Advancements in Retinal Tissue-Mimicking Optical Coherence Tomography Phantoms: Materials, Properties, and Applications
Abstract
1. Introduction
2. Phantom Matrix
2.1. Gelatin-Based Phantoms
2.2. Silicone Phantoms
2.3. Polydimethylsiloxane Phantoms
2.4. Polyvinyl Alcohol (PVA) Phantoms
2.5. Fibrin Phantoms
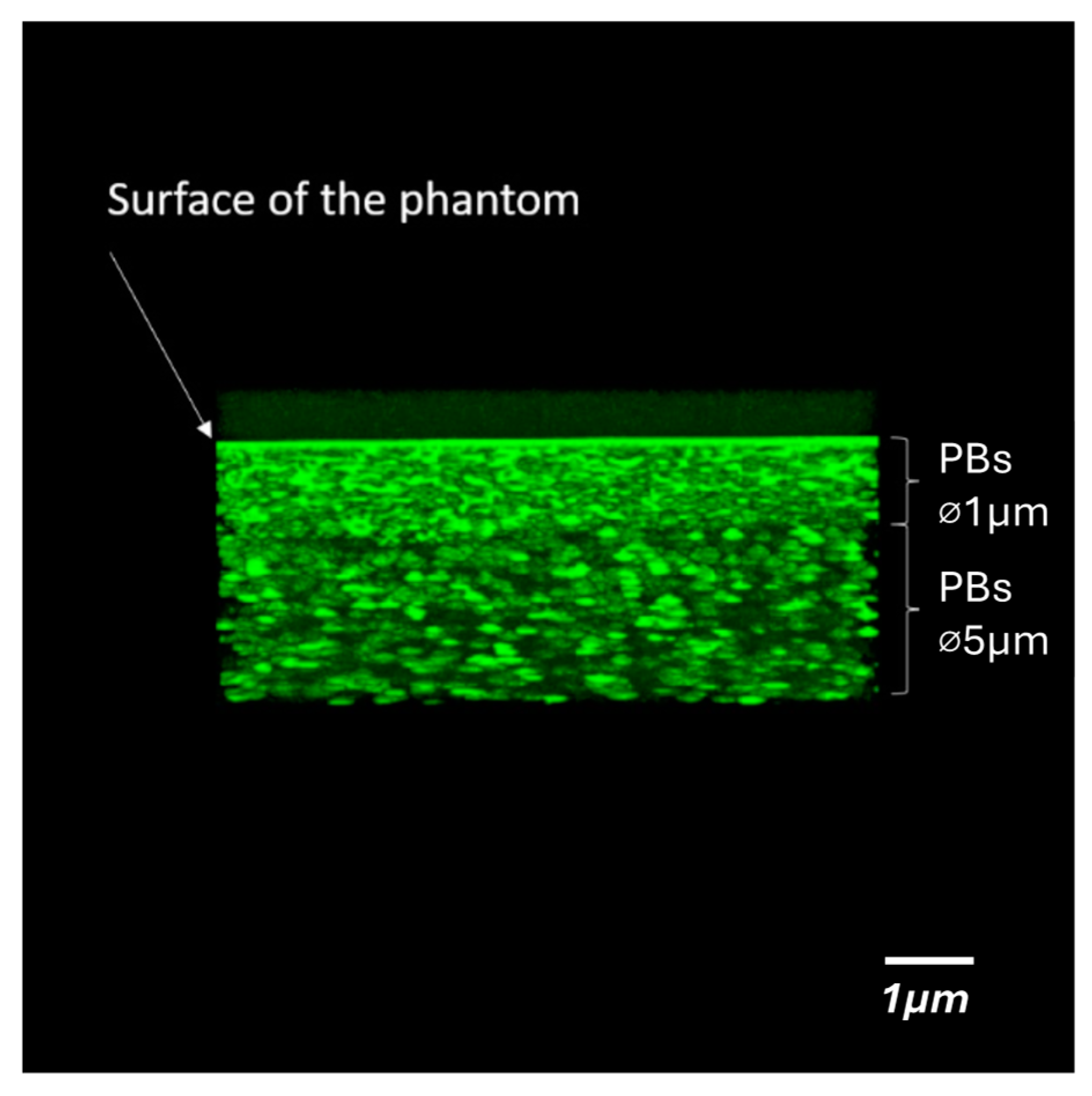
3. Scattering Particles
3.1. Polystyrene
3.2. Titanium Dioxide
3.3. Silica Microspheres
3.4. Gold Nanoshells
4. Molecular Mechanisms in OCT-Studied Retinal Diseases
4.1. Lipid Metabolism and RPE Dysfunction
4.2. Complement System in Photoreceptor Degeneration
4.3. Lysosomal Storage Disorders and Phagocytosis Defects
5. Discussion
Funding
Conflicts of Interest
References
- Baxi, J.; Calhoun, W.; Sepah, Y.J.; Hammer, D.X.; Ilev, I.; Pfefer, T.J.; Nguyen, Q.D.; Agrawal, A. Retina-simulating phantom for optical coherence tomography. J. Biomed. Opt. 2014, 19, 021106. [Google Scholar] [CrossRef] [PubMed]
- Culjat, M.O.; Goldenberg, D.; Tewari, P.; Singh, R.S. A review of tissue substitutes for ultrasound imaging. Ultrasound Med. Biol. 2010, 36, 861–873. [Google Scholar] [CrossRef]
- Pogue, B.W.; Patterson, M.S. Review of tissue simulating phantoms for optical spectroscopy, imaging and dosimetry. J. Biomed. Opt. 2006, 11, 041102. [Google Scholar] [CrossRef]
- Dunaief, J.L.; Dentchev, T.; Ying, G.S.; Milam, A.H. The role of apoptosis in age-related macular degeneration. Arch. Ophthalmol. 2002, 120, 1435–1442. [Google Scholar] [CrossRef]
- Vernazza, S.; Oddone, F.; Tirendi, S.; Bassi, A.M. Risk Factors for Retinal Ganglion Cell Distress in Glaucoma and Neuroprotective Potential Intervention. Int. J. Mol. Sci. 2021, 22, 7994. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.A.; Howell, G.R.; Barbay, J.M.; Braine, C.E.; Sousa, G.L.; John, S.W.; Morgan, J.E. Retinal Ganglion Cell Dendritic Atrophy in DBA/2J Glaucoma. PLoS ONE 2013, 8, e72282. [Google Scholar] [CrossRef]
- Williams, P.A.; Thirgood, R.A.; Oliphant, H.; Frizzati, A.; Littlewood, E.; Votruba, M.; Good, M.A.; Williams, J.; Morgan, J.E. Retinal ganglion cell dendritic degeneration in a mouse model of Alzheimer’s disease. Neurobiol. Aging 2013, 34, 1799–1806. [Google Scholar] [CrossRef] [PubMed]
- Beuthan, J.; Minet, O.; Helfmann, J.; Herrig, M.; Müller, G. The spatial variation of the refractive index in biological cells. Phys. Med. Biol. 1996, 41, 369–382. [Google Scholar] [CrossRef]
- Rafelski, S.; Marshall, W. Building the cell: Design principles of cellular architecture. Nat. Rev. Mol. Cell Biol. 2008, 9, 593–602. [Google Scholar] [CrossRef]
- Wiemerslage, L.; Lee, D. Quantification of mitochondrial morphology in neurites of dopaminergic neurons using multiple parameters. J. Neurosci. Methods 2016, 15, 56–65. [Google Scholar] [CrossRef]
- Kulmaganbetov, M.; Bevan, R.J.; Anantrasirichai, N.; Achim, A.; Erchova, I.; White, N.; Albon, J.; Morgan, J.E. Textural Feature Analysis of Optical Coherence Tomography Phantoms. Electronics 2022, 11, 669. [Google Scholar] [CrossRef]
- Kwon, I.H.; Kim, H.; Kim, D.Y.; Lee, H.; Lee, S. Utilizing Optical Phantoms for Biomedical-optics Technology: Recent Advances and Challenges. Curr. Opt. Photon. 2024, 8, 327–344. [Google Scholar] [CrossRef]
