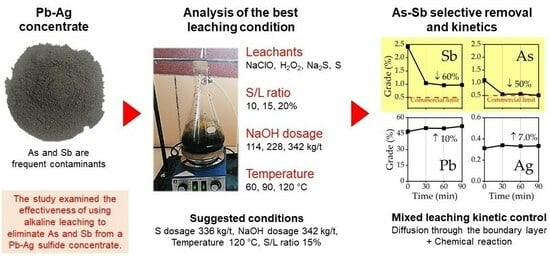
Selective Removal of Arsenic and Antimony from Pb-Ag Sulfide Concentrates by Alkaline Leaching: Thermodynamic and Kinetic Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Sulfurous Pb-Ag Concentrate
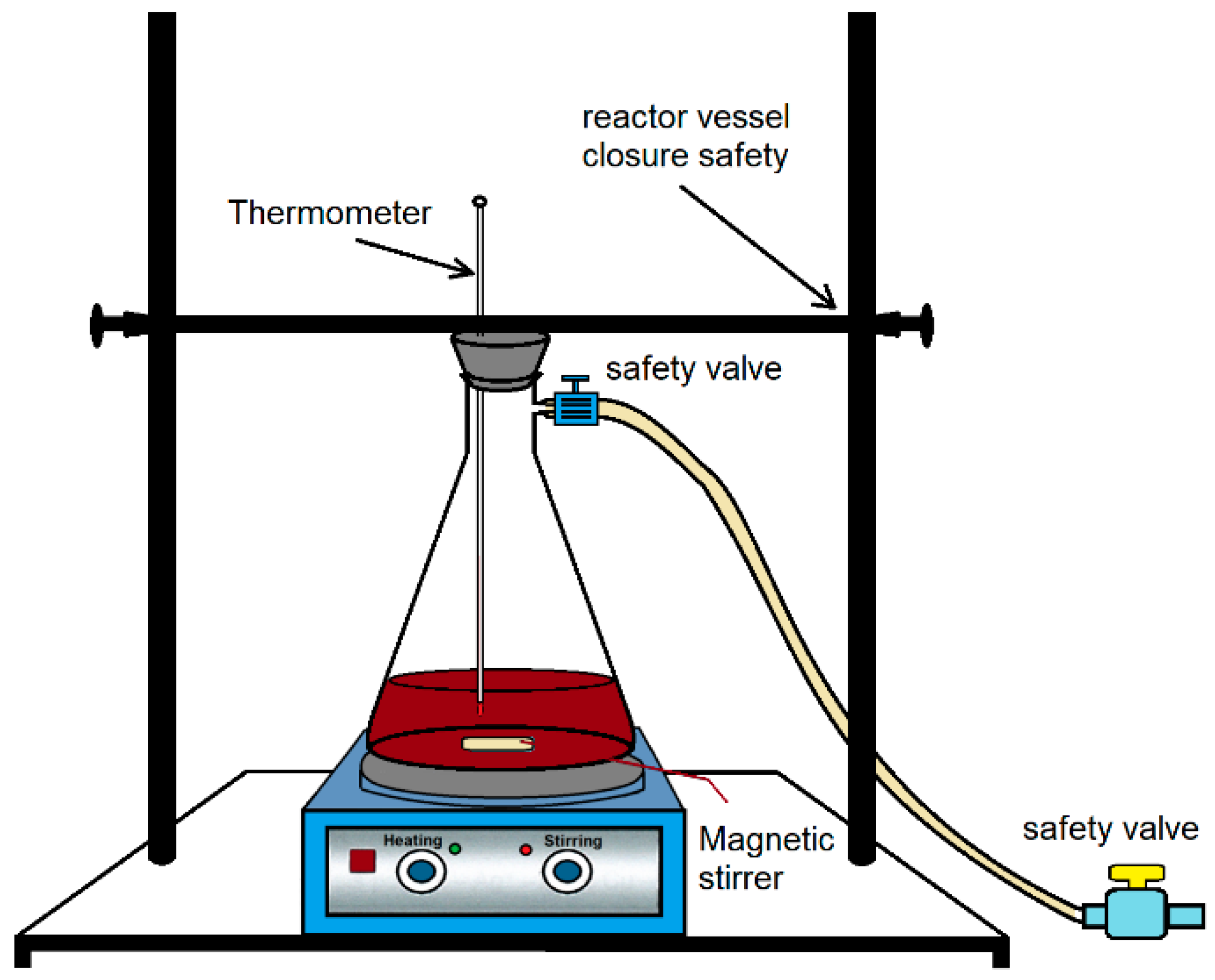
2.2. Hydrometallurgical Treatment of Concentrate
2.2.1. Exploratory Tests of Leaching Agents
2.2.2. Study of Leaching Operating Conditions
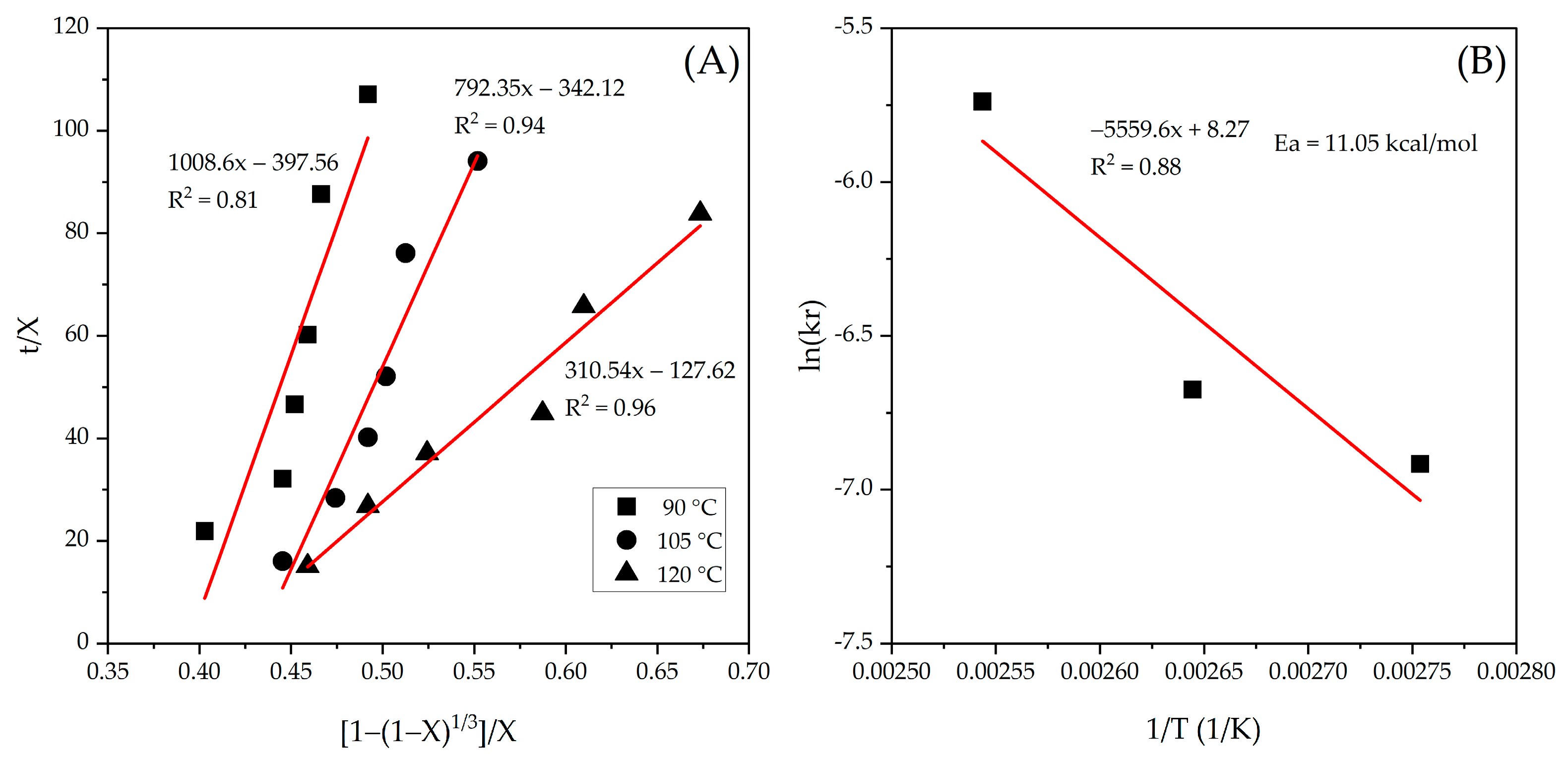
2.2.3. Kinetic Leaching Study
3. Results and Discussion
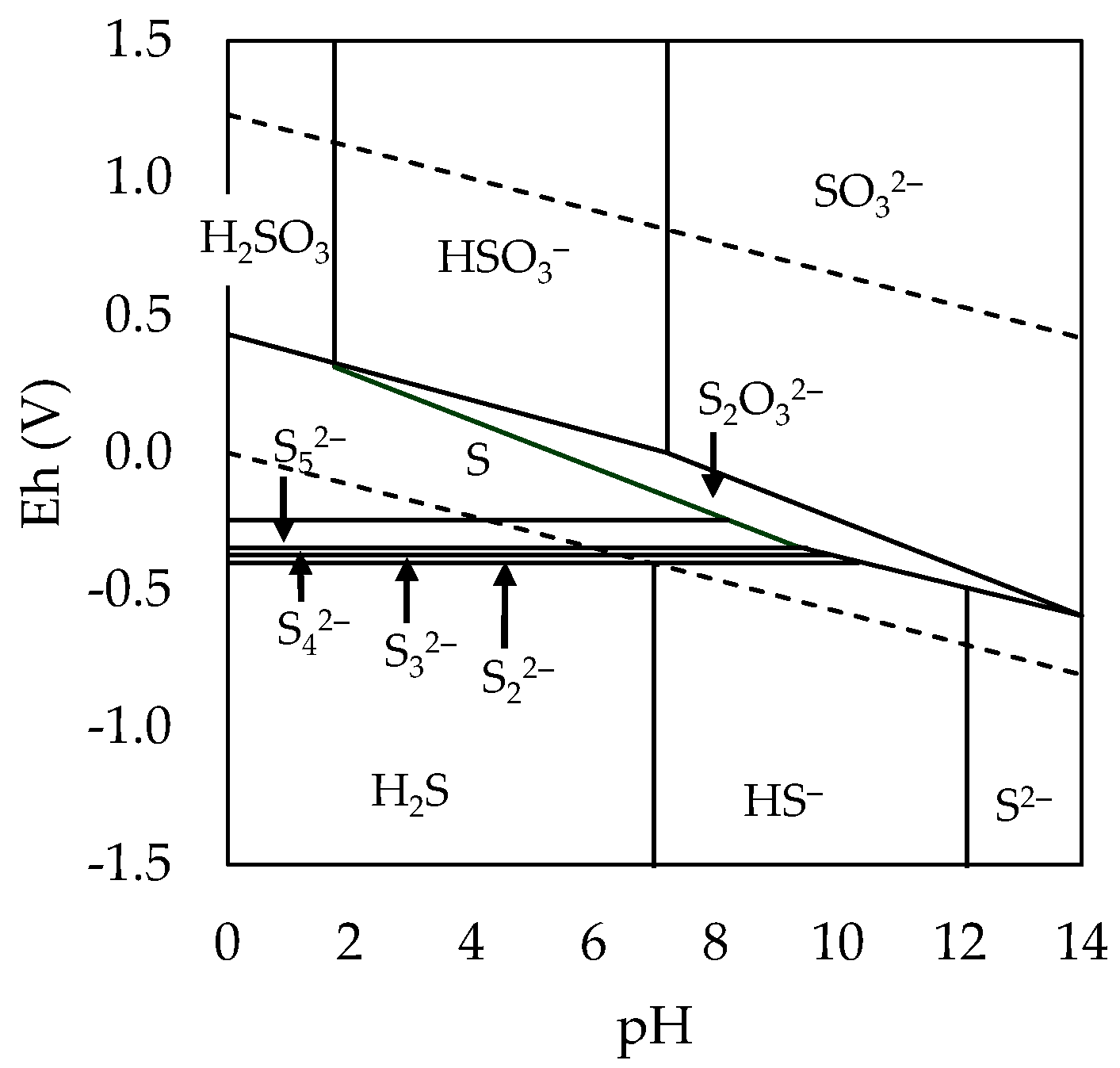
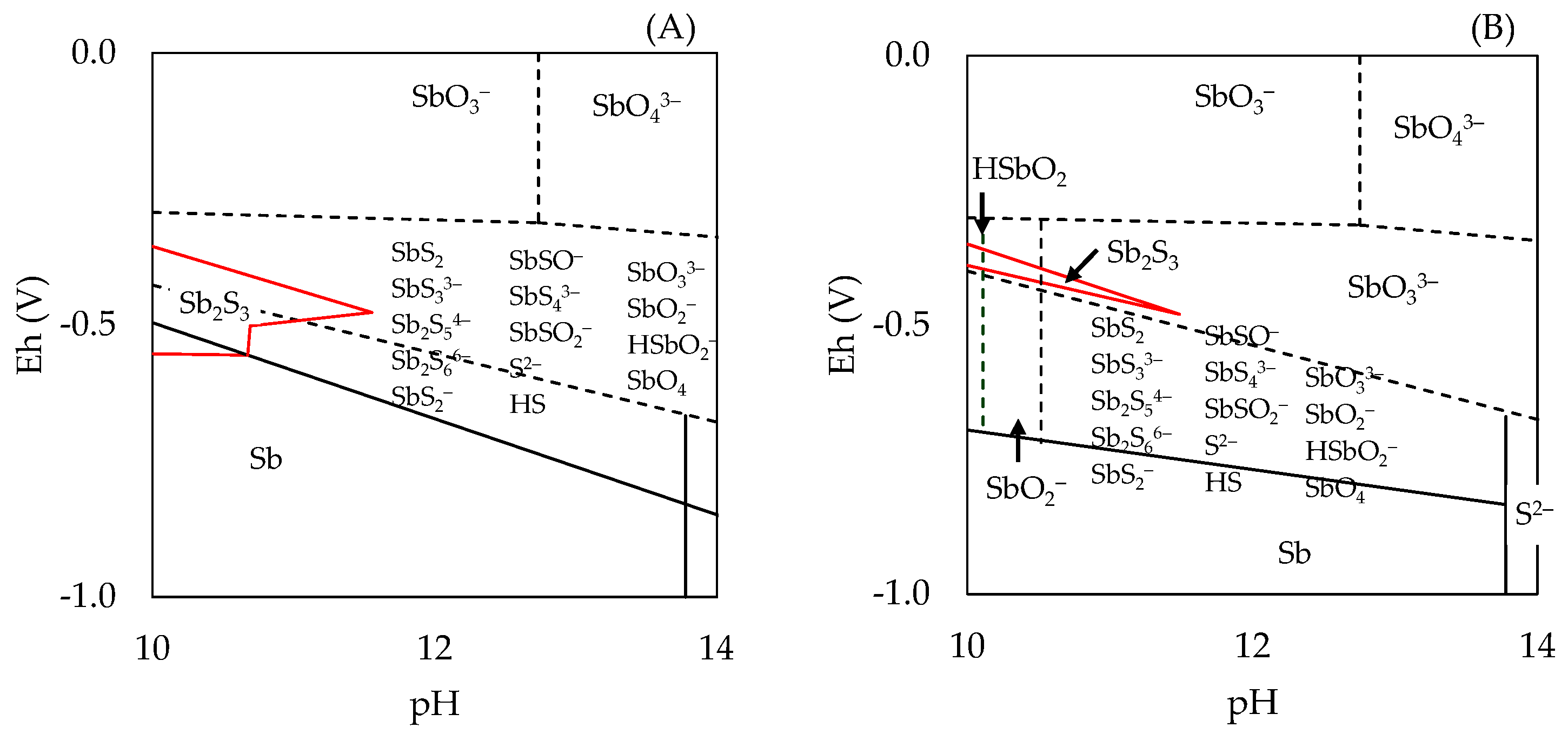
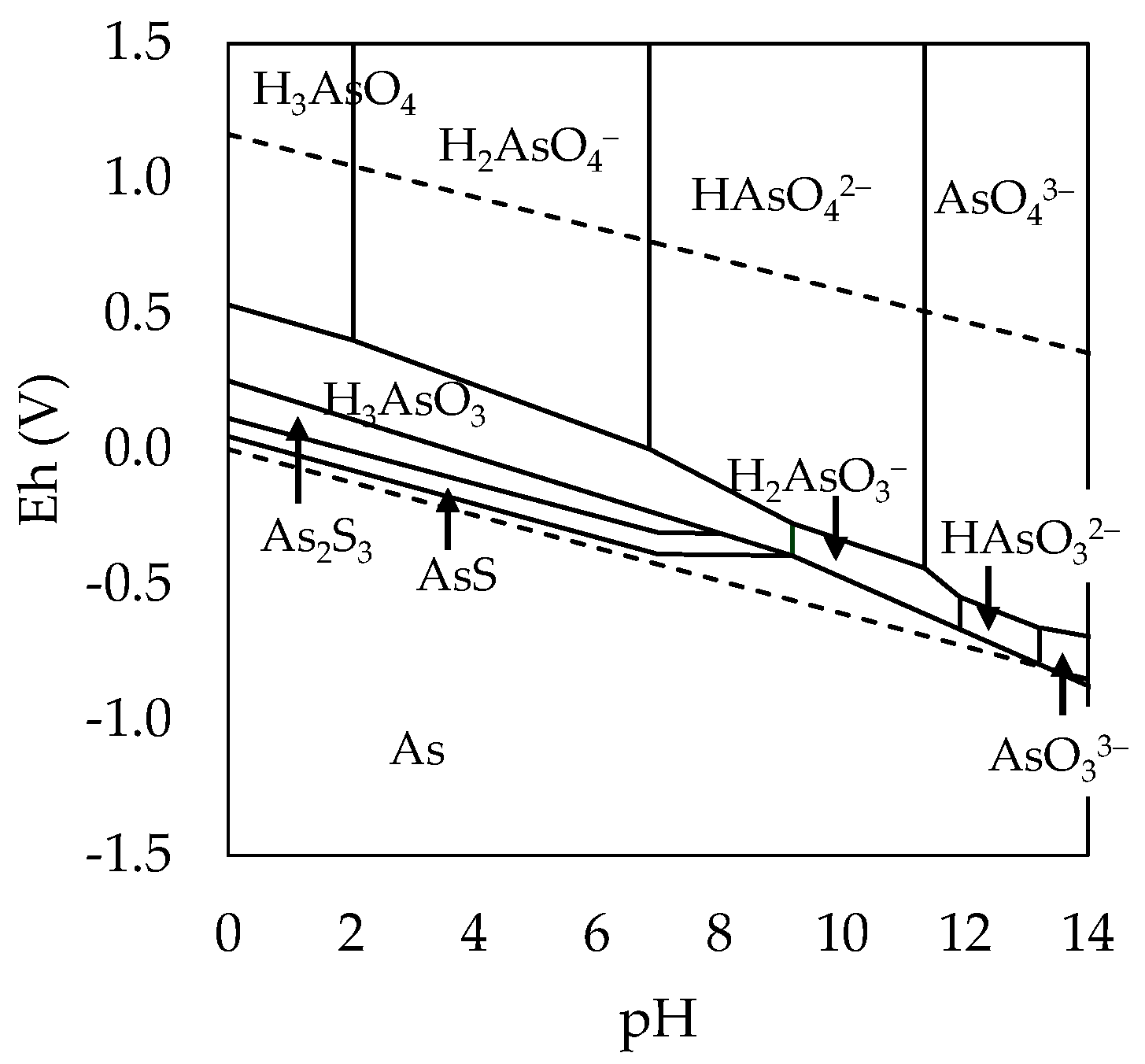
3.1. Exploration of Alternatives for the Removal of As and Sb
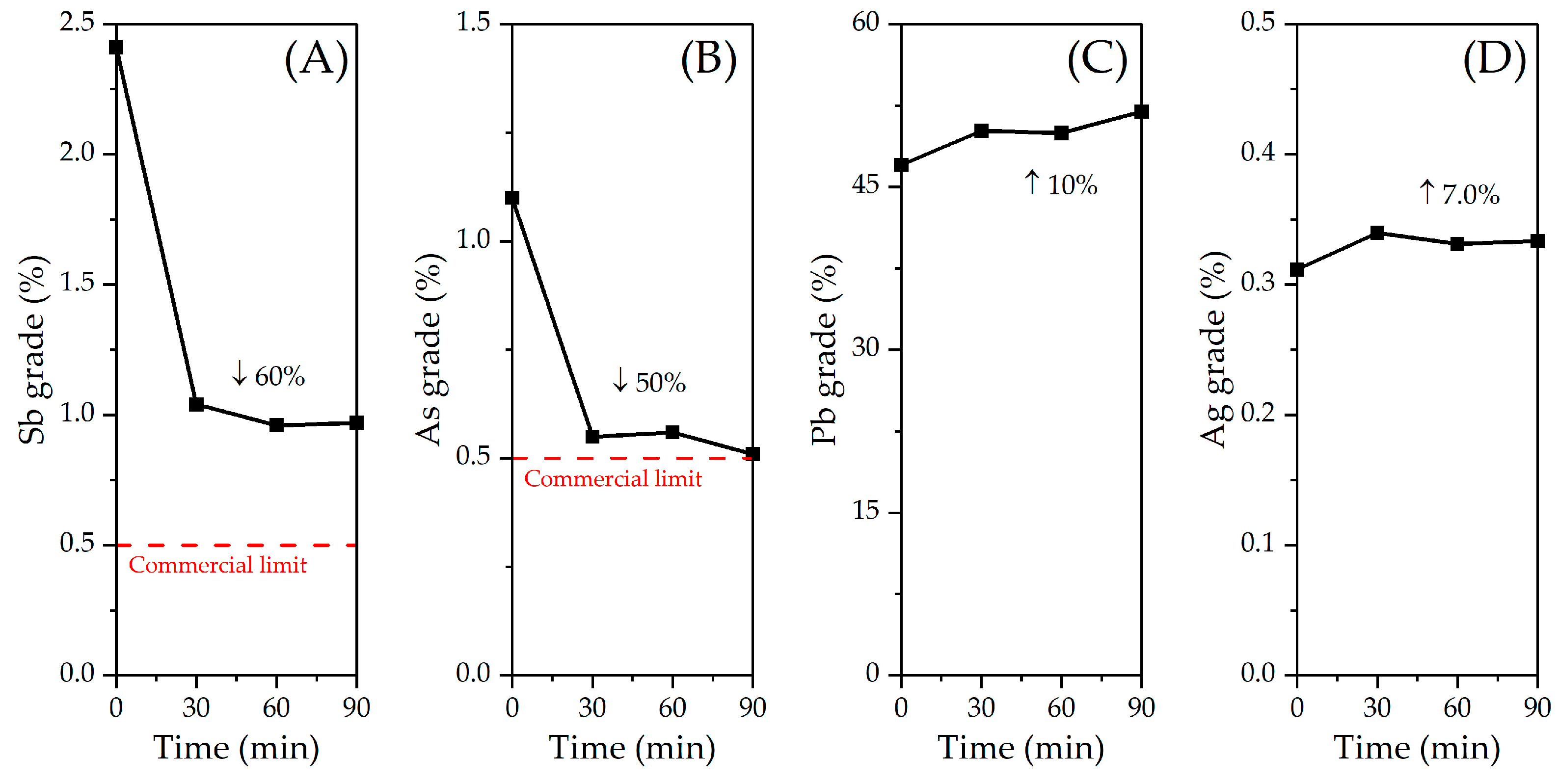
3.2. Effect of Variables in the Sulfur Digestion–Leaching Technique for Eliminating As and Sb from a Pb-Ag Concentrate
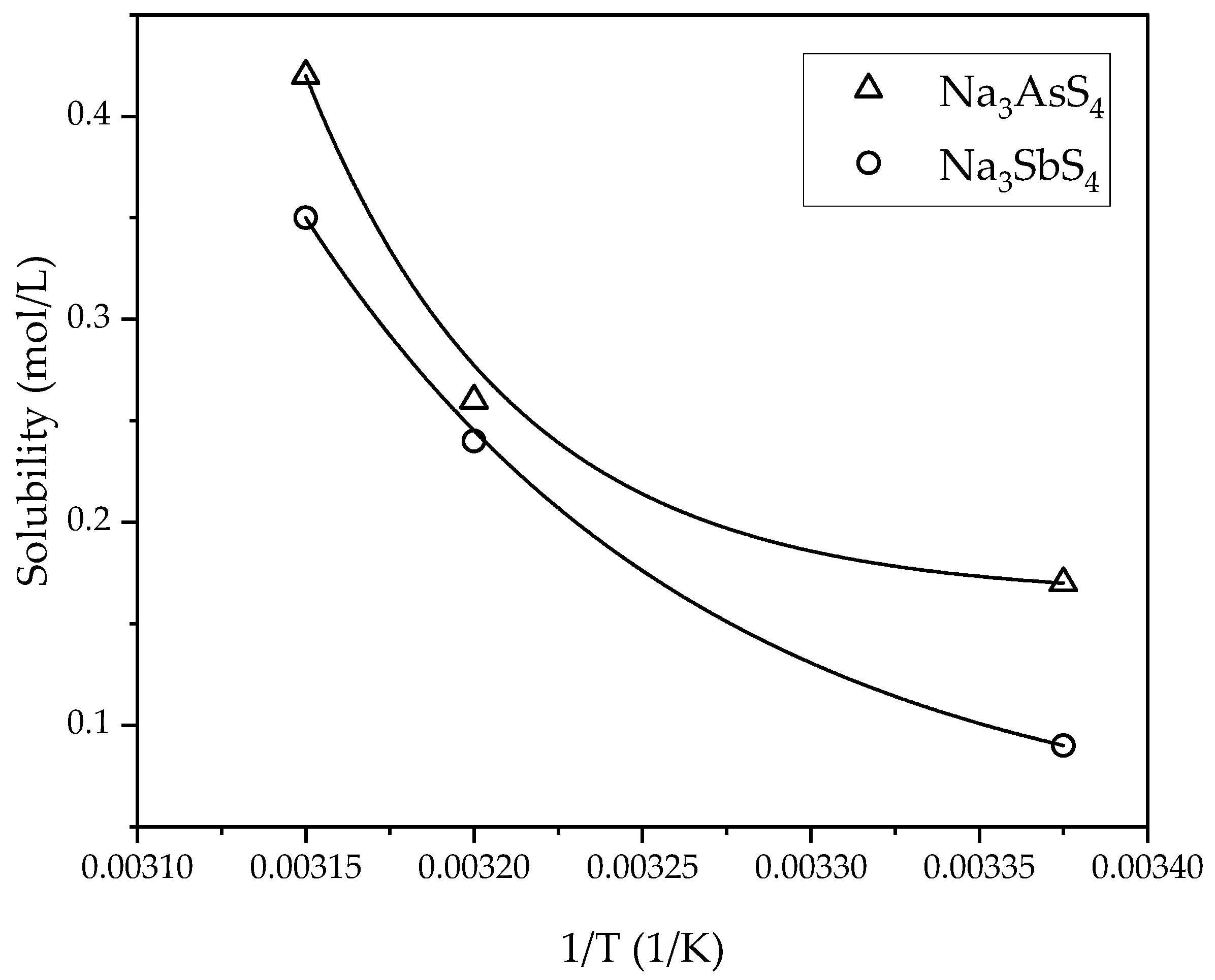
3.3. Kinetic Study of the Digestion–Leaching Technique for the Removal of As and Sb from a Pb-Ag Concentrate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mihajlovic, I.; Štrbac, N.; Živkovic, Z.; Kovacevic, R.; Stehernik, M. A potential method for arsenic removal from copper concentrates. Miner. Eng. 2007, 20, 26–33. [Google Scholar] [CrossRef]
- Sminčáková, E. Leaching of natural stibnite using sodium hydroxide solution. JOM 2009, 61, 32–35. [Google Scholar] [CrossRef]
