The field of structural biology has seen transformative advances that have significantly improved our understanding of protein misfolding and aggregation in Alzheimer’s disease (AD) [
28]. High-resolution techniques such as cryo-electron microscopy (cryo-EM), solid-state and solution nuclear magnetic resonance (NMR), and molecular dynamics simulations have illuminated the atomic-level architecture and dynamic behavior of amyloidogenic peptides [
27]. Recent high-resolution investigations have further elucidated the complex molecular architecture of amyloid deposits, with a focus on the nucleation and growth of amyloid-β (Aβ) fibrils. Advanced imaging techniques have enabled the visualization of the ordered and regular structure of Aβ
(25–35) fibrils grown epitaxially on various substrates, contrasting with the more polymorphic and less-defined aggregates typically observed in solution [
17]. This ordered growth behavior not only provides insight into the nucleation process but also underscores the critical role of substrate interactions in dictating fibril morphology. Complementary to these direct imaging studies, research employing tetramodal chemical imaging has revealed the significant influence of lipid–peptide interactions in amyloidogenesis. Studies have demonstrated that ganglioside GM1 promotes fibril growth and maturation by binding to Aβ peptides, implicating lipid microenvironments in the regulation of amyloid deposition [
39]. Furthermore, controlled deposition experiments on charged substrates, as reported in evaporation-driven liquid–liquid crystalline phase separation studies, have offered insight into the forces governing amyloid fibril assembly [
40]. Furthermore, the integration of polymer physics and structural biology has allowed for mechanical modeling of fibril stabilization, highlighting dominant roles of π–π stacking and hydrogen bonding [
4]. Intriguingly, exposed surface residues of fibrils can also act as nucleation sites for inorganic nanoparticles, potentially allowing the design of hybrid nanotherapeutics that both diagnose and modulate amyloid pathology [
23,
41]. Additionally, the exposure of functional groups on the surfaces of fibrils has been shown to serve as natural reduction sites for the nucleation of inorganic nanoparticles, hinting at potential hybrid therapeutic strategies aimed at modulating amyloid deposition [
41]. Together, these insights enhance understanding of pathological protein aggregation and can inform the rational design of nanostructured inhibitors. Building on these structural discoveries, the rational design of inhibitors targeting key epitopes on Aβ fibrils has emerged as a promising disease-modifying strategy. Molecular dynamics simulations have provided insights into the dynamic nature of Aβ42, specifically highlighting the flexibility of its “Greek key” motif spanning residues 18–26. This region has been observed to undergo conformational breathing, wherein its transient fluctuations create short-lived pockets that can serve as binding sites. Such dynamically induced pockets have significant implications for therapeutic intervention as they offer potential targets for gold nanoparticles (AuNPs) to bind, thereby disrupting the β-sheet stacking critical for amyloid fibril formation. Previous studies have underscored that those fluctuations in the central hydrophobic cluster can modulate the peptide’s overall structural ensemble and foster aggregation-prone states [
42,
43]. Furthermore, investigations employing replica exchange molecular dynamics simulations have demonstrated that interactions with gold nanoparticles can alter the conformational landscape of amyloid peptides by interfering with stabilizing intra- and intermolecular β-sheet contacts [
44,
45]. Collectively, these findings suggest that the inherent dynamic instability of the “Greek key” motif governs the early stages of aggregation and provides a mechanistic basis for the anti-amyloidogenic actions of gold nanoparticles.
4.1. Cryo-EM and NMR Insights into Amyloid-β (Aβ) and Tau Fibril Structures
Recent progress in structural biology has greatly enhanced our detailed understanding of Alzheimer’s disease. This improvement comes from using advanced methods such as cryogenic electron microscopy (cryo-EM) and nuclear magnetic resonance (NMR) spectroscopy. These powerful techniques allow us to clearly see the structures of the harmful proteins that play a role in Alzheimer’s. They show us not only the fully formed fiber-like structures but also the temporary, toxic protein clumps. These protein clumps are very important because they help us understand how the disease develops and gets worse over time.
