Application Potential of Lysinibacillus sp. UA7 for the Remediation of Cadmium Pollution
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of Urease-Producing Strains
2.2. Determination of Urease Activity
2.3. Optimization of Cultural Conditions
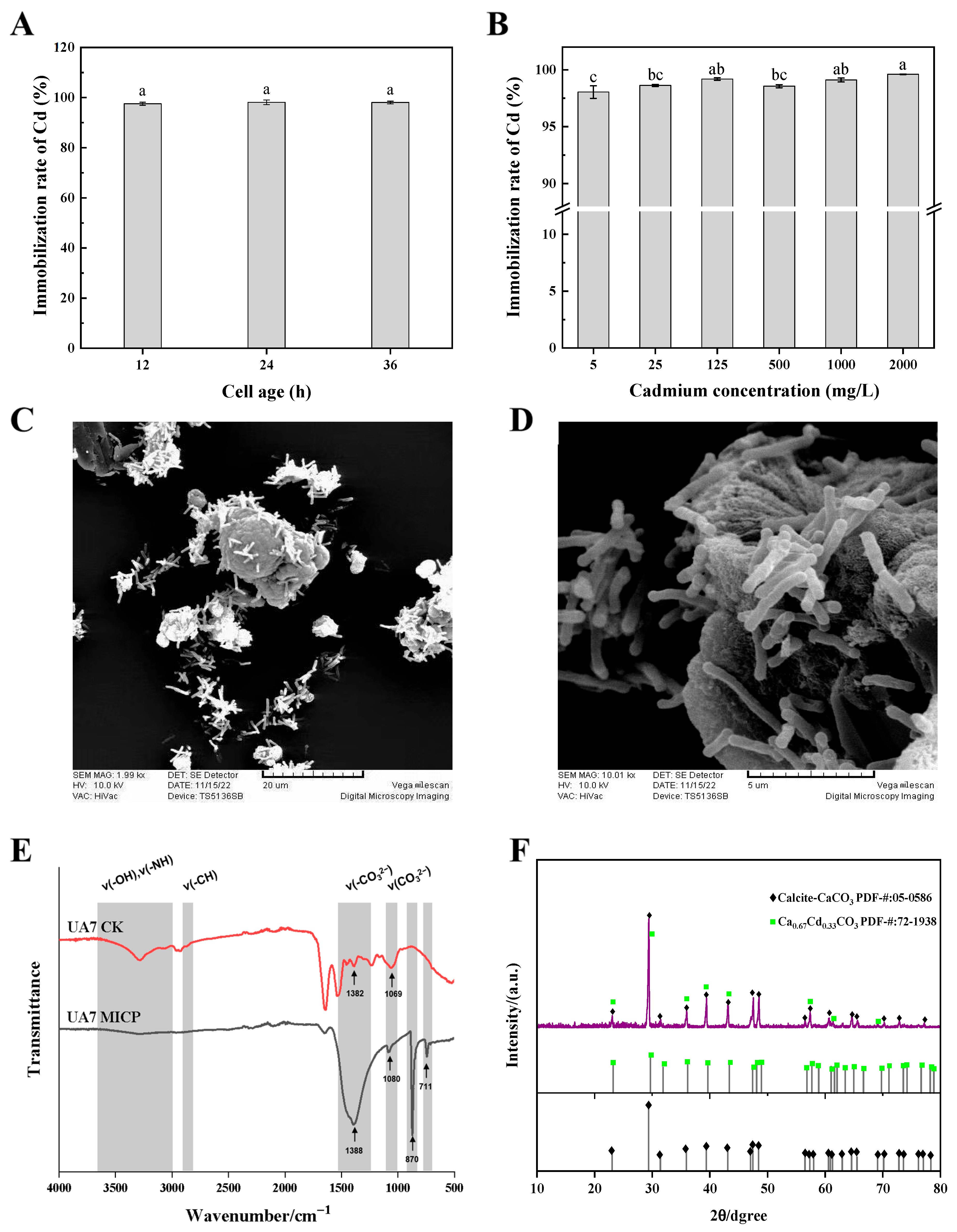
2.4. Determination of the Cd Immobilization Rate in Water
2.5. Characterization of Immobilized Products
2.6. Determination of the Cd Immobilization Rate in Soil
2.7. Planting Test
2.8. Data Analysis
3. Results
3.1. Isolation and Identification of Urease-Producing Strain
3.2. Urease Production of Strain UA7
3.3. Immobilization of Cd by Strain UA7 in Water
3.4. Immobilization of Cd by Strain UA7 in Soil
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pan, Z.W.; Gong, T.Y.; Liang, P. Heavy Metal Exposure and Cardiovascular Disease. Circ. Res. 2024, 134, 1160–1178. [Google Scholar] [CrossRef]
- Khan, M.A.; Khan, S.; Khan, A.; Alam, M. Soil contamination with cadmium, consequences and remediation using organic amendments. Sci. Total Environ. 2017, 601–602, 1591–1605. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.H. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The effects of cadmium toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef] [PubMed]
- Bravo, D.; Leon-Moreno, C.; Martínez, C.A.; Varón-Ramírez, V.M.; Araujo-Carrillo, G.A.; Vargas, R.; Quiroga-Mateus, R.; Zamora, A.; Rodríguez, E.A.G. The first national survey of cadmium in cacao farm soil in Colombia. Agronomy 2021, 11, 761. [Google Scholar] [CrossRef]
- Nde, S.C.; Felicite, O.M.; Aruwajoye, G.S.; Palamuleni, L.G. A meta-analysis and experimental survey of heavy metals pollution in agricultural soils. J. Trace Elem. Miner. 2024, 9, 100180. [Google Scholar] [CrossRef]
- Yuan, X.H.; Xue, N.D.; Han, Z.G. A meta-analysis of heavy metals pollution in farmland and urban soils in China over the past 20 years. J. Environ. Sci. 2021, 101, 217–226. [Google Scholar] [CrossRef]
- Kang, C.H.; Han, S.H.; Shin, Y.J.; Oh, S.J.; So, J.S. Bioremediation of Cd by microbially induced calcite precipitation. Appl. Biochem. Biotechnol. 2014, 172, 645. [Google Scholar] [CrossRef]
- Peng, D.H.; Qiao, S.Y.; Luo, Y.; Ma, H.; Zhang, L.; Hou, S.Y.; Wu, B.; Xu, H. Performance of microbial induced carbonate precipitation for immobilizing Cd in water and soil. J. Hazard. Mater. 2020, 400, 123116. [Google Scholar] [CrossRef]
- Ji, G.S.; Huan, C.C.; Zeng, Y.; Lyu, Q.Y.; Du, Y.L.; Liu, Y.; Xu, L.S.; He, Y.; Tian, X.P.; Yan, Z.Y. Microbiologically induced calcite precipitation (MICP) in situ remediated heavy metal contamination in sludge nutrient soil. J. Hazard. Mater. 2024, 473, 134600. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Wang, M.; Wang, H.; Tang, D.; Huang, J.; Sun, Y. Study on the remediation of Cd pollution by the biomineralization of urease-producing bacteria. Int. J. Environ. Res. Public Health 2019, 16, 268. [Google Scholar] [CrossRef] [PubMed]
