Abstract
Colorectal cancer (CRC) is a prevalent and deadly tumor worldwide. Understanding the molecular mechanisms underlying CRC development will improve treatment outcomes and patient survival. Natural molecules and metabolites from plants, such as Tillandsia usneoides, reduce tumor growth by modulating glucose metabolism and increasing reactive oxygen species (ROS). To shed light on the mechanism involved in the anti-tumor effects of T. usneoides, we evaluated the cytotoxic effect of the ethanolic extract of this plant on the colon cancer cell line SW480 through the activation of the peroxisome proliferator-activated receptor gamma (PPARγ), a nuclear receptor that plays a role on lipid metabolism and inflammation in cancer cells. To this end, we assessed the activation of PPARγ by T. usneoides extract in transactivation luciferase assays, as well as the cytotoxic effect of this extract on the SW480 cell line after knocking down PPARγ using shRNA. Our findings indicate that the T. usneoides extract exhibits cytotoxic effects on the SW480 cell line, potentially in the same way as PPARγ activator, pioglitazone, i.e., by increasing reactive oxygen species (ROS). In addition, both T. usneoides extract and pioglitazone exert lipogenic properties in the SW480 cells. Taken together, these results demonstrate that the T. usneoides extract decreases the viability of the colon cancer cell line SW480, at least in part, through the activation of PPARγ. This suggests the potential for further use of this plant in the treatment of other chronic diseases.
1. Introduction
In 2020, nearly 10 million deaths from cancer were reported worldwide, making it the second leading cause of death [1]. In the same year, colorectal cancer (CRC) was the third most commonly diagnosed malignancy and the second leading cause of cancer related death in both men and women [2]. By 2030, CRC incidence is estimated to increase by 60%, to 2.2 million new cases and 1.1 million deaths annually [3]. CRC is more common in Western countries and has a lower incidence in Asia, although this trend has been changing in recent years for a variety of reasons, including unhealthy dietary habits such as low consumption of vegetables or fiber, high consumption of processed red meat, reduced physical activity, and the prevalence of obesity in this population [4]. The current treatments for CRC vary depending on the stage of the disease. For example, in early-stage disease, endoscopic removal of malignant polyps is common, with tumor remission and a 5-year survival rate of 91%, whereas in more advanced disease stages, surgical resection of the tumor and chemotherapy are required [5]. However, the effectiveness of these strategies decreases in these patients, increasing the likelihood of cancer relapse by 40% and reducing 5-year survival by 27% [5]. Hence, there is a need for research into the different mechanisms involved in the development and progression of the disease, as well as new therapeutic options that will allow the development of more effective and targeted approaches for these patients [6]. Currently, a promising treatment for CRC is immunotherapy. Studies have shown that tumors with a high mutational burden, such as those exhibiting microsatellite instability (MSI) or mismatch repair deficiency (dMMR), are more responsive to immune checkpoint inhibitors like anti-PD-1 and anti-PD-L1 agents [7]. The U.S. Food and Drug Administration (FDA) has approved the use of pembrolizumab and nivolumab, either as monotherapy or in combination with ipilimumab, for the treatment of MSI-H/dMMR metastatic CRC [8]. However, patients without these molecular signatures often do not experience the same clinical benefits, underscoring the need to explore alternative therapeutic approaches for managing colorectal cancer, particularly in patients who lack the favorable molecular features that predict robust responses to immune checkpoint blockade [9].
Several molecular mechanisms have been identified in the development of CRC [10]. One such mechanism, linked to the origin, progression, and metastasis of the disease, is associated with Peroxisome proliferator-activated receptors (PPARs). These receptors are ligand-inducible transcription factors characterized by the transcriptional modulation of genes involved in processes such as energy metabolism [11], inflammation [12,13], and immunity [14]. In particular, evidence suggests an anti-tumor role of the PPARγ isotype, specifically leading to growth inhibition [15], modulation of oxidative stress [16,17], and induction of death in cancer cells of different origins [18]. In addition, patients with C161T polymorphism in PPARγ increase the risk of developing CRC [19]; then, the expression of tumor suppressor proteins (pRB, p16, and p21) were positively correlated with PPARγ expression in colon cancer from 86 patients by immunostaining procedures [20], and people who consumed pioglitazone, an agonist of PPARγ, for more than 36 months decreased their risk of colon cancer (HR 0.8 (0.4–1.5)) in a cohort study of 252,467 patients [21]. For immunotherapy approaches in cancer treatment, PPARγ becomes an excellent target because (1) it has been reported that this receptor enhances dendritic cell (DC) functionality by modulating antigen uptake, presentation, activation, and migration [22]; (2) it reduces proinflammatory cytokine release from tumor associated macrophages (TAMs) and induces polarization of these cells [23]; and (3) it reduces the expansion and inflammation of Myeloid-derived suppressor cells (MDSCs) by controlling the neutral lipid metabolism [24].
In addition to its role in the microenvironment, PPARγ activation exhibits antiproliferative properties in colon cancer cell lines [25,26] and in CX-1 cell tumor bearing nude mice [25]. Moreover, immunohistochemical analysis of colon adenocarcinoma from patients demonstrated that PPARγ expression is downregulated in tumors with high expression of proinflammatory markers associated with colon cancer such as cyclooxygenase-2, pc-JUN, CBP, and p-IκB-α [27]. Consistent with these findings, animal studies have shown that sustained activation of PPARγ results in a reduction of abnormal crypt foci formation in rats and a significant suppression of CRC [28].
New therapeutic avenues have arisen in the last decades, and natural therapy or phytotherapy has emerged as one of the most promising options. Phytotherapy is distinguished by the utilization of plant extracts containing a mixture of secondary metabolites, which can be targeted towards various therapeutic objectives, thereby facilitating improved disease management [29]. In that sense, assessing the anti-tumor effects of phytotherapeutics and deciphering the underlying molecular mechanisms responsible for their anti-tumoral capacity will provide scientific evidence for alternative cancer therapy options. T. usneoides, commonly known as Spanish moss, is a member of the Bromeliaceae family [30]. In North, Central, and South America, it is traditionally used for the treatment of gastritis, epilepsy, sore throat, and diabetes [31,32]. Recently, its anti-tumor activity has been reported in in vitro and in vivo models of breast cancer, where the ethanolic extract of T. usneoides caused cytotoxicity in 4T1 cells, increasing ROS production and altering the energetic metabolism; in vivo, it delayed tumor growth and impacted the distribution of tumor-infiltrating immune cells, notably increasing conventional dendritic cells (cDCs) and CD8α DC subpopulations compared to the control condition; these effects were not noted in the in vivo melanoma model, although the extract showed in vitro apoptosis induction and production of ROS as in the 4T1 model [33]. To date, there have been no reports on the anti-tumor activity of this plant in CRC. Chemical characterization of the ethanolic extract of T. usneoides showed an enrichment in triterpenes and flavonoid compounds, some of which have been reported as ligands of PPARγ [34,35]. Therefore, the aim of this study was to evaluate whether some of the anti-tumor effects of the ethanolic extract of T. usneoides on the colon cancer cell line SW480 are mediated by the modulation of PPARγ. Interestingly, we revealed that the ethanolic extract of T. usneoides activates the PPARγ receptor in transactivation assays, and this extract reduces the viability of the SW480 cell line by inducing oxidative stress just as the activation of PPARγ by pioglitazone does. Finally, we demonstrated that the effect of the ethanolic extract of T. usneoides on the viability of this cancer cell line is, at least in part, mediated through the activation of PPARγ.
2. Materials and Methods
2.1. Plant Material
The preparation and characterization of the extract of T. usneoides was carried out as described in [33]. The plant extract was obtained from fresh leaves collected in Boyacá, Colombia, and identified by the Javeriana University Herbarium (voucher COL 30547). The P2Et extract from Caesalpinia spinosa was prepared and characterized according to previous standardized protocols [36,37]. Fresh pods were collected in Boyacá, Colombia, and identified by Carlos Parra of the National Herbarium of Colombia (voucher COL 588448). The extract of Petiveria alliacea (Anamu-SC) was prepared and characterized according to the procedure described in [38]. Plant material was collected in Cundinamarca, Colombia. The extract of the plant material was identified by Antonio Luis Mejia of the National Herbarium of Colombia, voucher COL 333406. Finally, Piper nigrum extract was prepared and characterized, as previously described in [39]. Briefly, plant extract was obtained from fresh fruits collected in Putumayo, Colombia. Identification was performed by Nestor García at the Pontificia Universidad Javeriana Herbarium (voucher COL 30548). All extracts were dissolved in ethanol.
2.2. Cell Culture
The human colon cancer cell lines SW480 and HT29 (a gift from Tonny Williams Naranjo of the Biological Research Corporation, Medellín-Antioquia, Colombia) were cultured in DMEM media (Sigma-Aldrich, Saint Louis, MO, USA) supplemented with 10% heat-inactivated horse serum (HS) (Gibco, Thermo Fisher ScientificTM, Waltham, MA, USA) or with 10% heat-inactivated fetal bovine serum (FBS) (Eurobio Scientific, Toulouse, France), respectively, 2 mM L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, 0.01 M HEPES buffer, and 1 mM sodium pyruvate (Eurobio) and incubated in a humidified incubator at 37 °C in 5% CO2. Human embryonic kidney HEK 293T cells were cultured in the same way as the HT29 cell line. B16-F10 murine melanoma cells were cultured in RPMI-1640 medium (Eurobio) supplemented with 10% FBS, 2 mM L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, 0.01 M HEPES buffer, and 1 mM sodium pyruvate (Eurobio) and cultivated in a humidified incubator at 37 °C in 5% CO2. Prior to treatments, cells were pre-incubated in low serum media Opti-MEM (Gibco) to induce starvation.