Recent cryo-EM studies have provided near-atomic resolution views of tau fibrils, elucidating the detailed organization of the amyloid core and the conformational variability within neurofibrillary tangles. For example, Kuang et al. [
46] employed the cryo-EM structure of tau filaments extracted from AD brain tissue as a structural template for computational modeling and PET tracer binding studies, thereby correlating fibril architecture with in vivo diagnostic applications. Complementary to these findings, Mammeri et al. [
47] reported on phospho-mimetic tau constructs where cryo-EM, in conjunction with NMR measurements, allowed for the delineation of the rigid β-sheet core from the dynamically disordered “fuzzy coat” regions. These peripheral domains, although difficult to visualize in many cryo-EM maps, are significant for mediating interactions with small molecules and other cellular proteins, thus modulating fibril stability and propagation.
In a related investigation, Chakraborty et al. [
48] combined cryo-EM and solid-state NMR to study the co-factor-free aggregation of tau. Their work underscored the importance of intrinsic molecular features that enable tau to self-assemble into seeding-competent amyloid fibrils, drawing a connection between the atomic-level structure of tau and its observed neurotoxicity. Moreover, Savastano et al. [
49] exploited solid-state NMR to probe the involvement of the P2 region in tau amyloid fibrils, demonstrating that specific regions of the protein contribute to the rigid cross-β structure present in heparin-induced fibrils. Such detailed mapping is pivotal for understanding the structural determinants underlying both fibril formation and the toxic interactions of oligomeric species.
Turning to amyloid-β (Aβ) fibrils, atomic-resolution models derived from cryo-EM and NMR have shed light on the intricate β-sheet arrangement and the network of inter-residue contacts that stabilize these aggregates. Colvin et al. [
50] have provided one of the foremost atomic resolution structures of Aβ
42 fibrils, revealing how specific hydrogen bonding, side-chain packing, and β-sheet stacking reinforce fibril architecture. Beyond the fibrils themselves, advanced NMR methodologies have been instrumental in the atomic-level mapping of neurotoxic Aβ oligomers, species that precede fibril formation and are widely considered primary toxic agents in AD. Such residue-specific information illuminates early misfolding events and identifies potential binding sites for small-molecule inhibitors aimed at disrupting pathogenic aggregation pathways.
Using cryo-EM and NMR together gives us a full understanding of Alzheimer’s disease structures. Cryo-EM shows us how mature fibrils are built, while NMR lets us see how smaller groups of molecules, called oligomers, change shape [
51]. This combination helps us understand the disease better and aids in creating direct treatments. By focusing on key structural details, these methods assist in developing inhibitors and diagnostic tools that can precisely target the specific parts of fibrils or oligomers involved with Alzheimer’s [
52]. Importantly, these atomic-level insights are now being translated into real-world therapeutic and diagnostic innovations. For instance, the development of tau PET tracers such as [
18F]MK-6240 was directly guided by cryo-EM-derived models of tau filaments, enabling high-affinity binding to tau aggregates in vivo [
53]. Similarly, NMR-resolved structures of β-secretase (BACE1) informed the design of small-molecule inhibitors that have progressed to clinical evaluation [
54]. These examples underscore how atomic-resolution tools are not only elucidating pathogenic mechanisms but also accelerating the drug discovery pipeline for AD [
55]. Representative examples of such translational efforts are summarized in
Table 2. In summary, using cryo-EM and NMR together has changed our understanding of amyloid-β and tau structures in Alzheimer’s disease. Cryo-EM gives us a clear look at the detailed structure of mature fibril cores. NMR is useful for observing the small movements in temporary, harmful clusters called oligomers. This combination of technologies paves the way for developing new treatments. The goal of these treatments is to change how proteins clump together and slow down nerve-related damage in Alzheimer’s disease. These advancements are important for creating effective therapies for this condition.
4.2. Atomic-Level Mapping of Neurotoxic Oligomers
Recent advancements in mapping neurotoxic Aβ oligomers at the atomic level have greatly enhanced our understanding of Alzheimer’s disease. We have gained insights into the small structural traits that contribute to the toxicity of these oligomers and their interactions with receptors. High-resolution tools like solution and solid-state nuclear magnetic resonance (NMR) spectroscopy have become essential in this process. These technologies allow us to observe these temporary oligomer groupings at nearly the atomic scale, despite their tendency to be unstable and diverse in size and shape.
