Cytotoxic Effects of Indonesian Betel Quid Components on Oral Keratinocytes and Fibroblasts
Abstract
1. Introduction
2. Material and Methods
2.1. Preparation of Betel Quid Aqueous Extracts
2.2. Cells and Culture Conditions
2.3. Treatments
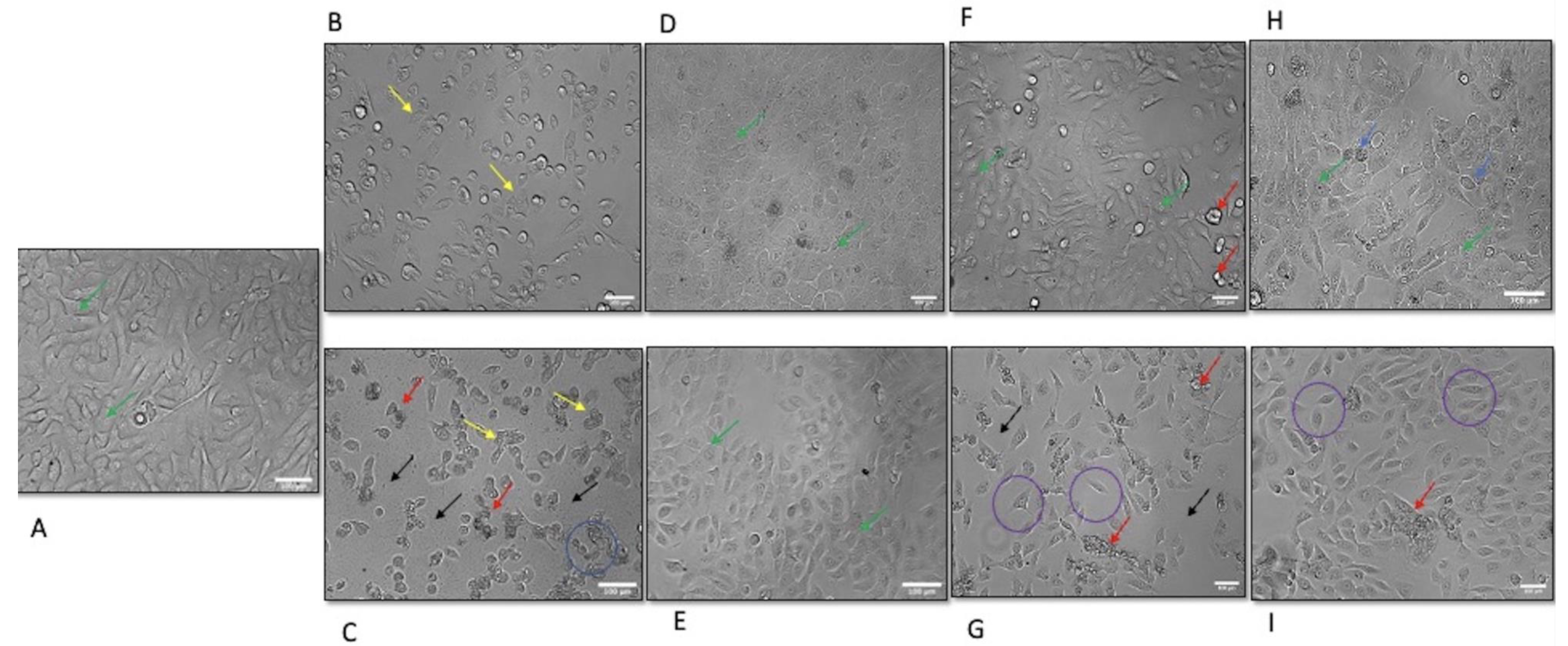
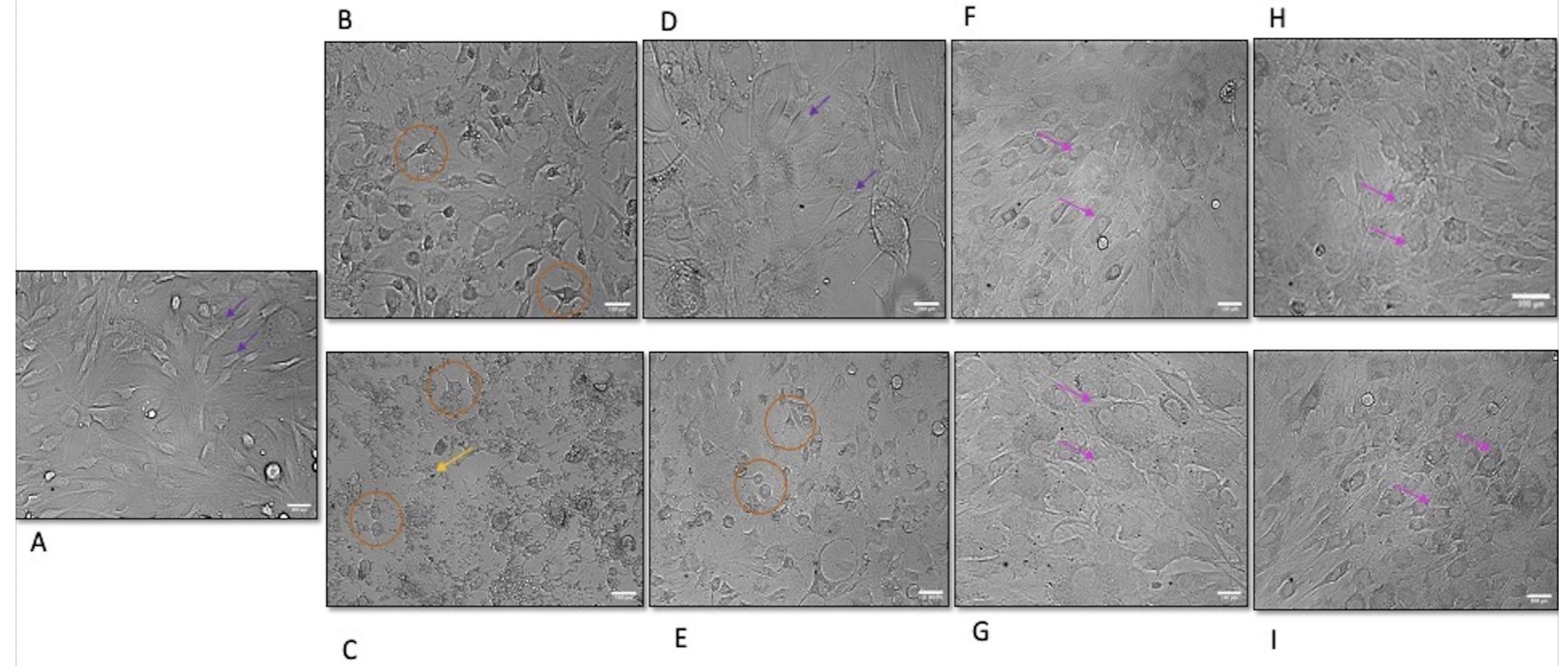