2.3. In Vitro Cytotoxicity Assay
The cytotoxic effect of the different plant extracts on tumor cells was evaluated using the methylthiazol tetrazolium (MTT) assay (Sigma-Aldrich). Briefly, 5 × 103 tumor cells were plated (96-well), incubated, and treated with different concentrations of plant extracts (250 to 1.9 µg/mL) (ethanol 0.02%) as well as pioglitazone (Tocris Bioscience, Bristol, UK) (100 to 0.78 µM) (DMSO 0.01%) for 48 h at 37 °C. After treatment, the cells were centrifuged, the media were removed, then cells were washed twice with phosphate-buffered saline (PBS) before the addition of MTT. Then, MTT (1 mg/mL) was added to RPMI medium without phenol red, and incubated for 4 h at 37 °C. DMSO was used to solubilize the formazan crystals. MTT results were read at 540 nm using a FluoStar Omega reader (BMG Labtech, Ortenberg, Germany). The IC50 value (50% inhibition of cell growth) was calculated using GraphPad Prism version 8.1.1 (GraphPad Software). The experiments were performed in triplicate.
2.4. Population Doubling Time (PDT)
PDT was determined by counting viable cells using the trypan blue dye exclusion method. For this, 1.5 × 104 cells were plated (12 wells) before treatment, with the IC50 calculated previously for the evaluation of the viability of each plant extract or pioglitazone assay for different time points, including 0, 24, 48, 72, and 96 h. For each time point, PDT was calculated using the exponential growth method (Mathusian) through GraphPad Prism version 8.1.1. Experiments were performed in triplicate.
2.5. Trypan Blue Cell Viability Assay
A quantity of 1.5 × 104 cells/well were seeded onto 12-well plates incubated and treated, with either the IC50 dose determined in MTT of T. usneoides or pioglitazone for 48 h. Then, cells were harvested and washed with phosphate-buffered saline (PBS). An equal volume of trypan blue solution (0.4% in PBS) and cell suspension were mixed and incubated for 3 min. After incubation, cells were loaded on a Neubauer chamber and counted under a microscope. Cell death was calculated as the percentage of trypan blue positive cells in the total population of stained and unstained cells. Experiments were performed in triplicate.
2.6. Cell Death Quantification by Propidium Iodide (PI)
A quantity of 1 × 105 cells/well were plated in 24 wells and treated, with the IC50 dose determined either in MTT of T. usneoides or pioglitazone at 37 °C for 48 h. Sample supernatants containing detached and dead cells were collected, and attached cells were trypsinized. Cell suspensions were stained with 10 µg/mL propidium iodide (PI) in PBS and quantified using a Millipore Guava EasyCyte Flow Cytometer. The percentage of dead cells was analyzed using GuavaSoft™ 3.1.1 software. Results are expressed as mean ± SEM. Experiments were performed in triplicate.
2.7. Luciferase Activity Assay
A quantity of 1.5 × 105 HEK 293T or SW480 cells/well were seeded (12 wells) and transfected with either a PPARγ overexpression plasmid (pSG5-PPARγ), PPARα overexpression plasmid (pSG5-PPARα), or PPARβ overexpression plasmid (pSG5-PPARβ) and a luciferase expression plasmid under the control of PPAR response elements (PPRE 3X-TK-Luc) and a GFP transfection control plasmid (pCMV6-AC-GFP from OrigeneTM) using Lipofectamine 2000 for HEK 293T or 3000 for SW480 (Invitrogen, Waltham, MA, USA) for 8 h in Opti-MEM media. The cells were then treated with sublethal doses of different plant extracts or agonists of the different PPARs (pioglitazone for PPARγ, WY14643 for PPARα, and GW501516 for PPARβ) as positive activation controls. Finally, the FluoStar Omega reader (BMG Labtech) was used to read the luminescence at 12, 24, and 48 h. The fluorescence emission of GFP was used to normalize the transfected cells. Results are expressed as mean ± SEM. Experiments were performed in triplicate for each treatment.
2.8. Interaction Assays of Pioglitazone and T. usneoides or P. nigrum Extracts
The synergistic effects of pioglitazone and T. usneoides or P. nigrum extracts were evaluated using the MTT assay. A dose–response matrix was created with eight concentrations of T. usneoides (ranging from 0 to 34 μg/mL) or P. nigrum (ranging from 0 to 8 μg/mL) and pioglitazone (ranging from 0 to 6.6 μM). Drug combination effects were estimated using R Synergy Finder Plus [40]. The Zero Interaction Potency (ZIP) model generated a synergy score matrix from a dose–response matrix [41]. Experiments were performed in triplicate.
2.9. Lipid Staining
Lipid droplet (LD) accumulation in tumor cells was assessed by staining the cells with Nile Red (Invitrogen). A total of 1.5 × 105 cells/well were plated in 24 wells and treated with either the IC50 dose determined in MTT of T. usneoides or pioglitazone at 37 °C for 48 h. The cells were then washed twice with PBS. Subsequently, the cells were stained with 1 µM Nile Red for 20 min at room temperature. After a round of washing with PBS, the cells were trypsinized and centrifuged at 200× g for 5 min. The cell pellet was resuspended in PBS and analyzed using a Millipore Guava EasyCyte Flow Cytometer. Data were analyzed using GuavaSoft™ 3.1.1 software. Results are expressed as mean ± SEM. Experiments were performed in triplicate.
2.10. Determination of Total Fatty Acids
Lipids were extracted according to a modified version of the method of Bligh and Dyer (1959) [42]. Briefly, treated cells were trypsinized, centrifuged at 200× g for 5 min, and counted. Freeze-dried cells were suspended in 0.8 mL PBS and transferred to an amber glass tube. Next, 3 mL of ice-cold chloroform/methanol solution (1:2) was added and mixed. Subsequently, 1 mL of chloroform and 1 mL of PBS were added and mixed to generate a two-phase solution. The mixture was centrifuged at 1000× g for 2 min. The lower phase was recovered and concentrated in a N2 stream. Fatty acid profiles were obtained by Gas Chromatography with Flame Ionization Detection (GC-FID) employing a GC-2014 instrument (Shimadzu, Kyoto, Japan). Fatty acids were analyzed in the form of fatty acid methyl esters (FAMEs). The derivatization of lipids and fatty acids was carried out using BF3/MeOH following the AOAC 925.41 protocol [43]. For the separation and quantification of FAMEs, a Rt-2560 column (100 m, 0.20 mm ID, 0.2 μ) (Restek, Centre County, PA, USA) was employed. Helium was used as the carrier gas and flowed at a rate of 1 mL/min. The temperature program included an initial phase of 100 °C for 4 min, followed by a temperature ramp of 4 °C/min until it reached 220 °C. Subsequently, a holding time of 30 min was applied at 220 °C. The total run time for the analysis was 74 min. The experiments were performed in triplicate. Results are expressed as the mean ± SEM of area of total lipids/number of lysed cells.
2.11. Determination of Reactive Oxygen Species
To evaluate ROS production, 8.5 × 104 cells/well were plated in 12-well plates and treated, with the IC50 and IC50/2 determined in MTT of T. usneoides extract or pioglitazone for 48 h at 37 °C; ethanol or DMSO, respectively, were used as a negative control of the treatment. Ascorbic acid (AA) (5 mM) was used as an antioxidant control, and doxorubicin 0.5 µM was used as a pro-oxidant control of the ROS detection probe. A known number of cells were stained with 1 μM 2′,7′-dichlorodihydrofluorescein diacetate (H2-DCFDA, Sigma-Aldrich) for 30 min at 37 °C as ROS detection probes. After washing with cold PBS, the centrifuged cell pellets were resuspended in PBS with propidium iodine 1 µg/mL before FACS analysis. Finally, samples were acquired by a FACS Aria II flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). Data were analyzed using FlowJo™ v10.8.1 software (BD). Experiments were performed in triplicate. Results are expressed as mean ± SEM.
2.12. Knocking Down of PPARγ on Human Colon Cancer Cell Line SW480
SW480 cells were transfected with a pLVTH_shRNA-PPARγ or shScramble negative control (pGFP-C-shLenti shRNA Vector from OriGeneTM, Rockville, MD, USA) using Lipofectamine 3000 (Invitrogen, Waltham, MA, USA). Transfection was performed in Opti-MEMTM (Thermo Fisher ScientificTM, Waltham, MA, USA) media for 48 h before to verify PPARγ expression by western blot or cytotoxicity by XTT procedures (Roche, Bale, Switzerland)
2.13. Western Blotting
SW480 cells treated with the IC50 determined in an MTT of T. usneoides extract or pioglitazone for 48 h at 37 °C, or transfected with negative control shScramble or shPPARγ, were lysed on ice for 20 min with RIPA buffer (150 mM NaCl, 50 mM Tris-HCl, pH 7.4, 1 mM EDTA, 1% Triton X-100, 0.1% SDS, and 0.5% sodium deoxycholate). Lysates were centrifuged, and the protein concentration was measured by bicinchoninic acid assay (Thermo Fisher Scientific™, Waltham, MA, USA). A total of 25 μg of total protein was resolved on a precast 4–12% polyacrylamide SDS-PAGE and transferred to a nitrocellulose membrane. The membranes were blocked for 1 h at room temperature in Tris-buffered salin with 0.1% Tween® 20 detergent (TBST) supplemented with 5% non-fat dry milk. The primary anti-PPARγ antibody (Cat. No: Y080086, abm®, Richmond, BC, Canada), Akt (pan) (11E7) (Cat. No: 4685, Cell Signaling Technology, Inc., Davers, MA, USA), or Phospho-Akt (Ser473) Antibody (Cat. No: 9271, Cell Signaling Technology) was incubated overnight at 4 °C, followed by incubation with a specific secondary antibody conjugated to horseradish peroxidase. Membrane-bound immunocomplexes were visualized using Super Signal West Pico chemiluminescent substrate (PierceTM, Thermo Fisher) in an iBright FL1500 imaging system (Thermo Fisher ScientificTM, Waltham, MA, USA). Densitometry of each protein was normalized to β-actin or total proteins on the western blot membrane stained with Coomassie Brilliant Blue. The densitometry was calculated using ImageJ software 1.53e.