For example, Ling et al. [
56] investigated the toxicity mechanism of Aβ42 oligomers by characterizing their interactions with the GABA_B R1a sushi1 domain and key fragments of the amyloid precursor protein. Their findings suggest that the Aβ42 oligomer engages in a substitution-like binding mechanism that results in significant conformational changes at the atomic level. Such detailed mapping provides essential clues toward understanding how these oligomers disrupt receptor function and contribute to synaptic dysfunction. This receptor-level insight is particularly valuable because it offers a mechanistic foundation for developing molecular interventions that can specifically target and neutralize the toxic species.
Complementing this work, Harilal et al. [
57] employed state-of-the-art high-resolution NMR techniques to directly observe low-abundance Aβ oligomers without the need for extensive purification protocols. Their study successfully delineated residue-specific interactions within these oligomeric ensembles, revealing the dynamic interfaces that are likely to mediate the neurotoxic activity of these species. By mapping inter-residue contacts and backbone conformations, the work by Harilal et al. [
57] not only confirms the existence of distinct oligomeric states, but also establishes a framework for correlating specific structural features with neurotoxicity. These atomic-level insights are essential for the rational design of inhibitors that can stabilize non-toxic conformers or prevent the formation of pathogenic oligomeric intermediates.
These studies highlight the importance of looking at the atomic details to identify parts of Aβ oligomers that harm the brain. By mapping these small, temporary structures, we gain in two ways. First, we understand more about how amyloid proteins come together and form clumps. Second, we improve the design of treatments that aim to reduce the harmful effects associated with Alzheimer’s disease. This deeper understanding can lead to more effective ways to combat the disease’s toxic processes.
4.3. Nanotechnology Innovations
4.3.1. Nanoparticle-Based Drug Delivery (e.g., Crossing the Blood–Brain Barrier)
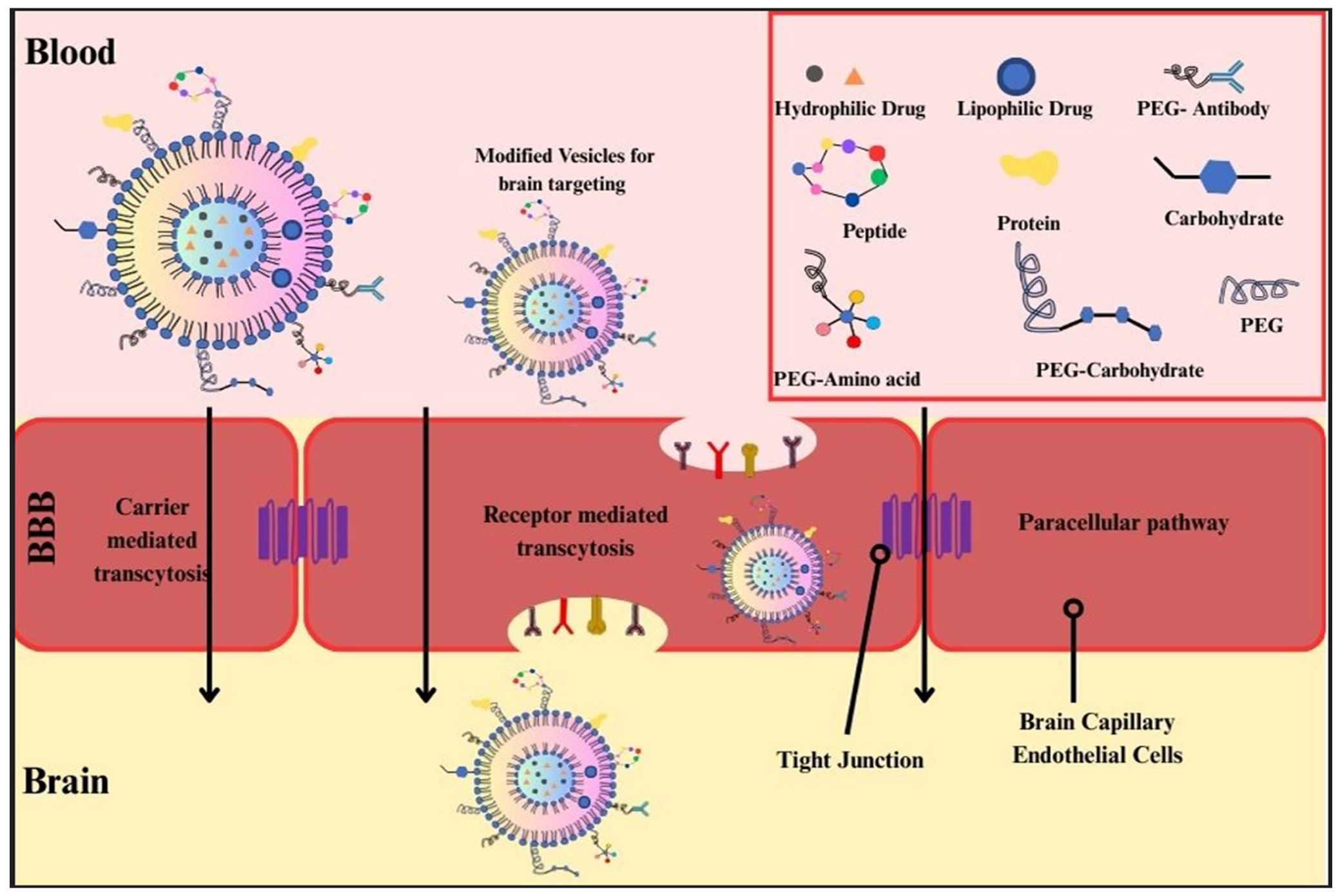
Nanoparticle-based drug delivery systems have emerged as a transformative strategy in the treatment of Alzheimer’s disease (AD), particularly in overcoming one of the most significant obstacles in neurotherapeutics: the blood–brain barrier (BBB). Nanotechnology offers unique approaches to ferry therapeutic agents across the BBB, thereby directly targeting pathological processes, such as β-amyloid aggregation and neurodegeneration.
