validation, G.J.B., D.T.P., P.D.M.P. and E.P.K.; formal analysis, F.P.d.V., W.M.K.L., G.J.B., M.K. and M.A.P.; investigation, F.P.d.V., W.M.K.L. and M.A.P.; resources, P.D.M.P. and E.P.K.; data curation, W.M.K.L., G.J.B. and M.K.; writing—original draft preparation, F.P.d.V., W.M.K.L., M.K. and M.A.P.; writing—review and editing, W.M.K.L., G.J.B., M.K., D.T.P., P.D.M.P. and E.P.K.; visualization, D.T.P., P.D.M.P. and E.P.K.; supervision, P.D.M.P. and E.P.K.; project administration, P.D.M.P. and E.P.K.; funding acquisition, P.D.M.P. and E.P.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by [Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil—CAPES], by [Conselho Nacional de Desenvolvimento Científico Tecnológico—CNPq] grant number [305910/2023-0], by [Financiadora de Estudos e Projetos - FINEP] and the APC was funded by [MDPI].
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Medina, G.S.; Farenzena, H.P.; Bruschi, G.J.; Rodrigues, B.A.; Silva, J.P.S.; Festugato, L.; Consoli, N.C. The Behaviour of Hydraulically Disposed Silty Iron Ore Tailings Under Static and Cyclic Loading. Geotech. Geol. Eng. 2024, 42, 4205–4223. [Google Scholar] [CrossRef]
- USGS. Mineral Commodities Summary 2024. U.S. Geological Survey: Reston, Virginia, USA, 2024. 212p. Available online: http://pubs.er.usgs.gov/publication/mcs2023 (accessed on 20 November 2024).
- Dauce, P.D.; de Castro, G.B.; Lima, M.M.F.; Lima, R.M.F. Characterisation and magnetic concentration of an iron ore tailings. J. Mater. Res. Technol. 2019, 8, 1052–1059. [Google Scholar] [CrossRef]
- Saldanha, R.B.; Caicedo, A.M.L.; de Araújo, M.T.; Scheuermann Filho, H.C.; Moncaleano, C.J.; Silva, J.P.S.; Consoli, N.C. Potential use of iron ore tailings for binder production: A life cycle assessment. Constr. Build. Mater. 2023, 365, 130008. [Google Scholar] [CrossRef]
- Hu, L.; Wu, H.; Zhang, L.; Zhang, P.; Wen, Q. Geotechnical Properties of Mine Tailings. J. Mater. Civ. Eng. 2017, 29, 04016220. [Google Scholar] [CrossRef]
- Goulart Bezerra, C.; Abelha Rocha, C.A.; de Siqueira, I.S.; Toledo Filho, R.D. Feasibility of iron-rich ore tailing as supplementary cementitious material in cement pastes. Constr. Build. Mater. 2021, 303, 124496. [Google Scholar] [CrossRef]
- Bruschi, G.J.; dos Santos, C.P.; Filho, H.C.S.; da Silva Martinatto, C.; Schulz, L.R.; Silva, J.P.S.; Consoli, N.C. Mechanical and Microstructural Response of Iron Ore Tailings under Low and High Pressures Considering a Wide Range of Molding Characteristics. Mining 2023, 3, 712–730. [Google Scholar] [CrossRef]
- Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2018, 114, 40. [Google Scholar] [CrossRef]
- Farenzena, H.P.; Bruschi, G.J.; Medina, G.S.; de Sousa Silva, J.P.; Lotero, A.; Consoli, N.C. Iron ore tailings stabilization with alternative alkali-activated cement for dry stacking: Mechanical and microstructural insights. Can. Geotech. J. 2024, 61, 649–667. [Google Scholar] [CrossRef]
- Lima, F.S.; Gomes, T.C.F.; Moraes, J.C.B. Effect of coffee husk ash as alkaline activator in one-part alkali-activated binder. Constr. Build. Mater. 2023, 362, 129799. [Google Scholar] [CrossRef]
- Bruschi, G.J.; dos Santos, C.P.; Tonini de Araújo, M.; Ferrazzo, S.T.; Marques, S.F.V.; Consoli, N.C. Green Stabilization of Bauxite Tailings: Mechanical Study on Alkali-Activated Materials. J. Mater. Civ. Eng. 2021, 33, 06021007. [Google Scholar] [CrossRef]
- Pereira dos Santos, C.; Bruschi, G.J.; Mattos, J.R.G.; Consoli, N.C. Stabilization of gold mining tailings with alkali-activated carbide lime and sugarcane bagasse ash. Transp. Geotech. 2022, 32, 100704. [Google Scholar] [CrossRef]
- Queiróz, L.C.; Batista, L.L.S.; Souza, L.M.P.; Lima, M.D.; Danieli, S.; Bruschi, G.J.; Bergmann, C.P. Alkali-activated system of carbide lime and rice husk for granular soil stabilisation. Proc. Inst. Civ. Eng. Gr. Improv. 2023, 176, 279–294. [Google Scholar] [CrossRef]
