Testicular Glycogen Metabolism: An Overlooked Source of Energy for Spermatogenesis?
Abstract
:1. Introduction
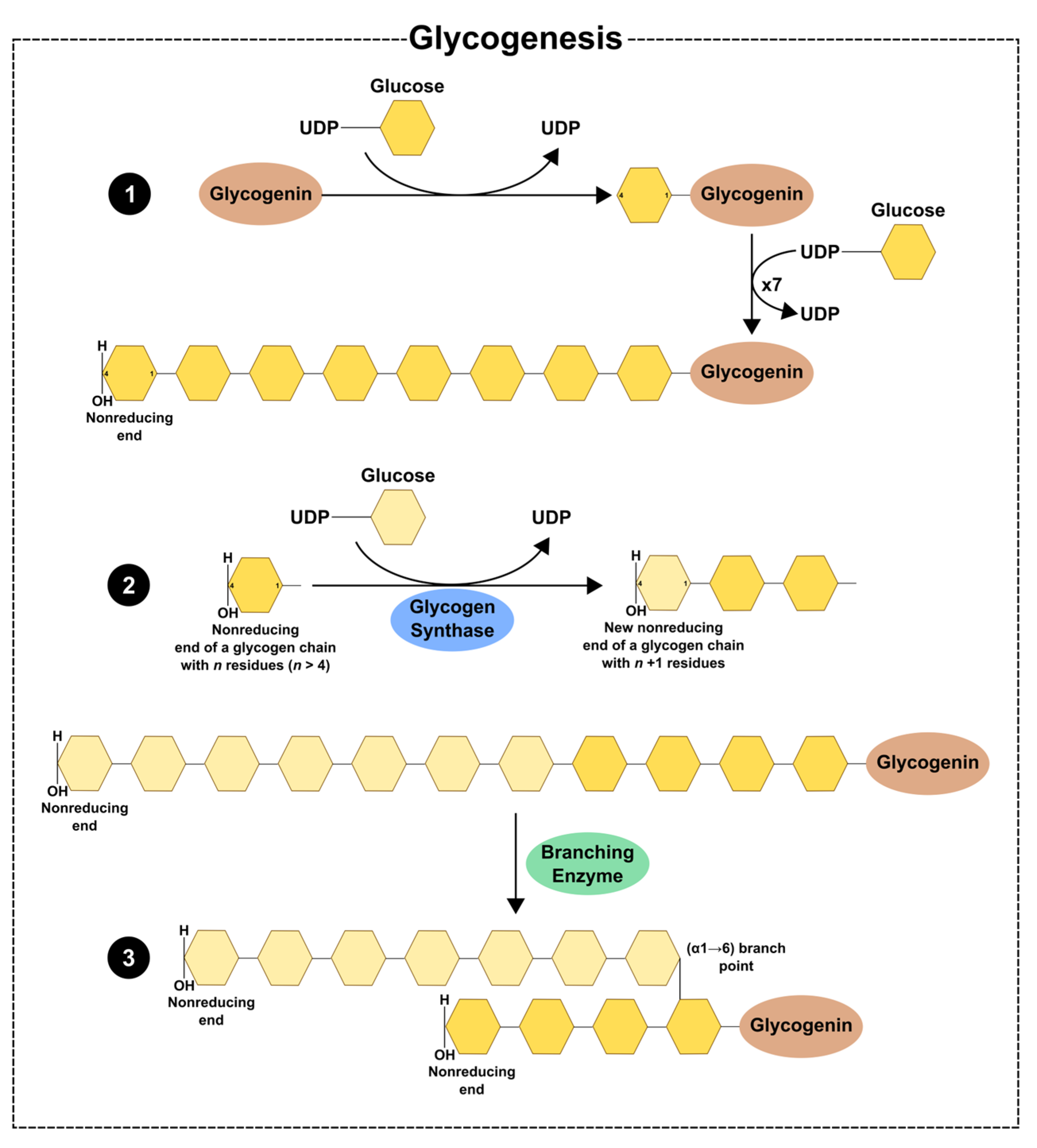
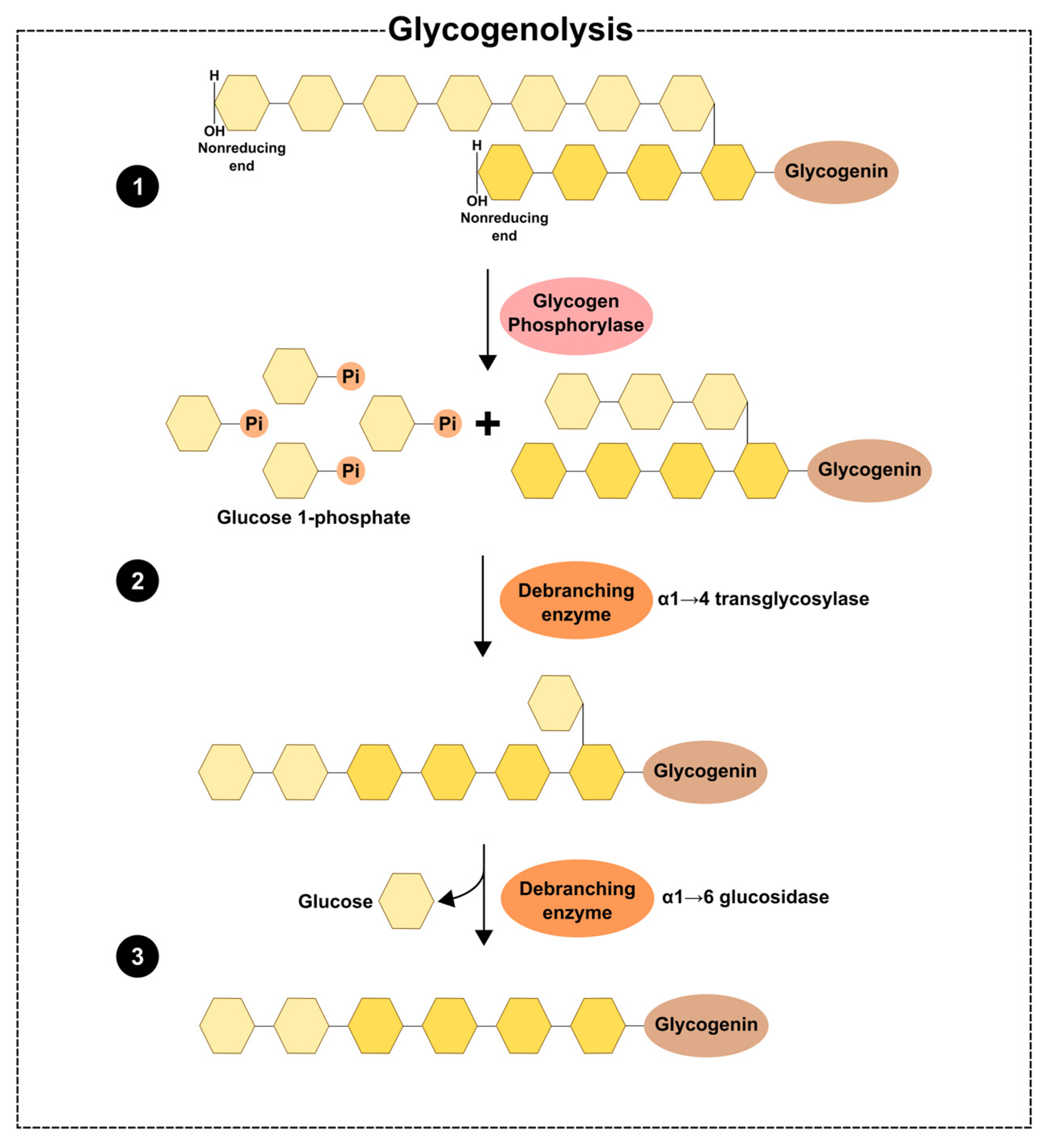
2. Glycogen Dynamics—Synthesis and Degradation
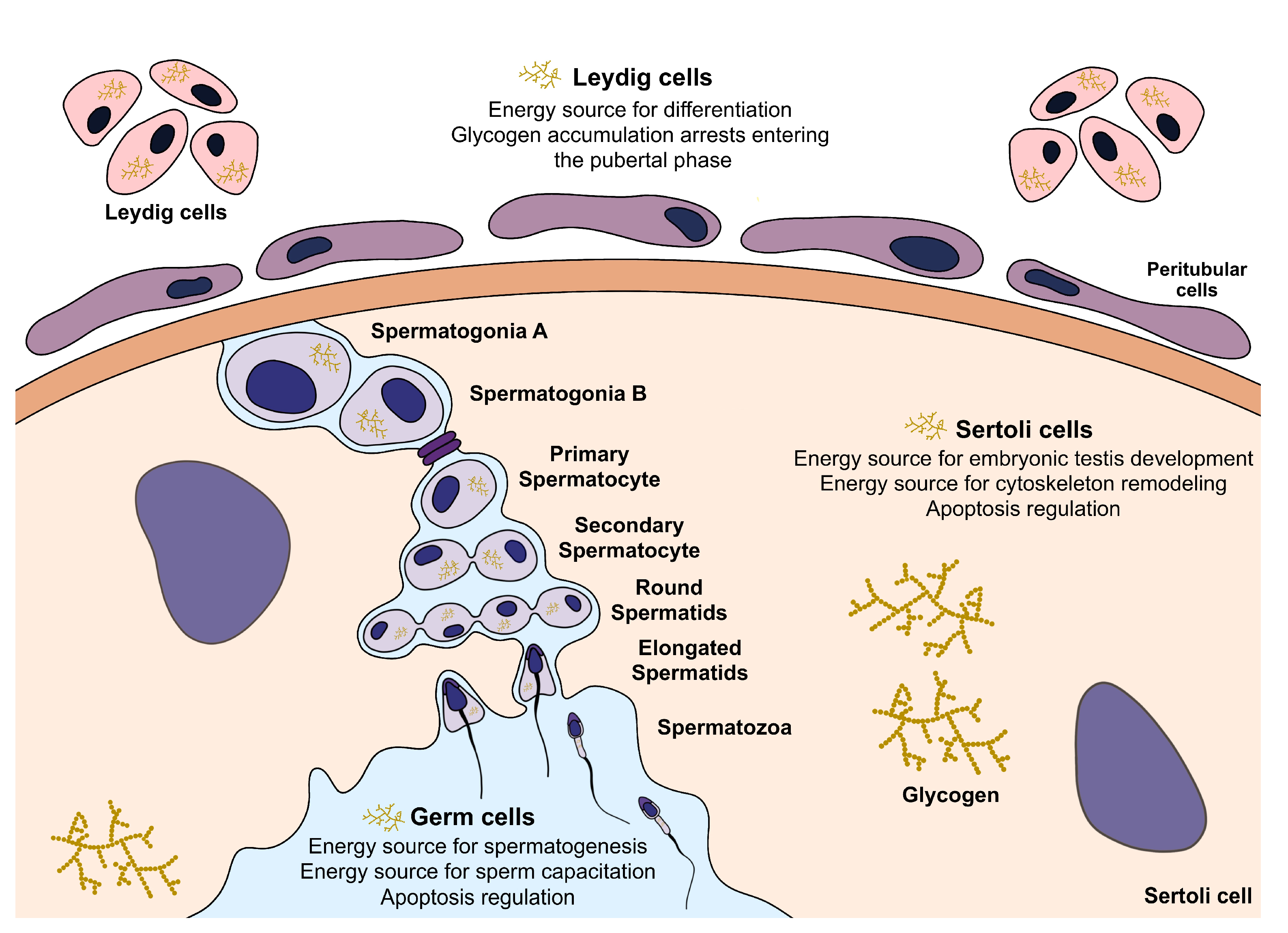
3. Glycogen in the Testicular Environment
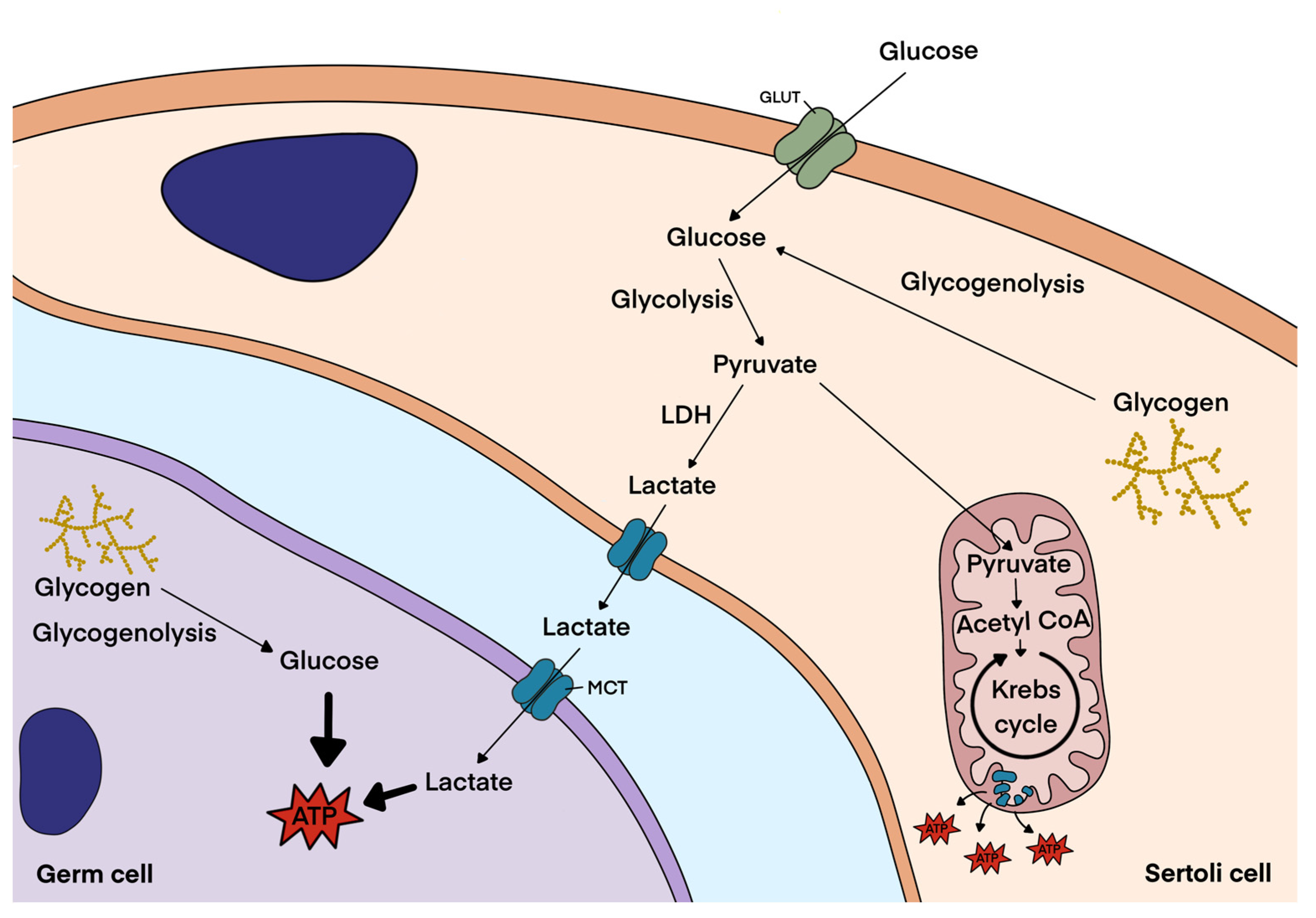
3.1. Glycogen Metabolism in Sertoli Cells
3.2. Glycogen Metabolism in Leydig Cells
3.3. Glycogen Metabolism in Germ Cells and Spermatozoa
4. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Jørgensen, N.; Andersen, A.G.; Eustache, F.; Irvine, D.S.; Suominen, J.; Petersen, J.H.; Andersen, A.N.; Auger, J.; Cawood, E.H.H.; Horte, A.; et al. Regional Differences in Semen Quality in Europe. Hum. Reprod. 2001, 16, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, N.; Carlsen, E.; Nermoen, I.; Punab, M.; Suominen, J.; Andersen, A.G.; Andersson, A.M.; Haugen, T.B.; Horte, A.; Jensen, T.K.; et al. East-West Gradient in Semen Quality in the Nordic-Baltic Area: A Study of Men from the General Population in Denmark, Norway, Estonia and Finland. Hum. Reprod. 2002, 17, 2199–2208. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.F.; Duran, I.; Olea, N.; Avivar, C.; Vierula, M.; Toppari, J.; Skakkebæk, N.E.; Jørgensen, N. Semen Quality and Reproductive Hormone Levels in Men from Southern Spain. Int. J. Androl. 