An exciting development in this field is the method of attaching specific molecules known as targeting ligands to nanoparticles. These targeting ligands are designed to help the nanoparticles use the body’s own natural transport systems to reach specific areas more effectively. This approach shows great promise in improving how precisely these nanoparticles can deliver drugs or other treatments to the exact places they are needed within the body. By using the body’s pathways, this method could potentially increase the effectiveness of treatments while reducing side effects, as it ensures that the nanoparticles are directed to the correct target areas. For example, Choi et al. [
58] demonstrated that iron oxide nanoparticles conjugated with transferrin and loaded with melittin can substantially mitigate β-amyloid pathology in a 5XFAD mouse model. The transferrin receptor is found on the blood–brain barrier. It plays a crucial role in a process called receptor-mediated transcytosis. This process is important because it lets specially designed nanoparticles pass through the barrier. By doing this, these nanoparticles can deliver treatment molecules right to the parts of the brain that need them. These molecules are aimed at helping or healing the brain’s damaged areas directly. Similarly, Topal et al. [
59] reported that solid lipid nanoparticles, when targeted with apolipoprotein E (ApoE) ligands, showed enhanced permeability across an in vitro model of the BBB. These new formulations make it easier for drugs like donepezil to reach their target.
Figure 2 provides a schematic overview of the various nanoparticle modifications—such as PEGylation, ligand conjugation, and charge alterations—used to enhance blood–brain barrier penetration through mechanisms like receptor-mediated and carrier-mediated transcytosis. This means that targeted lipid-based nanocarriers might be a very effective way to treat Alzheimer’s Disease, offering a reliable platform for improving drug delivery in such therapies.
4.3.2. Mechanisms of Nanoparticle Interaction with Alzheimer’s Pathology
Nanoparticles interact with AD pathology via multiple mechanisms, including:
Crossing the blood–brain barrier via receptor-mediated (e.g., transferrin, ApoE) or carrier-mediated (e.g., glucose transporter) transcytosis [
60].