- Tongamp, W.; Takasaki, Y.; Shibayama, A. Selective leaching of arsenic from enargite in NaHS-NaOH media. Hydrometallurgy 2010, 101, 64–68. [Google Scholar] [CrossRef]
- Sinclair, R.J. Extractive Lead Metallurgy; The Australasian Institute of Mining and Metallurgy: Carlton, Australia, 2009. [Google Scholar]
- Lin, S.; Liu, R.; Bu, Y.; Wang, C.; Wang, L.; Sun, W.; Hu, Y. Oxidative depression of arsenopyrite by using calcium hypochlorite and sodium humate. Minerals 2018, 8, 463. [Google Scholar] [CrossRef]
- Fernández, M.A.; Segarra, M.; Espiell, F. Selective leaching of arsenic and antimony contained in the anode slimes from copper refining. Hydrometallurgy 1996, 41, 255–267. [Google Scholar] [CrossRef]
- Viñals, J.; Roca, A.; Hernández, M.C.; Benavente, O. Topochemical transformation of enargite into copper oxide by hypochlorite leaching. Hydrometallurgy 2003, 68, 183–193. [Google Scholar] [CrossRef]
- Hernández, M.C.; Benavente, O.; Roca, A.; Melo, E.; Quezada, V. Selective Leaching of Arsenic from Copper Concentrates in Hypochlorite Medium. Minerals 2023, 13, 1372. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, L.; Tang, L.; Hu, J.; Xiao, Y.; Chen, Y.; Yin, Z. Removal of Metallic Impurities from Off-Grade Copper Concentrate in Alkaline Solution. Int. J. Nonferrous Metall. 2018, 07, 9–23. [Google Scholar] [CrossRef]