2012, 35, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nordkap, L.; Joensen, U.N.; Blomberg Jensen, M.; Jørgensen, N. Regional Differences and Temporal Trends in Male Reproductive Health Disorders: Semen Quality May Be a Sensitive Marker of Environmental Exposures. Mol. Cell. Endocrinol. 2012, 355, 221–230. [Google Scholar] [CrossRef]
- Sun, H.; Gong, T.-T.; Jiang, Y.-T.; Zhang, S.; Zhao, Y.-H.; Wu, Q.-J. Global, regional, and national prevalence and disability-adjusted life-years for infertility in 195 countries and territories, 1990–2017: Results from a global burden of disease study, 2017. Aging 2019, 11, 1990–2017. [Google Scholar] [CrossRef]
- Vander Borght, M.; Wyns, C. Fertility and infertility: Definition and epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef]
- Cavallini, G.; Beretta, G. Clinical Management of Male Infertility; Springer: London, UK, 2015; pp. 1–187. [Google Scholar]
- Nieschlag, E.; Behre, H.M.; Nieschlag, S. Andrology Male Reproductive Health and Dysfunction; Springer: Berlin/Heidelberg, Germany, 2010; pp. 87–92. [Google Scholar]
- Rato, L.; Alves, M.G.; Cavaco, J.E.; Oliveira, P.F. High-Energy Diets: A Threat for Male Fertility? Obes. Rev. 2014, 15, 996–1007. [Google Scholar]