- Zhang, T.; Yuan, S.; Xu, C.; Liu, P.; Chang, H.C.; Ng, S.H.C.; Ren, H.; Yuan, W. PneumaOCT: Pneumatic optical coherence tomography endoscopy for targeted distortion-free imaging in tortuous and narrow internal lumens. Sci. Adv. 2024, 10, eadp3145. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Lamont, A.C.; Liu, Z.; Sochol, R.; Hammer, D.X. Engineered Texture Phantom for Measuring Lateral Resolution of Adaptive Optics Ophthalmic Imaging Systems. In Proceedings of the Imaging and Applied Optics Congress, Vancouver, BC, Canada, 22–26 June 2020; OSA Technical Digest (Optica Publishing Group): Washington, DC, USA, 2020. Paper OTh5B.4. [Google Scholar] [CrossRef]
- Quan, K.M.; Christison, G.B.; MacKenzie, H.A.; Hodgson, P. Glucose determination by a pulsed photoacoustic technique: An experimental study using a gelatin-based tissue phantom. Phys. Med. Biol. 1993, 38, 1911–1922. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Birngruber, R.; Rosperich, J.; Engelhardt, R. Low-coherence optical tomography in turbid tissue: Theoretical analysis. Appl. Opt. 1995, 34, 6564–6574. [Google Scholar] [CrossRef]
- Schmitt, J.M.; Lee, S.L.; Yung, K.M. An optical coherence microscope with enhanced resolving power in thick tissue. Opt. Commun. 1997, 142, 203–207. [Google Scholar] [CrossRef]
- Cubeddu, R.; Pifferi, A.; Taroni, P.; Torricelli, A.; Valentini, G. A solid tissue phantom for photon migration studies. Phys. Med. Biol. 1997, 42, 1971–1979. [Google Scholar] [CrossRef]
- Catheline, S.; Gennisson, J.L.; Delon, G.; Fink, M.; Sinkus, R.; Abouelkaram, S.; Culioli, J. Measuring of viscoelastic properties of homogeneous soft solid using transient elastography: An inverse problem approach. J. Acoust. Soc. Am. 2004, 116, 3734–3741. [Google Scholar] [CrossRef]
- Brum, J.; Catheline, S.; Benech, N.; Negreira, C. Shear elasticity estimation from surface wave: The time reversal approach. J. Acoust. Soc. Am. 2008, 124, 3377–3380. [Google Scholar] [CrossRef]
- Chen, S.; Urban, M.W.; Pislaru, C.; Kinnick, R.; Zheng, Y.; Yao, A.; Greenleaf, J.F. Shearwave dispersion ultrasound vibrometry (SDUV) for measuring tissue elasticity and viscosity. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2009, 56, 55–62. [Google Scholar] [CrossRef]
- Schmitt, J.M. OCT elastography: Imaging microscopic deformation and strain of tissue. Opt. Exp. 1998, 3, 199–211. [Google Scholar] [CrossRef]
- Browne, J.E.; Ramnarine, K.V.; Watson, A.J.; Hoskins, P.R. Assessment of the acoustic properties of common tissue-mimicking test phantoms. Ultrasound Med. Biol. 2003, 29, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Qiang, B.; Greenleaf, J. Comparison of the surface wave method and the indentation method for measuring the elasticity of gelatin phantoms of different concentrations. Ultrasonics 2011, 51, 157–164. [Google Scholar] [CrossRef] [PubMed]
- De Grand, A.M.; Lomnes, S.J.; Lee, D.S.; Pietrzykowski, M.; Ohnishi, S.; Morgan, T.G.; Gogbashian, A.; Laurence, R.G.; Frangioni, J.V. Tissue-like phantoms for near-infrared fluorescence imaging system assessment and the training of surgeons. J. Biomed. Opt. 2006, 11, 014007. [Google Scholar] [CrossRef]
- Wagnières, G.; Cheng, S.; Zellweger, M.; Utke, N.; Braichotte, D.; Ballini, J.P.; van den Bergh, H. An optical phantom with tissue-like properties in the visible for use in PDT and fluorescence spectroscopy. Phys. Med. Biol. 1997, 42, 1415–1426. [Google Scholar] [CrossRef] [PubMed]
