Abstract
Skin is one of the organs most tested for toxicity and safety evaluation during the process of drug research and development and in the past has usually been performed in vivo using animals. Over the last few years, non-animal alternatives have been developed and validated epidermis models for human and rat skin are already available. Our goal was to develop a histotypical canine skin analog, suitable for non-animal biocompatibility and biosafety assessment. Canine keratinocytes were seeded in an air-lift culture using an adapted version of the CELLnTEC protocol. Corrosion and irritation protocols were adapted from human EpiSkinTM. For histological analysis, sample biopsies were fixed in neutral-buffered formalin, and paraffin slices were routinely processed and stained with hematoxylin and eosin. A canine multilayer and stratified epidermal-like tissue (cEpiderm), confirmed by histological analysis, was obtained. The cEpiderm tissue exhibited normal morphological and functional characteristics of epidermis, namely impermeability and an adequate response to stressors. The cEpiderm is a promising canine skin model for non-animal safety testing of veterinary pharmaceuticals and/or cosmetics, significantly contributing to reducing undesirable in vivo approaches. cEpiderm is therefore a valid canine skin model and may be made commercially available either as a service or as a product.
1. Introduction
Skin analogs or skin equivalents are extensively used in research and the testing of various products in different industries such as cosmetics, pharmaceuticals and skin care companies [1]. These analogs can also have applications in the treatment of acute and chronic wounds such as burns or diabetic wounds, additionally they aid in the research of new ways of treatments for various diseases such as melanoma and psoriasis [2,3].
Skin analogs have become quite popular because they are a sustainable and practical alternative compared to the use of live animals or mammalian skin explants, and they can also reduce significant errors and inaccuracies principally with regard to differences between species since the biochemistry and physiology of the skin varies from species to species [4].
Initially in vitro skin analogs consisted of a two-dimensional layer of cultured cells, mainly keratinocytes to represent the epidermal and outer layer of the skin, or fibroblasts that represented the dermal layer and were used mainly for toxicity assessment. However, these skin models lack the ability to mimic the penetration and absorption of different chemicals and materials of the live skin [5]. The development of new technologies and research has increased, especially in the case of human epidermal models capable of mimicking the in vivo skin, its morphology, its biomolecular and metabolic properties [6]. Skin analogs based on air-lift organotypic cultures have been developed, creating a three-dimensional skin model capable of simulating the permeability and absorption capacity of the in vivo skin. These 3D models may also incorporate components of a dermal layer, such as fibroblasts, or other skin cells, introducing complexity and versatility to these biosystems.
The skin barrier function is very complex and difficult to be artificially replicated because of the lack of skin equivalents including appendages, vasculature and lipids, thus the gold standard is still the use of in vivo testing. However, human skin equivalents are already being used by pharmaceutical and cosmetic companies, mainly in the preliminary phases for product development to screen the potential toxicity of unknown materials [7]. Most of the reconstructed human epidermal models commercialized today include EpiSkin®, SkinEthic®, and Epiderm®, while living skin equivalents are commercialized by GraftSkin®, EpidermFT®, and Phenion®. These human skin analogs are the standards used in the testing of irritation, corrosion and sensitization tests used in most companies and they may serve as models for the development of new skin analogs, for instance for animal skin analogs, since they are approved and regulated and follow the OECD test guidelines for chemicals [2,8,9,10].
Indeed, animal skin models are needed for the development of pharmaceutical and cosmetic products for veterinary use, yet there are no canine skin equivalents commercially available. Although, some methodologies were described to maintain canine skin biopsies in culture [11,12], to isolate dermal fibroblasts and keratinocytes and to obtain a 3D multilayer epidermis using natural canine skin explants [4,13,14], there is a lack of an easily standardizable methodology using culture-developed canine epidermis to test the efficacy and the biosafety of veterinary products. Thus, the aim of this study was to develop a canine epidermis analog based on CPEK (canine progenitor epithelial keratinocytes) expansion and differentiation in culture at the air–liquid surface. Tissue patches developed by this strategy are highly reproduceable and useful as a skin surrogate for veterinary product development.
