Adsorption and Sequential Extraction of Copper in Technosols Prepared from Unconsolidated Mining Wastes Rich in Limestone, Bentonite, and Organic Matter
Abstract
1. Introduction
2. Material and Methods
2.1. Composition of Technosols
2.2. “Batch” Experiment
- F1—Exchangeable Cu—extracted with 8 mL of MgCl2 1 M, at pH 7.0, with shaking for 1 h at room temperature. In this and the following steps, the extract was centrifuged at 3000 rpm for 15 min and filtered;
- F2—Cu associated with carbonates—extracted with 30 mL of a solution of 1 M NaOAc at pH 5.0, with 5 h of shaking at room temperature;
- F3—Cu associated with organic matter—extracted with 10 mL of 6% NaOCl, at pH 8.0, and shaking for 6 h at 25 °C. This procedure was repeated three times;
- F4—Cu associated with amorphous iron oxides—extracted with 30 mL of oxalic acid 0.2 M + ammonium oxalate 0.2 M, at pH 3, with shaking for 2 h in the dark;
- F5—Cu associated with crystalline iron oxides—extracted with a solution of 0.25 M sodium citrate + 0.11 M sodium bicarbonate + sodium dithionite (3 g), shaking for 30 min at 75 °C;
- F6—Cu associated with sulfides—extracted with 4 M HNO3 in a water bath for 16 h at 80 °C, with occasional shaking;
- F7—Residual—calculated by subtracting the total amount of Cu added via solution (500 ppm) by the values obtained in the previous 6 fractions + Cu content obtained in the supernatant.
2.3. Column Experiment
2.4. Analytical Procedures
2.5. Statistical Analysis
3. Results and Discussion
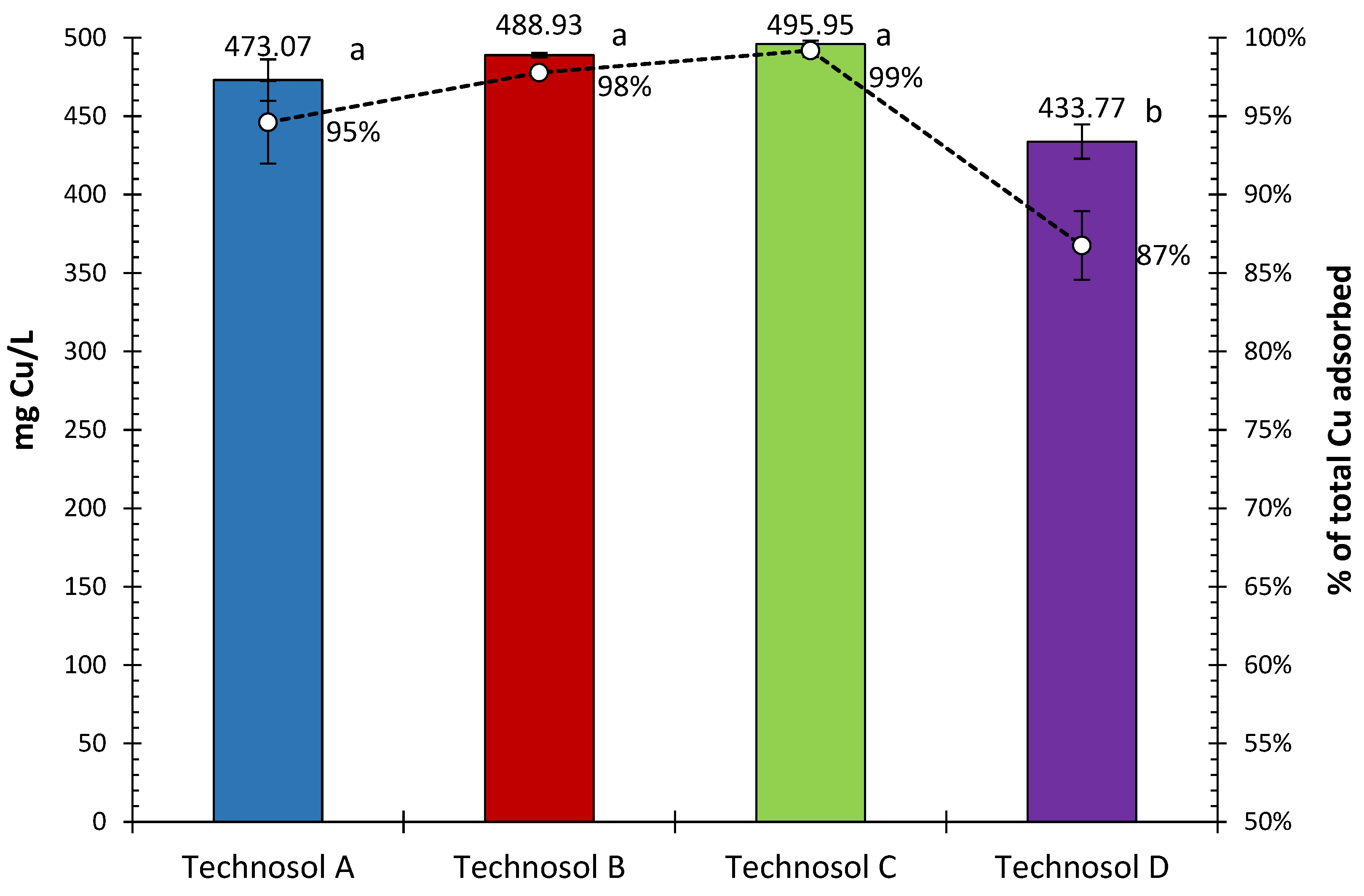
3.1. Total Concentration of Cu in Technosols in the Batch Experiment
3.2. Sequential Extraction
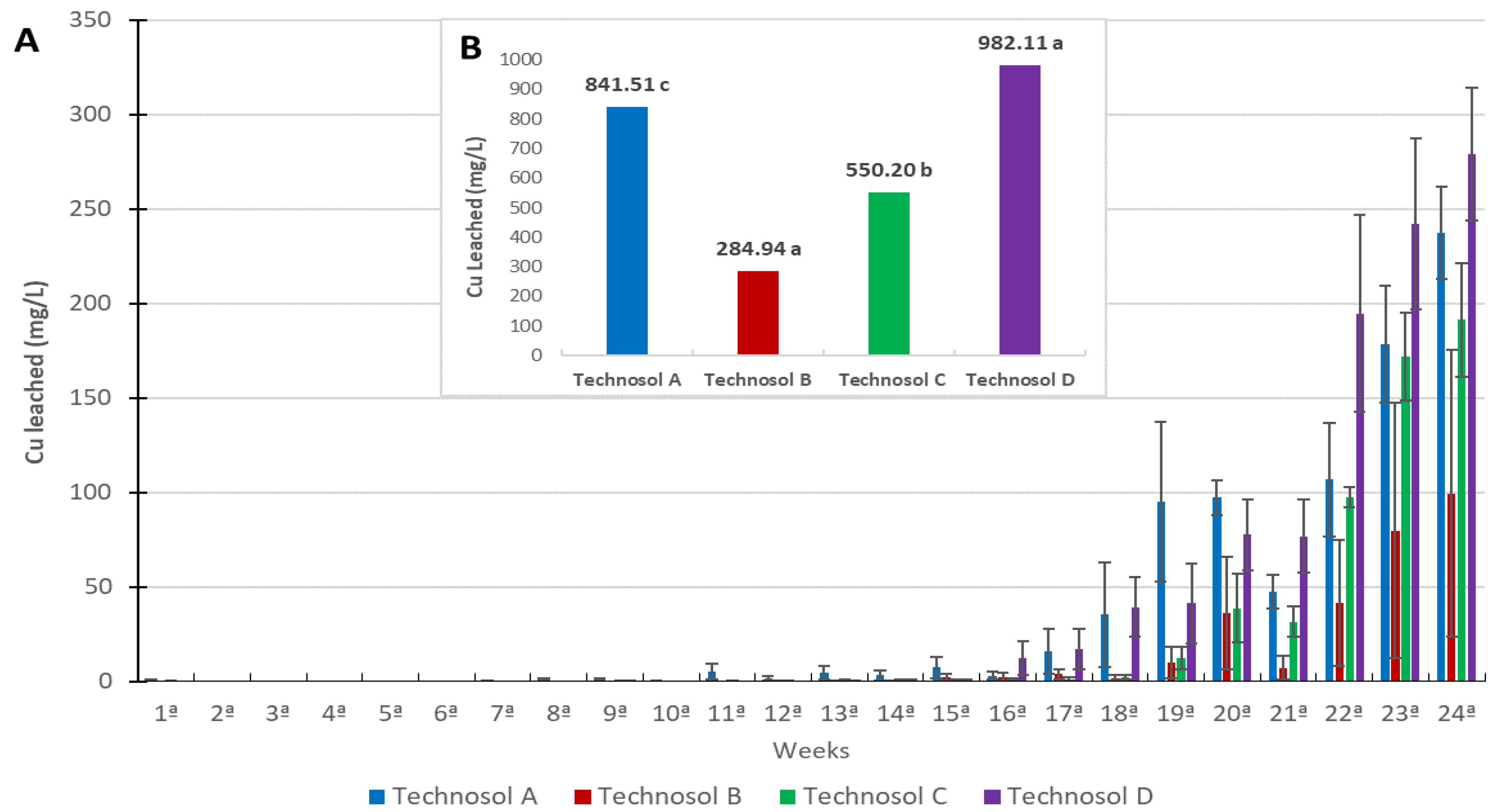
3.3. Column Experiment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, 2nd ed.; World Soil Resources Reports No. 103; FAO: Rome, Italy, 2015. [Google Scholar]
- Asensio, V.; Vega, F.A.; Singh, B.R.; Covelo, E.F. Effects of tree vegetation and waste amendments on the fractionation of Cr, Cu, Ni, Pb and Zn in polluted mine soils. Sci. Total Environ. 2013, 443, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ji, B.; Hu, Y.; Liu, R.; Sun, W. A review on in situ phytoremediation of mine tailings. Chemosphere 2017, 184, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Montiel-Rozas, M.M.; Domínguez, M.T.; Madejón, E.; Madejón, P.; Pastorelli, R.; Renella, G. Long-term effects of organic amendments on bacterial and fungal communities in a degraded Mediterranean soil. Geoderma 2018, 332, 20–28. [Google Scholar] [CrossRef]