2.14. In Vitro Cytotoxicity Assay in PPARγ Knocked Down SW480 Cells
The XTT colorimetric assay (Roche) was used to determine the changes on the cytotoxic activity of T. usneoides extract in the SW480 PPARγ knockdown colon cancer cell line. 5 × 103 cells were grown in 96-well plates for 1 d, transfected, and then treated with 50 μg/mL of T. usneoides extract or ethanol 0.02% as a negative control for 48 h at 37 °C. Cells were carefully washed twice with PBS before incubation with 50 μL of XTT solution in RPMI without phenol red for 8 h. After incubation with XTT, the metabolically active cells developed an orange formazan product, which was quantified at 450 nm using a FluoStar Omega reader (BMG Labtech, Ortenberg, Germany). The number of live cells was directly correlated to the amount of orange formazan formed. Three independent experiments were performed for each treatment.
2.15. Statistical Analysis
Statistical analysis of the significance between the two groups was calculated using a one- or two-tailed unpaired Student’s t-test for most of the statistical analyses, and a p-value of <0.05 was considered statistically significant. The specific statistical test results are indicated in each figure: * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. Statistical analyses were performed in GraphPad Prism version 8.1.1.
3. Results
3.1. Cytotoxic and Cytostatic Activity of Different Plant Extracts on the SW480 Cell Line
Previous studies have shown promising anti-tumor properties of standardized extracts of P. alliacea (Anamu-SC), C. spinosa (P2Et), T. usneoides, and P. nigrum in melanoma, breast cancer, and leukemia [33,44,45,46,47]. However, their effects on colon tumor cell lines have not been widely reported, except for P. nigrum [48,49]. Here, we evaluated the cytotoxic effect of these standardized extracts on the SW480 cell line for 48 h using the MTT assay. T. usneoides and P2Et extracts reduced cell viability in a dose-dependent manner, with a calculated IC50 of 8.6 ± 1.40 μg/mL and 6.1 ± 0.60 μg/mL, respectively. The P. nigrum extract decreased cell viability at all doses studied, with an IC50 of 0.06 ± 0.05 μg/mL. Anamu-SC did not show marked cytotoxic activity in the SW480 cell line, with an IC50 of 152.6 ± 11.2 μg/mL (Figure 1A). These results were consistent with a delay in the proliferative capacity after treatment with all extracts in comparision to ethanol (vehicle), although a more significant increment of the PDT was observed with the Anamu-SC and P. nigrum extracts (Figure 1B). Quantification of cell death by propidium iodide staining after treatment with the IC50 (determined in MTT) of different extracts or ethanol showed a significant increase in the number of PI-positive cells following treatment with all plant extracts compared to the ethanol (Figure 1C). This indicates that the plant extracts had not only a cytotoxic effect but also a mortality effect on the SW480 colon cancer cell line.
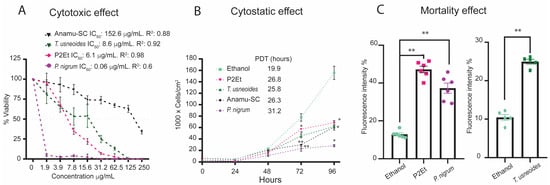
Figure 1.
Cytotoxic and cytostatic activities of different plant extracts on the SW480 cell line. (A) Cytotoxic activity for SW480 after treatment with Anamu-SC, T. usneoides, P2Et, and P. nigrum extracts at different concentrations (250 to 1.9 µg/mL) for 48 h. Each extract was dissolved in ethanol (vehicle). The IC50 was calculated in GraphPad Prism version 8.0 using a non-linear regression of the log (inhibitor) versus the normalized slope of the response variable. (B) Cytostatic activity was evaluated at 0, 24, 48, 72, and 96 h after treatment with the IC50 of different extracts or ethanol. The population doubling time (PDT) is shown for each treatment. (C) Quantification of cell death, by propidium iodide (PI) staining after treatment with the IC50 (determined in MTT) of different extracts or ethanol. In all cases, data are presented as the mean ± SEM. Data from three independent experiments are shown. * p < 0.05; ** p < 0.01.
3.2. T. usneoides Extract Induces Activation of the Nuclear Receptor PPARγ and Decreases p-AKT Levels in the SW480 Cell Line
The molecular mechanisms underlying the cytotoxicity and cytostatic effects of standardized extracts of Anamu-SC, P2Et, T. usneoides, and P. nigrum are not fully elucidated. Chemical analysis of these extracts revealed an abundance of terpenoids and flavonoids, which have been identified as potential PPARγ ligands [34,35]. To evaluate whether the cytotoxicity and cytostatic activity observed by these extracts treatment is related to the activation of PPARγ. We first confirmed the cytotoxic and cytostatic effects of the activation of this nuclear receptor using pioglitazone, a PPARγ agonist. Treatment with the PPARγ agonist showed cytotoxic activity in a dose-dependent manner, with a calculated IC50 of 4.8 µM ± 0.66 µM (Figure 2A). Additionally, analysis of the effects of pioglitazone on SW480 cells revealed a time-dependent reduction in proliferation (Figure 2B). Propidium iodide (PI) staining showed a significant increase in the number of PI-positive cells following treatment with the IC50 dose of pioglitazone, determined in MTT, compared to the DMSO control, suggesting that the pioglitazone treatment led to a substantial increase in the percentage of cells undergoing cell death (Figure 2C).
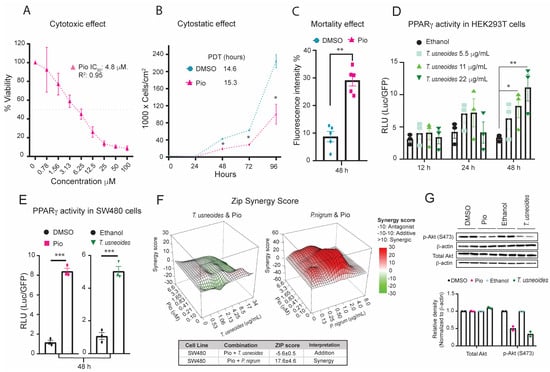
Figure 2.
Effects of T. usneoides extract on PPARγ activity in HEK 293T and SW480 cell lines. (A) Cytotoxic activity of SW480 cells after treatment with pioglitazone (100 to 0.78 µM) for 48 h. The IC50 was calculated in GraphPad Prism version 8.0 using a non-linear regression of the log (inhibitor) versus the normalized slope of the response variable. (B) Cytostatic activity of SW480 cells after treatment with pioglitazone IC50 or DMSO for 0, 24, 48, 72, and 96 h. PDT was calculated for pioglitazone treatment. (C) Quantification of cell death by propidium iodide (PI) staining after treatment with the IC50 of pioglitazone or DMSO. (D) Transcriptional activation of luciferase activity by PPARγ in HEK 293T cells co-transfected with pSG5-PPARγ, PPRE 3X-TK-Luc, and pCMV6-AC-GFP, and then treated with the with the IC50, IC50/2, or IC50×2 (MTT) of T. usneoides extract or ethanol for 12, 24 and 48 h. (E) Transcriptional activation of luciferase activity by PPARγ in SW480 cells treated with the IC50 of T. usneoides extract or the IC50 of pioglitazone with their respectively controls for 48 h. (F) Dose–response matrix containing eight concentrations of T. usneoides (0–34 μg/mL) or P. nigrum (0 to 8 μg/mL) and pioglitazone (0 to 6.6 μM). Drug combination effects were estimated using R Synergy Finder Plus [40]. (G) Representative images of western blot analysis: total AKT or p-AKT (S473) protein expression in SW480 cells treated with the IC50 of pioglitazone or DMSO or T. usneoides extract or ethanol for 48 h. Bar graphs were constructed according to the gray values of the protein bands. Data are presented as the mean ± SEM from three independent experiments. * p < 0.05; ** p < 0.01; *** p < 0.001.