Targeting β-amyloid plaques by direct disruption, sequestration, or inhibition of fibril elongation [
61].
Enhancing neuronal drug delivery through ligand-directed targeting to neuronal membranes (e.g., Tet-1 peptide) [
62].
Enabling alternative delivery routes, such as intranasal administration, that bypass the BBB entirely [
63].
Polymeric nanoparticles have also been widely investigated for their versatility and biodegradability. Mathew et al. [
64] developed curcumin-loaded PLGA nanoparticles conjugated with the Tet-1 peptide, which selectively targets neuronal cells. This formulation not only improves the bioavailability of curcumin—a compound with antioxidant and anti-amyloid properties—but also facilitates its transport across the BBB, thereby reducing amyloid burden in the brain. In another study, Radwan et al. [
65] exploited chitosan-based nanoparticles for the controlled delivery of memantine, an NMDA receptor antagonist approved for moderate to severe AD. The mucoadhesive properties and biocompatibility of chitosan enhance nasal absorption, presenting an alternative, non-invasive route for central nervous system drug delivery.
Furthermore, Jain and Sharma [
66] explored the development, characterization, and evaluation of lactoferrin-conjugated, memantine-loaded PEG-PLGA nanoparticles. The lactoferrin ligand uses a process called receptor-mediated transcytosis to enter the brain. This process involves lactoferrin receptors on the small blood vessels in the brain [
67]. By doing this, it helps to deliver drugs to the brain more effectively. These methods highlight the importance of designing the surface of nanoparticles in a way that improves targeting accuracy. This careful design allows the drugs to enter the brain better while reducing the risk of side effects in other parts of the body. A summary of major nanoparticle platforms under investigation for Alzheimer’s therapy—including their functions and representative examples—is presented in
Table 3.
In addition to systemic administration, novel administration routes—such as intranasal delivery—have gained traction due to their non-invasive nature and ability to bypass the BBB entirely. Dighe et al. [
68] reviewed advances and challenges in intranasal drug delivery systems employing nanoparticles. These systems can increase brain bioavailability by leveraging direct nose-to-brain pathways and provide controlled release profiles that ensure sustained therapeutic levels in the target tissue.
4.3.3. Quantum Dots and Biosensors for Early Aβ Detection
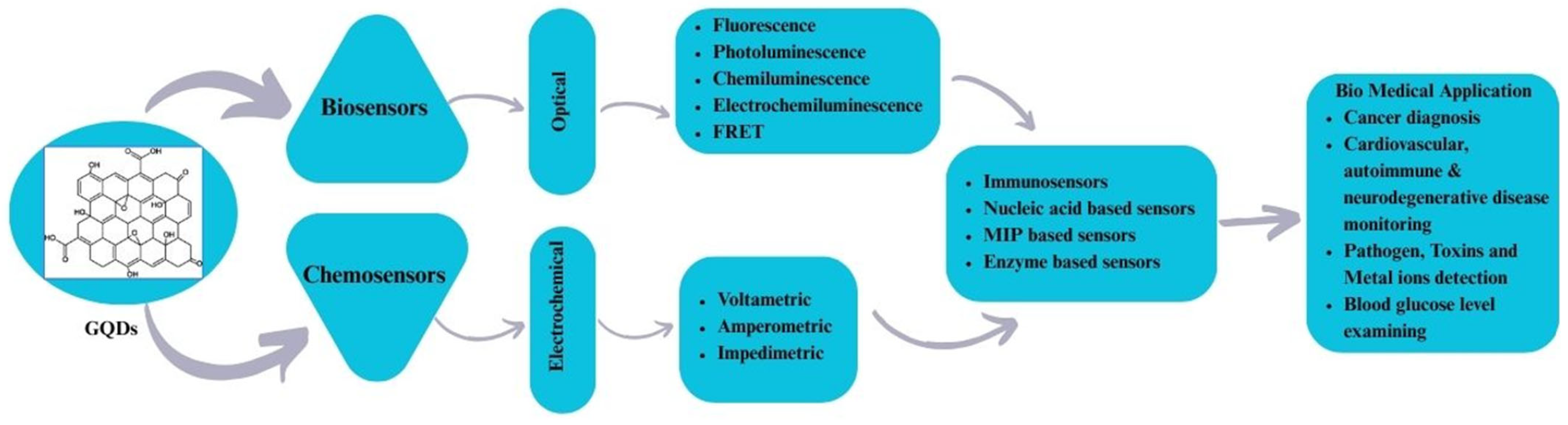
Recent progress in nanotechnology has led to the development of new biosensors and quantum dot (QD) platforms, which are expected to transform the early detection of amyloid-β (Aβ) in Alzheimer’s disease (AD) [
69,
70]. Quantum dots are tiny semiconductor crystals that have special optical properties. They can emit different colors of light based on their size, have high efficiency in producing light, and are very stable even when exposed to strong light. These features make them ideal for use in biosensors, as noted by Quan et al. [
71,
72]. These characteristics facilitate the design of highly sensitive and specific assays for Aβ detection, addressing the urgent clinical need to diagnose AD at its pre-symptomatic stage. The reviewed GQD-sensing techniques and their biomedical applications are outlined in
Figure 2 [
73]. The reviewed GQD-sensing techniques and their biomedical applications are illustrated in
Figure 3, highlighting the roles of graphene quantum dots as optical and electrochemical biosensors in detecting Aβ and related biomarkers.
