Biochemical Programming of the Fungal Cell Wall: A Synthetic Biology Blueprint for Advanced Mycelium-Based Materials
Abstract
1. Introduction: Fungi as Master Builders of Programmable Matter
2. The Fungal Cell Wall: A Hierarchical Nanocomposite Blueprint
2.1. The Chitin–Glucan Scaffold: The Structural Backbone
2.2. The Glycoprotein Matrix: A Functional and Adaptive Interface
2.3. Hydrophobins: Nature’s Amphipathic Surfactants
2.4. Architectural Plasticity and Interspecies Variation
2.5. Comparative Analysis: Fungal vs. Crustacean Chitin/Chitosan
3. Biochemical Levers for Tuning Mycelium Material Properties
3.1. Substrate-Driven Morphogenesis and Composition
3.2. The Fungal Secretome as an in Situ Modification Toolkit
3.3. Environmental Signaling as a Control Mechanism: The Role of pH
4. A Synthetic Biology Toolkit for Designing “Smart” Mycelium Materials
4.1. Rational Design Through Genetic Engineering of Cell Wall Architecture
4.2. Chemical Genetics: Dynamic and Reversible Control of Material Properties
4.3. Programming Functionality: Engineering Surfaces and Bioreceptivity
- Surface Display of Adhesion Ligands: Engineering the fungus to express and display specific peptides (like the RGD sequence) or proteins on its surface that promote the adhesion of specific human cell types (e.g., fibroblasts, osteoblasts).
- Controlled Release of Growth Factors: Modifying the fungus to synthesize and secrete human growth factors that stimulate cell proliferation and differentiation within the scaffold.
- Tuning Biodegradability: Altering the expression of cell wall cross-linking enzymes to control the rate at which the scaffold degrades in vivo, ensuring that it persists long enough to support tissue formation but is eventually cleared by the body.
4.4. Inducible Systems and Biosensors: Towards Living Functional Materials (LFMs)
5. The Translational Pathway: From Myco-Fabrication to Commercial Product
5.1. Sterilization and Post-Processing Imperatives
- Steam Sterilization (Autoclave): This method involves exposing the material to high temperatures (>121 °C) and pressure (>15 PSI) for a defined period. It has been shown to effectively inactivate mycelium while preserving the essential architecture of the cell wall, which is critical for maintaining mechanical properties [97].
- Gamma Irradiation: Gamma rays are highly penetrating and are a standard for sterilizing medical devices. However, a significant drawback for polymer-based materials is that gamma irradiation can cause “chain scission,” leading to a reduction in the material’s tensile strength and elongation [105,106].
- Ethylene Oxide (EtO): EtO is a low-temperature gaseous process ideal for heat-sensitive materials. The primary concern with this method is the potential for toxic residues, such as ethylene chlorohydrin, which must be rigorously validated to ensure compliance with standards like ISO 10993-7 for patient safety [107].
5.2. Biocompatibility, Regulations, and Standardization
- Biocompatibility: Materials intended for medical use must undergo a comprehensive biological evaluation to ensure they do not cause adverse reactions in the human body. This is governed by the ISO 10993 series of standards. Key tests include ISO 10993-5 [108] (cytotoxicity), which verifies the material is not toxic to cells, and ISO 10993-18 (chemical characterization), which identifies any leachable compounds [109].
- Standardization: The lack of standardized testing methods remains a major challenge [110]. For mycelium composites, a combination of existing industrial standards (e.g., ASTM, ISO) is necessary to evaluate properties like compressive strength and water absorption. Additionally, the adoption of Good Manufacturing Practices (GMPs) is crucial to ensure product quality and consistency across production batches.
- Mycotoxin Safety: A fundamental safety consideration is the potential for mycotoxin production by certain fungal species, such as Aspergillus and Penicillium [87]. For any application, especially in consumer or medical products, a rigorous screening process is essential to select non-mycotoxic strains to ensure public health and safety [87].
6. Future Perspectives and Recommendations
6.1. Development of Predictive Models
6.2. Expanding the Fungal Toolkit
6.3. Harnessing Multi-Kingdom Interactions
6.4. Addressing Scalability and Standardization
6.5. Ensuring Safety and Public Acceptance
7. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGS | α-1,3-glucan synthase |
| BFI | Bacterial-Fungal Interactions |
| CAZymes | Carbohydrate-Active enzymes |
| CHS | Chitin Synthases |
| DNMT | DNA Methyltransferase |
| ECM | Extracellular Matrix |
| FKS/GLS | Genes encoding β-(1,3)-glucan synthase |
| GAG | Galactosaminogalactan |
| GlcNAc | N-acetyl-D-glucosamine |
| HDAC | Histone Deacetylase |
| LFM | Living Functional Materials |
| NaOH | Sodium Hydroxide |
| PDB | Potato Dextrose Broth |
| PSK | Polysaccharide-K |
| PSP | Polysaccharopeptide |
| SAHA | Suberanilohydroxamic acid |