- Ramos, L.L.; Benet, A.S.; Suau, R.L.; Larrucea, A.A.; Campo, L.H.; Matilla, A.U. Field-testing and characterization of Technosols made from industrial and agricultural residues for restoring degraded slopes in semiarid SE Spain. J. Soils Sediments 2021, 21, 1989–1997. [Google Scholar] [CrossRef]
- Lebrun, M.; Miard, F.; Trakal, L.; Bourgerie, S.; Morabito, D. The reduction of the As and Pb phytotoxicity of a former mine technosol depends on the amendment type and properties. Chemosphere 2022, 300, 134592. [Google Scholar] [CrossRef]
- Bolaños-Guerrón, D.; Galarza, I.; Liumiquinga, E.; Toulkeridis, T.; Cumbal, L. Design and Construction of a Prototype for Arsenic Retention in Mining-Contaminated Waters by Application of Nanoparticles-Based Technosols. Sustainability 2023, 15, 1286. [Google Scholar] [CrossRef]
- Fandiño, V.A.; Cancelo, B.C.; Couce, M.L.A.; Veja, F.A.; Covelo, E.F. Efecto del Tratamiento com Tecnosoles en la Recuperación de Escombreras de Mina Ricas en Sulfuros Metálicos. Rev. Soc. Esp. Miner. 2008, 10, 107–110. [Google Scholar]
- Benidire, L.; Pereira, S.; Aboudrar, W.; Hafidi, M.; Castro, P.; Boularbah, A. Remediation of metal-contaminated mine tailings by the application of organic and mineral amendments. J. Soils Sediments 2022, 22, 482–495. [Google Scholar] [CrossRef]
- Yao, F.X.; Macias, F.; Virgel, S.; Blanco, F.; Jiang, X.; Camps-Arbestain, M. Chemical changes in heavy metals in the leachates from Technosoils. Chemosphere 2009, 77, 29–35. [Google Scholar] [CrossRef]
- Yao, F.X.; Macias, F.; Santesteban, A.; Virgel, S.; Blanco, F.; Jiang, X.; Camps-Arbestain, M. Influence of the acid buffering capacity of different types of Technosols on the chemistry of their leachates. Chemosphere 2009, 74, 250–258. [Google Scholar] [CrossRef]
- Camps-Arbestain, M.; Madinabeitia, Z.; Hortala, M.A.; Macias-Garcia, F.; Virgel, S.; Macias, F. Extractability and leachability of heavy metals in Technosols prepared from mixtures of unconsolidated wastes. J. Waste Manag. 2008, 28, 2653–2666. [Google Scholar] [CrossRef]
- Ruiz, F.; Perlatti, F.; Oliveira, D.P.; Ferreira, T.O. Revealing Tropical Technosols as an Alternative for Mine Reclamation and Waste Management. Minerals 2020, 10, 110. [Google Scholar] [CrossRef]
- Santorufo, L.; Joimel, S.; Auclerc, A.; Deremiens, J.; Grisard, G.; Hedde, M.; Nahmani, J.; Pernin, C.; Cortet, J. Early colonization of constructed technosol by microarthropods. Ecol. Eng. 2021, 162, 106174. [Google Scholar] [CrossRef]