One promising approach leverages quantum dot-based optical biosensors, which operate by conjugating QDs with targeting ligands, such as antibodies or aptamers, that selectively bind Aβ peptides. Quan et al. [
71] demonstrated that Aβ-targeted QDs can enhance both detection specificity and sensitivity relative to conventional fluorescent probes. This improvement is attributed to the robust fluorescence signal and resistance to photobleaching exhibited by QDs, allowing for reliable quantification of Aβ even at very low concentrations. Similarly, Balci et al. [
72] reported on a paper-based aptasensor that incorporates CdTe quantum dots for the simultaneous detection of Aβ(1–42) and phosphorylated tau (p-tau181) from plasma samples. The integration of QDs in this biosensor afforded a rapid, cost-effective, and non-invasive platform that holds significant potential for early AD diagnosis and large-scale screening.
In parallel to optical platforms, biosensor strategies based on advanced fluorescence lifetime imaging microscopy (FLIM) have been developed. Battisti [
74] introduced a FLIM-phasor analysis method that monitors Aβ-induced alterations in membrane order. This cell-based biosensor capitalizes on the sensitive fluorescence lifetime changes induced by Aβ oligomers interacting with the cell membrane. The approach facilitates real-time detection of early pathophysiological events related to AD and underscores the potential of nanotechnology-enabled biosensors to provide both qualitative and quantitative insights into Aβ dynamics [
75].
Using quantum dots in detection and biosensor engineering has many benefits. One key advantage is carrying out tests that detect different Aβ species or related biomarkers simultaneously. This technology can be built into portable diagnostic devices that are easy to use. By combining these systems with small-scale fluid systems and advanced data study methods, we can create fast and precise diagnostic tools that can be used directly where needed. These tools have the potential to greatly improve how we detect and manage various health conditions by bringing high-quality testing to more places in an easy and accessible way [
71,
72].
Although there have been promising improvements, some challenges still exist with quantum dot-based biosensors. These challenges include ensuring they remain compatible with living organisms over time, can be produced consistently, and manufactured on a large scale. More research and development are needed to resolve issues like potential toxicity of certain quantum dots and interference from complex biological environments. However, innovations in improving surface coatings and creating non-toxic quantum dots, such as those using graphene or carbon dots, are helping to advance these technologies toward clinical use [
71].
Table 4 summarizes key challenges associated with nanomedicine for AD—including blood–brain barrier permeability and QD toxicity—and outlines current solutions leveraging biocompatible nanocarriers and ligand-functionalized systems.
In a nutshell, recent advances in nanotechnology, particularly with quantum dots and biosensor platforms, have greatly enhanced our ability to detect early signs of Aβ biomarkers related to Alzheimer’s disease. Researchers leverage the special light-based qualities of quantum dots and integrate them with sensitive biosensor designs. This progress is leading toward the development of fast, easy-to-use, and very accurate tools for diagnosing Alzheimer’s. These advancements could significantly change the way doctors manage and treat Alzheimer’s disease.