2. Materials and Methods
For the development of the cEpiderm, epidermal keratinocyte progenitor cells supplied by CELLnTEC (Bern, Switzerland) were used. The 3D keratinocyte Starter Protocol [15] was followed with some adaptations. Briefly, 2 × 106 cells were seeded in uncoated inserts (VWR Internacional, Carnaxide, Portugal), in DMEM (Sigma-Aldrich, Darmstadt, Germany) supplemented with 10% fetal bovine serum (FBS; Biowest, Nuaille, France), 5 ng/mL insulin (Sigma-Aldrich, Darmstadt, Germany) and 10 ng/mL epithelial growth factor (EGF; Sino Biological, Eschborn, Germany). The culture medium inside and outside the inserts was changed every 2–3 days until confluence was reached, which typically occurred 7–10 days after seeding. After the confirmation of the confluence, the culture media was changed, inside and outside the insert, for differentiation medium (CnT-PR-3D, CELLnTEC). The following day, the inserts were air-lifted to promote differentiation, and changing of the medium under the insert was carried out every 2–3 days until an epidermis-like tissue was obtained (see Figure 1).
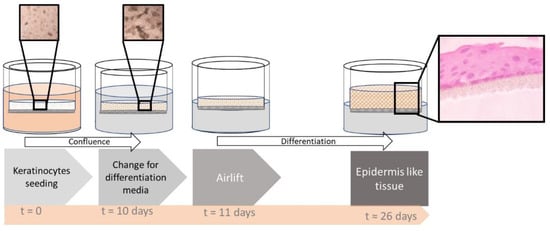
Figure 1.
Schematic representation of the keratinocyte differentiation process. Microscopic photographs of the cell culture in the upper boxes showing the cell density (at seeding time and after 10-day culture) and a histological image of the differentiated tissue in the side box (stained with eosin-hematoxylin).
For histological analysis, tissue patches were collected and fixed in 10% neutral-buffered formalin (VWR Internacional, Carnaxide, Portugal) and processed for examination by standard light microscopy techniques (Olympus Iberica, Llobregat, Spain). Paraffin (Panreac AppliChem, Darmstadt, Germany) sections were cut at 3 μm and stained with hematoxylin-eosin (Harries Hematoxylin modified solution for clinical diagnosis, Panreac AppliChem, Darmstadt, Germany; Eosin Y, Merk, Darmstadt, Germany) [16].
To assess cell barrier impermeability, 1% Triton-X100 (Sigma-Aladrich, Darmstadt, Germany) was added on top of the epidermal tissue and incubated over 4 h at 37 °C [14], and subsequently cell viability was measured with CCK-8 (Sigma Aldrich, Darmstadt, Germany) [17].
Corrosion and irritation tests were performed using a validated methodology for human skin models [8,9]. Briefly, stimuli were performed using 5% SDS (Himedia, Einhausen, Germany) and glacial acetic acid (Fisher Chemical, Porto Salvo, Portugal) for the irritation and the corrosion tests, respectively. PBS (phosphate buffer saline, in mM: Na2HPO4 10.0; KH2PO4 1.8; NaCl 137.0; KCl 2.7; pH 7.4) was used as control. Tissue patches were incubated with the stimuli for 42 min at room temperature for the irritation test, and 3 min and 60 min at 37 °C for the corrosion tests. After incubation, the inserts were washed with PBS to remove any traces of the stimuli, and CCK-8 was added to assess cell viability according to the supplier’s instructions [8,9,10,17].
3. Results and Discussion
An air-lift keratinocyte’s histotypical culture was produced and morphological, histological and functional assessments were performed to evaluate its canine epidermal-like (cEpiderm) properties.