- Lin, S.; Liu, R.; Sun, W.; Hu, Y.; Han, H. Effect of H2O2 on the separation of mo-bi-containing ore by flotation. Minerals 2018, 8, 402. [Google Scholar] [CrossRef]
- Gu, K.; Li, W.; Han, J.; Liu, W.; Qin, W.; Cai, L. Arsenic removal from lead-zinc smelter ash by NaOH-H2O2 leaching. Sep. Purif. Technol. 2019, 209, 128–135. [Google Scholar] [CrossRef]
- Filippou, D.; St-Germain, P.; Grammatikopoulos, T. Recovery of metal values from copper-arsenic minerals and other related resources. Miner. Process. Extr. Metall. Rev. 2007, 28, 247–298. [Google Scholar] [CrossRef]
- Awe, S.A.; Sandström, Å. Selective leaching of arsenic and antimony from a tetrahedrite rich complex sulphide concentrate using alkaline sulphide solution. Miner. Eng. 2010, 23, 1227–1236. [Google Scholar] [CrossRef]
- Tongamp, W.; Takasaki, Y.; Shibayama, A. Arsenic removal from copper ores and concentrates through alkaline leaching in NaHS media. Hydrometallurgy 2009, 98, 213–218. [Google Scholar] [CrossRef]
- Steudel, R. Inorganic Polysulfides Sn2− and Radical Anions Sn·−. In Elemental Sulfur and Sulfur-Rich Compounds II. Topics in Current Chemistry; Steudel, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 127–152. ISBN 9783540449515. [Google Scholar]
- Ackerman, J.B.; Nordwick, S.M.; Anderson, C.G.; Krys, L.E. Hydrometallurgy at the Sunshine Mine Metallurgical Complex. In Proceedings of the Hydrometallurgy–Fundamentals, Technology and Innovations, AIME, Salt Lake City, UT, USA, 1–5 August 1993; pp. 477–498. [Google Scholar]