- Sermondade, N.; Faure, C.; Fezeu, L.; Shayeb, A.G.; Bonde, J.P.; Jensen, T.K.; van Wely, M.; Cao, J.; Martini, A.C.; Eskandar, M.; et al. BMI in relation to sperm count: An updated systematic review and collaborative meta-analysis. Hum. Reprod. Update 2013, 19, 221–231. [Google Scholar] [CrossRef]
- Schisterman, E.F.; Mumford, S.L.; Chen, Z.; Browne, R.W.; Barr, D.B.; Kim, S.; Louis, G.M.B. Lipid concentrations and semen quality: The LIFE study. Andrology 2014, 2, 408–415. [Google Scholar] [CrossRef]
- Bener, A.; Al-Ansari, A.A.; Zirie, M.; Al-Hamaq, A.O. Is Male Fertility Associated with Type 2 Diabetes Mellitus? Int. Urol. Nephrol. 2009, 41, 777–784. [Google Scholar] [CrossRef]
- Rato, L.P.; Alves, M.G.; Dias, T.R.; Cavaco, J.E.; Oliveira, P.F. Testicular Metabolic Reprogramming in Neonatal Streptozotocin-Induced Type 2 Diabetic Rats Impairs Glycolytic Flux and Promotes Glycogen Synthesis. J. Diabetes Res. 2015, 2015, 973142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curi, S.M.; Ariagno, J.I.; Chenlo, P.H.; Mendeluk, G.R.; Pugliese, M.N.; Sardi Segovia, L.M.; Repetto, H.E.H.; Blanco, A.M. Asthenozoospermia: Analysis of a Large Population. Arch. Androl. 