| TSA | Trichostatin A |
| WCA | Water Contact Angle |
References
- Javadian, A.; Le Ferrand, H.; Hebel, D.E.; Saeidi, N. Application of Mycelium-Bound Composite Materials in Construction Industry: A Short Review. SOJ Mater. Sci. Eng. 2020, 7, 1–9. [Google Scholar] [CrossRef]
- Zou, G.; Li, T.; Mijakovic, I.; Wei, Y. Synthetic biology enables mushrooms to meet emerging sustainable challenges. Front. Microbiol. 2024, 15, 1337398. [Google Scholar] [CrossRef]
- Yang, L.; Park, D.; Qin, Z. Material Function of Mycelium-Based Bio-Composite: A Review. Front. Mater. 2021, 8, 737377. [Google Scholar] [CrossRef]
- Jo, C.; Zhang, J.; Tam, J.M.; Church, G.M.; Khalil, A.S.; Segrè, D.; Tang, T.-C. Unlocking the magic in mycelium: Using synthetic biology to optimize filamentous fungi for biomanufacturing and sustainability. Mater. Today Bio 2023, 19, 100560. [Google Scholar] [CrossRef]
- Billerbeck, S.; Oliveira, A.G.; Gonçalves, A.P. Editorial: Fungi as cell factories: Genetic engineering and applications. Front. Bioeng. Biotechnol. 2022, 10, 1109992. [Google Scholar] [CrossRef] [PubMed]
- Antinori, M.E.; Contardi, M.; Suarato, G.; Armirotti, A.; Bertorelli, R.; Mancini, G.; Debellis, D.; Athanassiou, A. Advanced mycelium materials as potential self-growing biomedical scaffolds. Sci. Rep. 2021, 11, 12630. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.G.; Westrick, N.M.; Baldwin, T.T. Fungal biotechnology: From yesterday to tomorrow. Front. Fungal Biol. 2023, 4, 1135263. [Google Scholar] [CrossRef] [PubMed]
- Meyer, V. Connecting materials sciences with fungal biology: A sea of possibilities. Fungal Biol. Biotechnol. 2022, 9, 5. [Google Scholar] [CrossRef]
- Goldman, G.H. New Opportunities for Modern Fungal Biology. Front. Fungal Biol. 2020, 1, 596090. [Google Scholar] [CrossRef]
- Corbu, V.M.; Gheorghe-Barbu, I.; Dumbravă, A.Ș.; Vrâncianu, C.O.; Șesan, T.E. Current Insights in Fungal Importance—A Comprehensive Review. Microorganisms 2023, 11, 1384. [Google Scholar] [CrossRef]
- Daâssi, D.; Bouassida, M.; Almaghrabi, F.; Chamkha, M. Mycoremediation: An Innovative and Sustainable Approach. In Bioremediation for Environmental Sustainability; Elsevier: Amsterdam, The Netherlands, 2025. [Google Scholar]
- Madusanka, C.; Udayanga, D.; Nilmini, R.; Rajapaksha, S.; Hewawasam, C.; Manamgoda, D.; Vasco-Correa, J. A review of recent advances in fungal mycelium based composites. Discov. Mater. 2024, 4, 13. [Google Scholar] [CrossRef]
- Elsacker, E.; Vandelook, S.; Brancart, J.; Peeters, E.; De Laet, L. Mechanical, physical and chemical characterisation of mycelium-based composites with different types of lignocellulosic substrates. PLoS ONE 2019, 14, e0213954. [Google Scholar] [CrossRef]
- Alemu, D.; Tafesse, M.; Mondal, A.K. Mycelium-Based Composite: The Future Sustainable Biomaterial. Int. J. Biomater. 2022, 2022, 8401528. [Google Scholar] [CrossRef]
- Antinori, M.E.; Ceseracciu, L.; Mancini, G.; Heredia-Guerrero, J.A.; Athanassiou, A. Fine-Tuning of Physicochemical Properties and Growth Dynamics of Mycelium-Based Materials. ACS Appl. Bio Mater. 2020, 3, 1044–1051. [Google Scholar] [CrossRef]
- Meyer, V.; Andersen, M.R.; Brakhage, A.A.; Braus, G.H.; Caddick, M.X.; Cairns, T.C.; de Vries, R.P.; Haarmann, T.; Hansen, K.; Hertz-Fowler, C.; et al. Current challenges of research on filamentous fungi in relation to human welfare and a sustainable bio-economy: A white paper. Fungal Biol. Biotechnol. 2016, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Cairns, T.C.; Nai, C.; Meyer, V. How a fungus shapes biotechnology: 100 years of Aspergillus niger research. Fungal Biol. Biotechnol. 2018, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Haneef, M.; Ceseracciu, L.; Canale, C.; Bayer, I.S.; Heredia-Guerrero, J.A.; Athanassiou, A. Advanced Materials from Fungal Mycelium: Fabrication and Tuning of Physical Properties. Sci. Rep. 2017, 7, 41292. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.; Mautner, A.; Luenco, S.; Bismarck, A.; John, S. Engineered mycelium composite construction materials from fungal biorefineries: A critical review. Mater. Des. 2020, 187, 108397. [Google Scholar] [CrossRef]
- Adrio, J.L.; Demain, A.L. Fungal biotechnology. Int. Microbiol. 2003, 6, 191–199. [Google Scholar] [CrossRef]
- van den Brink, J.; de Vries, R.P. Fungal enzyme sets for plant polysaccharide degradation. Appl. Microbiol. Biotechnol. 2011, 91, 1477–1492. [Google Scholar] [CrossRef]
- Gow, N.A.R.; Latge, J.-P.; Munro, C.A. The Fungal Cell Wall: Structure, Biosynthesis, and Function. Microbiol. Spectr. 2017, 5, 10.1128. [Google Scholar] [CrossRef]
- Garcia-Rubio, R.; de Oliveira, H.C.; Rivera, J.; Trevijano-Contador, N. The Fungal Cell Wall: Candida, Cryptococcus, and Aspergillus Species. Front. Microbiol. 2020, 10, 2993. [Google Scholar] [CrossRef] [PubMed]
- Padalia, K.B. Biology and Diversity of Viruses, Bacteria and Fungi (Paper Code: BOT 501). Available online: https://es.scribd.com/document/528627502/%D8%AD%D9%84%D9%88-%D9%81%D8%B7%D8%B1 (accessed on 10 August 2025).