- Rajasekar, A.; Wilkinson, S.; Moy, C.K.S. MICP as a potential sustainable technique to treat or entrap contaminants in the natural environment: A review. Environ. Sci. Technol. 2021, 6, 100096. [Google Scholar] [CrossRef] [PubMed]
- Jalilvand, N.; Akhgar, A.; Alikhani, H.A.; Rahmani, H.A.; Rejali, F. Removal of heavy metals zinc, lead, and cadmium by biomineralization of urease-producing bacteria isolated from Iranian mine calcareous soils. J. Soil Sci. Plant Nut. 2020, 20, 206–219. [Google Scholar] [CrossRef]
- Omoregie, A.I.; Khoshdelnezamiha, G.; Senian, N.; Ong, D.E.L.; Nissom, P.M. Experimental optimisation of various cultural conditions on urease activity for isolated Sporosarcina pasteurii strains and evaluation of their biocement potentials. Ecol. Eng. 2017, 109, 65–75. [Google Scholar] [CrossRef]
- Wang, T.J.; Wang, S.L.; Tang, X.C.; Fan, X.P.; Yang, S.; Yao, L.G.; Li, Y.D.; Han, H. Isolation of urease-producing bacteria and their effects on reducing Cd and Pb accumulation in lettuce (Lactuca sativa L.). Environ. Sci. Pollut. Res. 2020, 27, 8707–8718. [Google Scholar] [CrossRef]
- Mekonnen, E.; Kebede, A.; Nigussie, A.; Kebede, G.; Tafesse, M. Isolation and characterization of urease-producing soil bacteria. Int. J. Microbiol. 2021, 2021, 8888641. [Google Scholar] [CrossRef]
- Ma, B.; Song, W.L.; Zhang, X.X.; Chen, M.X.; Li, J.P.; Yang, X.Q.; Zhang, L. Potential application of novel cadmium-tolerant bacteria in bioremediation of Cd-contaminated soil. Ecotoxicol. Environ. Saf. 2023, 255, 114766. [Google Scholar] [CrossRef]
- Hu, X.; He, B.; Liu, Y.; Ma, S.; Yu, C. Genomic characterization of a novelureolytic bacteria, Lysinibacillus capsici TSBLM, and its application to the remediation of acidic heavy metal-contaminated soil. Sci. Total Environ. 2024, 927, 172170. [Google Scholar] [CrossRef]
- Chen, C.R.; Li, X.; Liang, J.T.; Yang, X.; Hu, Z.Y.; Li, J.Y.; Xue, Y.W. The role of Lysinibacillus fusiformis S01 in cadmium removal from water and immobilization in soil. J. Hazard. Mater. 2025, 485, 136828. [Google Scholar] [CrossRef]
- Diez-Marulanda, J.C.; Brandao, P.F.B. Isolation of urease-producing bacteria from cocoa farms soils in Santander, Colombia, for cadmium remediation. 3 Biotech 2023, 13, 98. [Google Scholar] [CrossRef]
- Weatherburn, M.W. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 1967, 39, 971–974. [Google Scholar] [CrossRef]
- Özkan, A.; Uygu, V. Determination of heavy metal concentrations in agricultural lands of amik plain with MP-AES. Fresen. Environ. Bull. 2019, 28, 416–425. [Google Scholar]
