Impact of Variability in Precipitation Patterns on the Geochemistry of Pyritic Uranium Tailings Rehabilitated with Saturated Cover Technology
Abstract
1. Introduction
2. Materials and Methods
2.1. Mineralogical and Physicochemical Characterization of U Tailings
2.1.1. Tailings and Cover Material
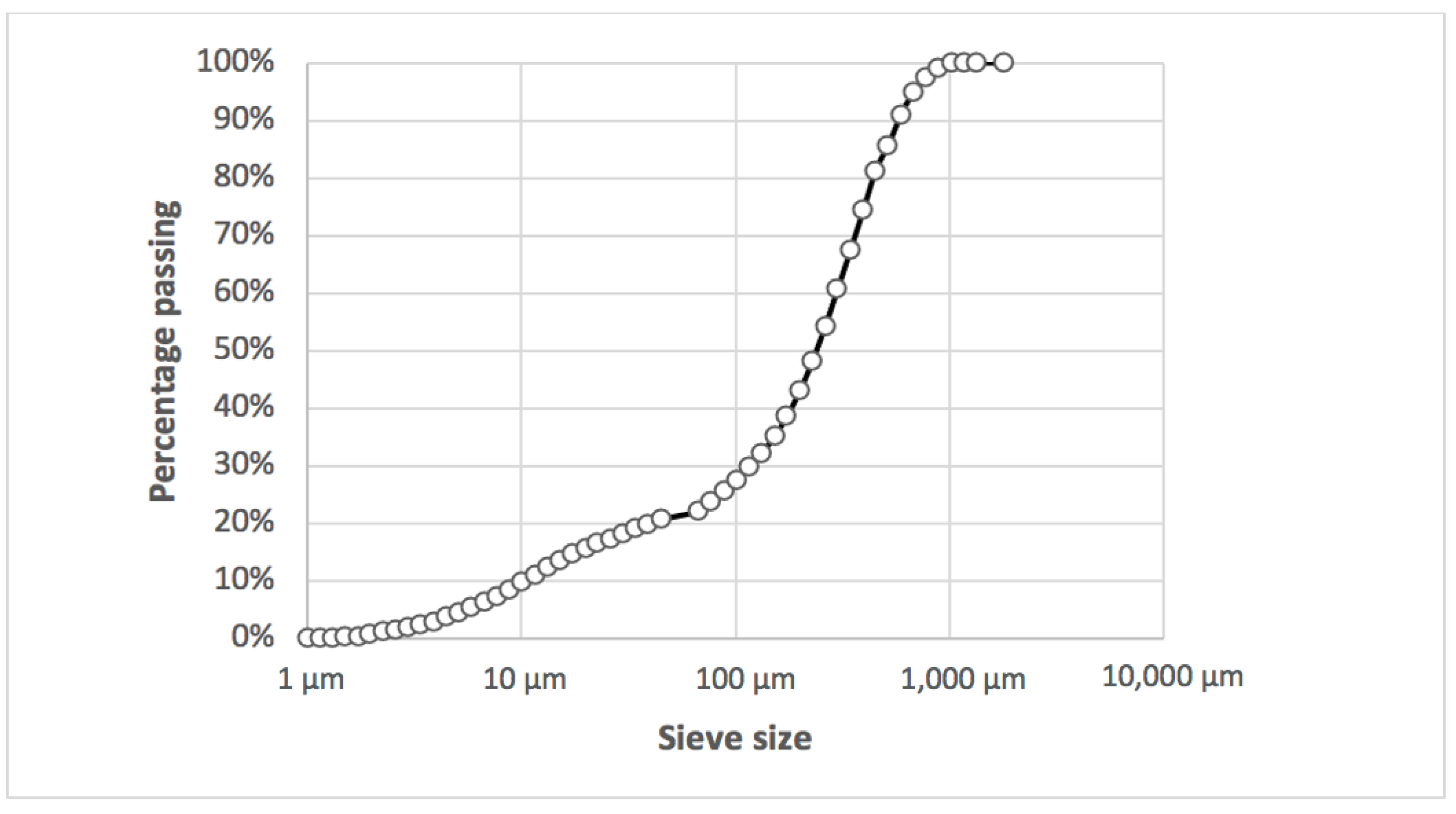
2.1.2. Physical Characterization
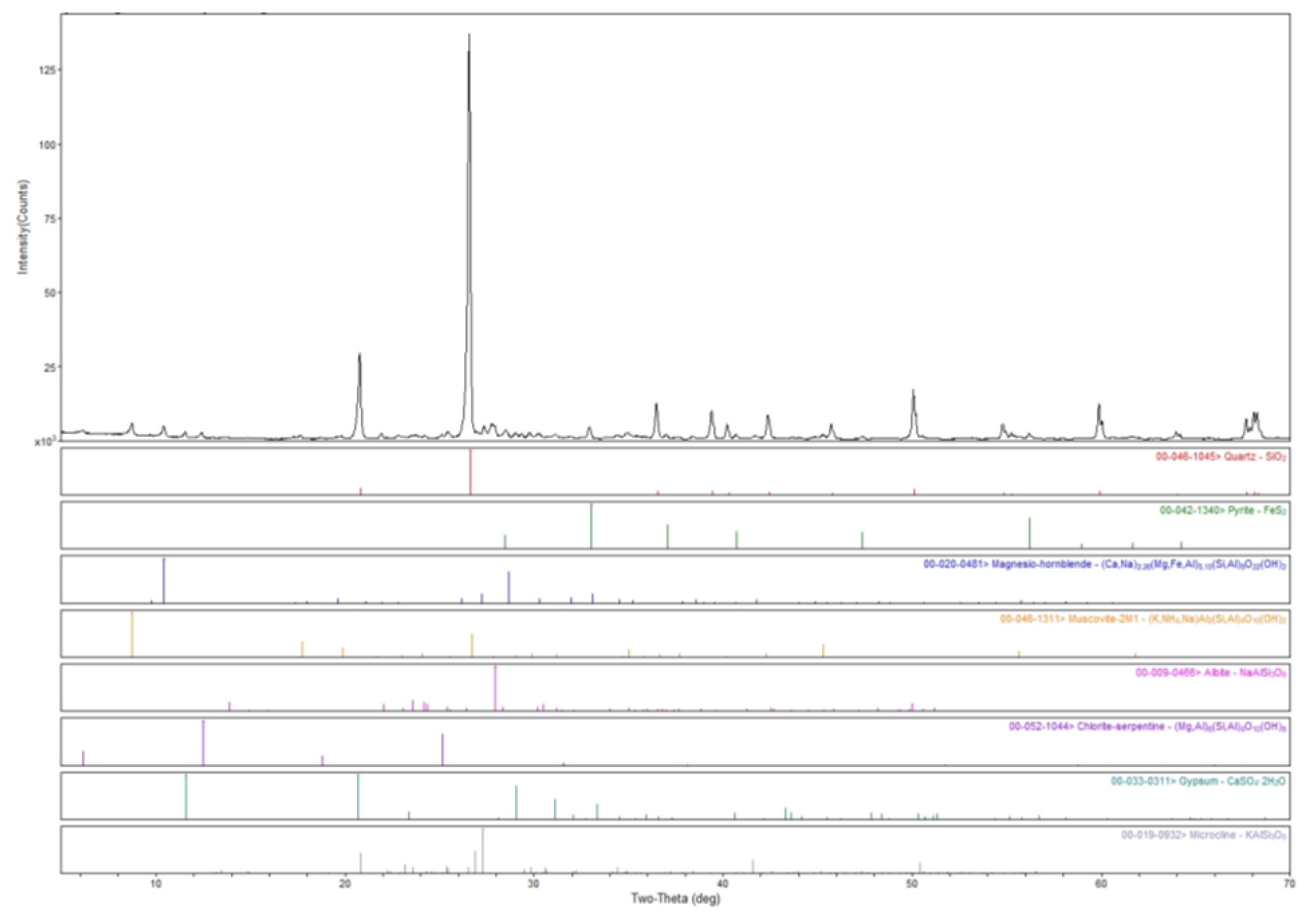
2.1.3. Mineralogy
2.1.4. Chemical Characterization
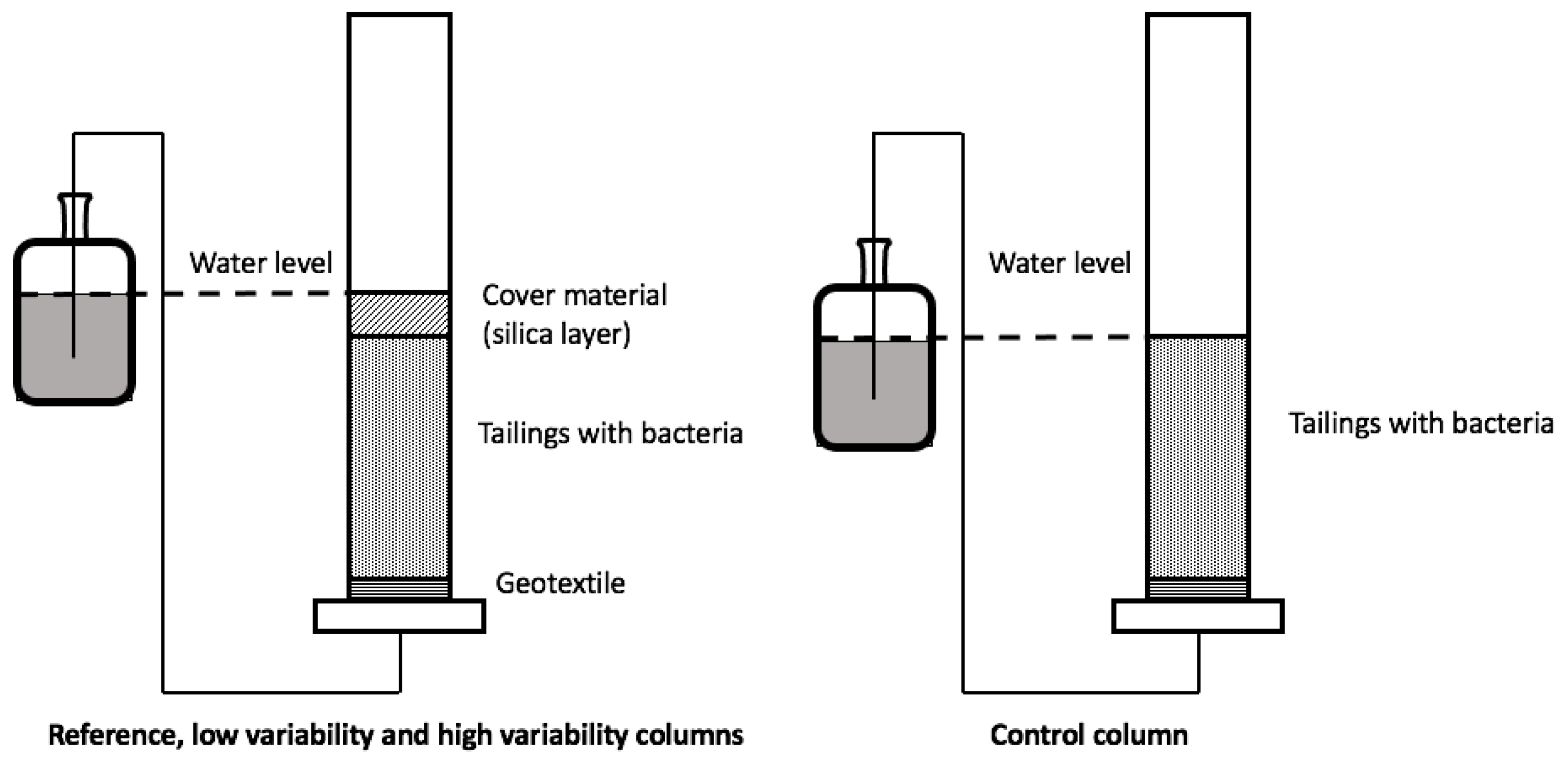
2.2. Column Study
2.2.1. Static Testing
2.2.2. Column Set-Up
2.2.3. Predicted Climate Change Conditions
2.2.4. Leachate Analysis
3. Results and Discussion
3.1. Characterization of BHP Tailings
3.2. Leachate Analysis
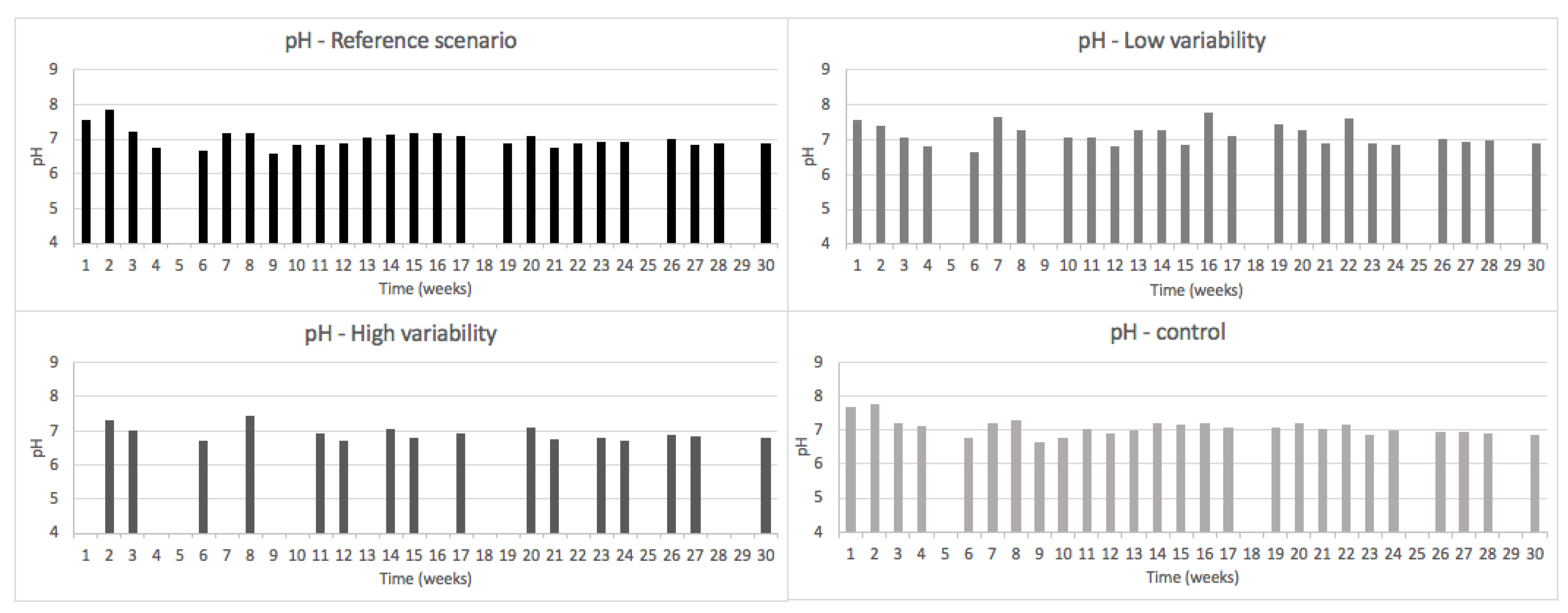
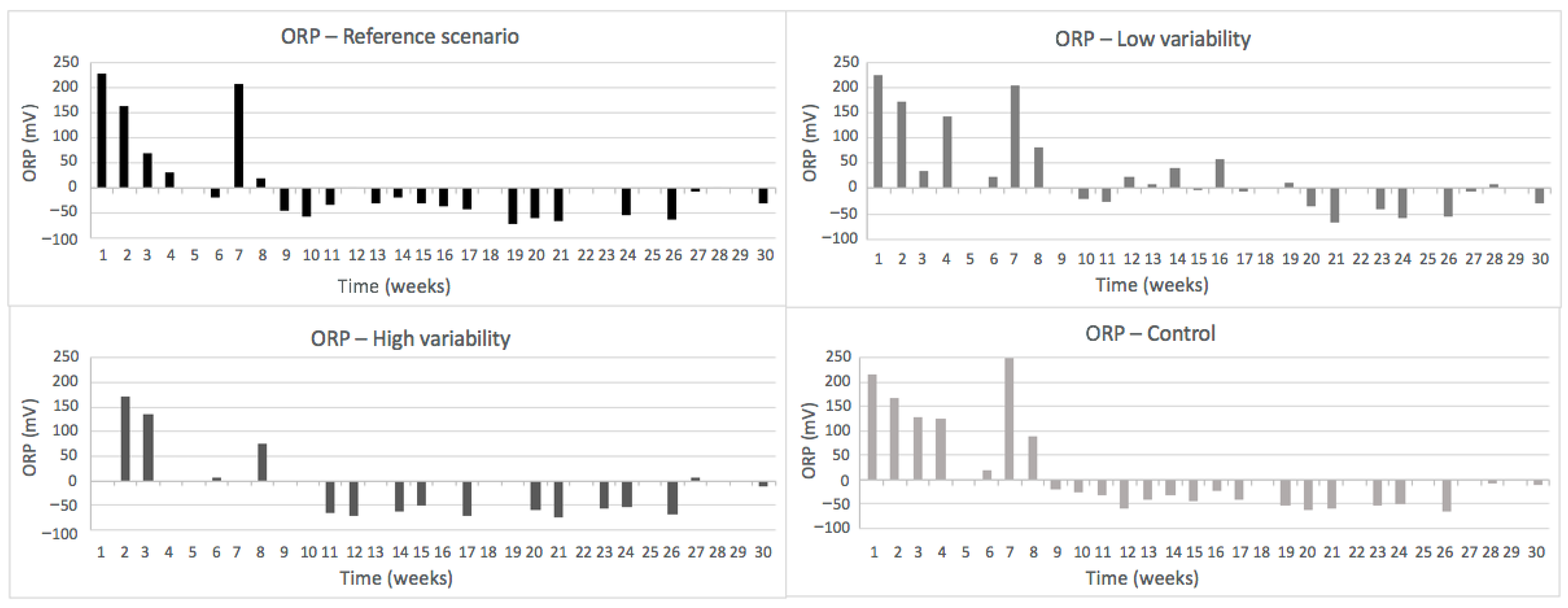
3.2.1. pH, ORP and EC
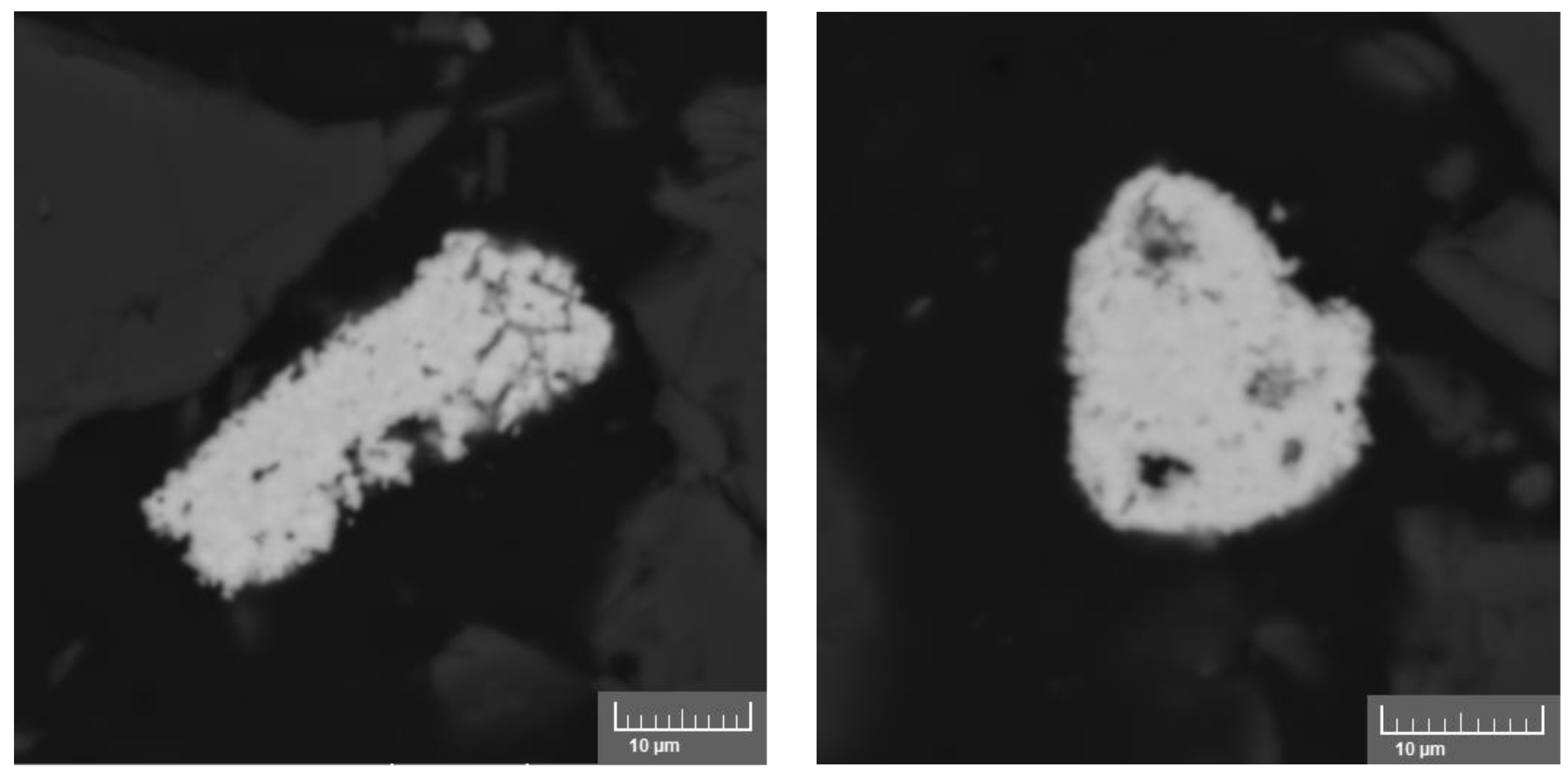
3.2.2. Leaching of Uranium
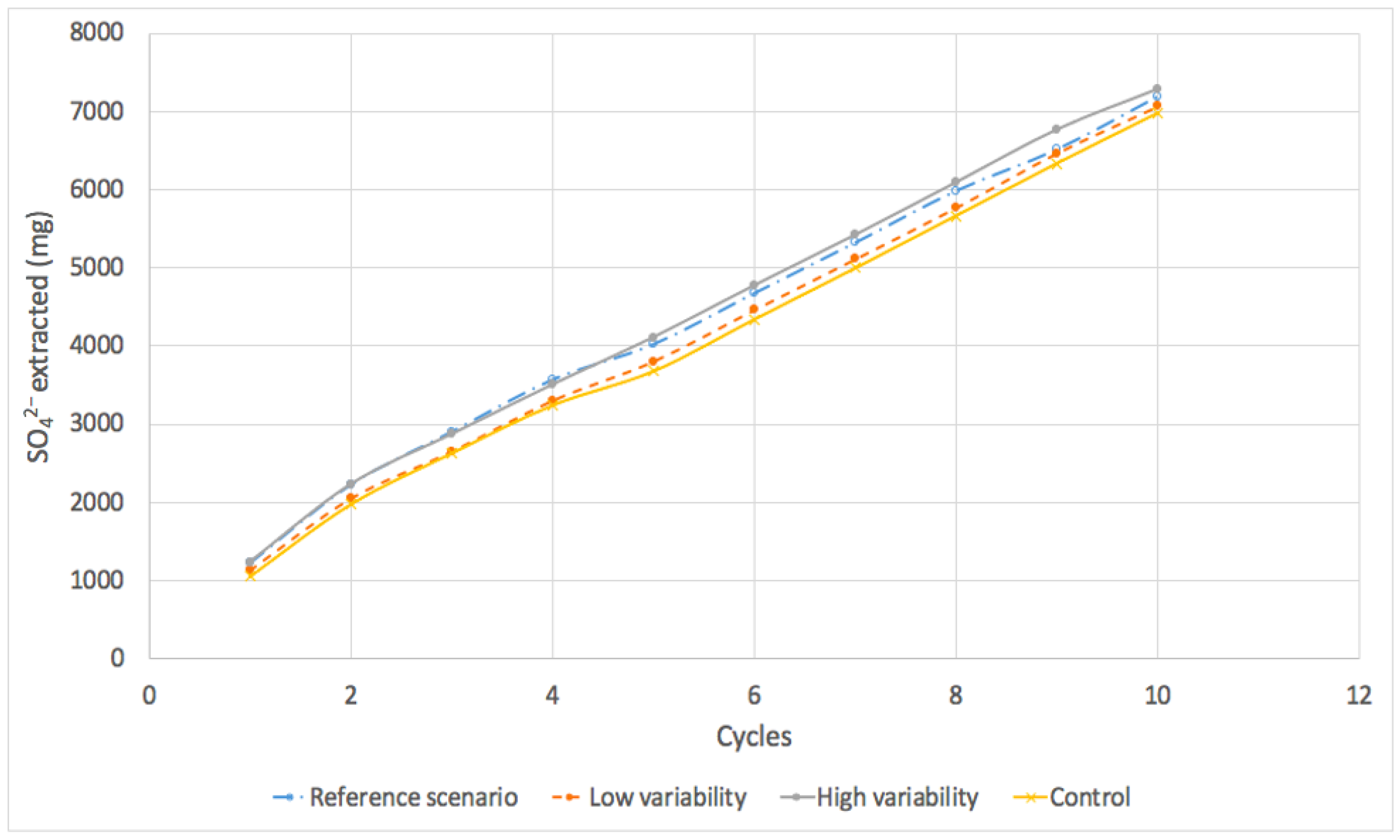
3.2.3. Carbonate Leaching
3.2.4. Leaching of Thorium
3.2.5. Dissolution of Iron Sulfide Minerals
3.2.6. Precipitation Patterns and Oxygen Flux