4.3.4. Comparative Performance of Nanocarriers in AD Therapy
Among various nanocarriers studied for Alzheimer’s disease (AD), PLGA nanoparticles, liposomes, and exosomes are the most prominent. Each exhibits distinct advantages in terms of blood–brain barrier (BBB) penetration, targeting, immunogenicity, and therapeutic efficacy.
PLGA nanoparticles are biodegradable and versatile, allowing for surface modification with ligands (e.g., Tet-1, lactoferrin) to enhance BBB transport [
76]. They offer high drug-loading capacity and have demonstrated reductions in amyloid burden and cognitive improvement in AD models, though circulation time can be limited without PEGylation [
77].
Liposomes mimic cell membranes and support both hydrophilic and lipophilic drug loading. PEGylated liposomes improve stability and brain uptake of drugs like curcumin and donepezil. However, moderate immunogenicity and rapid clearance remain concerns [
78].
Exosomes, due to their endogenous origin, naturally cross the BBB and show strong neuronal targeting with minimal immune activation [
79]. In preclinical studies, exosome-loaded siRNAs and proteins have effectively reduced plaques and improved cognition. Yet, scalability and standardization remain significant hurdles [
80].
Table 5 displays information on comparative features of nanocarriers in AD preclinical models.
Recent comparative studies suggest that exosomes outperform synthetic carriers in neuronal uptake and therapeutic outcomes, though PLGA NPs and liposomes remain superior in drug loading, stability, and scalability [
80]. Choosing the appropriate carrier depends on the specific therapeutic context and target delivery goals.
4.4. Therapeutic Breakthroughs
4.4.1. Nanomaterials Targeting Protein Aggregation (e.g., Graphene Oxide, Gold Nanoparticles)
Researchers are exploring nanomaterials as a potential new treatment for Alzheimer’s disease (AD). These tiny materials work by preventing harmful proteins such as amyloid-β (Aβ) and tau from clumping together, which can cause damage to the brain. In Alzheimer’s, when these proteins stick together, they create problems that affect memory and thinking. There is a lot of interest in using materials like graphene oxide derivatives and gold nanoparticles (AuNPs) for this purpose. These materials are attractive for research because they have special qualities that are both physical and chemical. Additionally, their surfaces can be modified in many ways to meet various medical needs, making them versatile tools in the fight against Alzheimer’s.
Graphene-based nanomaterials have a large surface area and adjustable electronic properties. These features help them connect effectively with the water-repellent parts of amyloidogenic peptides. For example, graphene quantum dots (GQDs) can prevent Aβ from coming together into clumps. GQDs attach to the peptide’s water-repelling core, which disrupts the starting and growing stages necessary for forming fibrils, helping to stop the accumulation process [
81,
82]. These interactions include π–π stacking, hydrophobic contacts, and electrostatic forces, which together help keep Aβ in non-toxic shapes. Graphene-based nanomaterials have very low toxicity to cells and are compatible with the body. This makes them great potential treatments. They can be designed to move through the blood–brain barrier with few side effects, which is an important quality for medicines targeting the brain [
81,
83].
Gold nanoparticles exemplify another class of nanomaterials with significant potential for modulating protein aggregation. Their plasmonic properties and ease of surface functionalization allow for the conjugation of targeting ligands that can selectively bind Aβ species. Araya et al. [
84] demonstrated that AuNPs, particularly when used in conjunction with microwave irradiation, can impede Aβ amyloidogenesis by interfering with the early stages of peptide aggregation. Such inhibition appears to stem from the disruption of intermolecular interactions required for fibril stabilization. Additionally, functionalized AuNPs have been utilized not only to inhibit aggregation but also to facilitate the disaggregation of preformed fibrils, thereby offering a dual therapeutic approach [
84].