Next, we tested the ability of the different plant extracts to induce activation of PPARγ. Luciferase reporter gene assays were performed using HEK 293T cells. The positive control (pioglitazone) showed significant activation of PPARγ, inducing luciferase reporter expression at every time point measured (Figure S1A). Conversely, HEK 293T cells treated with a sublethal dose of P. nigrum or Anamu-SC extracts did not show functional activation of the nuclear receptor. Additionally, all sublethal doses of P2Et extract decreased half-fold luciferase activity compared to ethanol (Figure S1B). These results suggest that the cytotoxic and cytostatic effects of these extracts (Figure 1A–C) are independent of PPARγ activation. Interestingly, treatment of HEK 293T cells with different sublethal doses of T. usneoides extract caused a 2-fold increase in luciferase activity after 24 h compared with ethanol, and this increase was dose-dependent after 48 h of treatment. Maximum activation of the reporter gene occurred at a concentration of 22 μg/mL of T. usneoides extract (Figure 2D). A similar activation of PPARγ was observed in SW480 cells treated with the IC50 of pioglitazone or the IC50 of T. usneoides extract for 48 h (Figure 2E). Although transactivation activity was observed in SW480 transfected with the PPARα overexpression or PPARβ overexpression plasmids, and treated with the IC50 of T. usneoides (Figure S1C,D), neither the PPARα agonist WY14643 (Figure S1E,G) nor the PPARβ agonist GW501516 (Figure S1F,G) showed any significant cytotoxic activity in the SW480 cell line. This suggests that part of the cytotoxic effect of T. usneoides in these cells is due to the specific activation of PPARγ and not to other PPAR isotypes. Then, we evaluated the interaction between the cytotoxic properties of T. usneoides extract and pioglitazone on SW480 cells and compared it with the combination of another extract that does not activate PPARγ, such as P. nigrum extract (Figure S1B) and pioglitazone (Figure 2F). The analysis of the synergic effect generated by the interaction of T. usneoides extract or P. nigrum extract and pioglitazone on the cells is shown in the 3D synergy map, where the intensity in red and increase in relief represents a score greater than 0, while the intensity in green and decrease in relief represents a score less than 0. A score between −10 and 10 indicates an additive interaction, whereas a score higher than 10 suggests a synergistic effect. In this case, T. usneoides with pioglitazone presented an additive interaction in the SW480 cell line (ZIP score − 5.6 ± 0.5). Conversely, P. nigrum extract and pioglitazone showed a synergistic effect (ZIP score 17.6 ± 4.6). This indicates that the cytotoxic effect of T. usneoides and pioglitazone seem to partially share the same target. In the case of P. nigrum extract and pioglitazone, the cytotoxic mechanisms involved in these treatments seemed to contribute differently. To further characterize the cytotoxic activity of T. usneoides, we examined the phosphorylation of an important kinase, AKT, at serine 473, which is implicated in cancer cell survival. After treatment with pioglitazone and the IC50 of T. usneoides extract, we observed that both treatments decreased p-AKT levels in SW480 cells (Figure 2G). All these results strongly suggested a correlation between T. usneoides cytotoxicity and the specific activation of PPARγ.
3.3. Correlation between PPARγ Expression and T. usneoides Extract Cytotoxicity on Different Cell Lines
To evaluate whether the cytotoxicity of T. usneoides is dependent on the level of PPARγ expression in cancer cell lines, we measured the cytotoxic activity and cell death of T. usneoides extract and pioglitazone on cells previously reported to have a weak expression of PPARγ, such as murine melanoma cells B16–F10 [50]. We then compared these effects to the cytotoxicity and cell death observed in SW480 cells, which are known to exhibit high levels of PPARγ expression [51]. Treatment with pioglitazone showed cytotoxic and cell death activity in both cell lines. However, SW480 cells were more sensitive to the agonist compared to B16-F10 melanoma cells (IC50 of 4.8 ± 0.66 μM vs. 26.8 ± 6.52 μM, respectively) (Figure 3A,C). Consistently, T. usneoides extract reduced the viability of SW480 and B16–F10 cells (Figure 3B,C). Moreover, similar to the effect of pioglitazone, SW480 cells were more sensitive to the plant extract than B16–F10 cells, with an IC50 of 8.6 ± 1.40 μg/mL vs. 46 ± 8.7 μg/mL, respectively (Figure 3B). These findings suggest that the cytotoxic effect of T. usneoides extract and pioglitazone is probably stronger in SW480 cells than in B16–F10 cells because of the differences in PPARγ expression levels in both cell lines (Figure 3D). In addition, no effects of T. usneoides on the expression of PPARγ were observed in either cancer cell line (Figure 3D).
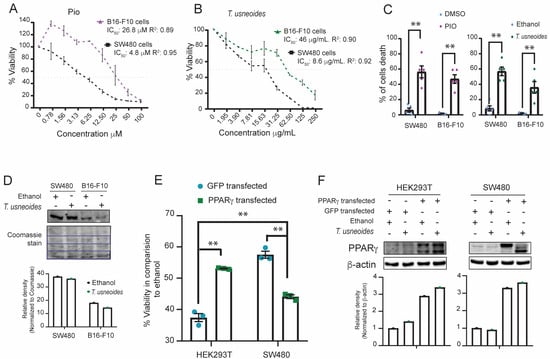
Figure 3.
Effect of PPARγ expression and T. usneoides extract cytotoxicity on different cell lines. (A) Cytotoxic activity for B16-F10 and SW480 after treatment with pioglitazone (100 to 0.78 µM) or (B) different concentrations (250 to 1.9 µg/mL) of T. usneoides extract for 48 h. The IC50 of the B16–F10 cell line was calculated in GraphPad Prism version 8.0 using a non-linear regression of the log (inhibitor) versus the normalized slope of the response variable. (C) Cell death quantification using trypan blue assay for B16–F10 and SW480 tumor cell lines after treatment with the IC50 of pioglitazone, DMSO, T. usneoides extract or ethanol for 48 h. (D) Representative images of western blot analysis: PPARγ protein expression in SW480 cells and B16–F10 treated with the IC50 of T. usneoides extract or ethanol for 48 h. Proteins on the western blot membrane were stained with Coomassie Brilliant Blue and shown as loading control in the lower panel. (E) Cytotoxic activity for HEK 293T and SW480 cells co-transfected with pSG5-PPARγ and pCMV6-AC-GFP and then treated with an 11 µg/mL of T. usneoides extract for 48 h. (F) Western blot analysis: PPARγ protein overexpression in HEK 293T and SW480 cells and treated with 11 µg/mL of T. usneoides extract or ethanol for 48 h. β-actin was used as the internal standard. Bar graphs were constructed according to the gray values of the protein bands. Data represent three independent experiments. ** p < 0.01.
To evaluate whether the cytotoxic effect occurs in non-tumoral cells expressing PPARγ [52], an experiment of viability using phytohaemagglutinin-activated peripheral blood mononuclear cells (PBMCs) treated with pioglitazone and the T. usneoides extract was performed. Low cytotoxicity was observed in PBMCs treated with IC50, IC50/2, or IC50×2 of pioglitazone or the T. usneoides extract compared to SW480 cells (Figure S2A,B). Furthermore, overexpression of PPARγ in HEK 293T and SW480 cells treated with 11 µg/mL of T. usneoides extract showed a specific cytotoxic effect in SW480 cells (Figure 3E,F). Additionally, in the HT29 colon cancer cell line, which express PPARγ (Figure S3C), it was noticed that pioglitazone and T. usneoides extract also exerted a cytotoxic effect (Figure S3A,B). These results could indicate that the cytotoxicity of T. usneoides extract depends on the expression of PPARγ and the tumor cell characteristics.
3.4. T. usneoides Promotes the Physiological Activation of PPARγ as Pioglitazone Does
Previously, the literature showed that activation of PPARγ by different agonists such as pioglitazone induces the accumulation of lipid droplets (LDs) [53]. We quantified LD formation using Nile Red staining to determine whether the physiological effect of PPARγ activation is similar to T. usneoides extract treatment in SW480 cells. As expected, the activation of PPARγ by pioglitazone induced a two-fold LD accumulation compared to the DMSO-treated control (p-value = 0.015); similarly, T. usneoides treatment presents almost five times more LDs than ethanol control treated cells (p-value = 0.0043) (Figure 4A). Furthermore, the fatty acid content, as determined by GC-MS, indicates that both treatments (pioglitazone and T. usneoides) enhance the content in SW480 cells to a similar extent compared with their respective controls (Figure 4B). Next, to analyze whether the synthesis of fatty acid is related to the decrease of viability induced by pioglitazone and T. usneoides extract, SW480 cells were treated with the T. usneoides extract (50 µg/mL) or pioglitazone (25 µM) in the presence or absence of C75 (1.97 or 3.95 µM), a fatty acid synthase (FAS) inhibitor. The results revealed a decrease in the pioglitazone-induced cytotoxic activity of approximately 50% and 70%, with 1.97 µM and 3.95 µM of C75, respectively (Figure 4C). In the same way, T. usneoides extract’s effect on viability was increased 50% and 75%, with 1.97 µM and 3.95 µM of C75, respectively (Figure 4D). According to these results, it is possible to suggest that a cytotoxic effect of T. usneoides extract implies an increase in fatty acid synthesis, a process mediated by the activation of PPARγ.
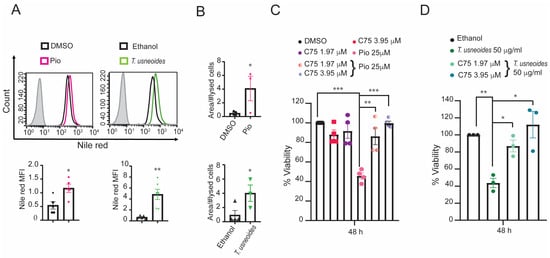
Figure 4.
Effect of T. usneoides in the physiological activation of PPARγ. (A) Quantification of lipid droplets (LDs) in SW480 cells after treatment with the IC50 determined in MTT of pioglitazone and T. usneoides for 48 h using Nile Red. Representative histograms are shown: negative controls (black line for DMSO or ethanol), treated cells (pink or green for pioglitazone or T. usneoides, respectively), and isotype control (gray solid histogram). (B) Fatty acid content obtained by Gas Chromatography with Flame Ionization Detection (GC-FID) of SW480 cells after treatment with the IC50 of pioglitazone or T. usneoides for 48 h. (C) Cytotoxic effect after 48 h of treatment with pioglitazone (25 µM) or (D) T. usneoides (50 µg/mL) in the presence or absence of C75 (1.97 or 3.95 µM). Data represent three independent experiments. * p < 0.05; ** p < 0.01; *** p < 0.001.