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aubertin, M.; Bussière, B.; Bernier, L. Effluents et rejets miniers. In Environnement et Gestion Des Résidus, 1st ed.; Presses Internationales de Polytechnique: Montréal, QC, Canada, 2003. [Google Scholar]
- Davé, N.K. Knowledge Gaps in Water Cover and Saturated Cover Mine Waste Management Technologies; CanmetMINING Internal report, Project: P-002496.001.02/RC 179; Natural Resources Canada: Ottawa, ON, Canada, 2016. [Google Scholar]
- Davé, N.K.; Lim, T.P.; Horne, D.; Boucher, Y. Water cover on reactive tailings and wasterock: Laboratory studies of oxidation and metal release characteristics. In Proceedings of the 4th International Conference on Acid Rock Drainage, Vancouver, BC, Canada, 31 May–6 June 1997; Volume 2, pp. 779–794. [Google Scholar]
- Eriksson, N.; Lindvall, M.; Sandberg, M. A quantitative evaluation of effectiveness of the water cover at the Stekenjokk tailings pond in northern Sweden: Eight years of follow-up. In Proceedings of the International Conference on Mining and the Environment, Skellefteå, Suède, 25 June 2001. [Google Scholar]
- Best Available Techniques for Management of Tailings and Waste-Rock in Mining Activities. Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC109657 (accessed on 31 January 2022).
- Aubertin, M.; Dionne, J.; Marcoux, L. Design Guidelines and Stability Criteria of Engineering Works for Water Covers. In Proceedings of the 4th International Conference on Acid Rock Drainage, Vancouver, BC, Canada, 31 May–6 June 1997; Volume 4, pp. 1851–1866. [Google Scholar]
- Bussière, B.; Guittonny, M. Long term evolution of reclamation performance. In Hard Rock Mine Reclamation from Prediction to Management of Acid Mine Drainage, 1st ed.; Bussière, B., Guittonny, M., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 351–356. [Google Scholar]
- Pabst, T. Elevated water table with monolayer covers. In Hard Rock Mine Reclamation from Prediction to Management of Acid Mine Drainage, 1st ed.; Bussière, B., Guittonny, M., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 187–200. [Google Scholar]