3.1. Morphological and Histological Characterization of the cEpiderm
The tissue patches obtained were constituted by a multilayer of canine keratinocytes, 3–4 cell layers thick, generating a stratified epidermal-like tissue, confirmed by histological analysis (Figure 2).
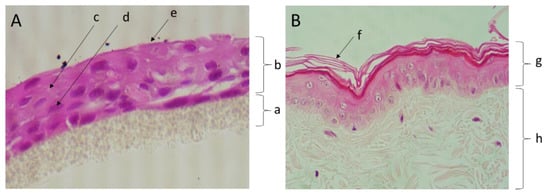
Figure 2.
Micrographs of a section of the canine epidermis-like culture (A) and a canine skin biopsy (B), both routinely processed and stained with hematoxylin and eosin (400× amplification). Legend: (a) insert porous membrane; (b) keratinocyte layers of the histotypical culture; (c) cell junctions; (d) keratinocyte nucleus; (e) stratum corneum-like layer; (f) stratum corneum; (g) epidermis; (h) dermis.
The epidermal-like tissue (Figure 2A) exhibited cohesive keratinocytes (single layer of keratinized cells) comparable to those observed in the squamous epithelial cells of skin epidermis (Figure 2B). The keratinocytes in the histotypical model are cuboidal or slightly flattened cells that are attached to adjacent cells via desmosomes, which is also a feature of canine epidermis.
Our results are in a good agreement with those observed by Yagihara and collaborators (2011), whereby using inserts coated with type-I collagen allowed for the observation that CPEK cells cultured at an air–liquid interface became stratified and formed characteristic epidermis layers [18].
3.2. Functional Characterization of the cEpiderm
Exposing the cEpiderm apical side to 0.1% Triton X-100 evoked a 45% reduction in cell viability (Figure 3A), revealing a good impermeability performance, an important feature of a functional epidermal-like tissue [9].
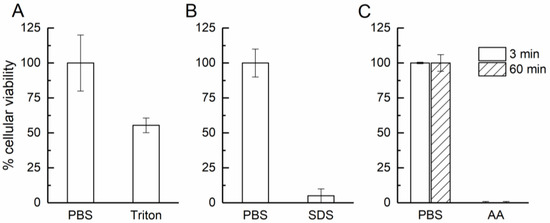
Figure 3.
Cellular viability accessed in permeability (A), irritation (B), and corrosion (C) tests. PBS was used as control; Triton: 0.1% Triton X-100; SDS: 5% SDS; AA: acetic acid (glacial).
For functional irritation response, the cEpiderm slices were exposed on the apical side to 5% SDS. The negatively charged detergent induced a loss of cell viability of 95% compared to controls (Figure 3B). Histological evaluation of the biopsies revealed that SDS acted by disrupting the structure of cEpiderm patches, causing detachments of the keratinocytes (Figure 4, upper panel).
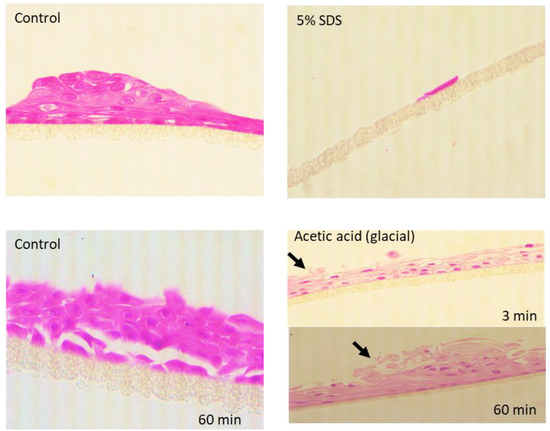
Figure 4.
Micrographs of sections of the cEpiderm untreated (Control) and upon irritation (5% SDS) or corrosion (acetic acid) insult. Arrows point to tissue disruption.
For this test, a known irritant (SDS) was used and the cEpiderm response was similar to the reference tables, corresponding to a loss of cell viability above 50% [8,10].