- Esmonde-White, F.W.L.; Esmonde-White, K.A.; Kole, M.R.; Goldstein, S.A.; Roessler, B.J.; Morris, M.D. Biomedical tissue phantoms with controlled geometric and optical properties for Raman spectroscopy and tomography. Analyst 2011, 136, 4437–4446. [Google Scholar] [CrossRef]
- Lamouche, G.; Kennedy, B.F.; Kennedy, K.M.; Bisaillon, C.E.; Curatolo, A.; Campbell, G.; Pazos, V.; Sampson, D.D. Review of tissue simulating phantoms with controllable optical, mechanical and structural properties for use in optical coherence tomography. Biomed. Opt. Express 2012, 3, 1381–1398. [Google Scholar] [CrossRef]
- Madsen, E.L.; Hobson, M.A.; Shi, H.; Varghese, T.; Frank, G.R. Tissue-mimicking agar/gelatin materials for use in heterogeneous elastography phantoms. Phys. Med. Biol. 2005, 50, 5597–5618. [Google Scholar] [CrossRef]
- Richardson, C.; Bernard, S.; Dinh, V.A. A Cost-effective, Gelatin-Based Phantom Model for Learning Ultrasound-Guided Fine-Needle Aspiration Procedures of the Head and Neck. J. Ultrasound Med. 2015, 34, 1479–1484. [Google Scholar] [CrossRef]
- Bolin, F.P.; Preuss, L.E.; Taylor, R.C.; Ference, R.J. Refractive index of some mammalian tissues using a fiber optic cladding method. Appl. Opt. 1989, 28, 2297–2303. [Google Scholar] [CrossRef]
- Bays, R.; Wagnières, G.; Robert, D.; Theumann, J.F.; Vitkin, A.; Savary, J.F.; Monnier, P.; van den Bergh, H. Three-dimensional optical phantom and its application in photodynamic therapy. Lasers Surg. Med. 1997, 21, 227–234. [Google Scholar] [CrossRef]
- Williams, D.R. Visual consequences of the foveal pit. Investig. Ophthalmol. Vis. Sci. 1980, 19, 653–667. [Google Scholar]
- Bisaillon, C.-E.; Lanthier, M.-M.; Dufour, M.L.; Lamouche, G. Durable coronary artery phantoms for optical coherence tomography. In Proceedings of the SPIE 7161, Photonic Therapeutics and Diagnostics V, San Jose, CA, USA, 24–29 January 2009; p. 71612E. [Google Scholar] [CrossRef][Green Version]
- Zoutenbier, V.S.; Amelink, A.; de Boer, J.F. Ex-corpus human blood flow system for flow and physiological control in retina-mimicking silicone phantoms. In Proceedings of the SPIE PC12147, Tissue Optics and Photonics II, Strasbourg, France, 3 April–23 May 2022; p. PC1214705. [Google Scholar]
- Potlov, A.Y.; Frolov, S.V.; Proskurin, S.G. Tissue-mimicking phantoms of human retina with consideration to blood circulation for Doppler optical coherence tomography. In Proceedings of the SPIE 11457, Saratov Fall Meeting 2019: Optical and Nano-Technologies for Biology and Medicine, Saratov, Russia, 23–27 September 2019; p. 114571S. [Google Scholar] [CrossRef]
- Ayers, F.; Grant, A.; Kuo, D.; Cuccia, D.J.; Durkin, A.J. Fabrication and characterization of silicone-based tissue phantoms with tunable optical properties in the visible and near infrared domain. In Proceedings of the SPIE 6870, Design and Performance Validation of Phantoms Used in Conjunction with Optical Measurements of Tissue, San Jose, CA, USA, 19–21 January 2008; p. 687007. [Google Scholar] [CrossRef]
- Long, R.; King, T.; Akl, T.; Ericson, M.N.; Wilson, M.; Coté, G.L.; McShane, M.J. Optofluidic phantom mimicking optical properties of porcine livers. Biomed. Opt. Exp. 2011, 2, 1877–1892. [Google Scholar] [CrossRef] [PubMed]
- Avigo, C.; Di Lascio, N.; Armanetti, P.; Kusmic, C.; Cavigli, L.; Ratto, F.; Meucci, S.; Masciullo, C.; Cecchini, M.; Pini, R.; et al. Organosilicon phantom for photoacoustic imaging. J. Biomed. Opt. 2015, 20, 046008. [Google Scholar] [CrossRef] [PubMed]