- Demers, I. Oxygen diffusion and consumption in low-sulphide tailings covers. Can. Geotech. J. 2009, 46, 454–469. [Google Scholar] [CrossRef]
- Ouangrawa, M.; Molson, M.; Aubertin, J.W.; Zagury, M.; Bussière, B. The effect of water table elevation on acid mine drainage from reactive tailings: A laboratory and numerical modelling study. In Proceedings of the 7th International Conference on Acid Rock Drainage, St. Louis, MO, USA, 26–30 March 2006; Volume 1, pp. 1473–1482. [Google Scholar]
- Ouangrawa, M. Étude Expérimentale et Analyse Numérique des Facteurs qui Influencent le Comportement Hydrogéochimique de Résidus Miniers Sulfureux Partiellement Saturés. Ph.D. Thesis, Polytechnique de Montréal, Montreal, QC, Canada, 2007. [Google Scholar]
- Dagenais, A.-M. Techniques de Contrôle du Drainage Minier Acide Basées sur les Effets Capillaires. Ph.D. Thesis, Université du Québec en Abitibi-Témiscamingue, Rouyn Noranda, QC, Canada, 2005. [Google Scholar]
- Demers, I.; Bussière, B.; Rousselle, M.; Aubertin, M.; Pabst, T.; Lacroix, R. Laboratory evaluation of reclamation scenarios for the spillage areas of the abandoned Manitou mine site using Goldex tailings. In Proceedings of the 23rd World Mining Congress, Montreal, QC, Canada, 1 August 2013. [Google Scholar]
- Pabst, T.; Aubertin, M.; Bussière, B.; Molson, J. Column tests to assess water flow and oxygen transport in monolayer covers for the reclamation of acid-generating tailings. In Proceedings of the Sixth International Conference on Mine Closure, Lake Louise, AB, Canada, 18–21 September 2011. [Google Scholar]
- Lieber, E. Influence des Facteurs Climatiques sur la Performance de la Nappe Phréatique Surélevée Combinée à un Recouvrement Monocouche. Master’s Thesis, Polytechnique de Montréal, Montréal, QC, Canada, 2019. [Google Scholar]
- Nordstrom, D.K.; Alpers, C.N. Geochemistry of acid mine waters. In The Environmental Geochemistry of Mineral Deposits, 1st ed.; Society of Economic Geologist: Littleton, CO, USA, 1999. [Google Scholar]
- Canadian Malartic. Available online: https://mern.gouv.qc.ca/mines/quebec-mines/2002-02/canadian-malartic.jsp (accessed on 9 April 2020).