- Wessels, J.G.H. Developmental regulation of fungal cell wall formation. Annu. Rev. Phytopathol. 1994, 32, 413–437. [Google Scholar] [CrossRef]
- Fuertes-Rabanal, M.; Rebaque, D.; Largo-Gosens, A.; Encina, A.; Mélida, H. Cell walls: A comparative view of the composition of cell surfaces of plants, algae, and microorganisms. J. Exp. Bot. 2025, 76, 2614–2645. [Google Scholar] [CrossRef]
- Nawawi, W.M.F.B.W.; Jones, M.; Murphy, R.J.; Lee, K.-Y.; Kontturi, E.; Bismarck, A. Nanomaterials Derived from Fungal Sources—Is It the New Hype? Biomacromolecules 2020, 21, 30–55. [Google Scholar] [CrossRef]
- Li, R.; Hsueh, P.-H.; Ulfadillah, S.A.; Wang, S.-T.; Tsai, M.-L. Exploring the Sustainable Utilization of Deep Eutectic Solvents for Chitin Isolation from Diverse Sources. Polymers 2024, 16, 3187. [Google Scholar] [CrossRef]
- Jones, M.; Huynh, T.; Dekiwadia, C.; Daver, F.; John, S. Mycelium Composites: A Review of Engineering Characteristics and Growth Kinetics. J. Bionanosci. 2017, 11, 241–257. [Google Scholar] [CrossRef]
- Guerriero, G.; Avino, M.; Zhou, Q.; Fugelstad, J.; Clergeot, P.-H.; Bulone, V. Chitin Synthases from Saprolegnia Are Involved in Tip Growth and Represent a Potential Target for Anti-Oomycete Drugs. PLoS Pathog. 2010, 6, e1001070. [Google Scholar] [CrossRef]
- Joo, S.-M.; Kim, Y.-G.; Kwak, Y.-J.; Yoo, D.J.; Jeong, C.-U.; Park, J.; Oh, M.-S. Enhanced Long-Term Reliability of Seal DeltaSpot Welded Dissimilar Joint between 6061 Aluminum Alloy and Galvannealed Steel via Excimer Laser Irradiation. Materials 2021, 14, 6756. [Google Scholar] [CrossRef]
- Cortina-Escribano, M.; Pihlava, J.-M.; Miina, J.; Veteli, P.; Linnakoski, R.; Vanhanen, H. Effect of Strain, Wood Substrate and Cold Treatment on the Yield and β-Glucan Content of Ganoderma lucidum Fruiting Bodies. Molecules 2020, 25, 4732. [Google Scholar] [CrossRef]
- Fazli Wan Nawawi, W.M.; Lee, K.-Y.; Kontturi, E.; Murphy, R.J.; Bismarck, A. Chitin Nanopaper from Mushroom Extract: Natural Composite of Nanofibers and Glucan from a Single Biobased Source. ACS Sustain. Chem. Eng. 2019, 7, 6492–6496. [Google Scholar] [CrossRef]
- Jiménez-Ortigosa, C.; Aimanianda, V.; Muszkieta, L.; Mouyna, I.; Alsteens, D.; Pire, S.; Beau, R.; Krappmann, S.; Beauvais, A.; Dufrêne, Y.F.; et al. Chitin Synthases with a Myosin Motor-Like Domain Control the Resistance of Aspergillus fumigatus to Echinocandins. Antimicrob. Agents Chemother. 2012, 56, 6121–6131. [Google Scholar] [CrossRef]
- Kleijburg, F.E.L.; Safeer, A.A.; Baldus, M.; Wösten, H.A.B. Binding of micro-nutrients to the cell wall of the fungus Schizophyllum commune. Cell Surf. 2023, 10, 100108. [Google Scholar] [CrossRef]
- Free, S.J. Fungal Cell Wall Organization and Biosynthesis. Adv. Genet. 2013, 81, 33–82. [Google Scholar]
- Universitat Politècnica de Catalunya. Book of Abstracts of All the Posters: Barcelona, Spain, 29th June–3rd July 2025: Precision Agriculture: A Reality for Everyone; Universitat Politècnica de Catalunya: Barcelona, Spain, 2025; ISBN 9791387613570. [Google Scholar]
- Kuştaş, S.; Gezer, E.D. Physical and mechanical properties of mycelium-based insulation materials produced from desilicated wheat straws—Part A. BioResources 2024, 19, 1330–1347. [Google Scholar] [CrossRef]
- Castoria, R.; De Curtis, F.; Lima, G.; De Cicco, V. β-1,3-glucanase activity of two saprophytic yeasts and possible mode of action as biocontrol agents against postharvest diseases. Postharvest Biol. Technol. 1997, 12, 293–300. [Google Scholar] [CrossRef]
- Ökmen, B.; Jaeger, E.; Schilling, L.; Finke, N.; Klemd, A.; Lee, Y.J.; Wemhöner, R.; Pauly, M.; Neumann, U.; Doehlemann, G. A conserved enzyme of smut fungi facilitates cell-to-cell extension in the plant bundle sheath. Nat. Commun. 2022, 13, 6003. [Google Scholar] [CrossRef] [PubMed]
- Terauchi, Y.; Nagayama, M.; Tanaka, T.; Tanabe, H.; Yoshimi, A.; Nanatani, K.; Yabu, H.; Arita, T.; Higuchi, T.; Kameda, T.; et al. Adsorption Kinetics and Self-Assembled Structures of Aspergillus oryzae Hydrophobin RolA on Hydrophobic and Charged Solid Surfaces. Appl. Environ. Microbiol. 2022, 88, e02087-21. [Google Scholar] [CrossRef] [PubMed]
- Zangi, R.; de Vocht, M.L.; Robillard, G.T.; Mark, A.E. Molecular Dynamics Study of the Folding of Hydrophobin SC3 at a Hydrophilic/Hydrophobic Interface. Biophys. J. 2002, 83, 112–124. [Google Scholar] [CrossRef]
- Chau, H.W.; Si, B.C.; Goh, Y.K.; Vujanovic, V. A novel method for identifying hydrophobicity on fungal surfaces. Mycol. Res. 2009, 113, 1046–1052. [Google Scholar] [CrossRef]
- Morris, V.K.; Ren, Q.; Macindoe, I.; Kwan, A.H.; Byrne, N.; Sunde, M. Recruitment of Class I Hydrophobins to the Air:Water Interface Initiates a Multi-step Process of Functional Amyloid Formation. J. Biol. Chem. 2011, 286, 15955–15963. [Google Scholar] [CrossRef] [PubMed]