- Bohndiek, S.E.; Bodapati, S.; Van De Sompel, D.; Kothapalli, S.R.; Gambhir, S.S. Development and application of stable phantoms for the evaluation of photoacoustic imaging instruments. PLoS ONE 2013, 8, e75533. [Google Scholar] [CrossRef]
- Zell, K.; Sperl, J.I.; Vogel, M.W.; Niessner, R.; Haisch, C. Acoustical properties of selected tissue phantom materials for ultrasound imaging. Phys. Med. Biol. 2007, 52, N475–N484. [Google Scholar] [CrossRef]
- Wu, Q.; Ren, W.; Yu, Z.; Dong, E.; Zhang, S.; Xu, R.X. Microfabrication of polydimethylsiloxane phantoms to simulate tumor hypoxia and vascular anomaly. J. Biomed. Opt. 2015, 20, 121308. [Google Scholar] [CrossRef]
- Saager, R.B.; Kondru, C.; Au, K.; Sry, K.; Ayers, F.; Durkin, A.J. Multilayer silicone phantoms for the evaluation of quantitative optical techniques in skin imaging. In Proceedings of the SPIE 7567, Design and Performance Validation of Phantoms Used in Conjunction with Optical Measurement of Tissue II, San Jose, CA, USA, 23–28 January 2010; p. 756706. [Google Scholar] [CrossRef]
- Wang, H.; Liu, W.; Hu, Z.; Li, X.; Li, F.; Duan, L. Model eye tool for retinal optical coherence tomography instrument calibration. J. Innov. Opt. Health Sci. 2021, 14, 2150010. [Google Scholar] [CrossRef]
- Al-Sadiq, H.; Walters, J.; Alshayeb, A.; Parekh, H.; Veidt, M. Tissue mimicking phantom of the acoustical and geometrical properties of the human eye. In Proceedings of the Conference of the Acoustical Society of New Zealand, Wellington, New Zealand, 31 October–2 November 2022. [Google Scholar]
- Chang, S.; Handwerker, J.; Giannico, G.A.; Chang, S.S.; Bowden, A.K. Birefringent tissue-mimicking phantom for polarization-sensitive optical coherence tomography imaging. J. Biomed. Opt. 2022, 27, 074711. [Google Scholar] [CrossRef]
- Fromageau, J.; Brusseau, E.; Vray, D.; Gimenez, G.; Delachartre, P. Characterization of PVA cryogel for intravascular ultrasound elasticity imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2003, 50, 1318–1324. [Google Scholar] [CrossRef]
- Mano, I.; Goshima, H.; Nambu, M.; Iio, M. New polyvinyl alcohol gel material for MRI phantoms. Magn. Reson. Med. 1986, 3, 921–926. [Google Scholar] [CrossRef]
- Chu, K.C.; Rutt, B.K. Polyvinyl alcohol cryogel: An ideal phantom material for MR studies of arterial flow and elasticity. Magn. Reson. Med. 1997, 37, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Brusseau, E.; Fromageau, J.; Finet, G.; Delachartre, P.; Vray, D. Axial strain imaging of intravascular data: Results on polyvinyl alcohol cryogel phantoms and carotid artery. Ultrasound Med. Biol. 2001, 27, 1631–1642. [Google Scholar] [CrossRef] [PubMed]
- Surry, K.J.; Austin, H.J.; Fenster, A.; Peters, T.M. Poly(vinyl alcohol) cryogel phantoms for use in ultrasound and MR imaging. Phys. Med. Biol. 2004, 49, 5529–5546. [Google Scholar] [CrossRef] [PubMed]
- Kharine, A.; Manohar, S.; Seeton, R.; Kolkman, R.G.; Bolt, R.A.; Steenbergen, W.; de Mul, F.F. Poly(vinyl alcohol) gels for use as tissue phantoms in photoacoustic mammography. Phys. Med. Biol. 2003, 48, 357–370. [Google Scholar] [CrossRef]
- Fogli, G.; Orsi, G.; De Maria, C.; Montemurro, F.; Palla, M.; Rizzo, S.; Vozzi, G. New eye phantom for ophthalmic surgery. J. Biomed. Opt. 2014, 19, 068001. [Google Scholar] [CrossRef]
- Kaetsu, H.; Uchida, T.; Shinya, N. Increased effectiveness of fibrin sealant with a higher fibrin concentration. Int. J. Adhes. Adhes. 2000, 20, 27–31. [Google Scholar] [CrossRef]