- Lv, X.D.; Li, G.; Xin, Y.T.; Yan, K.; Yi, Y. Selective Leaching of Arsenic from High-Arsenic Dust in the Alkaline System and its Prediction Model Using Artificial Neural Network. Mining, Metall. Explor. 2021, 38, 2133–2144. [Google Scholar] [CrossRef]
- Ruiz, M.C.; Daroch, F.; Padilla, R. Digestion kinetics of arsenic removal from enargite-tennantite concentrates. Miner. Eng. 2015, 79, 47–53. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, H.; Abashina, T.; Vainshtein, M. Review on arsenic removal from sulfide minerals: An emphasis on enargite and arsenopyrite. Miner. Eng. 2021, 172, 107133. [Google Scholar] [CrossRef]
- Ungureanu, G.; Santos, S.; Boaventura, R.; Botelho, C. Arsenic and antimony in water and wastewater: Overview of removal techniques with special reference to latest advances in adsorption. J. Environ. Manag. 2015, 151, 326–342. [Google Scholar] [CrossRef]
- Townsend, T.; Jang, Y.-C.; Tolaymat, T. A Guide to the Use of Leaching Tests in Solid Waste Management Decision Making; University of Florida: Gainesville, FL, USA, 2003. [Google Scholar]
- Marsden, J.; House, C.I. The Chemistry of Gold Extraction, 2nd ed.; Society for Mining, Metallurgy, and Exploration, Inc.: Littleton, CO, USA, 2006; ISBN 9780873352406. [Google Scholar]