2003, 49, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Xuan, W.; Lamhonwah, A.-M.; Librach, C.; Jarvi, K.; Tein, I. Characterization of organic cation/carnitine transporter family in human sperm. Biochem. Biophys. Res. Commun. 2003, 306, 121–128. [Google Scholar] [CrossRef]
- Liu, F.-J.; Liu, X.; Han, J.-L.; Wang, Y.-W.; Jin, S.-H.; Liu, X.-X.; Liu, J.; Wang, W.-T. Aged men share the sperm protein PATE1 defect with young asthenozoospermia patients. Hum. Reprod. 2015, 30, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Stanton, P.G. Regulation of the blood-testis barrier. Semin. Cell Dev. Biol. 2016, 59, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Thompson, L.A.; Dufour, J.M. Sertoli cells—Immunological sentinels of spermatogenesis. Semin. Cell Dev. Biol. 2014, 30, 36–44. [Google Scholar] [CrossRef]
- Park, Y.-J.; Pang, M.-G. Mitochondrial Functionality in Male Fertility: From Spermatogenesis to Fertilization. Antioxidants 2021, 10, 98. [Google Scholar] [CrossRef]
- Riera, M.F.; Meroni, S.B.; Schteingart, H.F.; Pellizzari, E.H.; Cigorraga, S.B. Regulation of lactate production and glucose transport as well as of glucose transporter 1 and lactate dehydrogenase A mRNA levels by basic fibroblast growth factor in rat Sertoli cells. J. Endocrinol. 2002, 173, 335–343. [Google Scholar] [CrossRef]