- Shi, Y.; Li, Y.; Wang, H. Eco-friendly solid waste-based cementitious material containing a large amount of phosphogypsum: Performance optimization, micro-mechanisms, and environmental properties. J. Clean. Prod. 2024, 471, 143335. [Google Scholar] [CrossRef]
- Levandoski, W.M.K.; Ferrazzo, S.T.; Bruschi, G.J.; Consoli, N.C.; Korf, E.P. Mechanical and microstructural properties of iron mining tailings stabilized with alkali-activated binder produced from agro-industrial wastes. Sci. Rep. 2023, 13, 15754. [Google Scholar] [CrossRef]
- Jiang, X.; Lang, L.; Liu, S.; Mu, F.; Wang, Y.; Zhang, Z.; Han, L.; Duan, S.; Wang, P.; Li, J. Stabilization of iron ore tailing with low-carbon lime/carbide slag-activated ground granulated blast-furnace slag and coal fly ash. Constr. Build. Mater. 2024, 413, 134946. [Google Scholar] [CrossRef]
- Yousaf, A.; Al Rashid, A.; Koç, M. 3D printing of alkali-activated geopolymers for sustainable and circular economy advancements. Circ. Econ. 2024, 3, 100101. [Google Scholar] [CrossRef]
- Komkova, A.; Habert, G. Optimal supply chain networks for waste materials used in alkali-activated concrete fostering circular economy. Resour. Conserv. Recycl. 2023, 193, 106949. [Google Scholar] [CrossRef]
- NBR 6459; Solo—Determinação do Limite de Liquidez. ABNT—Associação Brasileira de Normas Técnicas: Rio de Janeiro, Brazil, 2016.
- NBR 7180; Solo—Determinação do Limite de Plasticidade. ABNT—Associação Brasileira de Normas Técnicas: Rio de Janeiro, Brazil, 2016.
- NBR 6508; Grãos de Solos Que Passam na Peneira de 4,8 mm—Determinação de Massa Específica. ABNT—Associação Brasileira de Normas Técnicas: Rio de Janeiro, Brazil, 1984.
- NBR 7182; Solo—Ensaio de Compactação. ABNT—Associação Brasileira de Normas Técnicas: Rio de Janeiro, Brazil, 2016.
- ISO 13320; Particle Size Analysis—Laser Diffraction Methods. ISO—International Organization for Standardization: Geneva, Switzerland, 2020.
- NBR 16605; Cimento Portland e Outros Materiais em pó—Determinação da Massa Específica. ABNT—Associação Brasileira de Normas Técnicas: Rio de Janeiro, Brazil, 2017.
- Pelisser, G.; Ferrazzo, S.T.; Mota, J.D.; dos Santos, C.P.; Pelisser, C.; Rosa, F.D.; Korf, E.P. Rice husk ash-carbide lime as an alternative binder for waste foundry sand stabilization. Environ. Sci. Pollut. Res. 2023, 30, 42176–42191. [Google Scholar] [CrossRef]
- USEPA Method 1311; Toxicity Characteristic Leaching Procedure. Hazardous Waste Test Methods/SW-846. USEPA—United States Environmental Protection Agency: Washington, DC, USA, 1992.
- NBR 10004; Resíduos Sólidos—Classificação. ABNT—Associação Brasileira de Normas Técnicas: Rio de Janeiro, Brazil, 2004.
- NBR 10005; Procedimento Para Obtenção de Extrato Lixiviado de Resíduos Sólidos. ABNT—Associação Brasileira de Normas Técnicas: Rio de Janeiro, Brazil, 2004.
- NBR 10006; Procedimento Para Obtenção de Extrato Solubilizado de Resíduos Sólidos. ABNT—Associação Brasileira de Normas Técnicas: Rio de Janeiro, Brazil, 2004.
- Bruschi, G.J.; Santos, C.P.; Levandoski, W.M.K.; Ferrazzo, S.T.; Korf, E.P.; Saldanha, R.B.; Consoli, N.C. Leaching assessment of cemented bauxite tailings through wetting and drying cycles of durability test. Environ. Sci. Pollut. Res. 2022, 29, 59247–59262. [Google Scholar] [CrossRef]
- Falah, M.; Obenaus-Emler, R.; Kinnunen, P.; Illikainen, M. Effects of Activator Properties and Curing Conditions on Alkali-Activation of Low-Alumina Mine Tailings. Waste Biomass Valorizat. 2020, 11, 5027–5039. [Google Scholar] [CrossRef]
- ASTM D6276; Standard Test Method for Using pH to Estimate the Soil-Lime Proportion Requirement for Soil Stabilization. ASTM International: West Conshohocken, PA, USA, 2019.