- Valentin, E.; Herrero, E.; Pastor, F.I.J.; Sentandreu, R. Solubilization and Analysis of Mannoprotein Molecules from the Cell Wall of Saccharomyces cerevisiae. Microbiology 1984, 130, 1419–1428. [Google Scholar] [CrossRef]
- van den Brandhof, J.G.; Hansen, N.; Hou, C.; Broers, S.C.; Tegelaar, M.; Wösten, H.A.B. Characterization of pure mycelium materials from different mushroom-forming fungi. Antonie Leeuwenhoek 2025, 118, 121. [Google Scholar] [CrossRef]
- Gunaratnam, G.; Dudek, J.; Jung, P.; Becker, S.L.; Jacobs, K.; Bischoff, M.; Hannig, M. Quantification of the Adhesion Strength of Candida albicans to Tooth Enamel. Microorganisms 2021, 9, 2213. [Google Scholar] [CrossRef]
- Sousa, I.C.G.; Teixeira, S.C.; Souza, M.V.d.; Conde, M.B.M.; Bailon, G.R.; Cardoso, S.H.S.; Araújo, L.D.; Oliveira, E.B.d.; Ferreira, S.O.; Oliveira, T.V.d.; et al. Sustainable Extraction and Multimodal Characterization of Fungal Chitosan from Agaricus bisporus. Foods 2025, 14, 2785. [Google Scholar] [CrossRef]
- Habtemariam, S. Trametes versicolor (Synn. Coriolus versicolor) Polysaccharides in Cancer Therapy: Targets and Efficacy. Biomedicines 2020, 8, 135. [Google Scholar] [CrossRef]
- Macindoe, I.; Kwan, A.H.; Ren, Q.; Morris, V.K.; Yang, W.; Mackay, J.P.; Sunde, M. Self-assembly of functional, amphipathic amyloid monolayers by the fungal hydrophobin EAS. Proc. Natl. Acad. Sci. USA 2012, 109, E804–E811. [Google Scholar] [CrossRef]
- Tovar-Herrera, O.E.; Martha-Paz, A.M.; Pérez-LLano, Y.; Aranda, E.; Tacoronte-Morales, J.E.; Pedroso-Cabrera, M.T.; Arévalo-Niño, K.; Folch-Mallol, J.L.; Batista-García, R.A. Schizophyllum commune: An unexploited source for lignocellulose degrading enzymes. Microbiologyopen 2018, 7, e00637. [Google Scholar] [CrossRef]
- Adav, S.S.; Sze, S.K. Fungal Secretome for Biorefinery: Recent Advances in Proteomic Technology. Mass Spectrom. Lett. 2013, 4, 1–9. [Google Scholar] [CrossRef]
- Ballen Sierra, L.A.; Mendes-Pereira, T.; García, G.J.Y.; Werkhaizer, C.Q.; de Rezende, J.B.; Rodrigues, T.A.B.; Badotti, F.; Cardoso, E.S.d.C.; da Costa, A.M.; Uetanabaro, A.P.; et al. Current situation and future perspectives for the use of fungi in the biomaterial industry and proposal for a new classification of fungal-derived materials. PeerJ Mater. Sci. 2023, 5, e31. [Google Scholar] [CrossRef]
- Mattern, D.J.; Valiante, V.; Unkles, S.E.; Brakhage, A.A. Synthetic biology of fungal natural products. Front. Microbiol. 2015, 6, 775. [Google Scholar] [CrossRef]
- Varriale, L.; Ulber, R. Fungal-Based Biorefinery: From Renewable Resources to Organic Acids. ChemBioEng Rev. 2023, 10, 272–292. [Google Scholar] [CrossRef]
- Vieira, R.I.M.; Peixoto, A.d.S.; Monclaro, A.V.; Ricart, C.A.O.; Filho, E.X.F.; Miller, R.N.G.; Gomes, T.G. Fungal Coculture: Unlocking the Potential for Efficient Bioconversion of Lignocellulosic Biomass. J. Fungi 2025, 11, 458. [Google Scholar] [CrossRef] [PubMed]
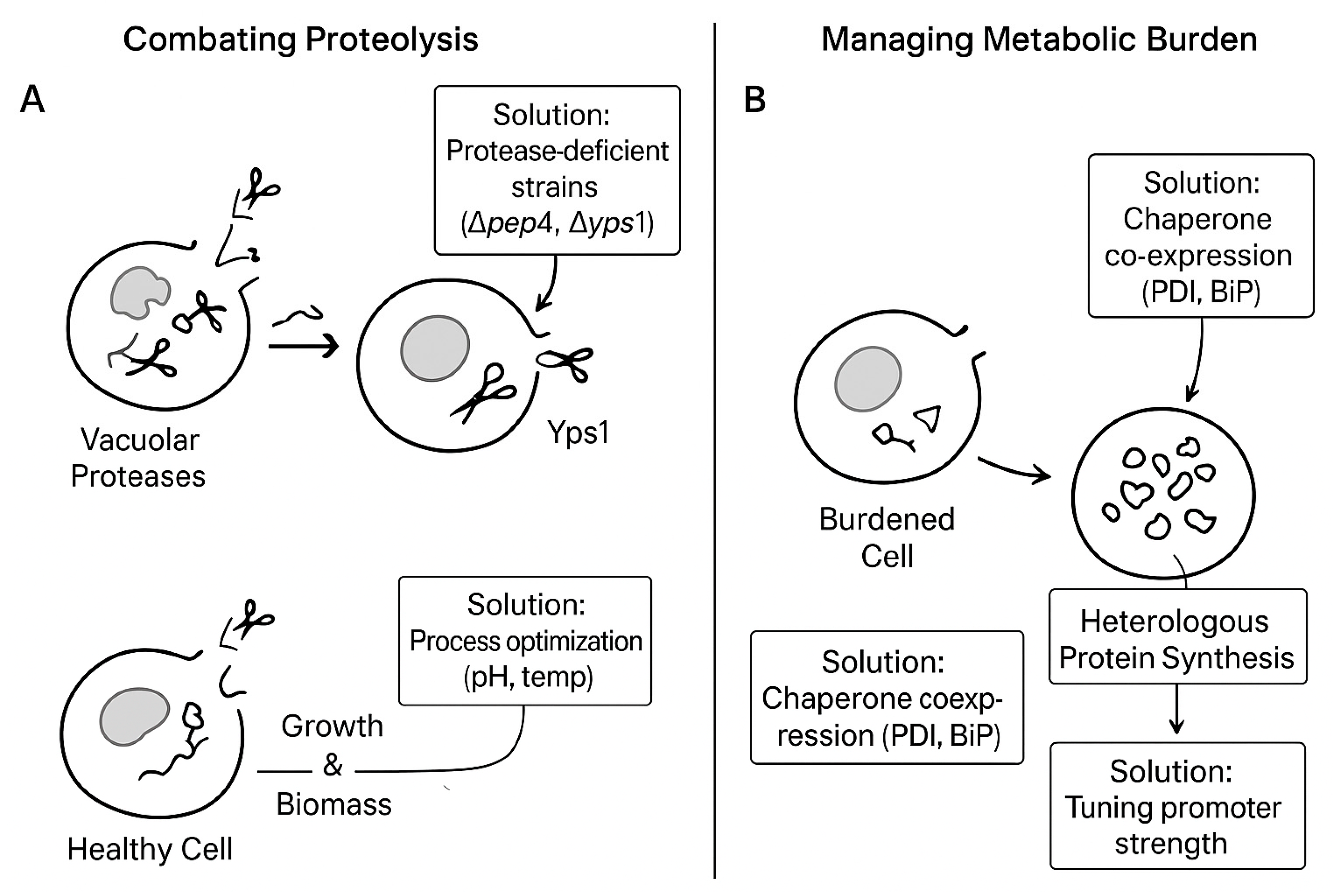
- Mattanovich, D.; Branduardi, P.; Dato, L.; Gasser, B.; Sauer, M.; Porro, D. Recombinant Protein Production in Yeasts. Methods Mol. Biol. 2012, 824, 329–358. [Google Scholar]
- Xu, L.; Bai, X.; Joong Oh, E. Strategic approaches for designing yeast strains as protein secretion and display platforms. Crit. Rev. Biotechnol. 2025, 45, 491–508. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Hirz, M.; Pichler, H.; Schwab, H. Protein expression in Pichia pastoris: Recent achievements and perspectives for heterologous protein production. Appl. Microbiol. Biotechnol. 2014, 98, 5301–5317. [Google Scholar] [CrossRef]
- Trinh, T.T.T.; Ngo, H.T.; Truong, P.Q. High cell density fermentation strategies for the production of bovine lactoferrin in Pichia pastoris. J. Appl. Biol. Biotechnol. 2023, 11, 58–65. [Google Scholar] [CrossRef]
- Schuster, E.; Dunn-Coleman, N.; Frisvad, J.C.; Van Dijck, P.W. On the safety of Aspergillus niger—A review. Appl. Microbiol. Biotechnol. 2002, 59, 426–435. [Google Scholar] [CrossRef]
- Latif, A.; Hassan, N.; Ali, H.; Niazi, M.B.K.; Jahan, Z.; Ghuman, I.L.; Hassan, F.; Saqib, A. An overview of key industrial product citric acid production by Aspergillus niger and its application. J. Ind. Microbiol. Biotechnol. 2024, 52, kuaf007. [Google Scholar] [CrossRef]
- Shen, S.C.; Lee, N.A.; Lockett, W.J.; Acuil, A.D.; Gazdus, H.B.; Spitzer, B.N.; Buehler, M.J. Robust myco-composites: A biocomposite platform for versatile hybrid-living materials. Mater. Horizons 2024, 11, 1689–1703. [Google Scholar] [CrossRef]