2.4. Morphological Evaluation
2.5. Viability Assay
2.6. Statistical Analysis
3. Results
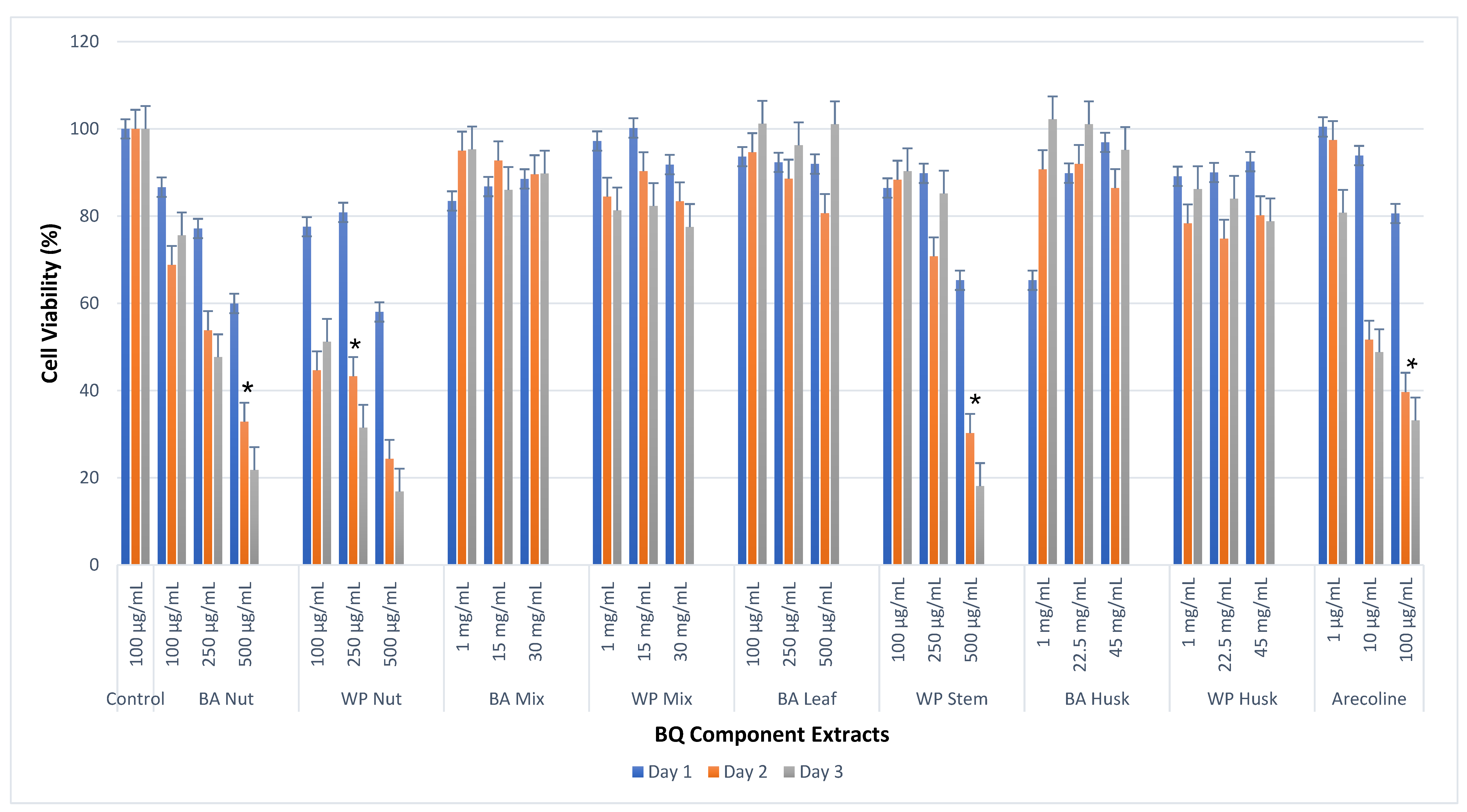
3.1. Cell Viability Effects of BQ Extracts on OKF-6
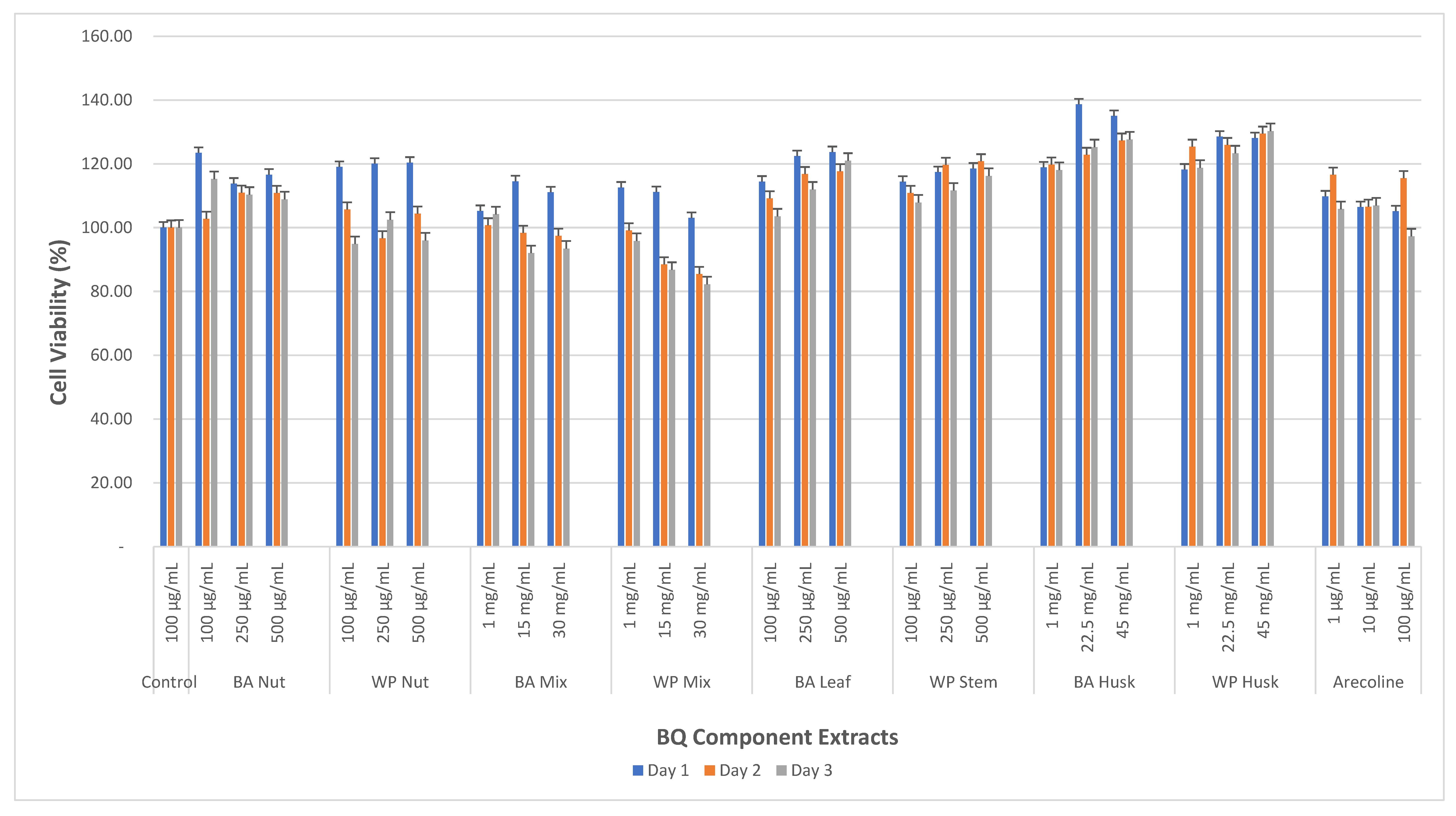
3.2. Cell Viability Effects of BQ Extracts on MMF-1
3.3. Morphological Alteration of OKF-6 Following Exposure to BQ Components from BA and WP Regions
3.4. Morphological Alteration of MMF-1 Following Exposure to BQ Components from BA and WP Regions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- IARC. Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans; IARC: Lyon, France, 1985; Volume 73.