- Riera, M.F.; Galardo, M.N.; Pellizzari, E.H.; Meroni, S.B.; Cigorraga, S.B. Molecular mechanisms involved in Sertoli cell adaptation to glucose deprivation. Am. J. Physiol. Metab. 2009, 297, E907–E914. [Google Scholar] [CrossRef]
- Jarow, J.P.; Zirkin, B.R. The Androgen Microenvironment of the Human Testis and Hormonal Control of Spermatogenesis. Ann. N. Y. Acad. Sci. 2005, 1061, 208–220. [Google Scholar] [CrossRef]
- Medar, M.L.J.; Marinkovic, D.Z.; Kojic, Z.; Becin, A.P.; Starovlah, I.M.; Kravic-Stevovic, T.; Andric, S.A.; Kostic, T.S. Dependence of Leydig Cell’s Mitochondrial Physiology on Luteinizing Hormone Signaling. Life 2020, 11, 19. [Google Scholar] [CrossRef]
- Rodríguez-Gil, J.E.; Bonet, S. Current knowledge on boar sperm metabolism: Comparison with other mammalian species. Theriogenology 2016, 85, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Pesini, E.; Díez-Sánchez, C.; López-Pérez, M.J.; Enríquez, J.A. The Role of the Mitochondrion in Sperm Function: Is There a Place for Oxidative Phosphorylation or Is This a Purely Glycolytic Process? Curr. Top. Dev. Biol. 2007, 77, 3–19. [Google Scholar] [PubMed]