- Garcia-Lodeiro, I.; Palomo, A.; Fernández-Jiménez, A. An overview of the chemistry of alkali-activated cement-based binders. In Handbook of Alkali-Activated Cements, Mortars and Concretes; Woodhead Publishing: Sawston, UK, 2015; pp. 19–47. ISBN 9781782422884. [Google Scholar]
- Pompermaier, C.L.; Ferrazzo, S.T.; Levandoski, W.M.K.; Bruschi, G.J.; Prietto, P.D.M.; Korf, E.P. Stabilization of waste foundry sand with alkali-activated binder: Mechanical behavior, microstructure and leaching. Constr. Build. Mater. 2024, 444, 137772. [Google Scholar] [CrossRef]
- ASTM C39/C39M; Standard Test Method for Compressive Strength of Cylindrical Concrete Specimens. ASTM International: West Conshohocken, PA, USA, 2020.
- Padilla-Encinas, P.; Palomo, A.; Blanco-Varela, M.T.; Fernández-Jiménez, A. Calcium sulfoaluminate clinker hydration at different alkali concentrations. Cem. Concr. Res. 2020, 138, 106251. [Google Scholar] [CrossRef]
- BRASIL. Conselho Nacional do Meio Ambiente. Resolução nº 460, de 30 de Dezembro de 2013; Diário Oficial da União: Brasília, Brazil, 2013; p. 153. [Google Scholar]
- VROM. Circular on Target Values and Intervention Values for Soil Remediation; Dutch Target and Intervention Values, 2000 (The New Dutch List); VROM—Ministry of Housing, Spatial Planning and the Environment: The Hague, The Netherlands, 2000. [Google Scholar]
- USEPA. Ground Water and Drinking Water: National Primary Drinking Water Regulations. USEPA—United States Environmental Protection Agency. 2024. Available online: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations#Organic (accessed on 20 November 2024).
- Lotero, A.; Moncaleano, C.J.; Consoli, N.C. Alkali-activated red ceramic wastes-carbide lime blend: An alternative alkaline cement manufactured at room temperature. J. Build. Eng. 2023, 65, 105663. [Google Scholar] [CrossRef]
- Consoli, N.C.; Pedroso de Oliveira, J.; Lotero, A.; Scheuermann Filho, H.C.; Nuñéz, V. One-part alkali-activated GGBFS as a cement for enhancing compacted filtered iron ore tailings disposal by stacking. Transp. Geotech. 2024, 48, 101306. [Google Scholar] [CrossRef]
- Yang, M.; Zheng, Y.; Li, X.; Yang, X.; Rao, F.; Zhong, L. Durability of alkali-activated materials with different C–S–H and N-A-S-H gels in acid and alkaline environment. J. Mater. Res. Technol. 2022, 16, 619–630. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Y.; Zhu, H.; Zhang, Y.; Provis, J.L.; Wang, H. Effect of drying procedures on pore structure and phase evolution of alkali-activated cements. Cem. Concr. Compos. 2019, 96, 194–203. [Google Scholar] [CrossRef]
- Elói, F.P.F. Ativação Alcalina do Rejeito de Barragem de Minério de Ferro Com Adição de Sílica Ativa. Ph.D. Thesis, Programa de Pós-Graduação em Engenharia Civil, Escola de Minas, Universidade Federal de Ouro Preto, Ouro Preto, Brazil, 2020. [Google Scholar]
- Geraldo, R.H. Aglomerante Álcali-Ativado de Parte Única: Obtenção, Composição, Propriedades e Durabilidade. Ph.D. Thesis, Arquitetura e Urbanismo, Faculdade de Engenharia Civil, Universidade Estadual de Campinas, Campinas, Brazil, 2020. [Google Scholar]
- Li, W.; Yi, Y. Use of carbide slag from acetylene industry for activation of ground granulated blast-furnace slag. Constr. Build. Mater. 2020, 238, 117713. [Google Scholar] [CrossRef]
- Oh, J.E.; Monteiro, P.J.M.; Jun, S.S.; Choi, S.; Clark, S.M. The evolution of strength and crystalline phases for alkali-activated ground blast furnace slag and fly ash-based geopolymers. Cem. Concr. Res. 2010, 40, 189–196. [Google Scholar] [CrossRef]
- Ben Haha, M.; Le Saout, G.; Winnefeld, F.; Lothenbach, B. Influence of activator type on hydration kinetics, hydrate assemblage and microstructural development of alkali activated blast-furnace slags. Cem. Concr. Res. 2011, 41, 301–310. [Google Scholar] [CrossRef]