- Araujo, J.H.R.; Pando-Bahuon, A.; Aroui-Boukbiga, H.; Desjardins, T.; Lerch, T.Z. Making Green(s) With Black and White: Constructing Soils for Urban Agriculture Using Earthworms, Organic and Mineral Wastes. Front. Ecol. Evol. 2022, 10, 884134. [Google Scholar] [CrossRef]
- Khlifa, R.; Rivest, D.; Grimond, L.; Bélanger, N. Stability of carbon pools and fluxes of a Technosol along a 7-year reclamation chronosequence at an asbestos mine in Canada. Ecol. Eng. 2023, 186, 106839. [Google Scholar] [CrossRef]
- Pinskii, D.L.; Minkina, T.M.; Bauer, T.V.; Nevidomskaya, D.G.; Shuvaeva, V.A.; Mandzhieva, S.S.; Tsitsuashvili, V.S.; Burachevskaya, M.V.; Chaplygin, V.A.; Barakhov, A.V.; et al. Identification of Heavy Metal Compounds in Technogenically Transformed Soils Using Sequential Fractionation, XAFS Spectroscopy, and XRD Powder Diffraction. Eurasian Soil Sci. 2022, 5, 600–614. [Google Scholar] [CrossRef]
- Perlatti, F.; Otero, X.L.; Macias, F.; Ferreira, T.O. Geochemical speciation and dynamics of copper in tropical semi-arid soils exposed to metal-bearing mine wastes. Sci Total Environ. 2014, 500–501, 91–102. [Google Scholar] [CrossRef]
- Nevidomskaya, D.G.; Minkina, T.M.; Soldatov, A.V.; Bauer, T.V.; Shuaeva, V.A.; Zubavichus, Y.V.; Trigub, A.L.; Mandzhieva, S.S.; Dorovatovskii, P.V.; Popov, Y.V. Speciation of Zn and Cu in Technosol and evaluation of a sequential extraction procedure using XAS, XRD and SEM–EDX analyses. Environ. Geochem. Health 2021, 43, 2301–2315. [Google Scholar] [CrossRef]
- Rodrigues-Rubio, P.; Morillo, E.; Madrid, L.; Undabeytia, T.; Maqueda, C. Retention of copper by a calcareous soil and its textural fractions: Influence of amendment with two agro industrial residues. Eur. J. Soil Sci. 2003, 54, 401–409. [Google Scholar] [CrossRef]
- Illera, V.; Garrido, F.; Serrano, S.; Garcia-Gonzales, M.T. Immobilization of the heavy metals Cd, Cu and Pb in an acid soil amended with gypsum- and lime-rich industrial by-products. Eur. J. Soil Sci. 2004, 55, 135–145. [Google Scholar] [CrossRef]
- Soler-Rovira, P.; Madejon, E.; Madejon, P.; Plaza, C. In situ remediation of metal contaminated soils with organic amendments: Role of humic acids in copper bioavailability. Chemosphere 2010, 79, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Pinskii, D.L.; Minkina, T.M.; Bauer, T.V.; Nevidomskaya, S.S.; Mandzhieva, S.S.; Burachevskaya, M.V. Copper Adsorption by Chernozem Soils and Parent Rocks in Southern Russia. Geochem. Int. 2018, 56, 266–275. [Google Scholar] [CrossRef]