- Yeh, C.-Y.; Chen, H.-M.; Chang, M.-C.; Kok, S.-H.; Lee, J.-J.; Chang, B.-E.; Jeng, P.-Y.; Chan, C.-P.; Jeng, J.-H. Cytotoxicity and transformation of C3H10T1/2 cells induced by areca nut components. J. Formos. Med. Assoc. 2016, 115, 108–112. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hosein, M.; Mohiuddin, S.; Fatima, N. Association Between Grading of Oral Submucous Fibrosis With Frequency and Consumption of Areca Nut and Its Derivatives in a Wide Age Group: A Multi-centric Cross Sectional Study From Karachi, Pakistan. J. Cancer Prev. 2015, 20, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Kearsley, J. Betel Quid and Oral Cancer: A Review. Eur. J. Cancer Oral Oncol. 1992, 298, 251–255. [Google Scholar] [CrossRef]
- Wang, M.; Xiao, C.; Ni, P.; Yu, J.J.; Wang, X.W.; Sun, H. Correlation of Betel Quid with Oral Cancer from 1998 to 2017: A Study Based on Bibliometric Analysis. Chin. Med. J. 2018, 131, 1975–1982. [Google Scholar] [CrossRef] [PubMed]
- Wollina, U.; Verma, S.B.; Ali, F.M.; Patil, K. Oral submucous fibrosis: An update. Clin. Cosmet. Investig. Dermatol. 2015, 8, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Talukder, G.; Sharma, A. Betel Cytotoxicity. J. Ethnopharmacol. 1989, 26, 217–247. [Google Scholar] [CrossRef]
- IARC. Betel-quid and Areca-nut Chewing and Some Areca-nut-derived Nitrosamines. In Monographs on the Evaluation of Carcinogenic Risks to Humans, 85th ed.; IARC Press: Lyon, France, 2004. [Google Scholar]
- Gupta, P.C.; Warnakulasuriya, S. Global epidemiology of areca nut usage. Addict. Biol. 2002, 7, 77–83. [Google Scholar] [CrossRef]
- Williams, S.; Malik, A.; Chowdhury, S.; Chauhan, S. Sociocultural aspects of areca nut use. Addict. Biol. 2002, 7, 147–154. [Google Scholar] [CrossRef]
- Sari, E.F.; Prayogo, G.P.; Loo, Y.T.; Zhang, P.; McCullough, M.J.; Cirillo, N. Distinct phenolic, alkaloid and antioxidant profile in betel quids from four regions of Indonesia. Sci. Rep. 2020, 10, 16254. [Google Scholar] [CrossRef]
- Zhang, P.; Sari, E.F.; McCullough, M.J.; Cirillo, N. Metabolomic Profile of Indonesian Betel Quids. Biomolecules 2022, 12, 1469. [Google Scholar] [CrossRef]
- Sari, E.F.; Johnson, N.W.; McCullough, M.J.; Cirillo, N. Global Burden of Disease Data for Indonesia. Lancet Glob. Health 2023, 11, e336. [Google Scholar] [CrossRef]
- Cirillo, N.; Duong, P.H.; Er, W.T.; Do, C.T.N.; De Silva, M.E.H.; Dong, Y.; Cheong, S.C.; Sari, E.F.; McCullough, M.J.; Zhang, P.; et al. Are There Betel Quid Mixtures Less Harmful than Others? A Scoping Review of the Association between Different Betel Quid Ingredients and the Risk of Oral Submucous Fibrosis. Biomolecules 2022, 12, 664. [Google Scholar] [CrossRef]
- Elias, S.T.; Diniz, J.; Rubens, S.S.; Almeida Alvarenga, N.; Simeoni, L.A.; Ferro, E.; Silva Guerra, E.N.; Motoyama, A.B. Cytotoxic effect of tobacco extract on human oral squamous cell carcinoma cell-line. Oral Oncol. 2010, 46, 869–873. [Google Scholar] [CrossRef]
- Harvey, W.; Scutt, A.; Meghji, S.; Canniff, J.P. Stimulation Of Human Buccal Mucosa Fibrobalst In Vitro by Betel Nut Alkaloids. Arch. Oral Biol. 1986, 31, 45–49. [Google Scholar] [CrossRef]