- Peñalva, M.A.; Tilburn, J.; Bignell, E.; Arst, H.N. Ambient pH gene regulation in fungi: Making connections. Trends Microbiol. 2008, 16, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Lara-Martínez, D.; Tristán-Flores, F.E.; Cervantes-Montelongo, J.A.; Silva-Martínez, G.A. Fungal Stress Responses and the Importance of GPCRs. J. Fungi 2025, 11, 213. [Google Scholar] [CrossRef] [PubMed]
- Cornet, M.; Gaillardin, C. pH Signaling in Human Fungal Pathogens: A New Target for Antifungal Strategies. Eukaryot. Cell 2014, 13, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Kováčová, K.; Degani, G.; Stratilová, E.; Farkaš, V.; Popolo, L. Catalytic properties of Phr family members of cell wall glucan remodeling enzymes: Implications for the adaptation of Candida albicans to ambient pH. FEMS Yeast Res. 2015, 15, fou011. [Google Scholar] [CrossRef]
- Brown, H.E.; Telzrow, C.L.; Saelens, J.W.; Fernandes, L.; Alspaugh, J.A. Sterol-Response Pathways Mediate Alkaline Survival in Diverse Fungi. mBio 2020, 11, e00719-20. [Google Scholar] [CrossRef]
- Jeennor, S.; Anantayanon, J.; Panchanawaporn, S.; Chutrakul, C.; Laoteng, K. Morphologically engineered strain of Aspergillus oryzae as a cell chassis for production development of functional lipids. Gene 2019, 718, 144073. [Google Scholar] [CrossRef]
- Appels, F.V.W.; Camere, S.; Montalti, M.; Karana, E.; Jansen, K.M.B.; Dijksterhuis, J.; Krijgsheld, P.; Wösten, H.A.B. Fabrication factors influencing mechanical, moisture- and water-related properties of mycelium-based composites. Mater. Des. 2019, 161, 64–71. [Google Scholar] [CrossRef]
- Izadi, H.; Asadi, H.; Bemani, M. Chitin: A comparison between its main sources. Front. Mater. 2025, 12, 1537067. [Google Scholar] [CrossRef]
- Sayfutdinova, A.; Samofalova, I.; Barkov, A.; Cherednichenko, K.; Rimashevskiy, D.; Vinokurov, V. Structure and Properties of Cellulose/Mycelium Biocomposites. Polymers 2022, 14, 1519. [Google Scholar] [CrossRef]
- Manan, S.; Ullah, M.W.; Ul-Islam, M.; Atta, O.M.; Yang, G. Synthesis and applications of fungal mycelium-based advanced functional materials. J. Bioresour. Bioprod. 2021, 6, 1–10. [Google Scholar] [CrossRef]
- Nwe, N.; Chandrkrachang, S.; Stevens, W.F.; Maw, T.; Tan, T.K.; Khor, E.; Wong, S.M. Production of fungal chitosan by solid state and submerged fermentation. Carbohydr. Polym. 2002, 49, 235–237. [Google Scholar] [CrossRef]
- Geoghegan, I.A.; Gurr, S.J. Chitosan Mediates Germling Adhesion in Magnaporthe oryzae and Is Required for Surface Sensing and Germling Morphogenesis. PLoS Pathog. 2016, 12, e1005703. [Google Scholar] [CrossRef] [PubMed]
- Guarro, J.; Gené, J.; Stchigel, A.M. Developments in Fungal Taxonomy. Clin. Microbiol. Rev. 1999, 12, 454–500. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, E.M.; Bernal, D. Iterative finite element model updating in the time domain. Mech. Syst. Signal Process. 2013, 34, 39–46. [Google Scholar] [CrossRef]
- Deveau, A.; Bonito, G.; Uehling, J.; Paoletti, M.; Becker, M.; Bindschedler, S.; Hacquard, S.; Hervé, V.; Labbé, J.; Lastovetsky, O.A.; et al. Bacterial–fungal interactions: Ecology, mechanisms and challenges. FEMS Microbiol. Rev. 2018, 42, 335–352. [Google Scholar] [CrossRef]
- Steffan, B.N.; Venkatesh, N.; Keller, N.P. Let’s Get Physical: Bacterial-Fungal Interactions and Their Consequences in Agriculture and Health. J. Fungi 2020, 6, 243. [Google Scholar] [CrossRef]
- Nogueira, F.; Sharghi, S.; Kuchler, K.; Lion, T. Pathogenetic Impact of Bacterial–Fungal Interactions. Microorganisms 2019, 7, 459. [Google Scholar] [CrossRef]
- Thornton, S.T.; Abdelmageed, G.; Kahwagi, R.F.; Koleilat, G.I. Progress towards lead-free, efficient, and stable perovskite solar cells. J. Chem. Technol. Biotechnol. 2022, 97, 810–829. [Google Scholar] [CrossRef]
- Mühlschlegel, F.A.; Fonzi, W.A. PHR2 of Candida Albicans Encodes a Functional Homolog of the pH-regulated Gene PHR1 with an Inverted Pattern of pH-Dependent Expression. Mol. Cell. Biol. 1997, 17, 5960–5967. [Google Scholar] [CrossRef]
- Corrêa-Moreira, D.; Baptista, B.d.O.; Giosa, D.; Oliveira, M.M.E. Editorial: Emerging fungal pathogens: Perspectives. Front. Fungal Biol. 2024, 5, 1369062. [Google Scholar] [CrossRef]
- El-Gendi, H.; Saleh, A.K.; Badierah, R.; Redwan, E.M.; El-Maradny, Y.A.; El-Fakharany, E.M. A Comprehensive Insight into Fungal Enzymes: Structure, Classification, and Their Role in Mankind’s Challenges. J. Fungi 2021, 8, 23. [Google Scholar] [CrossRef]
- Alapan, D.; Bisweswar, O.; Prasenjit, S.; Prasanjit, D.; Arkapal, B. Recent advances in the clinical development of antifungal vaccines: A narrative review. Front. Trop. Dis. 2024, 5, 1446477. [Google Scholar] [CrossRef]
- Eagan, J.L.; Keller, N.P. Fungal secondary metabolism. Curr. Biol. 2025, 35, R503–R508. [Google Scholar] [CrossRef]