- Erdem, B.; Özcan, A.; Gök, O.; Özcan, A.S. Immobilization of 2,2-dipyridyl onto bentonite and its adsorption behaviour of copper (II) ions. J. Hazard. Mater. 2009, 163, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Zhironga, L.; Uddinb, A.; Zhanxuea, S. FT-IR and XRD analysis of natural Na-bentonite and Cu(II)-loaded Na-bentonite. Spectrochim. Acta A Mol. 2011, 79, 1013–1016. [Google Scholar] [CrossRef] [PubMed]
- Asensio, V.; Flórido, F.G.; Ruiz, F.; Perlatti, F.; Otero, X.L.; Oliveira, D.P.; Ferreira, T.O. The potential of a Technosol and tropical native trees for reclamation of copper-polluted soils. Chemosphere 2019, 220, 892–899. [Google Scholar] [CrossRef]
- Karapinar, N.; Donat, R. Adsorption behaviour of Cu2+ and Cd2+ onto natural bentonite. Desalination 2009, 249, 123–129. [Google Scholar] [CrossRef]
- Ruiz, F.; Cherubin, M.R.; Ferreira, T.O. Soil quality assessment of constructed Technosols: Towards the validation of a promising strategy for land reclamation, waste management and the recovery of soil functions. J. Environ. Manag. 2020, 276, 111344. [Google Scholar] [CrossRef]
- Alloway, B.J. Soil processes and the behaviour of metals. Heavy Met. Soils. 1995, 13, 3488. [Google Scholar]
- Covelo, E.F.; Vega, F.A.; Andrade, M.L. Sorption and desorption of Cd, Cr, Cu, Ni, Pb and Zn by a Fibric Histosol and its organo-mineral fraction. J. Hazard. Mater. 2008, 159, 342–347. [Google Scholar] [CrossRef]
- Sartor, L.R.; Azevedo, A.C.; Andrade, G.R.P. Study of colloidal properties of natural and Al-pillared smectite and removal of copper ions from an aqueous solution. Environ Technol. 2015, 36, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, J.P. Visual MINTEQ; Jon Petter Gustafsson: Uppsala, Sweden, 2013. [Google Scholar]
- Tahervand, S.; Jalali, M. Sorption and desorption of potentially toxic metals (Cd, Cu, Ni and Zn) by soil amended with bentonite, calcite and zeolite as a function of pH. J. Geochem. Explor. 2017, 181, 148–159. [Google Scholar] [CrossRef]
- Minkina, T.M.; Pinskii, D.L.; Bauer, T.V.; Nevidomskaya, D.G.; Mandzhieva, S.S.; Sushkova, S.N. Sorption of Cu by chernozems in southern Russia. J. Geochem. Explor. 2017, 174, 107–112. [Google Scholar] [CrossRef]
- Zhizhaev, A.M.; Merkulova, E.N.; Bragin, I.V. Copper precipitation from sulfate solutions with calcium carbonates. Russ. J. Appl. Chem. 2007, 80, 1632–1635. [Google Scholar] [CrossRef]