- Lee, P.-H.; Chang, M.-C.; Chang, W.-H.; Wang, T.-M.; Wang, Y.-J.; Hahn, L.-J.; Ho, Y.-S.; Lin, C.-Y.; Jeng, J.-H. Prolonged exposure to arecoline arrested human KB epithelial cell growth: Regulatory mechanisms of cell cycle and apoptosis. Toxicology 2006, 220, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.-K.; Chang, M.-C.; Hsu, M.-L.; Su, C.-Y.; Chi, L.-Y.; Lan, W.-C.; Jeng, J.-H. Arecoline inhibits endothelial cell growth and migration and the attachment to mononuclear cells. J. Dent. Sci. 2014, 9, 258–264. [Google Scholar] [CrossRef][Green Version]
- Van Wyk, C.W.; Olivier, A.; De Miranda, C.M.; Van der Bijl, P.; Grobler-Rabie, A.F. Observation on the effect of areca nut extracts on oral fibroblast proliferation. J. Oral Pathol. Med. 1994, 23, 145–148. [Google Scholar] [PubMed]
- Kammerer, D.; Claus, A.; Carle, R.; Schieber, A. Polyphenol Screening of Pomace from Red and White Grape Varieties (Vitis vinifera L.) by HPLC-DAD. J. Agric. Food Chem. 2004, 52, 4360–4367. [Google Scholar] [CrossRef]
- Dickson, M.A.; Hahn, W.C.; Ino, Y.; Ronfard, V.; Wu, J.Y.; Weinberg, R.A.; Rheinwald, J.G. Human Keratinocytes That Express hTERT and Also Bypass a p16INK4a-Enforced Mechanism That Limits Life Span Become Immortal yet Retain Normal Growth and Differentiation Characteristics. Mol. Cell. Biol. 2000, 20, 1436–1447. [Google Scholar] [CrossRef] [PubMed]
- Gonzales-Reyes, S.; Guzman-Beltran, S.; Medine-Campos, O.N.; Pedraza-Chaverri, J. Curcumin pretreatment induces Nrf2 and an antioxidant response and prevents hemin-induced toxicity in primary cultures of cerebellar granule neurons of rats. Oxid. Med. Cell. Longev. 2013, 2013, 801418. [Google Scholar] [CrossRef]
- Prime, S.S.; Nixon, S.V.; Crane, I.J.; Stone, A.; Matthews, J.B.; Maitland, N.J.; Remnant, L.; Powell, S.K.; Game, S.M.; Scully, C. The behaviour of human oral squamous cell carcinoma in cell culture. J. Pathol. 1990, 160, 259–269. [Google Scholar] [CrossRef]
- Petti, S.; Scully, C. Polyphenols, oral health and disease: A review. J. Dent. 2009, 37, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Jeng, J.H.; Hahn, L.J.; Lin, B.R.; Hsieh, C.C.; Chan, C.P.; Chang, M.C. Effects of areca nut, inflorescence piper betle extracts and arecoline on cytotoxicity, total and unscheduled DNA synthesis in cultured gingival keratinocytes. Oral Pathol. Med. 1999, 28, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Wary, K.K.; Sharan, R.N. Aqueous extract of betel-nut in North-East India induces DNA-stiand breaks and enhanc- es rate of cell proliferation in vitro. J. Cancer Rex. Ctin. Oncol. 1988, 114, 579–582. [Google Scholar] [CrossRef] [PubMed]
- Awang, M.N. Fate of betel nut chemical constituents following nut treatment prior lo chewing anJ its relation to oral precancerous and cancerous lesions. Dent. J. Malays. 1988, 10, 33–37. [Google Scholar]
- Hwang, L.S.; Wang, C.K.; Sheu, M.J.; Kao, L.S. Phenolic compounds of Piper betle flower as flavoring and neuronal activity modulating agents. In Phenolic Compounds in Food and their Effects on Health, Analysis, Occurrence and Chemistry. Food and Their Effects on Health; Ho, C.T., Lee, C.Y., Huang, M.T., Eds.; American Chemical Society: Washington, DC, USA, 1992; Volume 1. [Google Scholar]
- IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans; Some Naturally Occurring Substances; IARC: Lyon, France, 1976.