- Göbel, K.; Eichler, S.; Wiendl, H.; Chavakis, T.; Kleinschnitz, C.; Meuth, S.G. The Coagulation Factors Fibrinogen, Thrombin, and Factor XII in Inflammatory Disorders—A Systematic Review. Front. Immunol. 2018, 9, 1731. [Google Scholar] [CrossRef]
- Kennedy, B.F.; Loitsch, S.; McLaughlin, R.A.; Scolaro, L.; Rigby, P.; Sampson, D.D. Fibrin phantom for use in optical coherence tomography. J. Biomed. Opt. 2010, 15, 030507. [Google Scholar] [CrossRef]
- Yu, H.; Jang, J.; Lim, J.; Park, J.H.; Jang, W.; Kim, J.Y.; Park, Y. Depth-enhanced 2-D optical coherence tomography using complex wavefront shaping. Opt. Exp. 2014, 22, 7514–7523. [Google Scholar] [CrossRef]
- Kim, M.; Im, S.; Park, I.; Kim, D.; Kim, E.S.; Joseph, J.; Yoon, J. Fabrication of agar-based tissue-mimicking phantom for the technical evaluation of biomedical optical imaging systems. Curr. Appl. Phys. 2024, 61, 80–85. [Google Scholar] [CrossRef]
- Khasawneh, A.; Kuroda, M.; Yoshimura, Y.; Sugianto, I.; Bamgbose, B.O.; Hamada, K.; Barham, M.; Tekiki, N.; Konishi, K.; Sugimoto, K.; et al. Development of a novel phantom using polyethylene glycol for the visualization of restricted diffusion in diffusion kurtosis imaging and apparent diffusion coefficient subtraction method. Biomed. Rep. 2020, 13, 52. [Google Scholar] [CrossRef]
- Nanda Kumar, Y.; Singh, Z.; Wang, Y.N.; Schade, G.R.; Kreider, W.; Bruce, M.; Vlaisavljevich, E.; Khokhlova, T.D.; Maxwell, A.D. Development of Tough Hydrogel Phantoms to Mimic Fibrous Tissue for Focused Ultrasound Therapies. Ultrasound Med. Bio. 2022, 48, 1762–1777. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Nazarenus, J.; Egeler, B.; Thode, T.; Osman, F.; Osmonov, D.; Bahr, J.; Kaps, S.; Siebert, F.A.; Koch, R.; et al. Hydrogel System with Independent Tailoring of Mechanics, CT, and US Contrasts for Affordable Medical Phantoms. ACS Mater. Lett. 2024, 6, 4847–4853. [Google Scholar] [CrossRef]
- Malektaj, H.; Drozdov, A.D.; deClaville Christiansen, J. Mechanical Properties of Alginate Hydrogels Cross-Linked with Multivalent Cations. Polymers 2023, 15, 3012. [Google Scholar] [CrossRef]
- Delpy, D.T.; Cope, M.; van der Zee, P.; Arridge, S.; Wray, S.; Wyatt, J. Estimation of optical pathlength through tissue from direct time of flight measurement. Phys. Med. Biol. 1988, 33, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Al Rawashdeh, W.; Kray, S.; Pich, A.; Pargen, S.; Balaceanu, A.; Lenz, M.; Spöler, F.; Kiessling, F.; Lederle, W. Polymeric nanoparticles as OCT contrast agents. J. Nanopart. Res. 2012, 14, 1255. [Google Scholar] [CrossRef]
- Chang, R.C.; Johnson, P.; Stafford, C.M.; Hwang, J. Fabrication and characterization of a multilayered optical tissue model with embedded scattering microspheres in polymeric materials. Biomed. Opt. Exp. 2012, 3, 1326–1339. [Google Scholar] [CrossRef][Green Version]
- Bao, Y.; Huang, Y.; Li, W.; Zhu, K. Comparison of the scattering properties between TiO2 and ITO clusters based on the particle superposition model. Opt. Mater. Express 2019, 9, 562–575. [Google Scholar] [CrossRef]
- Curatolo, A.; Kennedy, B.F.; Sampson, D.D. Structured three-dimensional optical phantom for optical coherence tomography. Opt. Exp. 2011, 19, 19480–19485. [Google Scholar] [CrossRef]