- Zhou, X.F.; Yang, Y.; Yin, Q.X.; Zhang, X.; Li, M.T. Application potential of Comamonas testosteroni ZG2 for vegetable cultivation in nickel and cadmium polluted soil. Environ. Technol. Innov. 2021, 23, 101626. [Google Scholar] [CrossRef]
- Huang, J.; Yan, L.; Cai, W.S.; Zhong, Q.S.; Wang, S.H. Distribution of do, substrate enzyme activities and operating performance of constructed wetlands for wastewater treatment. Fresen. Environ. Bull. 2013, 22, 2816–2822. [Google Scholar]
- Geisseler, D.; Scow, K.M. Long-term effects of mineral fertilizers on soil microorganisms—A review. Soil Boil. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- Yang, Q.; Liang, X.R.; Wang, L.; Yang, R.Y.; He, C.Z.; Tu, C.L.; Zhan, F.D.; He, Y.M. Urease-producing bacteria with plant growth-promoting ability that may tolerate and remove cadmium from aqueous solution. Int. J. Phytoremediat. 2024, 26, 2010–2020. [Google Scholar] [CrossRef] [PubMed]
- Su, N.N.; Wang, K.; Zhang, Z.T.; Yao, L.G.; Chen, Z.J.; Han, H. Urease-producing bacteria combined with pig manure biochar immobilize Cd and inhibit the absorption of Cd in lettuce (Lactuca sativa L.). Environ. Sci. Pollut. Res. 2024, 31, 45537–45552. [Google Scholar] [CrossRef]
- Wei, T.; Yashir, N.; An, F.Q.; Imtiaz, S.A.; Li, X.; Li, H. Study on the performance of carbonate-mineralized bacteria combined with eggshell for immobilizing Pb and Cd in water and soil. Environ. Sci. Pollut. Res. 2022, 29, 2924–2935. [Google Scholar] [CrossRef]
- Whiffin, V.S.; van Paassen, L.A.; Harkes, M.P. Microbial carbonate precipitation as a soil improvement technique. Geomicrobiol. J. 2007, 24, 417–423. [Google Scholar] [CrossRef]
- Do, H.; Wang, Y.Q.; Long, Z.H.; Ketehouli, T.; Li, X.; Zhao, Z.J.; Li, M.T. A psychrotolerant Ni-resistant Bacillus cereus D2 induces carbonate precipitation of nickel at low temperature. Ecotoxicol. Environ. Saf. 2020, 198, 110672–110680. [Google Scholar] [CrossRef]
- Ahsan, N.; Shimizu, M. Lysinibacillus species: Their potential as effective bioremediation, biostimulant, and biocontrol agents. Rev. Agric. Sci. 2021, 9, 103–116. [Google Scholar] [CrossRef]
- Zhou, W.; Zhu, Y.Q.; Achal, V. Synergistic mechanisms of humic acid and biomineralization in cadmium remediation using Lysinibacillus fusiformis. Environ. Microbiol. Rep. 2024, 16, e70037. [Google Scholar] [CrossRef]



| Bacterial Strains | Urease Activity (U/mL) | Initial Cd Concentration, Immobilization Rate, and Treatment Duration | Reference |
|---|---|---|---|
| Enterobacter bugandensis TJ6 | ~35.6 ① | 5 mg/L, 84.9%, 24 h | Wang et al., 2020 [15] |
| Bacillus megaterium HD8 | ~54.2 ① | 5 mg/L, 93.2%, 24 h | |