- Baldasquin-Caceres, B.; Gomez-Garcia, F.J.; Lopez-Jornet, P.; Castillo-Sanchez, J.; Vicente-Ortega, V. Chemopreventive potential of phenolic compounds in oral carcinogenesis. Arch. Oral Biol. 2014, 59, 1101–1107. [Google Scholar] [CrossRef]
- Amonkar, A.J.; Padma, P.R.; Bhide, S.V. Protective effect of hydroxychavicol, a phenolic component of betel leaf, against the tobacco-specific carcinogens. Mutat. Res. 1989, 210, 249–253. [Google Scholar] [CrossRef]
- Steven, J.; Thomas, R.M. Slaked lime and betel nut cancer in Papua New Guinea. Lancet 1992, 340, 577–578. [Google Scholar]
- Dunham, L.J.; Muir, C.S.; Hamner, J.E. Epithelial atypia in hamster cheek pouches treated repeatedly with calcium hydroxide. Br. J. Cancer 1966, 20, 586–593. [Google Scholar] [CrossRef]
- Sari, E.F.; McCullough, M.; Cirillo, N. Oral Pre-malignant and malignant lesion detection among Indonesian: The Prevalence and Risk Factors. Head Neck 2017, 39, E58. [Google Scholar]
- Muruganandam, L.; Krishna, A.; Reddy, J.; Nirmala, G.S. Optimization studies on extraction of phytocomponents from betel leaves. Resour.-Effic. Technol. 2017, 3, 385–393. [Google Scholar]
- Chen, C.-L.; Chi, C.-W.; Chang, K.-W.; Liu, T.-Y. Safrole-like DNA adducts in oral tissue from oral cancer patients with a betel quid chewing history. Carcinogenesis 1999, 20, 2331–2334. [Google Scholar] [CrossRef] [PubMed]
- Franke, A.A.; Biggs, L.; Yew, J.Y.; Lai, J.F. Areca alkaloids measured from buccal cells using DART-MS serve as accurate biomarkers for areca nut chewing. Drug Test. Anal. 2019, 11, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Shivapurkar, N.M.; Ranadive, S.N.; Gothoskar, S.V.; Bhide, S.V.; Ranadive, K.J. Tumorigenic effect of aqueous and polyphenolic fractions of betel nut in Swiss strain mice. Indian J. Exp. Biol. 1980, 18, 1159–1161. [Google Scholar] [PubMed]
- Patek, R.S.; Rajorhia, G.S. Antioxidative role of curry (Murray koenigi) and betel (Piper betle) leaves in ghee. J. Food Sci. Technol. 1991, 16, 158–160. [Google Scholar]
- Sethi, S.C.; Aggarwal, J.S. Stabilization of edible fats by spices. Part II. A new antioxidant from betel leaf. J. Sci. Ind. Res. 1956, 15, 34–36. [Google Scholar]
- Arakeri, G.; Rai, K.K.; Hunasgi, S.; Merkx, M.A.W.; Gao, S.; Brennan, P.A. Oral submucous fibrosis: An update on current theories of pathogenesis. J. Oral Pathol. Med. 2017, 46, 406–412. [Google Scholar] [CrossRef]
- Reichart, P.A.; Van Wyk, C.W.; Becker, J.; Schuppan, D. Distribution of procollagen type III, collagen type IV and tenascin in oral submucous fibrosis. J. Oral Pathol. Med. 1994, 23, 394–398. [Google Scholar] [CrossRef]
- Angadi, P.V.; Rao, S.S. Areca nut in pathogenesis of oral submucous fibrosis: Revisited. Oral Maxillofac. Surg. 2011, 15, 1–9. [Google Scholar] [CrossRef]