- Liba, O.; Lew, M.D.; SoRelle, E.D.; Dutta, R.; Sen, D.; Moshfeghi, D.M.; Chu, S.; de la Zerda, A. Speckle-modulating optical coherence tomography in living mice and humans. Nat. Commun. 2017, 8, 15845. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, A.; Muyo, G.; van Hemert, J.; Gorman, A.; Harvey, A.R. Application of a wide-field phantom eye for optical coherence tomography and reflectance imaging. J. Mod. Opt. 2015, 62, 1828–1838. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Samiudin, N.M.; Lee, T.G.; Doh, I.; Lee, S.W. Retina phantom for the evaluation of optical coherence tomography angiography based on microfluidic channels. Biomed. Opt. Express 2019, 10, 5535–5548. [Google Scholar] [CrossRef] [PubMed]
- Lamont, A.C.; Restaino, M.A.; Alsharhan, A.T.; Liu, Z.; Hammer, D.X.; Sochol, R.D.; Agrawal, A. Direct laser writing of a titanium dioxide-laden retinal cone phantom for adaptive optics-optical coherence tomography. Opt. Mater. Express 2020, 10, 2757–2767. [Google Scholar] [CrossRef]
- Kuttippurath, V.; Slijkhuis, N.; Liu, S.; van Soest, G. Spectroscopic optical coherence tomography at 1200 nm for lipid detection. J. Biomed. Opt. 2023, 28, 096002. [Google Scholar] [CrossRef]
- Motipally, S.I.; Kolson, D.R.; Guan, T.; Kolandaivelu, S. Aberrant lipid accumulation and retinal pigmental epithelium dysfunction in PRCD-deficient mice. Exp. Eye Res. 2024, 246, 110016. [Google Scholar] [CrossRef]
- Philipp, H.R.; Taft, E.A. Optical Constants of Silicon in the Region 1 to 10 ev. Phys. Rev. 1960, 120, 37–38. [Google Scholar] [CrossRef]
- Bisaillon, C.-E.; Lamouche, G.; Maciejko, R.; Dufour, M.; Monchalin, J.P. Deformable and durable phantoms with controlled density of scatterers. Phys. Med. Biol. 2008, 53, N237–N247. [Google Scholar] [CrossRef]
- Ki, J.; Lee, H.; Lee, T.G.; Lee, S.W.; Wi, J.S.; Na, H.K. Visualization Materials Using Silicon-Based Optical Nanodisks (ViSiON) for Enhanced NIR Imaging in Ophthalmology. Adv. Healthc. Mater. 2024, 13, e2303713. [Google Scholar] [CrossRef]
- Zabotnov, S.V.; Kashaev, F.V.; Shuleiko, D.V.; Gongalsky, M.B.; Golovan, L.A.; Kashkarov, P.K.; Loginova, D.A.; Agrba, P.D.; Sergeeva, E.A.; Kirillin, M.Y. Silicon nanoparticles as contrast agents in the methods of optical biomedical diagnostics. Quantum Electron. 2017, 47, 638–646. [Google Scholar] [CrossRef]
- Loo, C.; Lin, A.; Hirsch, L.; Lee, M.H.; Barton, J.; Halas, N.; West, J.; Drezek, R. Nanoshell-enabled photonics-based imaging and therapy of cancer. Technol. Cancer Res. Treat. 2004, 3, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Tuersun, P.; Han, X.; Ren, K.F. Backscattering Properties of Gold Nanoshells: Quantitative Analysis and Optimization for Biological Imaging. Procedia Eng. 2015, 102, 1511–1519. [Google Scholar] [CrossRef][Green Version]
- Agrawal, A.; Huang, S.; Wei Haw Lin, A.; Lee, M.H.; Barton, J.K.; Drezek, R.A.; Pfefer, T.J. Quantitative evaluation of optical coherence tomography signal enhancement with gold nanoshells. J. Biomed. Opt. 2006, 11, 041121. [Google Scholar] [CrossRef] [PubMed]
- Kah, J.C.; Chow, T.H.; Ng, B.K.; Razul, S.G.; Olivo, M.; Sheppard, C.J. Concentration dependence of gold nanoshells on the enhancement of optical coherence tomography images: A quantitative study. Appl. Opt. 2009, 48, D96–D108. [Google Scholar] [CrossRef]
- Zagaynova, E.V.; Shirmanova, M.V.; Kirillin, M.Y.; Khlebtsov, B.N.; Orlova, A.G.; Balalaeva, I.V.; Sirotkina, M.A.; Bugrova, M.L.; Agrba, P.D.; Kamensky, V.A. Contrasting properties of gold nanoparticles for optical coherence tomography: Phantom, in vivo studies and Monte Carlo simulation. Phys. Med. Biol. 2008, 53, 4995–5009. [Google Scholar] [CrossRef]