3.5. T. usneoides Extract and Pioglitazone Increased ROS Production in the Colon Cancer Cell Line SW480
It has been reported that PPARγ activation can induce apoptosis and cell death in cancer cell lines through excessive ROS generation [54]. We investigated whether T. usneoides extract and pioglitazone mediate ROS generation in SW480 cells using the H2DCFDA probe. Both T. usneoides extract and pioglitazone induced an increase in ROS production compared to its control (Figure 5A–C). Furthermore, this pro-oxidant effect was mitigated by the presence of antioxidant substances, such as ascorbic acid (AA).
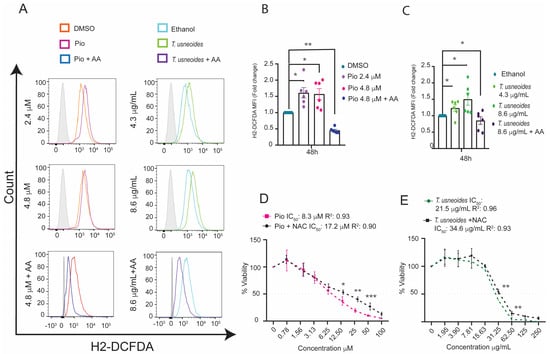
Figure 5.
Effect of T. usneoides extract and pioglitazone on oxidative stress in the colon cancer cell line SW480. (A) ROS levels were evaluated in cells treated with the IC50 or IC50/2 determined in MTT of T. usneoides or pioglitazone ± 5 mM of ascorbic acid (AA) for 48 h. The cells were harvested and labeled with 1 µM of H2-DCFDA. Representative histograms are shown: negative controls (orange or cyan line for DMSO or ethanol, respectively), treated cells (pink or green for pioglitazone or T. usneoides, respectively; blue or purple for pioglitazone + AA or T. usneoides + AA, respectively), and isotype control (gray solid histogram). (B) Fold change in H2DCFDA mean fluorescent intensity (MFI) after treatment with IC50 and IC50/2 of pioglitazone or (C) IC50 and IC50/2 of T. usneoides extract for 48 h in SW480 cells. In all cases, the fold change was determined using the MFI of each treatment relative to the vehicle (DMSO or ethanol). (D) Cytotoxic effect after 48 h of treatment with different concentrations of pioglitazone (100 to 0.78 µM) or (E) T. usneoides (250 to 1.9 µg/mL) or in the presence or absence of NAC 2.5 mM. The IC50 was calculated in GraphPad Prism version 8.0 using a non-linear regression of the log (inhibitor) versus the normalized slope of the response variable. Data represent three independent experiments. * p < 0.05; ** p < 0.01; *** p < 0.001.
Moreover, antioxidants such as N-acetylcysteine (NAC) play a pivotal role in regulating oxidative stress and its subsequent effects on cell death [55]. Our findings revealed that NAC treatment reduced cytotoxic activity in both cases. In the case of pioglitazone, this antioxidant raises up the IC50 value from 8.3 ± 2.4 μM to 17.2 ± 2.8 μM, and for T. usneoides, NAC increases in the IC50 from 21.5 ± 6.8 μg/mL to 34.6 ± 2.6 μg/mL (Figure 5D,E), corresponding to a reduction of cytotoxic activity of 52% and 38% for pioglitazone and T. usneoides extract, respectively. These findings demonstrate that T. usneoides may affect the viability of SW480 cells through ROS generation as pioglitazone does as well.
3.6. Deficient Expression of PPARγ Suppresses the Cytotoxic Effect of T. usneoides in the SW480 Cell Line
We evaluated whether the cytotoxic effect of T. usneoides was altered by knocking down PPARγ expression by shRNA in SW480 cells. First, western blot analysis demonstrated that SW480 cells transfected with shPPARγ plasmid showed a 2-fold decrease in PPARγ expression compared to cells transfected with shScramble plasmid (Figure 6A). Consequently, this reduction in expression is congruent with the less toxic effect of T. usneoides extract on viability in SW480 cells transfected with shPPARγ compared to those transfected with shScramble (Figure 6B). PPARγ deficiency in SW480 cells allow us to conclude that T. usneoides extract is exerting, at least in part, its cytotoxic effect through the activation of PPARγ.
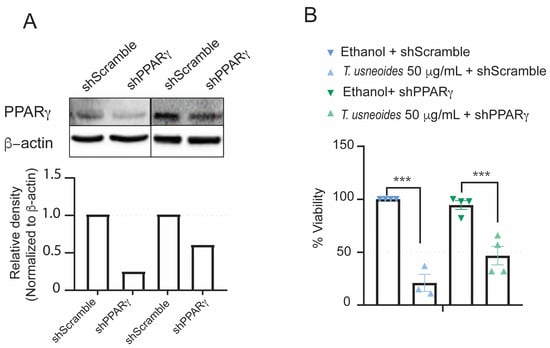
Figure 6.
Effect of PPARγ knocking down in the SW480 cell line on the cytotoxic activity of T. usneoides extract. (A) Images of western blot analysis: PPARγ protein expression in SW480 cells transfected with control shScramble and shPPARγ plasmids (two different replicates are shown). (B) Loss of cytotoxic activity of T. usneoides extracts on SW480 cells bearing shPPARγ compared to cells transfected with shScramble plasmid using XTT assay. Data are shown from three independent experiments. *** p < 0.001.
4. Discussion
Previous studies have shown promising anti-tumor properties of standardized extracts of P. alliacea (Anamu-SC), C. spinosa (P2Et), T. usneoides, and P. nigrum in melanoma, breast cancer, and leukemia. Some of them act by boosting the immune system against the tumor [33,44,45] or by killing the cancer cells themselves [46,47,56,57,58]. T. usneoides has been shown to reduce tumor size in 4T1 tumor-bearing mice while also decreasing myeloid-derived suppressor-like cells, increasing antigen-presenting dendritic cells and significantly reducing regulatory T cells in tumor-draining lymph nodes [33]. However, no effect of these extracts on colon tumor cell lines has been reported except for P. nigrum [48,49]. Therefore, this study reports for the first time that the ethanolic extract of T. usneoides has a cytotoxic effect on the colon cancer cell line SW480, at least in part through the activation of PPARγ. One of the cellular mechanisms implicated in this cytotoxic effect is the increase of ROS levels, which has been previously reported by T. usneoides in the breast cancer cell line 4T1 [33] and by the PPARγ ligand ciglitazone in non-small cell lung cancer [54]. Our findings demonstrate that the accumulation of lipid droplets (LDs) induced by T. usneoides extract or pioglitazone is responsible for the observed cytotoxic effects (Figure 4C,D). In precedent reports, it has been shown that PPARγ activation by rosiglitazone induces lipid accumulation and a reduction of viability dependent on PTEN expression in MCF7 cells [59]. Similarly, results have been reported in endometrial cancer cells where oleic acid induces lipogenesis and cell cycle arrest [60]. The aforementioned results are in accordance with an increase in oxidative stress, probably due to a reduction in the intracellular NADPH pool used for fatty acid synthesis. This phenomenon was observed in the increase of ROS in NCI-H2347 and NCI-H1993 lung cancer cells after 24 h of treatment with pioglitazone. This was accompanied by an enhancement of the lipid synthesis gene transcribed by PPARγ in the chromatin immuno-precipitation followed by deep sequencing experiments [61]. The enhanced ROS production that reduces SW480 cell viability was observed in cells treated with pioglitazone or T. usneoides extract (Figure 5D,E); interestingly, the viability of non-tumor cells, PBMCs, was not affected by these substances, even at high concentrations of pioglitazone (177–350 µM) [62]. One potential explanation for this phenomenon is that cancer cells exhibit a high basal level of ROS, rendering them susceptible to any further increase in ROS levels beyond a certain protective threshold [63].
Our results demonstrate that extracts of C. spinosa (P2Et), T. usneoides, and P. nigrum decrease the viability of colon cancer SW480 cells and only T. usneoides involves the activation of PPARγ in this anti-tumoral effect, as this is shown in the reduced effect of the extract on the PPARγ-deficient SW480 cells (Figure 6B). Similarly, melanoma B16-F10 cells show a lower expression of PPARγ than SW480 and exhibit a lower toxic effect of T. usneoides (Figure 3B,C). This is consistent with the reduced effect of T. usneoides extract on tumor size in B16-F10 tumor-bearing mice [33]. The results of our study demonstrate that the toxic effect of T. usneoides extract depends on the basal level of PPARγ expression, because this plant extract does not increase the expression of this receptor (Figure 3D and Figure S3C), suggesting that the extract induces the synthesis of PPARγ ligands, as it could be assumed by the dose–response effect of the extract on luciferase activity after 48 h of incubation (Figure 2D). Interestingly, a GeneSet analysis (https://www.gsea-msigdb.org/, accessed on 8 July 2024) from transcriptomic data on the effect of β-sitosterol (a phytosterol contained in T. usneoides extract) on the hippocampus of mice [64] shows an enrichment score of 0.46 for the set of KEGG_ARACHIDONIC_ACID_METABOLISM (nominal p-value = 0.11). This hypothesis could explain the activation of PPARα and PPARβ for the T. usneoides extract in the transactivation assay (Figure S1C,D), because as a gamma, these other isotypes of PPARs are activated by ligands synthesized from this metabolic pathway. Even though T. usneoides extract induces the activation of PPARα and PPARβ overexpressed in SW480, it does not seem to have an important role for the endogenous proteins, at least in the decrease of the viability of the cells (Figure S1E–G).