| Serratia marcescens C5-6 | 804.51 ③ | 5 mg/L, 69.83%, 48 h | Yang et al., 2024 [26] |
| Lysinibacillus fusiformis S01 | NA | 5 mg/L, 80%, 2 d | Chen et al., 2025 [19] |
| Enterobacter sp. TJ6 | 36.1 ① | 10 mg/L, 61.3%, 5 d | Su et al., 2024 [27] |
| Enterobacter sp. | NA | 20 mg/L, 99.50%, 7 d | Peng et al., 2020 [9] |
| Paenarthrobactor nitroguajacolicus | NA | 100 mg/L, 46%, 144 h | Ma et al., 2023 [17] |
| Cupriavidus sp. CZW-2 | 51.6 ① | 2 mM, 80.10%, 120 h | Zhao et al., 2019 [11] |
| Stenotrophomonas rhizophila A323 | 1.65 ① | 2 mM, 71.3%, 72 h | Jalilvand et al., 2019 [13] |
| Variovorax boronicumulans C113 | 1.46 ① | 2 mM, 73.45%, 72 h | |
| Stenotrophomonas pasteurii | 11.08 ① | 2 mM, 97.15%, 72 h | |
| Bacillus sp. UR21 | 55.2 ① | 2 mM, 65.0%, 72 h | Wei et al., 2022 [28] |
| Lysinibacillus sp. UA7 | 188 ② | 2000 mg/L (7.57 mM), 99.61%, 36 h | This study |
| Comamonas testosteroni ZG2 | ~200 ② | ~11.9 mM, 98.4%, 48 h | Zhou et al., 2021 [23] |
| Bacterial Strains | Urease Activity (U/mL) | Initial Cd Concentration, Immobilization Rate and Treatment Duration | Reference |
|---|---|---|---|
| Comamonas testosteroni ZG2 | ~200 ② | 0.448 mg/kg, 42.86%, 1 week + 30 days | Zhou et al., 2021 [23] |
| Enterobacter sp. TJ6 | 36.1 ① | 3.14 mg/kg, 49.1%, 56 days | Su et al., 2024 [27] |
| Lysinibacillus fusiformis S01 | NA | 3.504 mg/kg, 76.96%, 7 days | Chen et al. 2025 [19] |
| Cupriavidus sp. CZW-2 | 51.6 ① | 5.10 mg/kg, 53.30%, 2 weeks + 1 month | Zhao et al., 2019 [11] |
| Lysinibacillus fusiformis S01 | NA | 9.324 mg/kg, 66.43%, 7 days | Chen et al. 2025 [19] |
| Bacillus sp. UR21 | 55.2 ① | 10 mg/kg, 25.2%, 40 days | Wei et al., 2022 [28] |
| Enterobacter sp. | NA | 20 mg/kg, 56.10%, 40 days | Peng et al., 2020 [9] |
| Lysinibacillus sp. UA7 | 188 ② | 20 mg/kg, 70.25%, 10 days | This study |
| 50 mg/kg, 63.37%, 10 days |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.; Zhao, P.; Shi, H.; Xue, F. Application Potential of Lysinibacillus sp. UA7 for the Remediation of Cadmium Pollution. BioChem 2025, 5, 34. https://doi.org/10.3390/biochem5040034
Liang Y, Zhao P, Shi H, Xue F. Application Potential of Lysinibacillus sp. UA7 for the Remediation of Cadmium Pollution. BioChem. 2025; 5(4):34. https://doi.org/10.3390/biochem5040034
Chicago/Turabian StyleLiang, Yue, Peng Zhao, Haoran Shi, and Feiyan Xue. 2025. "Application Potential of Lysinibacillus sp. UA7 for the Remediation of Cadmium Pollution" BioChem 5, no. 4: 34. https://doi.org/10.3390/biochem5040034
APA StyleLiang, Y., Zhao, P., Shi, H., & Xue, F. (2025). Application Potential of Lysinibacillus sp. UA7 for the Remediation of Cadmium Pollution. BioChem, 5(4), 34. https://doi.org/10.3390/biochem5040034