After a corrosion insult with acetic acid (glacial), the cell viability declined to 0% after 3 min of exposure, remaining at 0% after 60 min (Figure 3C). Histological evaluation has shown that the cell patches remain attached but show a progressive disruption of the tissue, characterized by a loss of integrity at the tissue surface (see arrows in Figure 4, lower panel).
Acetic acid (glacial) is a known subcategory 1A corrosive chemical which causes an acute massive loss of cell viability (>95%) [9,10], consistent with the response of the cEpiderm model.
The cEpiderm responded as expected to corrosion, irritation and permeability assessment tests, and in accordance with the observations in equivalent human in vitro tests [8]. These are good indicators of cEpiderm’s functional barrier and response to stressors [10], suggesting that it is suitable to be used in biosafety and biocompatibility screening studies. This canine epidermis model presents the properties to be used for other common tests in drug and cosmetics development, but also for other stimuli assessment (e.g., phototoxicity, response to UV-lights), permeability and/or skin sensitivity (evaluating the epithelial immune activation). Considering the above, the canine epidermis model described in this paper has the potential to greatly contribute to the reduction of animal testing in the development of veterinary products. On the other hand, the cEpiderm skin model lacks a circulation system, thus having limited applicability to evaluate angiogenesis, a process involved in wound healing in vivo [5].
One limitation of this work was the absence of reference tables for the irritation and corrosion in vitro tests for canine skin or epidermis, such as those already available for human skin models [19]. In the future, guidelines for corrosion and irritation tests appliable for in vitro canine epidermis should be defined, so the cEpiderm may be validated as a canine skin surrogate.
4. Conclusions
A stratified epidermal-like tissue, cEpiderm, which responds to irritation and corrosion insults, according to the OECD guidelines for human skin models, was successfully developed using commercially available keratinocytes (CPEK) grown in air-lift uncoated inserts. The cEpiderm has a high repeatability potential and is suitable for large-scale use.
The use of species-specific histotypical models for epidermis may contribute to more accurate safety results and to a significative reduction in animal testing in the development of veterinary pharmaceuticals and cosmetic products. The high standardization potential of this epidermis model makes it suitable for the future development of guidelines for animal-free canine skin testing.
Author Contributions
Conceptualization, A.R.C. and C.M.A.; methodology, J.N. and M.M.; validation, S.B. and L.M.; formal analysis, J.N. and M.M.; investigation, B.U., L.F., J.N. and M.M.; resources, A.J.B., C.F. and L.M.; data curation, A.R.C. and C.M.A.; writing—original draft preparation, A.R.C., C.M.A., J.N. and M.M.; writing—review and editing, A.J.B., A.R.C. and C.M.A., S.B.; supervision, A.R.C. and C.M.A.; project administration, A.J.B., C.F. and L.M.; funding acquisition, A.C.C. and A.J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Alentejo 2020 Programme, grant number ALT20-03-247-FEDER-033578, and co-financed by FEDER Funds through the Operational Programme for Competitiveness Factors—COMPETE 2020 and by National Funds through FCT—Foundation for Science and Technology under the Project UIDB/50006/2020, UIDP/5006/2020, UIDB/04683/2020 and UIDP/04683/2020.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Netzlaff, F.; Kaca, M.; Bock, U.; Haltner-Ukomadu, E.; Meiers, P.; Lehr, C.-M.; Schaefer, U.F. Permeability of the reconstructed human epidermis model Episkin in comparison to various human skin preparations. Eur. J. Pharm. Biopharm. 2007, 66, 127–134. [Google Scholar] [CrossRef] [PubMed]
- MacNeil, S. Progress and opportunities for tissue-engineered skin. Nature 2007, 445, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Supp, D.M.; Boyce, S.T. Engineered skin substitutes: Practices and potentials. Clin. Dermatol. 2005, 23, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Souci, L.; Denesvre, C. 3D skin models in domestic animals. Vet. Res. 2021, 52, 21. [Google Scholar] [CrossRef] [PubMed]
- Rittié, L. Cellular mechanisms of skin repair in humans and other mammals. J. Cell Commun. Signal. 2016, 10, 103–120. [Google Scholar] [CrossRef]
- Suhail, S.; Sardashti, N.; Jaiswal, D.; Rudraiah, S.; Misra, M.; Kumbar, S.G. Engineered Skin Tissue Equivalents for Product Evaluation and Therapeutic Applications. Biotechnol. J. 2019, 14, e1900022. [Google Scholar] [CrossRef]
- Martínez-Santamaría, L.; Guerrero-Aspizua, S.; Del Río, M. Skin bioengineering: Preclinical and clinical applications. Actas Dermosifiliogr. 2012, 103, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Skin Irritation Protocol SkinEthic RHE, (Validated) [Internet]. Lyon, FRANCE: Episkin. Available online: https://www.episkin.com/skin-irritation (accessed on 2 May 2022).