Another important finding in our study is that the toxic effect of T. usneoides does not imply PPARγ, as it is observed in a 60% decreased viability of non-tumoral HEK293T without overexpression of PPARγ; however, once PPARγ is overexpressed, the effect of the extract is an increase of viability, opposite to tumor SW480 cells, where the overexpression of PPARγ decreases viability (Figure 3E). This result is encouraging considering that PPARγ is a crucial receptor in the treatment of other chronic diseases, such as diabetes. In fact, aqueous extracts of T. usneoides have been shown to effectively reduce blood glucose levels in diabetes murine models [65]. On the other hand, to identify the component of the extract responsible for the observed decrease in viability in SW480 cell lines, the whole ethanolic extract was fractionated into the following polarity-increased fractions: hexane, dichloromethane, acetyl acetate, and dihydroethanol fractions. Next, GC-MS analysis revealed that the dicholoromethane fraction is enriched in fatty alcohols and terpenoids, and Ultra-Performance Liquid Chromatography analysis of acetyl acetate and dihydroethanol fractions showed that the main components of this fraction are flavonoids (unpublished results); interestingly, this polar fraction exhibits lower IC50 in the viability of SW480 cells (IC50 18.8 ± 0.5 µg/mL) in contrast to the less polar fraction, the hexane, which did not affect the viability of these cells (128 ± 1.4 µg/mL). This suggests that it is the more polar fraction that could be responsible for the PPARγ activity; however, more studies should be performed. Collectively, these results and reports suggest that T. usneoides extract probably triggers the same metabolic pathway controlled by PPARγ, which is promising because it is possible to use T. usneoides extract as a new therapeutic concept against several metabolic diseases with the implication of PPARγ activation.
5. Conclusions
The cytotoxic effect of T. usneoides on the SW480 colon cancer cell line extract has been reported for the first time. This effect is believed to be partly caused by the activation of PPARγ, which induces fatty acid synthesis, the accumulation of LDs, and ultimately leads to oxidative stress. However, is necessary to study the implications of PPARγ activation using T. usneoides extract and the consequent effects on lipid metabolism in in vivo colon cancer models as well as the anti-tumor immune response too. Here, we are opening perspectives about the potential targeting of PPARγ and cancer cell metabolism to hijack tumor growth. The report that T. usneoides activates PPARγ opens up new therapeutic opportunities to treat metabolic diseases related to lipid metabolism, such as obesity, heart disease, diabetes, and many types of cancers.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biochem4030011/s1, Figure S1: Effects of different plant extracts on the activation of PPARs. (A). Transcriptional activation of luciferase activity by PPARγ in HEK 293T cells co-transfected with pSG5-PPARγ, PPRE 3X-TK-Luc, and pCMV6-AC-GFP and then treated with the PPARγ agonist (pioglitazone 50 μM) or DMSO as a positive control of the assay or (B) the IC50 determined in MTT of different plant extracts or vehicles (ethanol dashed horizontal line) for 48 h. (C). Transcriptional activation of luciferase activity by PPARα in SW480 cells co-transfected with pSG5-PPARα, PPRE 3X-TK-Luc, and pCMV6-AC-GFP and then treated with the IC50 determined in MTT of T. usneoides extract or ethanol or the PPARα agonist (WY14643 50 μM) or DMSO for 48 h. (D). Transcriptional activation of luciferase activity by PPARβ in SW480 cells co-transfected with pSG5-PPARβ, PPRE 3X-TK-Luc, and pCMV6-AC-GFP and then treated with the IC50 of T. usneoides extract or ethanol, or the PPARβ agonist (GW501516 100 nM) or DMSO for 48 h. The luciferase activity of the reporter plasmid was normalized to the fluorescence emission of the GFP plasmid. (E). Cytotoxic activity of SW480 cells after treatment with Wy14643 (100 to 0.7 µM) or (F) GW501516 (200 to 1.5 nM) for 48 h. (G). Quantification of cell death by propidium iodide (PI) staining after treatment with 50 μM of WY14643, 100 nM of GW501516 or DMSO. Data are presented as the mean ± SEM from three independent experiments. * p < 0.05; ** p < 0.01; *** p < 0.001. Figure S2: (A). Viability of phytohaemagglutinin-activated human peripheral blood mononuclear cells (PBMCs) treated with the IC50, IC50/2, or IC50×2 of pioglitazone for 24 h compared with the dose–response viability curve of SW480 cells treated with different concentrations of pioglitazone (100 to 0.78 µM) for 48 h. (B). Viability of phytohaemagglutinin-activated human PBMCs treated with IC50, IC50/2 or IC50×2 of T. usneoides extract for 24 h and compared with the dose–response viability curve of SW480 cells treated with different concentrations of T. usneoides extract (250 a 1.9 μg/mL) for 48 h. Viability was determined by the MTT method described in Materials and Methods. Figure S3: (A). Cytotoxic activity of HT29 cells after treatment with pioglitazone (100 to 0.78 µM) or (B) different concentrations (250 to 1.9 µg/mL) of T. usneoides extract for 48 h. The IC50 was calculated in GraphPad Prism version 8.0 using a non-linear regression of the log (inhibitor) versus the normalized slope of the response variable. (C). Western blot analysis: PPARγ protein expression in HT29 cells treated with the IC50 of T. usneoides extract or ethanol for 48 h. β-actin was used as the internal standard. Bar graphs were constructed according to the gray values of the protein bands.
Author Contributions
J.I. designed the experiments, analyzed the results, constructed the manuscript, and obtained financing; S.F. participated in the discussion of results, revised the manuscript, and obtained financing; M.C.J. participated in the design of ROS experiments and flow cytometry analysis; J.E.C. participated in the development of FAMES analysis by GC-FID; L.R. performed the characterization of T. usneoides extract, which was important to answer the reviewers’ inquiries. M.P.L. participated in the design and execution of the experiments, analysis of the results, and construction of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Pontificia Universidad Javeriana, Ministerio de Ciencia, Tecnología e Innovación, Ministerio de Educación Nacional, Ministerio de Industria, Comercio y Turismo and ICETEX, 2ª Convocatoria Ecosistema Científico—Colombia Científica 792–2017, Program “Generación de alternativas terapéuticas en cáncer a partir de plantas a través de procesos de investigación y desarrollo traslacional, articulados en sistemas de valor sostenibles ambiental y económicamente” (Contract no. FP44842–221–2018). In addition, the funds from project 00006406 financed by the Pontificia Universidad Javeriana were also necessary to perform this work.
Data Availability Statement
The data presented in this study are available in this article (and Supplementary Materials).
Acknowledgments
We would like to thank the Pontificia Universidad Javeriana and the Colombian Environmental Ministry for granting access to genetic resources via Contract No. 220 of 2018 for Colombian plant material. We also thank Walter Wahli, Liliane Michalik, and Paul Grimaldi of the Centre Integratif Genomique University of Lausanne (Lausanne-Switzerland) for kindly providing pSG5-PPARα, pSG5-PPARβ, pSG5-PPARγ, and PPRE 3X-TK-Luc, as well as pLVTH_shRNA-PPARγ and pCMV6-AC-GFP plasmids. Additionally, we thank Mauro Montanaro of Instituto de Investigaciones Bioquímicas de la Plata from Universidad de la Plata, La Plata-Argentina, for the donated pGFP-C-shLenti shRNA Vector. Finally, we are grateful to Geison Modesti Costa and Luis Carlos Chitiva, (Phytochemistry Research Group, Pontificia Universidad Javeriana, Bogotá-Colombia), Sonia Albarracin, and Wilson Leonardo Villareal. (Experimental and Computational Biochemistry, Research Group, Pontificia Universidad Javeriana, Bogotá-Colombia) for providing the different extracts of T. usneoides and P. nigrum.
Conflicts of Interest
S.F. is the owner of a patent related to the P2Et extract and Anamu-SC. Additionally, they are a partner of the DreemBio Company, which was a licensee of related patents. No competing interests are declared by the other authors.