- Brookins, D.G. Eh-pH Diagrams for Geochemistry; Springer: Berlin/Heidelberg, Germany, 1988; Volume 53, ISBN 978-3-642-73095-5. [Google Scholar]
- Anderson, C.G.; Twidwell, L.G. The alkaline sulfide hydrometallurgical separation, recovery and fixation of tin, arsenic, antimony, mercury and gold. In Proceedings of the The Southern African Institute of Mining and Metallurgy Lead & Zinc 2008, Durban, South Africa, 25–29 February 2008; pp. 121–132. [Google Scholar]
- Burriel Martí, F.; Lucena Conde, F.; Arribas Jimeno, S.; Hernández Méndez, J. Química Analítica Cualitativa, 18th ed.; Paraninfo Cengage Learning: Madrid, Spain, 2008; ISBN 9788497321402. [Google Scholar]
- Nadkarni, R.M.; Kusik, C.L. Hydrometallurgical removal of arsenic from copper concentrates. In Arsenic Metallurgy Fundamentals and Applications; Reddy, R.G., Hendrix, J.L., Queneau, P.B., Eds.; The Metallurgical Society: Pittsburgh, PA, USA, 1988; pp. 263–286. [Google Scholar]
- Briones, R.; Lapidus, G.T. The leaching of silver sulfide with the thiosulfate-ammonia-cupric ion system. Hydrometallurgy 1998, 50, 243–260. [Google Scholar] [CrossRef]
- Feng, D.; Van Deventer, J.S.J. The role of heavy metal ions in gold dissolution in the ammoniacal thiosulphate system. Hydrometallurgy 2002, 64, 231–246. [Google Scholar] [CrossRef]