- Min-Ho, L.; Nammalvar, V.; Gobin, A.; Barton, J.; West, J.; Drezek, R. Nanoshells as contrast agents for scatter-based optical imaging. In Proceedings of the 3rd IEEE International Symposium on Biomedical Imaging: Nano to Macro, Arlington, WA, USA, 6–9 April 2006; pp. 371–374. [Google Scholar] [CrossRef]
- Guo, L.; Zhao, Q.; Wang, M. Core–Shell Microspheres with Encapsulated Gold Nanoparticle Carriers for Controlled Release of Anti-Cancer Drugs. J. Funct. Biomater. 2024, 15, 277. [Google Scholar] [CrossRef]
- Wilczyński, T.; Zalejska-Fiolka, J.; Sapeta-Wieckowska, S.; Sarnat-Kucharczyk, M.; Rokicki, W. In situ oxidative stress in patients with epiretinal membrane. Acta Biochim. Pol. 2024, 71, 13581. [Google Scholar] [CrossRef]
- Wang, T.; Song, Y.; Bell, B.A.; Anderson, B.D.; Lee, T.T.; Yu, W.; Dunaief, J.L. Complement C3 knockout protects photoreceptors in the sodium iodate model. Exp. Eye Res. 2025, 250, 110161. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Chear, S.; Talbot, J.; Swier, V.; Booth, C.; Reuben-Thomas, C.; Dalvi, S.; Weimer, J.M.; Hewitt, A.W.; Cook, A.L.; et al. Genetic and Cellular Basis of Impaired Phagocytosis and Photoreceptor Degeneration in CLN3 Disease. Investig. Ophthalmol. Vis. Sci. 2024, 65, 23. [Google Scholar] [CrossRef]
- Olufsen, M.E.; Hannibal, J.; Sørensen, N.B.; Christiansen, A.T.; Christensen, U.; Pertile, G.; Steel, D.H.; Heegaard, S.; Kiilgaard, J.F. Wound Healing in a Porcine Model of Retinal Holes. Investig. Ophthalmol. Vis. Sci. 2024, 65, 35. [Google Scholar] [CrossRef]
- Peng, N.; Xu, C.; Shen, Y.; Yuan, W.; Yang, X.; Qi, C.; Qiu, H.; Gu, Y.; Chen, D. Accurate attenuation characterization in optical coherence tomography using multi-reference phantoms and deep learning. Biomed. Opt. Express 2024, 15, 6697–6714. [Google Scholar] [CrossRef] [PubMed]
- Heikka, T.; Ometto, G.; Montesano, G.; Rowe, S.; Jansonius, N.M.; Crabb, D.P. Testing a phantom eye under various signal-to-noise ratio conditions using eleven different OCT devices. Biomed. Opt. Express 2020, 11, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- Zakir, R.; Iqbal, K.; Hassaan Ali, M.; Tariq Mirza, U.; Mahmood, K.; Riaz, S.; Hashmani, N. The outcomes and usefulness of Intraoperative Optical Coherence Tomography in vitreoretinal surgery and its impact on surgical decision making. Rom. J. Ophthalmol. 2022, 66, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Toygar, O.; Riemann, C. Intraoperative optical coherence tomography in macula involving rhegmatogenous retinal detachment repair with pars plana vitrectomy and perfluoron. Eye 2016, 30, 23–30. [Google Scholar] [CrossRef]
- Borrelli, E.; Palmieri, M.; Aharrh-Gnama, A.; Ciciarelli, V.; Mastropasqua, R.; Carpineto, P. Intraoperative optical coherence tomography in the full-thickness macular hole surgery with internal limiting membrane inverted flap placement. Int. Ophthalmol. 2019, 39, 929–934. [Google Scholar] [CrossRef]
- Hwang, J.; Goldfain, A.M.; Zhang, H.; Briggman, K. Material Characterization of Phantom Standards Developed by NIST for Quantitative Optical Medical Imaging Applications. Available online: https://cormusa.org/wp-content/uploads/2024/01/1-CORM2023-OPM-Hwang.pdf (accessed on 1 April 2025).