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.E.; Johnson, B. The reality of early-onset colorectal cancer: Highlighting the needs in a unique but emerging population. Dig. Med. Res. 2021, 4, 63. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y. Rectal Cancer in Asian vs. Western Countries: Why the Variation in Incidence? Curr. Treat. Options Oncol. 2017, 18, 64. [Google Scholar] [CrossRef]
- Kuipers, E.J.; Grady, W.M.; Lieberman, D.; Seufferlein, T.; Sung, J.J.; Boelens, P.G.; van de Velde, C.J.H.; Watanabe, T. Colorectal cancer. Nat. Rev. Dis. Primers 2015, 1, 15065. [Google Scholar] [CrossRef]
- Xie, Y.-H.; Chen, Y.-X.; Fang, J.-Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target. Ther. 2020, 5, 22. [Google Scholar] [CrossRef]
- Nikolouzakis, T.K.; Chrysos, E.; Docea, A.O.; Fragkiadaki, P.; Souglakos, J.; Tsiaoussis, J.; Tsatsakis, A. Current and Future Trends of Colorectal Cancer Treatment: Exploring Advances in Immunotherapy. Cancers 2024, 16, 1995. [Google Scholar] [CrossRef]
- Motta, R.; Cabezas-Camarero, S.; Torres-Mattos, C.; Riquelme, A.; Calle, A.; Figueroa, A.; Sotelo, M.J. Immunotherapy in microsatellite instability metastatic colorectal cancer: Current status and future perspectives. J. Clin. Transl. Res. 2021, 7, 511–522. [Google Scholar]
- Wang, D.-R.; Wu, X.-L.; Sun, Y.-L. Therapeutic targets and biomarkers of tumor immunotherapy: Response versus non-response. Signal Transduct. Target. Ther. 2022, 7, 331. [Google Scholar] [CrossRef]
- Malki, A.; ElRuz, R.A.; Gupta, I.; Allouch, A.; Vranic, S.; Al Moustafa, A.E. Molecular Mechanisms of Colon Cancer Progression and Metastasis: Recent Insights and Advancements. Int. J. Mol. Sci. 2020, 22, 130. [Google Scholar] [CrossRef]
- Hong, F.; Pan, S.; Guo, Y.; Xu, P.; Zhai, Y. PPARs as Nuclear Receptors for Nutrient and Energy Metabolism. Molecules 2019, 24, 2545. [Google Scholar] [CrossRef]
- Korbecki, J.; Bobiński, R.; Dutka, M. Self-regulation of the inflammatory response by peroxisome proliferator-activated receptors. Inflamm. Res. 2019, 68, 443–458. [Google Scholar] [CrossRef]
- Wahli, W. A gut feeling of the PXR, PPAR and NF-kappaB connection. J. Intern. Med. 2008, 263, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Christofides, A.; Konstantinidou, E.; Jani, C.; Boussiotis, V.A. The role of peroxisome proliferator-activated receptors (PPAR) in immune responses. Metabolism 2021, 114, 154338. [Google Scholar] [CrossRef]
- Annicotte, J.S.; Iankova, I.; Miard, S.; Fritz, V.; Sarruf, D.; Abella, A.; Berthe, M.L.; Noël, D.; Pillon, A.; Iborra, F.; et al. Peroxisome proliferator-activated receptor gamma regulates E-cadherin expression and inhibits growth and invasion of prostate cancer. Mol. Cell Biol. 2006, 26, 7561–7574. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.W.; Seo, C.Y.; Han, H.; Han, J.Y.; Jeong, J.S.; Kwak, J.Y.; Park, J.I. 15d-PGJ2 induces apoptosis by reactive oxygen species-mediated inactivation of Akt in leukemia and colorectal cancer cells and shows in vivo antitumor activity. Clin. Cancer Res. 2009, 15, 5414–5425. [Google Scholar] [CrossRef]
- Liu, L.; Yang, Z.; Xu, Y.; Li, J.; Xu, D.; Zhang, L.; Sun, J.; Xia, S.; Zou, F.; Liu, Y. Inhibition of oxidative stress-elicited AKT activation facilitates PPARγ agonist-mediated inhibition of stem cell character and tumor growth of liver cancer cells. PLoS ONE 2013, 8, e73038. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-I.; Kwak, J.-Y. The Role of Peroxisome Proliferator-Activated Receptors in Colorectal Cancer. PPAR Res. 2012, 2012, 876418. [Google Scholar] [CrossRef]
- Kurnaz-Gomleksiz, O.; Torun, B.C.; Isbir, T.; Bulut, T.; Sokucu, N.; Yilmaz-Aydogan, H.; Canbay, E. The Role of PPAR-gamma C161T Polymorphism in Colorectal Cancer Susceptibility. In Vivo 2022, 36, 1911–1915. [Google Scholar] [CrossRef]
- Theocharis, S.; Giaginis, C.; Parasi, A.; Margeli, A.; Kakisis, J.; Agapitos, E.; Kouraklis, G. Expression of peroxisome proliferator-activated receptor-gamma in colon cancer: Correlation with histopathological parameters, cell cycle-related molecules, and patients’ survival. Dig. Dis. Sci. 2007, 52, 2305–2311. [Google Scholar] [CrossRef]
- Ferrara, A.; Lewis, J.D.; Quesenberry, C.P., Jr.; Peng, T.; Strom, B.L.; Van Den Eeden, S.K.; Ehrlich, S.F.; Habel, L.A. Cohort study of pioglitazone and cancer incidence in patients with diabetes. Diabetes Care 2011, 34, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, M.; Suh, J.M.; Hah, N.; Liddle, C.; Atkins, A.R.; Downes, M.; Evans, R.M. PPARγ signaling and metabolism: The good, the bad and the future. Nat. Med. 2013, 19, 557–566. [Google Scholar] [CrossRef]
- Gionfriddo, G.; Plastina, P.; Augimeri, G.; Catalano, S.; Giordano, C.; Barone, I.; Morelli, C.; Giordano, F.; Gelsomino, L.; Sisci, D.; et al. Modulating Tumor-Associated Macrophage Polarization by Synthetic and Natural PPARγ Ligands as a Potential Target in Breast Cancer. Cells 2020, 9, 174. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Du, H.; Blum, J.S.; Yan, C. Critical role of PPARγ in myeloid-derived suppressor cell-stimulated cancer cell proliferation and metastasis. Oncotarget 2016, 7, 1529–1543. [Google Scholar] [CrossRef]
- Sarraf, P.; Mueller, E.; Jones, D.; King, F.J.; DeAngelo, D.J.; Partridge, J.B.; Holden, S.A.; Chen, L.B.; Singer, S.; Fletcher, C.; et al. Differentiation and reversal of malignant changes in colon cancer through PPARgamma. Nat. Med. 1998, 4, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, S.; Safaroghli-Azar, A.; Pourbagheri-Sigaroodi, A.; Salari, S.; Gharehbaghian, A.; Hamidpour, M.; Bashash, D. Stimulation of peroxisome proliferator-activated receptor-gamma (PPARγ) using pioglitazone decreases the survival of acute promyelocytic leukemia cells through up-regulation of PTEN expression. Anti-Cancer Agents Med. Chem. 2021, 21, 108–119. [Google Scholar] [CrossRef]
- Konstantinopoulos, P.A.; Vandoros, G.P.; Sotiropoulou-Bonikou, G.; Kominea, A.; Papavassiliou, A.G. NF-kappaB/PPAR gamma and/or AP-1/PPAR gamma ‘on/off’ switches and induction of CBP in colon adenocarcinomas: Correlation with COX-2 expression. Int. J. Color. Dis. 2007, 22, 57–68. [Google Scholar] [CrossRef]
- Osawa, E.; Nakajima, A.; Wada, K.; Ishimine, S.; Fujisawa, N.; Kawamori, T.; Matsuhashi, N.; Kadowaki, T.; Ochiai, M.; Sekihara, H.; et al. Peroxisome proliferator-activated receptor gamma ligands suppress colon carcinogenesis induced by azoxymethane in mice. Gastroenterology 2003, 124, 361–367. [Google Scholar] [CrossRef]
- Haefeli, W.E.; Carls, A. Drug interactions with phytotherapeutics in oncology. Expert. Opin. Drug Metab. Toxicol. 2014, 10, 359–377. [Google Scholar] [CrossRef]
- Garth, R.E. The Ecology of Spanish Moss (Tillandsia usneoides): Its Growth and Distribution. Ecology 1964, 45, 470–481. [Google Scholar] [CrossRef]
- Keller, W.J.; Bourn, W.M.; Bonfiglio, J.F. A Folk Medicine for Diabetes Mellitus. Q. J. Crude Drug Res. 1981, 19, 49–51. [Google Scholar] [CrossRef]
- Hornung-Leoni, C. Avances sobre Usos Etnobotánicos de las Bromeliaceae en Latinoamérica [Progress on ethnobotanical uses of Bromeliaceae in Latin America]. Bol. Latinoam. Caribe Plantas Med. Aromat. 2011, 10, 297–314. [Google Scholar]
- Lasso, P.; Rojas, L.; Arévalo, C.; Urueña, C.; Murillo, N.; Barreto, A.; Costa, G.M.; Fiorentino, S. Tillandsia usneoides Extract Decreases the Primary Tumor in a Murine Breast Cancer Model but Not in Melanoma. Cancers 2022, 14, 5383. [Google Scholar] [CrossRef]
- Wang, L.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Blunder, M.; Liu, X.; Malainer, C.; Blazevic, T.; Schwaiger, S.; Rollinger, J.M.; Heiss, E.H.; et al. Natural product agonists of peroxisome proliferator-activated receptor gamma (PPARγ): A review. Biochem. Pharmacol. 2014, 92, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, J.K.; Cho, M.C.; Shin, S.; Yoon, D.Y.; Heo, Y.S.; Kim, Y. Cytotoxic flavonoids as agonists of peroxisome proliferator-activated receptor gamma on human cervical and prostate cancer cells. J. Nat. Prod. 2010, 73, 1261–1265. [Google Scholar] [CrossRef]