- Ekanayaka, R.P.; Tilakaratne, W.M. Oral submucous fibrosis: Review on mechanisms of pathogenesis and malignant transformation. J. Carcinog. Mutagen. 2013, S5, 002. [Google Scholar] [CrossRef]
- Banerjee, A.; Kamath, V.V.; Kotrashetti, V.; Bhatt, K. Fibroblastic phenotype in oral submucous fibrosis—A cell culture analysis. Saudi J. Pathol. Microbiol. 2017, 2, 36–47. [Google Scholar]
| BQ Components | Concentration | 1-Day Cell Viability (%) ± SD | 2-Day Cell Viability (%) ± SD | 3-Day Cell Viability (%) ± SD | p-Value |
|---|---|---|---|---|---|
| Control | 0 | 100 | 100 | 100 | |
| BA-AN | 100 μg/mL | 86.6 ± 3.0 | 68.8 ± 4.2 | 75.6 ± 7.8 | <0.05 |
| 250 μg/mL | 77.1 ± 3 | 53.8 ± 2.0 | 47.6 ± 4.4 | ||
| 500 μg/mL | 59.9 ± 3.4 | 32.8 ± 1.4 | 21.8 ± 1.0 | ||
| WP-AN | 100 μg/mL | 77.5 ± 5.8 | 44.6 ± 4.7 | 51.1 ± 2.3 | <0.05 |
| 250 μg/mL | 80.8 ± 3.6 | 43.3 ± 0.7 | 31.48 ± 2.1 | ||
| 500 μg/mL | 58 ± 4.6 | 24.4 ± 2.1 | 16.86 ± 0.5 | ||
| BA-BQ Mixture | 1 mg/mL | 83.5 ± 6.9 | 95.0 ± 3.1 | 95.3 ± 7.7 | <0.05 |
| 15 mg/mL | 86.8 ± 4.6 | 92.8 ± 4 | 86.0 ± 8.4 | ||
| 30 mg/mL | 88.5 ± 1.84 | 89.6 ± 2.8 | 89.8 ±3.1 | ||
| WP-BQ Mixture | 1 mg/mL | 97.2 ± 4.3 | 84.4 ± 3 | 81.3 ± 6.7 | <0.05 |
| 15 mg/mL | 100.2 ± 2.5 | 90.3 ± 4.2 | 82.3 ± 5.2 | ||
| 30 mg/mL | 91.8 ± 3 | 83.4 ± 5.0 | 77.5 ± 1.8 | ||
| BA-Leaf | 100 μg/mL | 93.6 ± 8.4 | 94.7 ± 5.7 | 101.2 ± 4.3 | <0.05 |
| 250 μg/mL | 92.3 ± 2.6 | 88.6 ± 2.8 | 96.2 ± 4 | ||
| 500 μg/mL | 91.9 ± 6.3 | 80.7 ± 4.6 | 101.1 ± 6.3 | ||
| WP-SI | 100 μg/mL | 86.4 ± 5.3 | 88.33 ± 10.1 | 90.3 ± 2.1 | <0.05 |
| 250 μg/mL | 89.8 ± 3.4 | 70.7 ± 6.9 | 85.2 ± 4.1 | ||
| 500 μg/mL | 65.3 ± 1.4 | 30.3 ± 0.7 | 18.1 ± 1.3 | ||
| BA-Husk | 1 mg/mL | 93.4 ± 5.8 | 90.7 ± 2.3 | 102.2 ± 1.1 | <0.05 |
| 22.5 mg/mL | 89.8 ± 5.0 | 91.9 ± 7.6 | 101.1 ± 4.6 | ||
| 45 mg/mL | 96.9 ± 2.7 | 86.4 ± 1.2 | 95.2 ± 5.8 | ||
| WP-Husk | 1 mg/mL | 89.1 ± 6.8 | 78.3 ± 4.7 | 86.2 ± 3.6 | <0.05 |
| 22.5 mg/mL | 90 ± 4.9 | 74.8 ± 10.6 | 84 ± 3.4 | ||
| 45 mg/mL | 92.5 ± 2.7 | 80.2 ± 5 | 78.8 ± 5.2 | ||
| Arecoline | 1 μg/mL | 100.5 ± 7.9 | 97.4 ± 0.4 | 80.8 ± 18.2 | <0.05 |
| 10 μg/mL | 93.9 ± 11.8 | 51.63 ± 1 | 48.8 ± 1.5 | ||
| 100 μg/mL | 80.6 ± 8.4 | 39.7 ± 5.5 | 33.2 ± 1.7 | ||
| Cytotoxic mean values (IC50) at the earliest time and lowest concentration on OKF-6 | |||||
| Significantly different mean values (number of OKF-6) compared to control value | |||||
| Not significantly different mean values (number of OKF-6) compared to control value | |||||
| BQ Components | Concentration | 1-Day Cell Viability (%) ± SD | 2-Day Cell Viability (%) ± SD | 3-Day Cell Viability (%) ± SD | p-Value |
|---|---|---|---|---|---|
| Control | 0 | 100 | 100 | 100 | |
| BA-AN | 100 μg/mL | 123.4 ± 2.8 | 102.8 ± 7.7 | 115.2 ± 5.1 | <0.05 |