- Sheppard, S.C.; Sheppard, M.I.; Gallerand, M.O.; Sanipelli, B. Derivation of ecotoxicity thresholds for uranium. J. Environ. Radioactiv. 2005, 79, 55–83. [Google Scholar] [CrossRef] [PubMed]
- Goulet, R.R.; Fortin, C.; Spry, D. Uranium. In Homeostasis and Toxicology of Non-Essential Metals; Wood, C.M., Farrell, A.P., Brauner, C.J., Eds.; Academic Press: Cambridge, MA, USA, 2011; Volume 31B, pp. 391–428. [Google Scholar] [CrossRef]
- Van Dam, R.A.; Trenfield, M.A.; Markich, S.J.; Harford, A.J.; Humphrey, C.L.; Hogan, A.C.; Stauber, J.L. Reanalysis of uranium toxicity data for selected freshwater organisms and the influence of dissolved organic carbon. Environ. Toxicol. Chem. 2012, 31, 2606–2614. [Google Scholar] [CrossRef] [PubMed]
- Goulet, R.; Thompson, P.A.; Serben, K.C.; Eickhoff, C.V. Impact of environmentally based chemical hardness on uranium speciation and toxicity in six aquatic species. Envirol. Toxicol. Chem. 2015, 34, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Goulet, R.R.; Thompson, P.A. Bioaccumulation and Toxicity of Uranium, Arsenic and Nickel to Juvenile and Adult Hyalella Azteca in Spiked Sediment Bioassays. Environ. Toxicol. Chem. 2018, 37, 2340–2349. [Google Scholar] [CrossRef] [PubMed]
- The Environmental Behavior of Radium: Revised Edition. Available online: https://www.iaea.org/publications/10478/the-environmental-behaviour-of-radium-revised-edition (accessed on 20 February 2022).
- The Environmental Behavior of Polonium. Available online: https://www.iaea.org/publications/10845/the-environmental-behaviour-of-polonium (accessed on 20 February 2022).
- Beaugelin-Seiller, K.; Goulet, R.R.; Mihok, S.; Beresford, N.S. Should we ignore U-235 series contribution to dose? J. Environ. Radioactiv. 2015, 151, 114–125. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abdelouas, A. Uranium Mill Tailings: Geochemistry, Mineralogy, and Environmental Impact. Elements 2006, 2, 335–341. [Google Scholar] [CrossRef]
- Thorium 232 et Environnement. Available online: https://www.irsn.fr/FR/Larecherche/publications-documentation/fiches-radionucleides/Documents/environnement/Thorium_Th232_v1.pdf (accessed on 12 April 2022).
- Dalencourt, C. Stratégies Analytiques Pour L’extraction Séquencée du Th, U, Ra, Pb, Po dans des Matrices Environmentales. Ph.D. Thesis, Laval University, Laval, QC, Canada, 2019. [Google Scholar]
- Altmaier, M.; Neck, V.; Muller, R.; Fanghanel, T. Solubility of ThO2xH2)(Am) in Carbonate Solution and the Formation of Ternary Th(IV) Hydroxide-Carbonate Complexes. Radiochim. Acta 2005, 93, 83–92. [Google Scholar] [CrossRef]
- Martin, A.J.; McNee, J.; Crusius, J.; Yanful, E.K. Metal Mobility in Pre-Oxidized Subaqueous Uranium Mine Tailings. J. Appl. Geochem. 2003, 145, 307–339. [Google Scholar]
- Davé, N.K. Wet Barriers on Pyritic Uranium Mine Tailings. Part III. Laboratory Diffusion Lysimeter Studies of Uranium Tailings Deposited under a Shallow Water Cover. Mine Environment Neutral Drainage Program; Report 2.13.1b; Natural Resources Canada: Ottawa, ON, Canada, 1998. [Google Scholar]
- Decommissioning of Uranium Mine Tailings. Available online: https://publications.gc.ca/site/archivee-archived.html?url=https://publications.gc.ca/collections/collection_2017/acee-ceaa/En105-52-1996-eng.pdf (accessed on 6 April 2022).
- Abdelouas, A.; Lutze, W.; Nuttall, E. Chemical reactions of uranium in groundwater at a mill tailings site. J. Contam. Hydrol. 1998, 34, 343–361. [Google Scholar] [CrossRef]
- The Long Term Stabilization of Uranium Mill Tailings. Available online: https://www-pub.iaea.org/MTCD/Publications/PDF/te_1403_web.pdf (accessed on 6 April 2022).
- Peacey, V.; Yanful, E.K.; Payne, R. Field study of geochemistry and solute fluxes in flooded uranium mine tailings. Can. Geotech. J. 2002, 39, 357–376. [Google Scholar] [CrossRef]
- Paktunc, A.D.; Davé, N.K. Formation of Secondary Pyrite and Carbonate Minerals in the Lower Williams Lake Tailings Basin, Elliot Lake, Ontario, Canada. Am. Mineral. 2002, 87, 593–602. [Google Scholar] [CrossRef]
- Flint, L.E.; Flint, A.L. Porosity. In Methods of Soil Analysis: Part 4 Physical Methods, 5.4, 1st ed.; Topp, C.G., Ed.; Soil Science Society of America, Inc.: Madison, WI, USA, 2002; p. 241. [Google Scholar]
- Multi-Acid (4-Acid) Digestions. Available online: https://www.sgs.ca/en/mining/analytical-services/geochemistry/digestion-and-fusion/multi-acid-4acid-digestions (accessed on 31 January 2022).
- Field and Laboratory Methods Applicable to Overburden and Mine Soils. Available online: https://www.resolutionmineeis.us/sites/default/files/references/sobek-1978.pdf (accessed on 2 April 2021).
- Determination of Neutralizing Potential for Acid Rock Drainage Prediction. Available online: http://mend-nedem.org/wp-content/uploads/2013/01/1.16.3.pdf (accessed on 2 April 2021).
- Prediction Manual for Drainage Chemistry from Sulphidic Geologic Materials. Available online: http://www.abandoned-mines.org/pdfs/MENDPredictionManual-Jan05.pdf (accessed on 29 May 2020).