- Skin Corrosion Protocol Episkin (Validated) [Internet]. Lyon, FRANCE: Episkin. Available online: https://www.episkin.com/skin-corrosion (accessed on 2 May 2022).
- Netzlaff, F.; Lehr, C.M.; Wertz, P.W.; Schaefer, U.F. The human epidermis models EpiSkin®, SkinEthic® and EpiDerm®: An evaluation of morphology and their suitability for testing phototoxicity, irritancy, corrosivity, and substance transport. Eur. J. Pharm. Biopharm. 2005, 60, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Abramo, F.; Pirone, A.; Lenzi, C.; Vannozzi, I.; Della Valle, M.F.; Miragliotta, V. Establishment of a 2-week canine skin organ culture model and its pharmacological modulation by epidermal growth factor and dexamethasone. Ann. Anat. 2016, 207, 109–117. [Google Scholar] [CrossRef][Green Version]
- Bauhammer, I.; Sacha, M.; Haltner, E. Establishment of an in vitro model of cultured viable human, porcine and canine skin and comparison of different media supplements. PeerJ 2019, 7, e7811. [Google Scholar] [CrossRef]
- Serra, M.; Brazís, P.; Puigdemont, A.; Fondevila, D.; Romano, V.; Torre, C.; Ferrer, L. Development and characterization of a canine skin equivalent. Exp. Dermatol. 2007, 16, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Cerrato, S.; Ramió-Lluch, L.; Fondevila, D.; Rodes, D.; Brazis, P.; Puigdemont, A. Effects of Essential Oils and Polyunsaturated Fatty Acids on Canine Skin Equivalents: Skin Lipid Assessment and Morphological Evaluation. J. Vet. Med. 2013, 2013, 231526. [Google Scholar] [CrossRef] [PubMed]
- 3D Keratinocyte Starter Kit (without Primary Cells) [Internet]. BERN, SWITZERLAND: CELLnTEC. Available online: https://cellntec.com/wp-content/uploads/pdf/3D_Starter_Kit_K.pdf (accessed on 2 May 2022).
- Histological Processing and Staining [Internet]. BERN, SWITZERLAND. Available online: https://cellntec.com/wp-content/uploads/pdf/RoutineHistology_Staining.pdf (accessed on 2 May 2022).
- Cell Counting Kit-8 [Internet]. Darmstadt, Germany: Sigma-Aldrich. Available online: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/product/documents/381/017/96992dat.pdf (accessed on 2 May 2022).
- Yagihara, H.; Okumura, T.; Shiomi, E.; Shinozaki, N.; Kuroki, S.; Sasaki, Y.; Ito, K.; Ono, K.; Washizu, T.; Bonkobara, M. Reconstruction of stratum corneum in organotypically cultured canine keratinocyte-derived CPEK cells. Vet. Res. Commun. 2011, 35, 433–437. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 431: In vitro skin corrosion: Reconstructed human epidermis (RHE) test method. In OECD Guidelines for the Testing of Chemicals, Section 4; OECD: Paris, France, 2019. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).