- Kordana, N.; Johnson, A.; Quinn, K.; Obar, J.J.; Cramer, R.A. Recent developments in Aspergillus fumigatus research: Diversity, drugs, and disease. Microbiol. Mol. Biol. Rev. 2025, 89, e00011-23. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, K.; Yoshimi, A.; Sano, M.; Tabata, F.; Sugahara, A.; Kasahara, S.; Koizumi, A.; Yano, S.; Nakajima, T.; Abe, K. Both Galactosaminogalactan and α-1,3-Glucan Contribute to Aggregation of Aspergillus oryzae Hyphae in Liquid Culture. Front. Microbiol. 2019, 10, 2090. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, M.; Shivarathri, R.; Aptekmann, A.A.; Chowdhary, A.; Kuchler, K.; Desai, J.V.; Chauhan, N. The Gcn5 lysine acetyltransferase mediates cell wall remodeling, antifungal drug resistance, and virulence of Candida auris. mSphere 2025, 10, e00069-25. [Google Scholar] [CrossRef] [PubMed]
- Mapplebeck, J.C.S.; Lorenzo, L.-E.; Lee, K.Y.; Gauthier, C.; Muley, M.M.; De Koninck, Y.; Prescott, S.A.; Salter, M.W. Chloride Dysregulation through Downregulation of KCC2 Mediates Neuropathic Pain in Both Sexes. Cell Rep. 2019, 28, 590–596.e4. [Google Scholar] [CrossRef]
- Borin, G.P.; Oliveira, J.V.d.C. Assessing the intracellular primary metabolic profile of Trichoderma reesei and Aspergillus niger grown on different carbon sources. Front. Fungal Biol. 2022, 3, 998361. [Google Scholar] [CrossRef]
- Mouyna, I.; Dellière, S.; Beauvais, A.; Gravelat, F.; Snarr, B.; Lehoux, M.; Zacharias, C.; Sun, Y.; de Jesus Carrion, S.; Pearlman, E.; et al. What Are the Functions of Chitin Deacetylases in Aspergillus fumigatus? Front. Cell. Infect. Microbiol. 2020, 10, 28. [Google Scholar] [CrossRef]
- Banks, I.R.; Specht, C.A.; Donlin, M.J.; Gerik, K.J.; Levitz, S.M.; Lodge, J.K. A Chitin Synthase and Its Regulator Protein Are Critical for Chitosan Production and Growth of the Fungal Pathogen Cryptococcus neoformans. Eukaryot. Cell 2005, 4, 1902–1912. [Google Scholar] [CrossRef]
- Krüger, W.; Vielreicher, S.; Kapitan, M.; Jacobsen, I.; Niemiec, M. Fungal-Bacterial Interactions in Health and Disease. Pathogens 2019, 8, 70. [Google Scholar] [CrossRef]
- Baert, K.; de Geest, B.G.; de Rycke, R.; da Fonseca Antunes, A.B.; de Greve, H.; Cox, E.; Devriendt, B. β-glucan microparticles targeted to epithelial APN as oral antigen delivery system. J. Control. Release 2015, 220, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Caruso, D.J.; Palombo, E.A.; Moulton, S.E.; Zaferanloo, B. Exploring the Promise of Endophytic Fungi: A Review of Novel Antimicrobial Compounds. Microorganisms 2022, 10, 1990. [Google Scholar] [CrossRef] [PubMed]
- Vadivel, D.; Cartabia, M.; Scalet, G.; Buratti, S.; Di Landro, L.; Benedetti, A.; Auricchio, F.; Babbini, S.; Savino, E.; Dondi, D. Innovative chitin-glucan based material obtained from mycelium of wood decay fungal strains. Heliyon 2024, 10, e28709. [Google Scholar] [CrossRef] [PubMed]
- de Lima Batista, A.C.; de Souza Paiva, W.; de Souza Neto, F.E. Chitosan. In Polysaccharides of Microbial Origin; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–18. [Google Scholar]
- Martínez, J.P.; Gil, M.L.; López-Ribot, J.L.; Chaffin, W.L. Serologic Response to Cell Wall Mannoproteins and Proteins of Candida albicans. Clin. Microbiol. Rev. 1998, 11, 121–141. [Google Scholar] [CrossRef]
- Güler, P.; Kutluer, F.; Kunduz, İ. Screening to Mycelium Specifications of Ganoderma lucidum (Fr.) Karst (Reishi). Hacet. J. Biol. Chem. 2011, 39, 397–401. [Google Scholar]
- Xue, M.; Hou, X.; Fu, J.; Zhang, J.; Wang, J.; Zhao, Z.; Xu, D.; Lai, D.; Zhou, L. Recent Advances in Search of Bioactive Secondary Metabolites from Fungi Triggered by Chemical Epigenetic Modifiers. J. Fungi 2023, 9, 172. [Google Scholar] [CrossRef]
- Angelova, G.; Brazkova, M.; Mihaylova, D.; Slavov, A.; Petkova, N.; Blazheva, D.; Deseva, I.; Gotova, I.; Dimitrov, Z.; Krastanov, A. Bioactivity of Biomass and Crude Exopolysaccharides Obtained by Controlled Submerged Cultivation of Medicinal Mushroom Trametes versicolor. J. Fungi 2022, 8, 738. [Google Scholar] [CrossRef]
- Shin, H.-J.; Ro, H.-S.; Kawauchi, M.; Honda, Y. Review on mushroom mycelium-based products and their production process: From upstream to downstream. Bioresour. Bioprocess. 2025, 12, 3. [Google Scholar] [CrossRef]
- Pearce, D.; Dora, M.; Wesana, J.; Gellynck, X. Toward sustainable primary production through the application of lean management in South African fruit horticulture. J. Clean. Prod. 2021, 313, 127815. [Google Scholar] [CrossRef]
- Guzman-Puyol, S.; Benítez, J.J.; Heredia-Guerrero, J.A. Sustainable Bio-Based Polymers: Towards a Circular Bioeconomy. Polymers 2021, 14, 22. [Google Scholar] [CrossRef]