- Sandoval, T.A.; Urueña, C.P.; Llano, M.; Gómez-Cadena, A.; Hernández, J.F.; Sequeda, L.G.; Loaiza, A.E.; Barreto, A.; Li, S.; Fiorentino, S. Standardized Extract from Caesalpinia spinosa is Cytotoxic Over Cancer Stem Cells and Enhance Anticancer Activity of Doxorubicin. Am. J. Chin. Med. 2016, 44, 1693–1717. [Google Scholar] [CrossRef]
- Castañeda, D.M.; Pombo, L.M.; Urueña, C.P.; Hernandez, J.F.; Fiorentino, S. A gallotannin-rich fraction from Caesalpinia spinosa (Molina) Kuntze displays cytotoxic activity and raises sensitivity to doxorubicin in a leukemia cell line. BMC Complement. Altern. Med. 2012, 12, 38. [Google Scholar] [CrossRef]
- Ballesteros-Ramírez, R.; Aldana, E.; Herrera, M.V.; Urueña, C.; Rojas, L.Y.; Echeverri, L.F.; Costa, G.M.; Quijano, S.; Fiorentino, S. Preferential Activity of Petiveria alliacea Extract on Primary Myeloid Leukemic Blast. Evid. Based Complement. Altern. Med. 2020, 2020, 4736206. [Google Scholar] [CrossRef]
- Bravo-Chaucanés, C.P.; Vargas-Casanova, Y.; Chitiva-Chitiva, L.C.; Ceballos-Garzon, A.; Modesti-Costa, G.; Parra-Giraldo, C.M. Evaluation of Anti-Candida Potential of Piper nigrum Extract in Inhibiting Growth, Yeast-Hyphal Transition, Virulent Enzymes, and Biofilm Formation. J. Fungi 2022, 8, 784. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Wang, W.; Aldahdooh, J.; Malyutina, A.; Shadbahr, T.; Tanoli, Z.; Pessia, A.; Tang, J. SynergyFinder Plus: Toward Better Interpretation and Annotation of Drug Combination Screening Datasets. Genom. Proteom. Bioinform. 2022, 20, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Yadav, B.; Wennerberg, K.; Aittokallio, T.; Tang, J. Searching for Drug Synergy in Complex Dose-Response Landscapes Using an Interaction Potency Model. Comput. Struct. Biotechnol. J. 2015, 13, 504–513. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Cavonius, L.R.; Carlsson, N.-G.; Undeland, I. Quantification of total fatty acids in microalgae: Comparison of extraction and transesterification methods. Anal. Bioanal. Chem. 2014, 406, 7313–7322. [Google Scholar] [CrossRef]
- Lasso, P.; Gomez-Cadena, A.; Urueña, C.; Donda, A.; Martinez-Usatorre, A.; Romero, P.; Barreto, A.; Fiorentino, S. An Immunomodulatory Gallotanin-Rich Fraction from Caesalpinia spinosa Enhances the Therapeutic Effect of Anti-PD-L1 in Melanoma. Front. Immunol. 2020, 11, 584959. [Google Scholar] [CrossRef]
- Urueña, C.; Sandoval, T.A.; Lasso, P.; Tawil, M.; Barreto, A.; Torregrosa, L.; Fiorentino, S. Evaluation of chemotherapy and P2Et extract combination in ex-vivo derived tumor mammospheres from breast cancer patients. Sci. Rep. 2020, 10, 19639. [Google Scholar] [CrossRef]
- Urueña, C.; Cifuentes, C.; Castañeda, D.; Arango, A.; Kaur, P.; Asea, A.; Fiorentino, S. Petiveria alliacea extracts uses multiple mechanisms to inhibit growth of human and mouse tumoral cells. BMC Complement. Altern. Med. 2008, 8, 60. [Google Scholar] [CrossRef]
- Lasso, P.; Rojas, L.; Arévalo, C.; Urueña, C.; Murillo, N.; Nossa, P.; Sandoval, T.; Chitiva, L.C.; Barreto, A.; Costa, G.M.; et al. Piper nigrum extract suppresses tumor growth and enhances the antitumor immune response in murine models of breast cancer and melanoma. Cancer Immunol. Immunother. 2023, 72, 3279–3292. [Google Scholar] [CrossRef]
- Jaidee, W.; Maneerat, T.; Rujanapun, N.; Paojumroon, N.; Duangyod, T.; Banerjee, S.; Kar, A.; Mukherjee, P.K.; Charoensup, R. Metabolite fingerprinting of Piper nigrum L. from different regions of thailand by UHPLC-QTOF-MS approach and in vitro bioactivities. J. Trends Sci. 2022, 19, 1520. [Google Scholar] [CrossRef]
- Wu, R.; Zhao, J.; Wei, P.; Tang, M.; Ma, Z.; Zhao, Y.; Du, L.; Wan, L. Piper nigrum Extract Inhibits the Growth of Human Colorectal Cancer HT-29 Cells by Inducing p53-Mediated Apoptosis. Pharmaceuticals 2023, 16, 1325. [Google Scholar] [CrossRef] [PubMed]
- Maresca, V.; Flori, E.; Camera, E.; Bellei, B.; Aspite, N.; Ludovici, M.; Catricalà, C.; Cardinali, G.; Picardo, M. Linking αMSH with PPARγ in B16-F10 melanoma. Pigment. Cell Melanoma Res. 2013, 26, 113–127. [Google Scholar] [CrossRef]
- Lin, P.C.; Lin, Y.J.; Lee, C.T.; Liu, H.S.; Lee, J.C. Cyclooxygenase-2 expression in the tumor environment is associated with poor prognosis in colorectal cancer patients. Oncol. Lett. 2013, 6, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Sadeghabadi, Z.A.; Nourbakhsh, M.; Alaee, M.; Larijani, B.; Razzaghy-Azar, M. Peroxisome proliferator-activated receptor gamma expression in peripheral blood mononuclear cells and angiopoietin-like protein 4 levels in obese children and adolescents. J. Endocrinol. Investig. 2018, 41, 241–247. [Google Scholar] [CrossRef]
- Patel, J.J.; Butters, O.R.; Arnett, T.R. PPAR agonists stimulate adipogenesis at the expense of osteoblast differentiation while inhibiting osteoclast formation and activity. Cell Biochem. Funct. 2014, 32, 368–377. [Google Scholar] [CrossRef]
- Kim, T.W.; Hong, D.-W.; Hong, S.H. CB13, a novel PPARγ ligand, overcomes radio-resistance via ROS generation and ER stress in human non-small cell lung cancer. Cell Death Dis. 2020, 11, 848. [Google Scholar] [CrossRef]
- Kalyanaraman, B. NAC, NAC, Knockin’ on Heaven’s door: Interpreting the mechanism of action of N-acetylcysteine in tumor and immune cells. Redox Biol. 2022, 57, 102497. [Google Scholar] [CrossRef]
- Urueña, C.; Mancipe, J.; Hernandez, J.; Castañeda, D.; Pombo, L.; Gomez, A.; Asea, A.; Fiorentino, S. Gallotannin-rich Caesalpinia spinosa fraction decreases the primary tumor and factors associated with poor prognosis in a murine breast cancer model. BMC Complement. Altern. Med. 2013, 13, 74. [Google Scholar] [CrossRef]
- Hernández, J.F.; Urueña, C.P.; Cifuentes, M.C.; Sandoval, T.A.; Pombo, L.M.; Castañeda, D.; Asea, A.; Fiorentino, S. A Petiveria alliacea standardized fraction induces breast adenocarcinoma cell death by modulating glycolytic metabolism. J. Ethnopharmacol. 2014, 153, 641–649. [Google Scholar] [CrossRef]
- Hernández, J.F.; Urueña, C.P.; Sandoval, T.A.; Cifuentes, M.C.; Formentini, L.; Cuezva, J.M.; Fiorentino, S. A cytotoxic Petiveria alliacea dry extract induces ATP depletion and decreases β-F1-ATPase expression in breast cancer cells and promotes survival in tumor-bearing mice. Rev. Bras. Farm. 2017, 27, 306–314. [Google Scholar] [CrossRef]
- Kim, K.Y.; Kim, S.S.; Cheon, H.G. Differential anti-proliferative actions of peroxisome proliferator-activated receptor-gamma agonists in MCF-7 breast cancer cells. Biochem. Pharmacol. 2006, 72, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Kong, W.; Suo, H.; Shen, X.; Newton, M.A.; Burkett, W.C.; Zhao, Z.; John, C.; Sun, W.; Zhang, X.; et al. Oleic Acid Exhibits Anti-Proliferative and Anti-Invasive Activities via the PTEN/AKT/mTOR Pathway in Endometrial Cancer. Cancers 2023, 15, 5407. [Google Scholar] [CrossRef]
- Srivastava, N.; Kollipara, R.K.; Singh, D.K.; Sudderth, J.; Hu, Z.; Nguyen, H.; Wang, S.; Humphries, C.G.; Carstens, R.; Huffman, K.E.; et al. Inhibition of cancer cell proliferation by PPARγ is mediated by a metabolic switch that increases reactive oxygen species levels. Cell Metab. 2014, 20, 650–661. [Google Scholar] [CrossRef]
- Haskins, J.R.; Rowse, P.; Rahbari, R.; de la Iglesia, F.A. Thiazolidinedione toxicity to isolated hepatocytes revealed by coherent multiprobe fluorescence microscopy and correlated with multiparameter flow cytometry of peripheral leukocytes. Arch. Toxicol. 2001, 75, 425–438. [Google Scholar] [CrossRef][Green Version]
- Ndombera, F.T. Anti-cancer agents and reactive oxygen species modulators that target cancer cell metabolism. Pure Appl. Chem. 2017, 89, 1333–1348. [Google Scholar] [CrossRef]
- Panayotis, N.; Freund, P.A.; Marvaldi, L.; Shalit, T.; Brandis, A.; Mehlman, T.; Tsoory, M.M.; Fainzilber, M. β-sitosterol reduces anxiety and synergizes with established anxiolytic drugs in mice. Cell Rep. Med. 2021, 2, 100281. [Google Scholar] [CrossRef]
- Witherup, K.M.; McLaughlin, J.L.; Judd, R.L.; Ziegler, M.H.; Medon, P.J.; Keller, W.J. Identification of 3-hydroxy-3-methylglutaric acid (HMG) as a hypoglycemic principle of Spanish moss (Tillandsia usneoides). J. Nat. Prod. 1995, 58, 1285–1290. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).