| 250 μg/mL | 113.8 ± 4.1 | 110.9 ± 0.8 | 110.3 ± 5.6 | ||
| 500 μg/mL | 116.6 ± 5.7 | 110.9 ± 4.6 | 108.9 ± 2.5 | ||
| WP-AN | 100 μg/mL | 119.0 ± 1.0 | 105.7 ± 3.3 | 94.8 ±3.7 | <0.05 |
| 250 μg/mL | 120.0 ± 1.1 | 96.7 ± 13.9 | 102.4 ± 2.4 | ||
| 500 μg/mL | 120.4 ± 0.2 | 104.4 ± 2.6 | 96 ± 3.3 | ||
| BA-BQ Mixture | 1 mg/mL | 105.2 ± 5.2 | 100.7 ± 5.6 | 104.2 ± 3.9 | <0.05 |
| 15 mg/mL | 114.5 ± 1.3 | 98.4 ± 4.5 | 92 ± 3 | ||
| 30 mg/mL | 111.2 ± 0.9 | 97.4 ± 6.1 | 93.4± 3.0 | ||
| WP-BQ Mixture | 1 mg/mL | 112.6 ± 2.7 | 99.1 ± 2.5 | 95.8 ± 6.6 | <0.05 |
| 15 mg/mL | 111.2 ± 0.5 | 88.4 ± 5.2 | 86.7 ± 0.7 | ||
| 30 mg/mL | 103 ± 2.6 | 85.5 ± 1 | 82.2 ± 2.9 | ||
| BA-Leaf | 100 μg/mL | 114.4 ± 3.9 | 109.2 ±3.7 | 103.5 ± 6.6 | <0.05 |
| 250 μg/mL | 122.4 ± 8.8 | 116.8 ± 1.6 | 111.9 ± 5.2 | ||
| 500 μg/mL | 123.7 ± 6.5 | 117.7 ± 1.2 | 121 ± 3.7 | ||
| WP-SI | 100 μg/mL | 114.4 ± 4.2 | 107.8 ± 4.9 | 110.9 ± 2.6 | <0.05 |
| 250 μg/mL | 117.4 ± 3.3 | 111.6 ± 0.8 | 119.7 ± 2.9 | ||
| 500 μg/mL | 118.5 ± 2.1 | 116.2 ± 2.6 | 120.8 ± 3.5 | ||
| BA-Husk | 1 mg/mL | 118.9 ± 4.9 | 118.1 ± 1.2 | 119.8 ± 4.8 | <0.05 |
| 22.5 mg/mL | 138.7 ± 2.3 | 125.2 ± 6.4 | 122.8 ± 5.7 | ||
| 45 mg/mL | 135.0 ± 2.7 | 127.6 ± 7.8 | 127.3 ± 6.5 | ||
| WP-Husk | 1 mg/mL | 118.2 ± 3.4 | 118.8 ± 2.6 | 125.4 ± 0.9 | <0.05 |
| 22.5 mg/mL | 128.5 ± 0.9 | 123.3 ± 2.4 | 125.9 ± 0.6 | ||
| 45 mg/mL | 128.1 ± 9.2 | 130.3 ± 2.1 | 129.5 ± 2.6 | ||
| Arecoline | 1 μg/mL | 109.8 ± 2.5 | 116.6 ± 6.4 | 105.8 ± 5.7 | <0.05 |
| 10 μg/mL | 107.4 ± 4.2 | 106.5 ± 5.1 | 106.9 ± 5.3 | ||
| 100 μg/mL | 105.9 ± 2.2 | 115.5 ± 3.8 | 97.3 ± 5.9 | ||
| Significantly higher cell proliferation | |||||
| Significantly lower cell proliferation | |||||
| Not significantly different | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sari, E.F.; Mohammed, A.I.; Celentano, A.; McCullough, M.J.; Cirillo, N. Cytotoxic Effects of Indonesian Betel Quid Components on Oral Keratinocytes and Fibroblasts. BioChem 2023, 3, 153-169. https://doi.org/10.3390/biochem3040011
Sari EF, Mohammed AI, Celentano A, McCullough MJ, Cirillo N. Cytotoxic Effects of Indonesian Betel Quid Components on Oral Keratinocytes and Fibroblasts. BioChem. 2023; 3(4):153-169. https://doi.org/10.3390/biochem3040011
Chicago/Turabian StyleSari, Elizabeth Fitriana, Ali I. Mohammed, Antonio Celentano, Michael John McCullough, and Nicola Cirillo. 2023. "Cytotoxic Effects of Indonesian Betel Quid Components on Oral Keratinocytes and Fibroblasts" BioChem 3, no. 4: 153-169. https://doi.org/10.3390/biochem3040011
APA StyleSari, E. F., Mohammed, A. I., Celentano, A., McCullough, M. J., & Cirillo, N. (2023). Cytotoxic Effects of Indonesian Betel Quid Components on Oral Keratinocytes and Fibroblasts. BioChem, 3(4), 153-169. https://doi.org/10.3390/biochem3040011