- Liu, X.; Camilleri, E.T.; Li, L.; Gaihre, B.; Rezaei, A.; Park, S.; Miller, A.L., II; Tilton, M.; Waletzki, B.E.; Terzic, A.; et al. Injectable catalyst-free “click” organic-inorganic nanohybrid (click-ON) cement for minimally invasive in vivo bone repair. Biomaterials 2021, 276, 121014. [Google Scholar] [CrossRef]
- ISO 10993-7:2008; Biological Evaluation of Medical Devices—Part 7: Ethylene Oxide Sterilization Residuals. ISO: Geneva, Switzerland, 2008.
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- ISO 10993-18:2020; Biological Evaluation of Medical Devices—Part 18: Chemical Characterization of Medical Device Materials Within a Risk Management Process. ISO: Geneva, Switzerland, 2020.
- Jordá, T.; Rozès, N.; Puig, S. Sterol Composition Modulates the Response of Saccharomyces cerevisiae to Iron Deficiency. J. Fungi 2021, 7, 901. [Google Scholar] [CrossRef]
- Asadollahzadeh, M.; Mahboubi, A.; Taherzadeh, M.J.; Åkesson, D.; Lennartsson, P.R. Application of Fungal Biomass for the Development of New Polylactic Acid-Based Biocomposites. Polymers 2022, 14, 1738. [Google Scholar] [CrossRef]


| Production Platform | Morphology | Secretion Capacity | Glycosylation Pattern | Key Advantages | Key Disadvantages | Typical Applications | Key References |
|---|---|---|---|---|---|---|---|
| Saccharomyces cerevisiae | Unicellular | Low natively, though improvable with engineering. | High-mannose (immunogenic to humans). | Well-established fermentation, robust genetic tools, GRAS status. | Crabtree effect (directs carbon to ethanol), low native secretion capacity. | Bioethanol, vaccines, therapeutic proteins. | [57,58] |
| Pichia pastoris | Unicellular | High (comparable to filamentous fungi). | Shorter, less immunogenic mannose-type glycosylation. | High cell density, strong inducible promoters, GRAS status. | Requires methanol for expression induction. | Industrial and pharmaceutical recombinant protein production. | [59,60] |
| Aspergillus niger | Filamentous | Exceptional, high native secretion capacity. | More complex glycosylation patterns, similar to mammalian ones. | High production (grams/liter), GRAS status, ability to biodegrade lignin. | Filamentous morphology can hinder oxygen transfer at scale. | Industrial enzyme production (amylase, glucoamylase), organic acids. | [61,62] |
| Filamentous fungi (in general) | Filamentous (hyphae) | Very high, with powerful secretory pathways. | Ability to perform complex post-translational modifications of eukaryotic proteins. | Self-assemble into a fibrous network, can biodegrade lignocellulose, GRAS sources. | Complex morphology hinders scale-up and product purification. | Myco-fabrication (composite materials), bioremediation, enzymes. | [9,63] |
| Fungal Species | Primary Hyphal System | Key Cell Wall Components | Reported Mechanical Properties (Example Values) | Reported Physical/Thermal Properties (Example Values) | Key References |
|---|---|---|---|---|---|
| Ganoderma lucidum | Dimitic/Trimitic | High β-glucan content, complex branching; chitin; triterpenoids. | Superior physical and mechanical properties compared to P. ostreatus. Compressive strength can be tuned by substrate. | Hydrophobic (WCA ~120°). Mycelium mats can be tuned for porosity and density. | [15,18,38] |
| Pleurotus ostreatus | Monomitic | β-1,3 and β-1,6 glucans; chitin; high density of surface functional groups. | Lower mechanical properties than G. lucidum. Compressive strength of composites: 0.03–0.3 MPa | High water absorption capacity. Effective for biosorption of heavy metals. | [14,38] |
| Trametes versicolor | Trimitic | Polysaccharide-K (PSK) and Polysaccharopeptide (PSP); high glucan content. | Trimitic system implies high intrinsic strength. Used for strong composites. Flexural modulus (pressed): ~34–80 MPa depending on substrate. | Good insulation and fire-retardant properties. | [29,70] |
| Schizophyllum commune | Monomitic | High levels of secreted hydrolytic enzymes (xylanases, glucanases). Cell wall binds various micronutrients. | Tensile strength (pure sheet): ~9.5 MPa. Mechanical properties depend heavily on substrate and processing. | Cell wall acts as a pH-dependent ion-exchange material. | [35,46] |
| Agaricus bisporus | Monomitic | Chitin–glucan complexes. Chitosan derived from it has lower crystallinity than crustacean source. | Nanopapers from its chitin–glucan have tensile strength > 200 MPa. | Chitosan films show good film-forming ability and porosity. | [33] |
| Feature | Fungal Chitin/Chitosan | Crustacean Chitin/Chitosan | Key References |
|---|---|---|---|
| Source Type | Fungi (e.g., Agaricus bisporus) | Crustacean Shells (e.g., shrimp, crab) | [71] |
| Purity/Composition | Higher purity (low mineral content) but often inseparable from β-glucans. | High purity after chemical extraction, but co-exists with high mineral/protein content in the raw source. | [71] |