- Tian-Cong, Z. The Metallurgy of Antimony; Central South University of Technology Press: Changsha, China, 1988; ISBN 7810201522. [Google Scholar]
- Limpo, J.L. Lixiviación oxidante de sulfuros complejos en soluciones de cloruro amónico: Cinética de la lixiviación de la esfalerita [Oxidative leaching of complex sulfide ore in ammonium chloride solutions: Kinetics leaching of sphalerite]. Rev. Met. Madrid 1997, 33, 258–270. [Google Scholar] [CrossRef]
- Levenspiel, O. Chemical Reaction Engineering; John Wiley and Sons: New York, NY, USA, 1999; ISBN 047125424X. [Google Scholar]
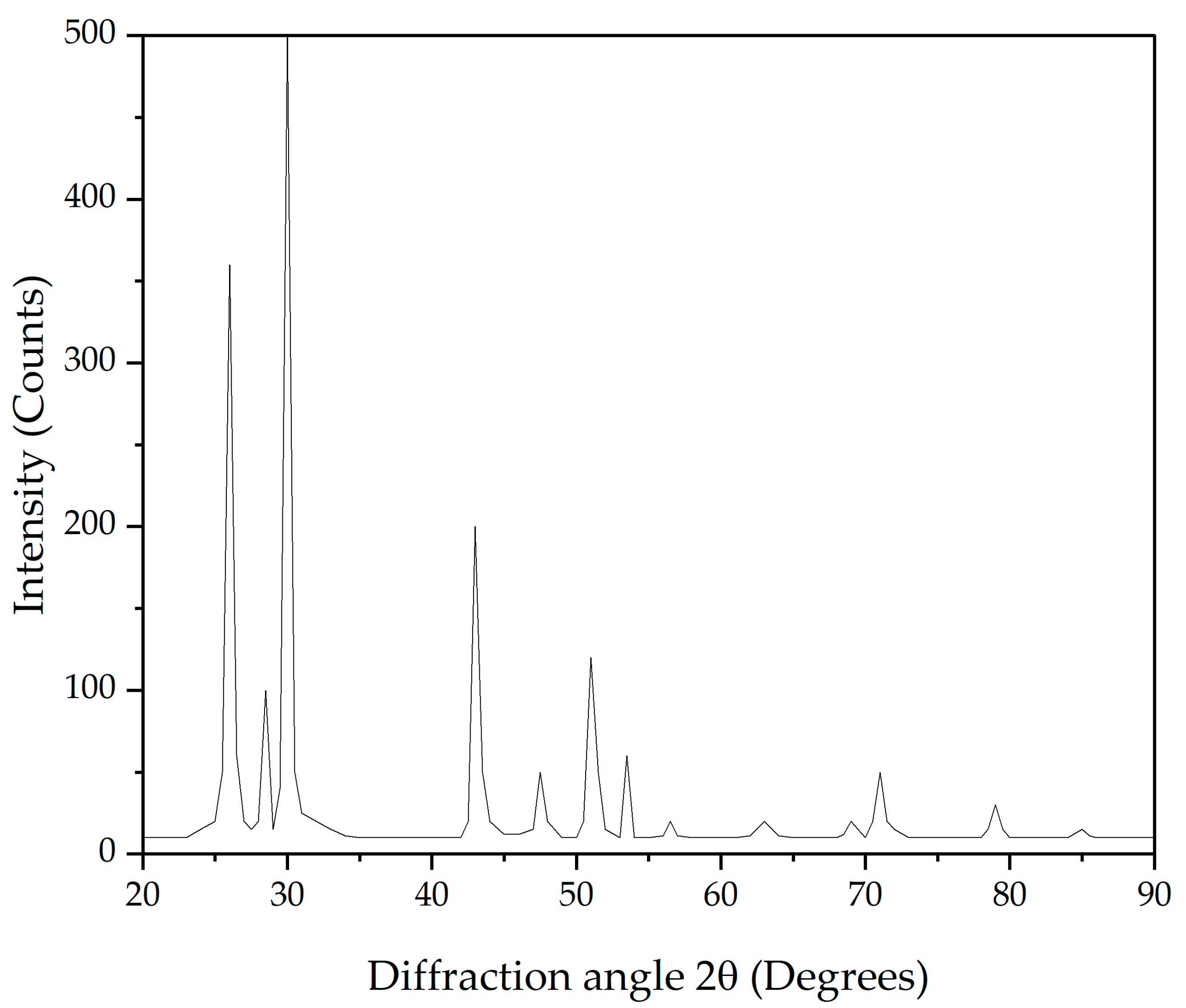


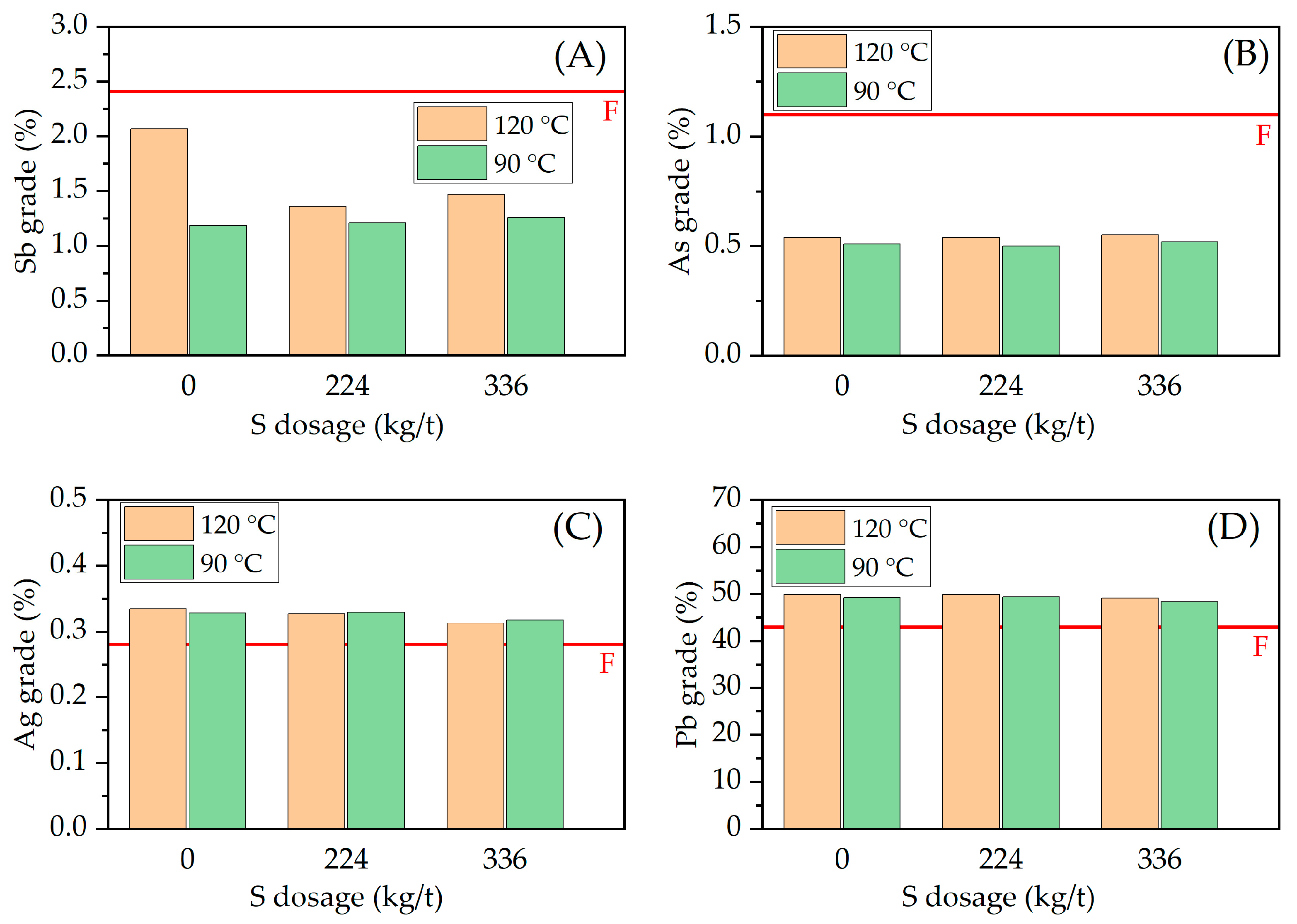
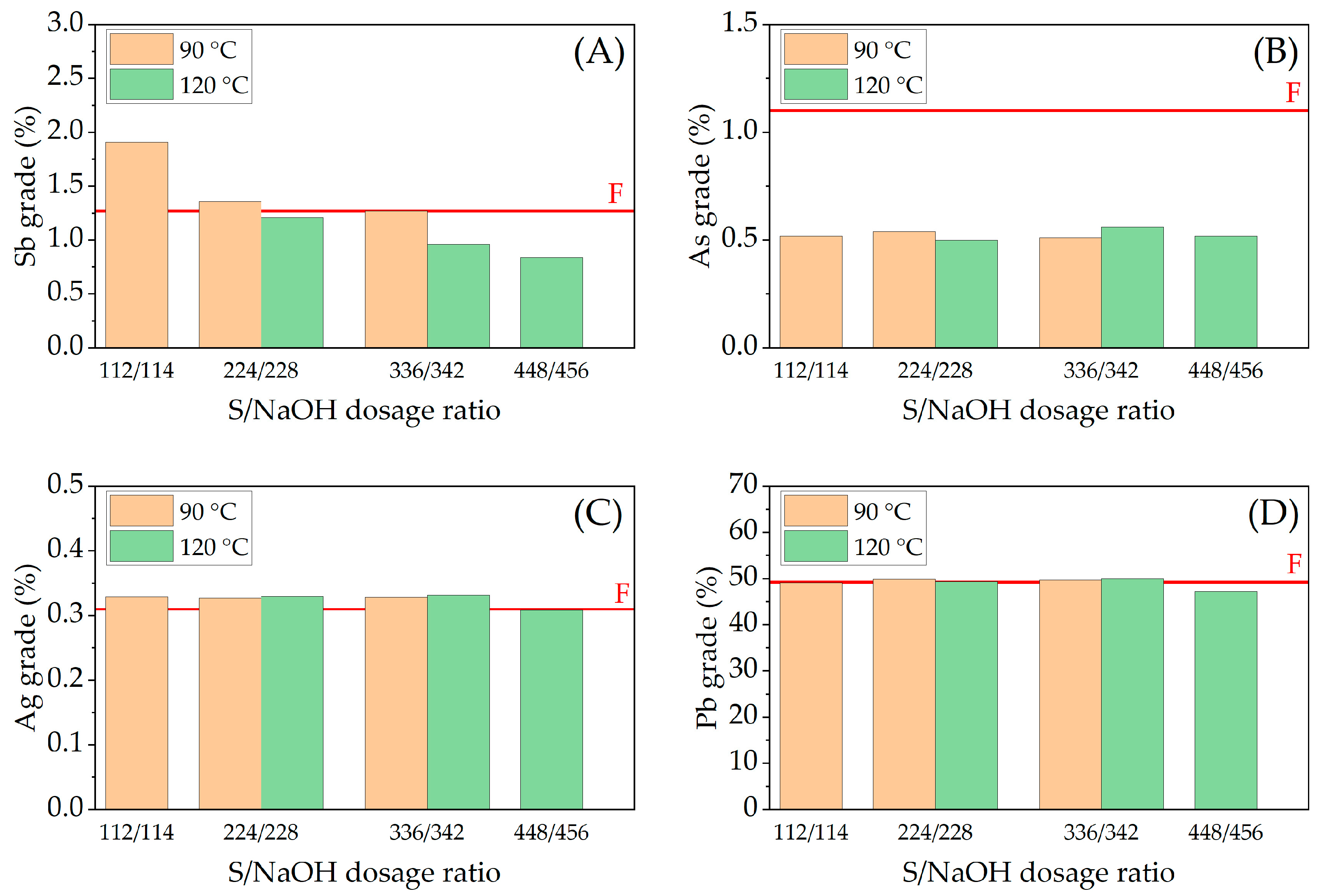
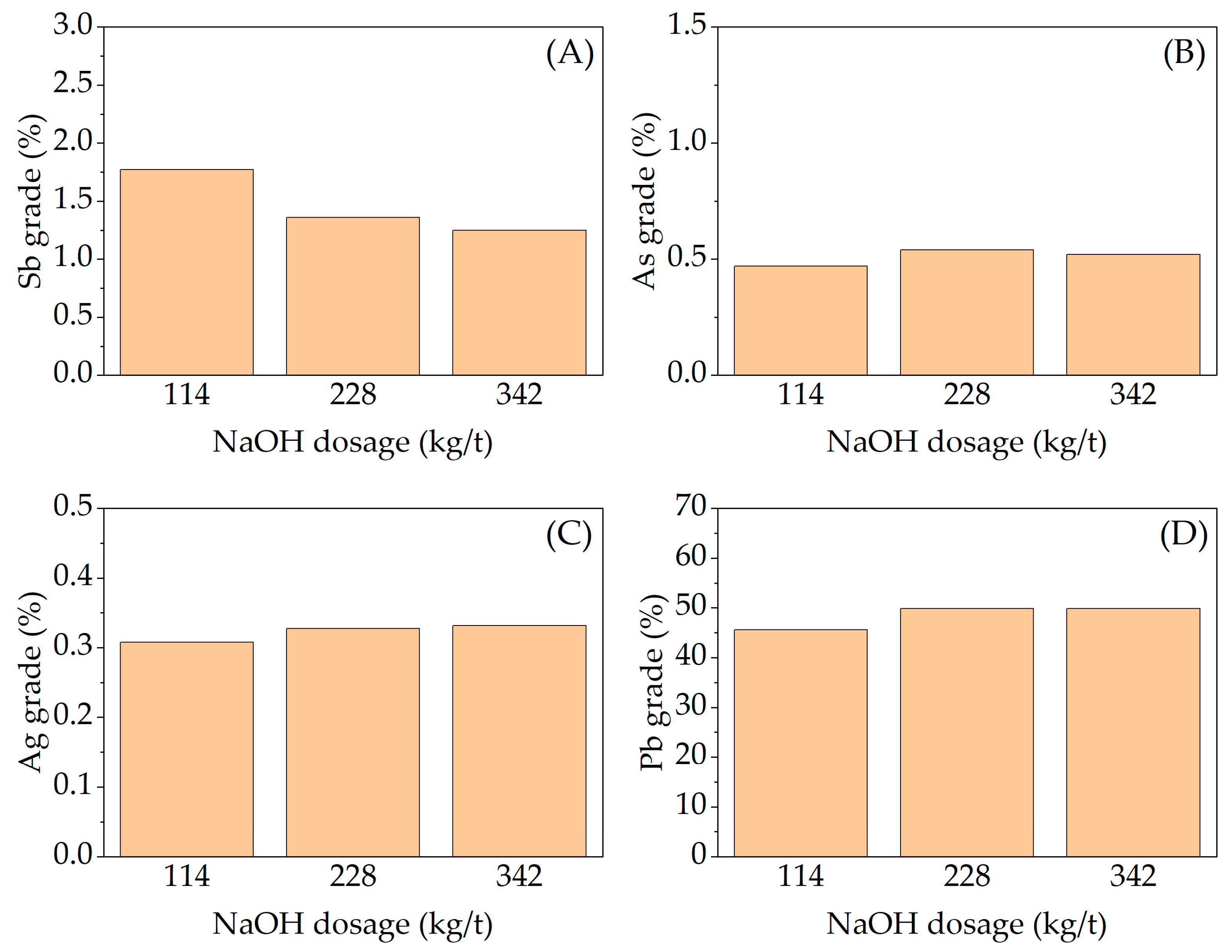
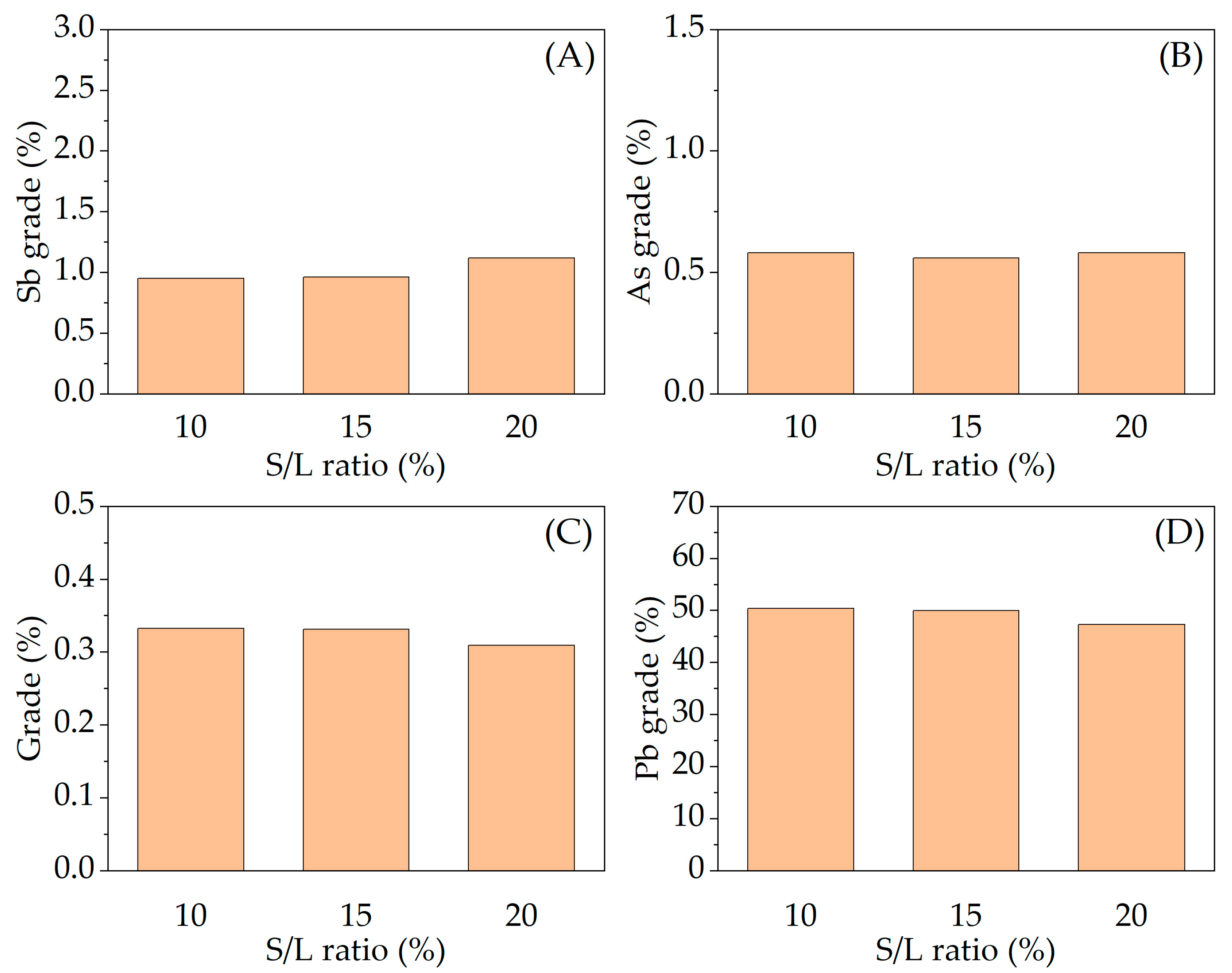

| Pb | S | Ag | Fe | SiO2 | Zn | Sb | Sn | As | Bi |
|---|---|---|---|---|---|---|---|---|---|
| 41.4 | 18.93 | 0.32 | 6.98 | 8.94 | 7.65 | 2.48 | 1.29 | 1.15 | 0.02 |
| Test | Leachant | Leachant Dosage (kg/t Concentrate) | NaOH Dosage (kg/t Concentrate) | Temperature (°C) | Digestion Time (h) | Leaching Time (h) |
|---|---|---|---|---|---|---|
| 1 | NaClO | 1.42 | 137 | 60 | 0 | 4 |
| 2 | NaClO | 1.42 | 7 mL H2SO4/100 g | 60 | 0 | 4 |
| 3 | H2O2 | 1.44 | 137 | 60 | 0 | 4 |
| 4 | Na2S | 505 | 137 | 90 | 0 | 4 |
| 5 | Na2S | 505 | 137 | 90 | 0.33 | 1 |
| 6 | S (solution) | 112 | 228 | 120 | 0 | 4 |
| 7 | S (solution) | 224 | 228 | 120 | 0 | 4 |