- Moukannaa, S.; Bagheri, A.; Benzaazoua, M.; Sanjayan, J.G.; Pownceby, M.I.; Hakkou, R. Elaboration of alkali activated materials using a non-calcined red clay from phosphate mines amended with fly ash or slag: A structural study. Mater. Chem. Phys. 2020, 256, 123678. [Google Scholar] [CrossRef]
- Provis, J.L.; Deventer, J.S.J. Geopolymers: Structure, Processing, Properties and Industrial Applications; Woodhead Publishing Limited: Cambridge, UK, 2009; pp. 1–454. [Google Scholar]
- Robayo, R.A.; Mulford, A.; Munera, J.; Mejía de Gutiérrez, R. Alternative cements based on alkali-activated red clay brick waste. Constr. Build. Mater. 2016, 128, 163–169. [Google Scholar] [CrossRef]
- Figueiredo, R.A.M.; Silveira, A.B.M.; Melo, E.L.P.; Costa, G.Q.G.; Brandão, P.R.G.; Aguilar, M.T.P.; Henriques, A.B.; Mazzinghy, D.B. Mechanical and chemical analysis of one-part geopolymers synthesised with iron ore tailings from Brazil. J. Mater. Res. Technol. 2021, 14, 2650–2657. [Google Scholar] [CrossRef]
- Chukanov, N.V. IR Spectra of Minerals and Reference Samples Data. In Infrared Spectra of Mineral Species; Springer: Dordrecht, The Netherlands, 2014; pp. 21–1701. ISBN 9789400771284. [Google Scholar]
- Queiróz, L.C.; Miguel, G.D.; Bruschi, G.J.; de Lima, M.D.S. Macro–Micro Characterization of Green Stabilized Alkali-Activated Sand. Geotech. Geol. Eng. 2022, 40, 3763–3778. [Google Scholar] [CrossRef]
- Kaze, R.C.; Beleuk à Moungam, L.M.; Cannio, M.; Rosa, R.; Kamseu, E.; Melo, U.C.; Leonelli, C. Microstructure and engineering properties of Fe2O3(FeO)-Al2O3-SiO2 based geopolymer composites. J. Clean. Prod. 2018, 199, 849–859. [Google Scholar] [CrossRef]
- Levandoski, W.M.K.; Ferrazzo, S.T.; Piovesan, M.A.; Bruschi, G.J.; Consoli, N.C.; Korf, E.P. Long-term performance: Strength and metal encapsulation in alkali-activated iron ore tailings. Environ. Sci. Pollut. Res. 2024, 31, 47071–47083. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Fernández-Jiménez, A.; Blanco, M.T.; Palomo, A. FTIR study of the sol-gel synthesis of cementitious gels: C-S-H and N-A-S-H. J. Sol-Gel. Sci. Technol. 2008, 45, 63–72. [Google Scholar] [CrossRef]
- Sun, K.; Ali, H.A.; Xuan, D.; Poon, C.S. Sulfuric acid resistance behaviour of alkali-activated slag and waste glass powder blended precursors. Cem. Concr. Compos. 2024, 145, 105319. [Google Scholar] [CrossRef]
- Srinivasamurthy, L.; Chevali, V.S.; Zhang, Z.; Wang, H. Effect of fly ash to slag ratio and Na2O content on leaching behaviour of fly Ash/Slag based alkali activated materials. Constr. Build. Mater. 2023, 383, 131234. [Google Scholar] [CrossRef]
- Provis, J.L.; Bernal, S.A. Geopolymers and related alkali-activated materials. Annu. Rev. Mater. Res. 2014, 44, 299–327. [Google Scholar] [CrossRef]
- Puertas, F.; Palacios, M.; Manzano, H.; Dolado, J.S.; Rico, A.; Rodríguez, J. A model for the C-A-S-H gel formed in alkali-activated slag cements. J. Eur. Ceram. Soc. 2011, 31, 2043–2056. [Google Scholar] [CrossRef]
- Li, C.; Sun, H.; Yi, Z.; Li, L. Innovative methodology for comprehensive utilization of iron ore tailings. Part 2: The residues after iron recovery from iron ore tailings to prepare cementitious material. J. Hazard. Mater. 2010, 174, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Ferrazzo, S.T.; de Araújo, M.T.; Bruschi, G.J.; Chaves, H.M.; Korf, E.P.; Consoli, N.C. Mechanical and environmental behavior of waste foundry sand stabilized with alkali-activated sugar cane bagasse ash-eggshell lime binder. Constr. Build. Mater. 2023, 383, 131313. [Google Scholar] [CrossRef]
- Komonweeraket, K.; Cetin, B.; Benson, C.H.; Aydilek, A.H.; Edil, T.B. Leaching characteristics of toxic constituents from coal fly ash mixed soils under the influence of pH. Waste Manag. 2015, 38, 174–184. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).