| Crystallinity | Lower crystallinity; more porous surface morphology. | Higher crystallinity; highly ordered, tough structure. | [71] |
| Extraction | Simpler process, but separation from glucans is challenging. | Complex, chemical-intensive extraction (acid/alkali). | [71] |
| Sustainability/Ethics | Vegan, cruelty-free; consistent supply. | Non-vegan; seasonal; high waste production. | [71] |
| Key Application | Biomedical scaffolds, food industry. | High-strength films, industrial biopolymers. | [71] |
| Biochemical Lever | Mechanism of Action | Impact on Material Properties | Key References |
|---|---|---|---|
| Substrate Engineering | Modulating physicochemical cues presented to the fungus. | Controls hyphal network density, tortuosity, and interfacial adhesion, thereby tuning bulk mechanical (stiffness, strength) and physical (porosity, thermal conductivity) properties. Chopped fibers < 5 mm and substrate pre-compression improve compressive strength and stiffness. | [13,38] |
| pH Triggers | Activating conserved signal transduction pathways (e.g., pH-responsive PacC/Rim101) to dynamically regulate cell wall gene expression. | Allows for spatiotemporal control over cell wall composition and cross-linking, enabling the fabrication of functionally graded materials. The expression of key wall-modifying genes, like PHR1 and PHR2, is directly regulated by ambient pH. | [64,66] |
| Secretome Modulation | Directing the enzymatic toolkit secreted by the fungus (e.g., via co-culturing or elicitors) to perform in situ substrate modification. | Enables in situ composite reinforcement through enzymatic modification of lignocellulose, creating tailored inter- and intra-fiber bonding. White-rot fungi selectively degrade lignin, strengthening the final material by preserving cellulose fibers. | [51,56] |
| Modulator Category | Specific Target | Agent/Method | Observed Biochemical Effect on Cell Wall | Potential Impact on Material Properties | Key References |
|---|---|---|---|---|---|
| Genetic | Gcn5 Lysine Acetyltransferase | Gene Deletion (GCN5Δ) | ↑ β-glucan exposure, ↑ chitin content, altered expression of FKS1 and adhesins. | Altered adhesion, stress response, and potentially flexibility/strength. Broad-spectrum control. | [90] |
| α-1,3-Glucan Synthase | Gene Deletion (agsΔ) | ↓ α-1,3-glucan content. | Altered morphology (e.g., smaller pellets or dispersed growth, species-dependent); ↓ culture viscosity, improved bioprocessing. | [69,88] | |
| Chitin Synthase (Class V/VII) | Gene Deletion (csmΔ) | Disorganization of wall structure, altered surface rodlet layer. | Altered surface properties (e.g., hydrophilicity), modified mechanical integrity. | [34] | |
| pH-Sensing Pathway (PacC/Rim101) | Gene Deletion (pacCΔ) | Inability to adapt wall structure to ambient pH, defective expression of pH-regulated enzymes. | Loss of pH-dependent programmability, defects in material formation under specific pH. | [83] | |
| Chemical | Chitin Synthases (all classes) | Nikkomycin Z, Polyoxins | Competitive inhibition of chitin synthesis at hyphal tips. | Localized growth inhibition, creation of zones of weakness/flexibility, patterned growth. | [95] |
| Histone Deacetylases (HDACs) | SAHA, Trichostatin A (TSA) | Chromatin de-repression, activation of silent biosynthetic gene clusters. | Induction of novel secondary metabolites (pigments, polymers), adding new functionalities. | [101] | |
| DNA Methyltransferases (DNMTs) | 5-Azacytidine | DNA demethylation, activation of silent gene clusters. | Similarly to HDAC inhibitors; induction of novel chemical functionalities in the material. | [101] | |
| β-1,3-Glucan Synthase | Echinocandins (e.g., Caspofungin) | Inhibition of β-1,3-glucan synthesis. | Weakened cell wall, increased sensitivity to stress, potential for controlled lysis or softening. | [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coca-Ruiz, V. Biochemical Programming of the Fungal Cell Wall: A Synthetic Biology Blueprint for Advanced Mycelium-Based Materials. BioChem 2025, 5, 33. https://doi.org/10.3390/biochem5040033
Coca-Ruiz V. Biochemical Programming of the Fungal Cell Wall: A Synthetic Biology Blueprint for Advanced Mycelium-Based Materials. BioChem. 2025; 5(4):33. https://doi.org/10.3390/biochem5040033
Chicago/Turabian StyleCoca-Ruiz, Víctor. 2025. "Biochemical Programming of the Fungal Cell Wall: A Synthetic Biology Blueprint for Advanced Mycelium-Based Materials" BioChem 5, no. 4: 33. https://doi.org/10.3390/biochem5040033
APA StyleCoca-Ruiz, V. (2025). Biochemical Programming of the Fungal Cell Wall: A Synthetic Biology Blueprint for Advanced Mycelium-Based Materials. BioChem, 5(4), 33. https://doi.org/10.3390/biochem5040033