| 8 | S (solution) | 224 | 228 | 120 | 0.33 | 1 |
| 9 | S (solid) | 224 | 228 | 120 | 0 | 4 |
| 10 | S (solid) | 224 | 228 | 120 | 0.33 | 1 |
| Test | Levels | |||
|---|---|---|---|---|
| Temperature (°C) | 90 | 105 | 120 | |
| S dosage (kg/t) | 112 | 224 | 336 | |
| NaOH dosage (kg/t) | 114 | 228 | 342 | 456 |
| S/L ratio (%) | 10 | 15 | 20 |
| Reaction | ∆G0, kcal/mol | |||
|---|---|---|---|---|
| 25 °C | 90 °C | 105 °C | 120 °C | |
| 8S + 14OH− = 5S2− + SO42− + S2O32− + 7H2O | −69.582 | −76.700 | −78.462 | −80.213 |
| 13S + 14OH− = 5S22− + SO42− + S2O32− + 7H2O | −77.045 | −84.937 | −86.071 | −87.720 |
| 18S + 14OH− = 5S32− + SO42− + S2O32− + 7H2O | −84.036 | −91.806 | −93.615 | −95.325 |
| 23S + 14OH− = 5S42− + SO42− + S2O32− + 7H2O | −89.377 | −97.574 | −99.469 | −101.234 |
| 28S + 14OH− = 5S52− + SO42− + S2O32− + 7H2O | −93.218 | −01.839 | −103.820 | −105.637 |
| 33S + 14OH− = 5S62− + SO42− + S2O32− + 7H2O | −93.186 | −89.849 | −88.795 | −87.452 |
| Reaction | ∆G0 298°K, kcal/mol | |
|---|---|---|
| Ag+ + S2O32− = Ag(S2O3)− | 9.2 | −12.550 |
| Ag+ + 2S2O32− = Ag(S2O3)23− | 12.5 | −17.051 |
| Ag+ + 3S2O32− = Ag(S2O3)35− | 14.4 | −19.643 |
| 2Ag+ + 3S2O32− = Ag2(S2O3)34− | 12.8 | −17.461 |
| 2Ag+ + 4S2O32− = Ag2(S2O3)46− | 26.3 | −35.876 |
| 3Ag+ + 5S2O32− = Ag3(S2O3)57− | 39.8 | −54.291 |
| 6Ag+ + 8S2O32− = Ag6(S2O3)810− | 78.6 | −107.219 |