- Strawn, D.G.; Baker, L.L. Molecular characterization of copper in soils using X-ray absorption spectroscopy. Environ. Pollut. 2009, 157, 2813–2821. [Google Scholar] [CrossRef] [PubMed]
- Dold, B. Speciation of the most soluble phases in a sequential extraction procedure adapted for geochemical studies of copper sulfide mine waste. J. Geochem. Explor. 2003, 80, 55–68. [Google Scholar] [CrossRef]
- Qunaibit, M.H.; Mekhemer, W.K.; Zaghloul, A.A. The adsorption of Cu (II) ions on bentonite—A kinetic study. J Colloid Interface Sci. 2005, 283, 316–321. [Google Scholar] [CrossRef]
- Ferhat, M.; Kadouche, S.; Drouiche, N.; Messaoudi, K.; Lounici, H. Competitive adsorption of toxic metals on bentonite and use of chitosan as flocculent coagulant to speed up the settling of generated clay suspensions. Chemosphere 2016, 165, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Visa, M. Tailoring fly ash activated with bentonite as adsorbent for complex wastewater treatment. Appl. Surf. Sci. 2015, 263, 753–762. [Google Scholar] [CrossRef]
- Gonçalves, J.O.; Fruto, C.M.; Barranco, M.J.; Oliveira, M.L.S.; Ramos, C.G. Recovery of Degraded Areas through Technosols and Mineral Nanoparticles: A Review. Sustainability 2022, 14, 993. [Google Scholar] [CrossRef]
- Otremba, K.; Kozlowski, M.; Tatusko-Krygier, N.; Pajak, M.; Kolodziej, B.; Bryk, M. Impact of alfalfa and NPK fertilization in agricultural reclamation on the transformation of Technosols in an area following lignite mining. Land Degrad. Dev. 2021, 32, 1179–1191. [Google Scholar] [CrossRef]
- Abbruzzini, T.F.; Mora, L.; Prado, B. Evaluation of Technosols constructed with construction and excavation debris for greenhouse production of ornamental plants. J. Soils Sediments 2022, 22, 745–756. [Google Scholar] [CrossRef]
- Colombini, G.; Watteau, F.; Auclerc, A. Technosol rehabilitation strategies drive soil physico-chemical properties and fauna diversity on a former coking plant area. Appl. Soil Ecol. 2022, 177, 104542. [Google Scholar] [CrossRef]
- Soria, R.; Gonzázel-Perez, J.A.; Rosa, J.M.; Emeterio, L.M.S.; Domene, M.A.; Oertega, R.; Miralles, I. Effects of technosols based on organic amendments addition for the recovery of the functionality of degraded quarry soils under semiarid Mediterranean climate: A field study. Sci. Total. Environ. 2022, 816, 151572. [Google Scholar] [CrossRef]