Collectively, these nanomaterials operate via mechanisms that include multivalent binding to amyloidogenic sequences, inhibition of nucleation, and disruption of fibril elongation. The high surface-to-volume ratios inherent to nanomaterials enable them to sequester misfolded proteins effectively, thereby preventing the cascade of toxic aggregation events that underlies AD pathology. Moreover, ongoing efforts in surface modification and conjugation strategies are improving the selectivity and delivery of these nanomaterials, potentially facilitating their translation into clinically viable therapies.
In summary, the integration of graphene oxide derivatives and gold nanoparticles into therapeutic platforms illustrates a significant advancement toward directly mitigating pathological protein aggregation. These nanomaterials harness unique interfacial interactions to disrupt the aggregation processes of Aβ and tau, thereby providing a promising direction for disease modification strategies in Alzheimer’s disease. Continued interdisciplinary research is essential for optimizing these systems to enhance bioavailability, minimize toxicity, and ultimately achieve clinical efficacy in neurodegenerative disease treatment.
4.4.2. Gene-Editing Tools (CRISPR-Nano) for APOE4 Modulation
Therapeutic breakthroughs in Alzheimer’s disease are increasingly being driven by innovative gene-editing strategies that combine CRISPR technology with nanoscale delivery systems, specifically targeting genetic risk factors such as the APOE4 allele. The APOE4 gene variant is a well-established genetic risk factor for late-onset Alzheimer’s disease, and its modulation represents a promising approach to mitigate disease progression. Recent studies have suggested that employing CRISPR-based systems could potentially be used to modify the APOE4 genotype and, thereby, reduce amyloid accumulation and associated neurodegeneration.
For instance, Teter et al. [
85] reported progress using a synthetic exosome-delivered CRISPR complex, administered intravenously in Alzheimer model mice. Their system successfully edited the APOE4 allele, converting it to the less pathogenic APOE3 isoform within the brain [
86]. Although this study highlights the potential precision of CRISPR-Cas9 in targeting disease-relevant mutations, detailed efficacy data, longitudinal behavioral outcomes, and off-target analysis remain limited in preclinical contexts [
87].
Compared to conventional adeno-associated virus (AAV)-based delivery systems, CRISPR-nano platforms offer several potential advantages [
88]. AAVs are limited by fixed payload capacity, immunogenicity risks, and persistent expression of Cas9, which increases the risk of unintended gene edits over time [
89]. In contrast, nanoparticle-mediated CRISPR delivery—such as lipid nanoparticles or exosome mimetics—enables transient Cas9 expression, reducing the window for off-target mutagenesis [
90]. Additionally, nanoparticles can be engineered to avoid immune detection, tuned for controlled release, and designed to cross the blood–brain barrier without viral capsids.
Off-target effects remain a major concern in genome editing. These unintended edits, often resulting from imperfect guide RNA specificity or prolonged Cas9 activity, can lead to genomic instability or oncogenesis [
91]. To mitigate this, current CRISPR-nano approaches are exploring high-fidelity Cas variants (e.g., SpCas9-HF1, eCas9) and shorter half-life Cas9 mRNA/protein complexes to limit exposure and increase targeting precision [
92].
Immune responses to both the CRISPR system and delivery vehicles also require careful consideration. While AAVs are known to elicit neutralizing antibodies, especially in patients with prior viral exposure, non-viral nano-delivery systems are increasingly favored for their lower immunogenic profiles and greater biocompatibility, particularly in the context of brain-targeted therapies [
93]. Lipid nanoparticles and exosomes, in particular, have shown promise in avoiding detection by the innate immune system, although repeated dosing strategies still need further investigation [
94].
Recent preclinical advancements include a tandem peptide-lipid CRISPR-Cas9 platform developed by Rahmanto et al. [
95], which targets both APP and APOE4 genes. This dual-targeting approach achieved greater specificity and improved uptake in neuronal cultures, suggesting a broader impact on AD pathology.
Although clinical trials of CRISPR-nano platforms in Alzheimer’s are not yet registered, their successful use in early human trials for other conditions (e.g., transthyretin amyloidosis, sickle cell disease) validates the translational feasibility of nanoparticle-mediated CRISPR delivery in systemic and even CNS-related diseases [
96]. It is anticipated that first-in-human trials targeting APOE4 via CRISPR-nano will emerge following validation in large-animal models and safety profiling [
97].