| Pb2+ + S2O32− = PbS2O3(aq) | 2.42 | −3.301 |
| Pb2+ + 2S2O32− = Pb(S2O3)22− | 4.86 | −6.630 |
| Pb2+ + 3S2O32− = Pb(S2O3)34− | 6.2 | −8.457 |
| Pb2+ + 4S2O32− = Pb(S2O3)46− | 6.2 | −8.457 |
| Element | Grade before (%) | Grade after (%) |
|---|---|---|
| Sb | 2.72 | 0.96 |
| As | 0.96 | 0.56 |
| Pb | 47.70 | 49.97 |
| Ag | 0.32 | 0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco-Vino, W.; Zamora, G.; Ordóñez, J.I. Selective Removal of Arsenic and Antimony from Pb-Ag Sulfide Concentrates by Alkaline Leaching: Thermodynamic and Kinetic Studies. Mining 2024, 4, 284-301. https://doi.org/10.3390/mining4020017
Blanco-Vino W, Zamora G, Ordóñez JI. Selective Removal of Arsenic and Antimony from Pb-Ag Sulfide Concentrates by Alkaline Leaching: Thermodynamic and Kinetic Studies. Mining. 2024; 4(2):284-301. https://doi.org/10.3390/mining4020017
Chicago/Turabian StyleBlanco-Vino, Walter, Gerardo Zamora, and Javier I. Ordóñez. 2024. "Selective Removal of Arsenic and Antimony from Pb-Ag Sulfide Concentrates by Alkaline Leaching: Thermodynamic and Kinetic Studies" Mining 4, no. 2: 284-301. https://doi.org/10.3390/mining4020017
APA StyleBlanco-Vino, W., Zamora, G., & Ordóñez, J. I. (2024). Selective Removal of Arsenic and Antimony from Pb-Ag Sulfide Concentrates by Alkaline Leaching: Thermodynamic and Kinetic Studies. Mining, 4(2), 284-301. https://doi.org/10.3390/mining4020017