- McCready, R.G.L.; Wadden, D.; Marchbank, A. Nutrient requirements for the in-place leaching of uranium by Thiobacillus ferrooxidans. Hydrometallurgy 1986, 17, 61–71. [Google Scholar] [CrossRef]
- Lottermoser, B.G.; Ashley, P.M. Tailings dam seepage at the rehabilited Mary Kathleen uranium mine, Australia. J. Geochem. Explor. 2005, 85, 119–137. [Google Scholar] [CrossRef]
- Reynier, N.; Gagné-Turcotte, R.; Coudert, L.; Costis, S.; Cameron, R.; Blais, J.-F. Bioleaching of Uranium Tailings as Secondary Sources for Rare Earth Elements Production. Minerals 2021, 11, 302. [Google Scholar] [CrossRef]
- Davé, N.K. Assessment of Physico-Chemical Stability of the Lower William Lake Tailings Basin, Denison Mines, Elliot Lake, Ontario; Section 3: Table 3; CanmetMINES Internal Report, Report 2000-057; Natural Resources Canada: Ottawa, ON, Canada, 2001. [Google Scholar]
- Grenthe, I.; Drozdzynski, J.; Fujino, T.; Buck, E.C.; Albrecth-Schmitt, T.E.; Wolf, S.F. Uranium. In The Chemistry of the Actinide and Transactinide Elements, 3rd ed.; Morss, L.R., Edelstein, N., Fuger, J., Katz, J.J., Eds.; Springer: Dordrecht, The Netherlands, 2006; p. 253. [Google Scholar]
- Maya, L. Hydrolysis and carbonate complexation of dioxouranium(VI) in the neutral-pH range at 25 degrees-C. Inorg. Chem. 1982, 21, 2895–2898. [Google Scholar] [CrossRef]
- Blowes, D.W.; Ptacek, C.J.; Jambor, J.L.; Weisener, C.G.; Paktunc, D.; Gould, W.D.; Johnson, D.B. The geochemistry of Acid Mine Drainage. In Treatise on Geochemistry, 1st ed.; Sherwood Lollar, B., Holland, D.H., Turekian, K.K., Eds.; Elsevier: Burlington, ON, Canada, 2003; Volume 9, pp. 149–204. [Google Scholar]
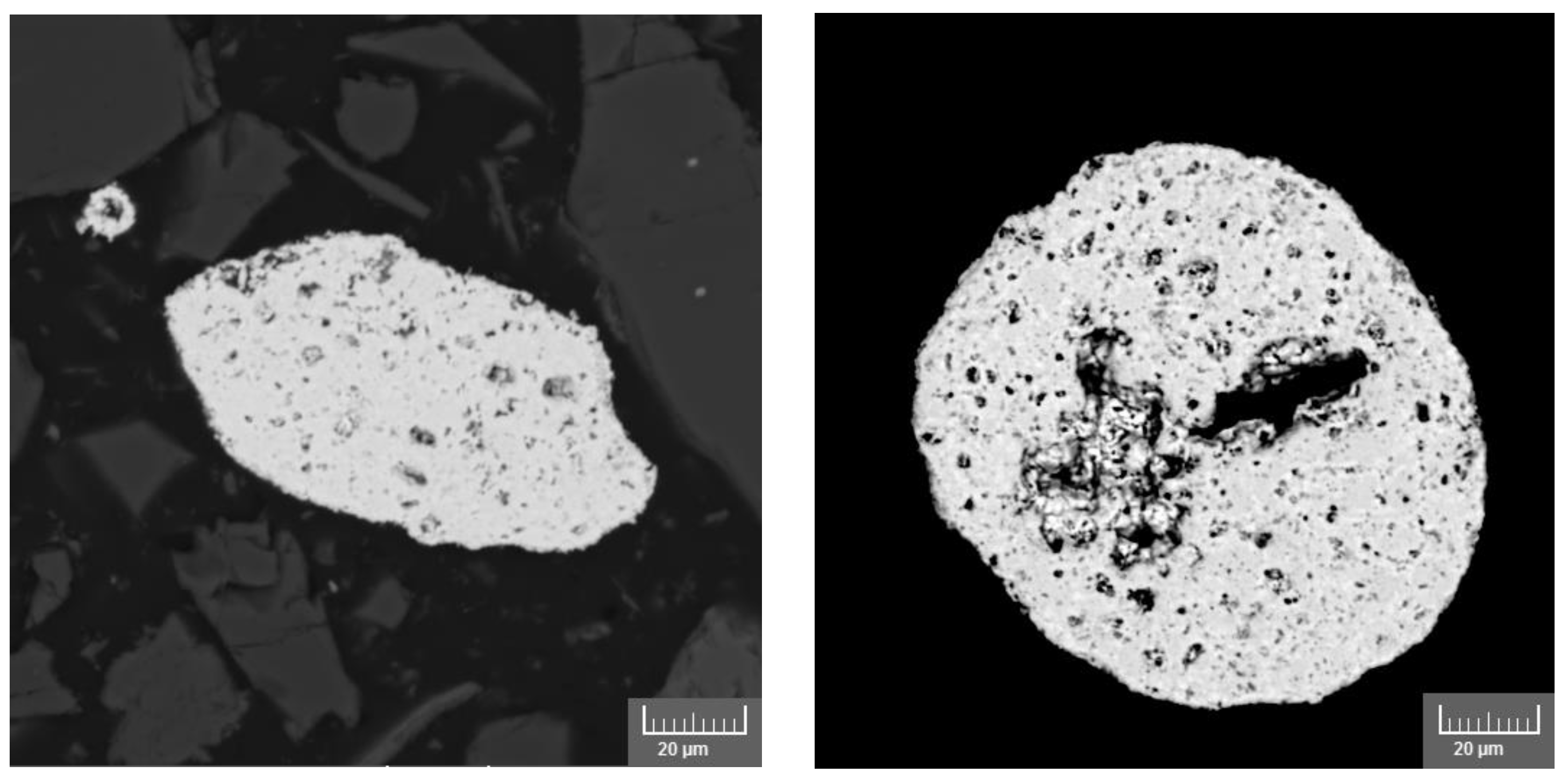

| Mineralogical Characteristics | Lower William Lake (Core Identified as LWL-4-3 M-2) [36] | BHP Tailings |
|---|---|---|
| Average pyrite content | 5.9% | 5.5% |
| Mineral phases (mass > 1%) | Quartz, K-feldspar, albite, muscovite, monazite, Fe-oxyhydroxide, pyrite, chalcopyrite | Quartz, muscovite, pyrite, orthoclase, anorthite, albite, calcite, kaersutite, hematite/magnetite |
| Porosity | 52.5% | 42.1% |
| Average Precipitation (mm) | |||||
|---|---|---|---|---|---|
| Time Period | Annual | Winter | Spring | Summer | Fall |
| 1961–1990 | 910 | 177 | 225 | 263 | 245 |
| 2025 | 936 (+2.9%) | 195 | 221 | 248 | 272 |
| 2055 | 961 (+5.5%) | 199 | 244 | 230 | 288 |
| 2085 | 960 (+5.5%) | 215 | 250 | 190 | 305 |
| Time Period | Column 1 and 2 * Reference Scenario | Column 3 and 4 * Low Rainfall Variability | Column 5 and 6 * High Rainfall Variability | Control | Blank |
|---|---|---|---|---|---|
| Week 1 | 145 mL (0.33) | 64 mL (0.15) | 0 mL (0) | 145 mL (0.33) | 145 mL (0.33) |
| Week 2 | 145 mL (0.33) | 145 mL (0.33) | 113 mL (0.26) | 145 mL (0.33) | 145 mL (0.33) |
| Week 3 | 145 mL (0.33) | 226 mL (0.52) | 322 mL (0.74) | 145 mL (0.33) | 145 mL (0.33) |
| Total—per cycle (mL) | 435 mL (1) | 435 mL (1) | 435 mL (1) | 435 mL (1) | 435 mL (1) |
| Mineral | Weight (wt% of the Phase) | Mineral | Weight (wt% of the Phase) |
|---|---|---|---|
| Quartz Amphibole Orthoclase | 55.9 | Uraninite | 0.29 |
| 9.7 | Monazite | 0.26 | |
| 4.7 | Ilmenite | 0.10 | |
| Muscovite | 4.7 | Pentlandite | 0.08 |
| Pyrite | 4.6 | Chalcopyrite | 0.08 |
| Anorthite | 3.5 | Zircon | 0.07 |