- Kulmaganbetov, M.; Bevan, R.; Want, A.; Anantrasirichai, N.; Achim, A.; Albon, J.; Morgan, J. SVM-Based Optical Detection of Retinal Ganglion Cell Apoptosis. Photonics 2025, 12, 128. [Google Scholar] [CrossRef]
- Kulmaganbetov, M.; Morgan, J.E. Application of Texture Analysis in Retinal OCT Imaging. In Handbook of Texture Analysis; CRC Press: Boca Raton, FL, USA, 2024; pp. 154–179. [Google Scholar]
- Inkarbekov, M.; Kulmaganbetov, M.; Bazarbekova, G.; Baiyrkhanova, A. Assessment of Postoperative Retinal Complications and the Possibility of Optical Diagnostics Using Support Vector Machine. Salud Cienc. Tecnol. 2024, 4, 1359. [Google Scholar] [CrossRef]
| Substances | RI | Advantages | Disadvantages | PD, µm | SC, cm−1 | AC, cm−1 | MS, MPa | DT | Applications |
|---|---|---|---|---|---|---|---|---|---|
| Gelatin-based phantoms | 1.35 |
|
| 500–2000 | 5–20 | 0.1–1 | 0.01–0.1 | days to weeks |
|
| Silicone phantoms | 1.41 |
|
| 1000–2000 | 10–15 | <1 | 0.1–3 | years |
|
| Polydimethylsiloxane (PDMS) phantoms | 1.41 |
|
| 1000–2000 | 5–10 | <0.1 | 0.5–3 | years |
|
| Polyvinyl alcohol (PVA) phantoms | 1.48 |
|
| 1000–2000 | 5–15 | <1 | 0.01–1 | months to years |
|
| Fibrin phantoms | 1.38 |
|
| 1000–2000 | 5–20 | <1 | 0.001–0.01 | days to weeks |
|
| Substances | RI | Advantages | Disadvantages | SC, cm−1 | AC, cm−1 | MS, GPa | Applications |
| Polystyrene | 1.57 |
|
| 50–100 | <0.01 | 3–3.5 |
|
| Titanium dioxide (TiO2) | 2.49 |
|
| 100–200 | <0.1 | 230–280 |
|
| Silica microspheres (SiO2) | 3.6 |
|
| 50–150 | <0.01 | 70–75 |
|
| Gold nanoshells | 2.59 |
|
| 50–150 | 1–10 | 70–80 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulmaganbetov, M. Advancements in Retinal Tissue-Mimicking Optical Coherence Tomography Phantoms: Materials, Properties, and Applications. BioChem 2025, 5, 6. https://doi.org/10.3390/biochem5020006
Kulmaganbetov M. Advancements in Retinal Tissue-Mimicking Optical Coherence Tomography Phantoms: Materials, Properties, and Applications. BioChem. 2025; 5(2):6. https://doi.org/10.3390/biochem5020006
Chicago/Turabian StyleKulmaganbetov, Mukhit. 2025. "Advancements in Retinal Tissue-Mimicking Optical Coherence Tomography Phantoms: Materials, Properties, and Applications" BioChem 5, no. 2: 6. https://doi.org/10.3390/biochem5020006
APA StyleKulmaganbetov, M. (2025). Advancements in Retinal Tissue-Mimicking Optical Coherence Tomography Phantoms: Materials, Properties, and Applications. BioChem, 5(2), 6. https://doi.org/10.3390/biochem5020006