- Cerqueira, B.; Covelo, E.F.; Andrade, L.; Veja, F.A. The influence of soil properties on the individual and competitive sorption and desorption of Cu and Cd. Geoderma 2011, 162, 20–26. [Google Scholar] [CrossRef]
- Seda, N.N.; Koenigsmark, F.; Vadas, T.M. Sorption and coprecipitation of copper to ferrihydrite and humic acid organomineral complexes and controls on copper availability. Chemosphere 2016, 147, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Zamulina, I.V.; Gorovtsov, A.V.; Minkina, T.M.; Mandzhieva, S.S.; Bauer, T.V.; Burachevskaya, M.V. The influence of long-term Zn and Cu contamination in Spolic Technosols on water-soluble organic matter and soil biological activity. Ecotoxicol. Environ. Saf. 2021, 208, 111471. [Google Scholar] [CrossRef]
- Ramírez-Pérez, A.M.; Paradelo, M.; Nóvoa-Muñoz, J.C.; Arias-Estévez, N.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñes-Delgado, A. Heavy metal retention in copper mine soil treated with mussel shells: Batch and column experiments. J. Hazard. Mater. 2013, 248–249, 122–130. [Google Scholar] [CrossRef] [PubMed]



| Wastes | Technosol A | Technosol B | Technosol C | Technosol D |
|---|---|---|---|---|
| % | ||||
| Limestone waste rock | 33.3 | 50.0 | 25.0 | 25.0 |
| Organic Compost | 33.3 | 25.0 | 50.0 | 25.0 |
| Bentonite + Sand (4:1) | 33.3 | 25.0 | 25.0 | 50.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perlatti, F.; Ruiz, F.; Otero, X.L.; Ferreira, T.O. Adsorption and Sequential Extraction of Copper in Technosols Prepared from Unconsolidated Mining Wastes Rich in Limestone, Bentonite, and Organic Matter. Mining 2023, 3, 151-162. https://doi.org/10.3390/mining3010009
Perlatti F, Ruiz F, Otero XL, Ferreira TO. Adsorption and Sequential Extraction of Copper in Technosols Prepared from Unconsolidated Mining Wastes Rich in Limestone, Bentonite, and Organic Matter. Mining. 2023; 3(1):151-162. https://doi.org/10.3390/mining3010009
Chicago/Turabian StylePerlatti, Fabio, Francisco Ruiz, Xosé Luis Otero, and Tiago Osório Ferreira. 2023. "Adsorption and Sequential Extraction of Copper in Technosols Prepared from Unconsolidated Mining Wastes Rich in Limestone, Bentonite, and Organic Matter" Mining 3, no. 1: 151-162. https://doi.org/10.3390/mining3010009
APA StylePerlatti, F., Ruiz, F., Otero, X. L., & Ferreira, T. O. (2023). Adsorption and Sequential Extraction of Copper in Technosols Prepared from Unconsolidated Mining Wastes Rich in Limestone, Bentonite, and Organic Matter. Mining, 3(1), 151-162. https://doi.org/10.3390/mining3010009