| Plagioclase | 2.8 | Anhydrite | 0.06 |
| Pyrrhotite | 1.7 | Apatite | 0.05 |
| Enstatite-(Fe) | 1.0 | Titanite | 0.04 |
| Albite | 1.0 | Allanite-(Ce) | 0.03 |
| Hematite/magnetite | 0.64 | Baryte | 0.02 |
| Calcite | 0.41 | Dolomite | 0.02 |
| Ankerite | 0.34 | Biotite | 0.01 |
| Ferrosaponite | 0.33 | Jacobsite | 0.01 |
| Rutile | 0.32 | Other | 7.3 |
| Elements | Average Concentration |
|---|---|
| Major elements (%) | |
| Mg | 0.43 ± 0.04 |
| Ca | 0.13 ± 0.05 |
| Fe | 3.06 ± 0.00 |
| Si | 31.81 ± 0.81 |
| S (total) | 2.26 ± 0.01 |
| C (total) | 0.16 ± 0.00 |
| C (inorg) | 0.16 ± 0.00 |
| Minor elements (mg/kg) | |
| Mn | 111.2 ± 17.3 |
| Ni | 161.8 ± 11.6 |
| Th | 122.1 ± 4.9 |
| U | 378.0 ± 19.1 |
| Sc | 4.8 ± 0.3 |
| Y | 18.9 ± 1.6 |
| La | 201.5 ± 13.3 |
| Ce | 645.0 ± 74.7 |
| Pr | 64.9 ± 7.2 |
| Nd | 205.3 ± 22.9 |
| Sm | 28.5 ± 2.9 |
| Eu | 1.3 ± 0.1 |
| Gd | 15.1 ± 1.5 |
| Tb | 1.3 ± 0.1 |
| Dy | 4.6 ± 0.3 |
| Ho | 0.6 ± 0.0 |
| Er | 1.1 ± 0.2 |
| Tm | 0.2 ± 0.0 |
| Yb | 1.0 ± 0.1 |
| Lu | 0.1 ± 0.0 |
| Minerals | Pristine Tailings (wt% of the Phase) | Leached Tailings (wt% of the Phase) |
|---|---|---|
| Quartz Amphibole Orthoclase | 55.9 | 62.6 |
| 9.7 | 7.6 | |
| 4.7 | 3.9 | |
| Muscovite | 4.7 | 5.0 |
| Pyrite | 4.6 | 4.7 |
| Anorthite | 3.5 | 2.0 |
| Plagioclase | 2.8 | 1.7 |
| Pyrrhotite | 1.7 | 0.9 |
| Enstatite-(Fe) | 1.0 | 0.8 |
| Albite | 1.0 | 0.8 |
| Hematite/Magnetite | 0.64 | 0.59 |
| Calcite | 0.41 | 0.00 |
| Ankerite | 0.34 | 0.21 |
| Ferrosaponite | 0.33 | 0.19 |
| Rutile | 0.32 | 0.30 |
| Uraninite | 0.29 | 0.00 |
| Monazite | 0.26 | 0.44 |
| Ilmenite | 0.10 | 0.09 |
| Chalcopyrite | 0.08 | 0.04 |
| Pentlandite | 0.08 | 0.26 |
| Zircon | 0.07 | 0.12 |
| Anhydrite | 0.06 | 0.01 |
| Apatite | 0.05 | 0.02 |
| Titanite | 0.04 | 0.02 |
| Allanite-(Ce) | 0.03 | 0.05 |
| Baryte | 0.02 | 0.05 |
| Dolomite | 0.02 | 0.00 |
| Biotite | 0.01 | 0.01 |
| Jacobsite | 0.01 | 0.02 |
| Unclassified | 7.3 | 7.5 |
| Total | 100.0 | 100.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gagné-Turcotte, R.; Reynier, N.; Larivière, D.; Zagrtdenov, N.R.; Goulet, R.; Huntsman, P. Impact of Variability in Precipitation Patterns on the Geochemistry of Pyritic Uranium Tailings Rehabilitated with Saturated Cover Technology. Mining 2022, 2, 385-401. https://doi.org/10.3390/mining2020020
Gagné-Turcotte R, Reynier N, Larivière D, Zagrtdenov NR, Goulet R, Huntsman P. Impact of Variability in Precipitation Patterns on the Geochemistry of Pyritic Uranium Tailings Rehabilitated with Saturated Cover Technology. Mining. 2022; 2(2):385-401. https://doi.org/10.3390/mining2020020
Chicago/Turabian StyleGagné-Turcotte, Roselyne, Nicolas Reynier, Dominic Larivière, Nail R. Zagrtdenov, Richard Goulet, and Philippa Huntsman. 2022. "Impact of Variability in Precipitation Patterns on the Geochemistry of Pyritic Uranium Tailings Rehabilitated with Saturated Cover Technology" Mining 2, no. 2: 385-401. https://doi.org/10.3390/mining2020020
APA StyleGagné-Turcotte, R., Reynier, N., Larivière, D., Zagrtdenov, N. R., Goulet, R., & Huntsman, P. (2022). Impact of Variability in Precipitation Patterns on the Geochemistry of Pyritic Uranium Tailings Rehabilitated with Saturated Cover Technology. Mining, 2(2), 385-401. https://doi.org/10.3390/mining2020020