- Adeva-Andany, M.M.; González-Lucán, M.; Donapetry-García, C.; Fernández-Fernández, C.; Ameneiros-Rodríguez, E. Glycogen metabolism in humans. BBA Clin. 2016, 5, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Chikwana, V.M.; Khanna, M.; Baskaran, S.; Tagliabracci, V.S.; Contreras, C.J.; DePaoli-Roach, A.; Roach, P.J.; Hurley, T.D. Structural basis for 2′-phosphate incorporation into glycogen by glycogen synthase. Proc. Natl. Acad. Sci. USA 2013, 110, 20976–20981. [Google Scholar] [CrossRef]
- Preiss, J.; Walsh, D.A. The Comparative Biochemistry of Glycogen and Starch; Ginsbg, V., Robbins, P., Eds.; John Wiley Sons: New York, NY, USA, 1981; pp. 199–314. [Google Scholar]
- Roach, P.J. Glycogen and Its Metabolism. Curr. Mol. Med. 2002, 2, 101–120. [Google Scholar] [CrossRef]
- Barbetti, F.; Rocchi, M.; Bossolasco, M.; Cordera, R.; Sbraccia, P.; Finelli, P. The Human Skeletal Muscle Glycogenin Gene: CDNA, Tissue Expression, and Chromosomal Localization. Biochem. Biophys. Res. Commun. 1996, 220, 72–77. [Google Scholar] [CrossRef]
- Mu, J.; Roach, P.J.; Chem, J.B. Characterization of Human Glycogenin-2, a Self-Glucosylating Initiator of Liver Glycogen Metabolism. J. Biol. Chem. 1998, 273, 34850–34856. [Google Scholar] [CrossRef]
- Villarroel-Espíndola, F.; Maldonado, R.; Mancilla, H.; Stelt, K.V.; Acuña, A.I.; Covarrubias, A.; López, C.; Angulo, C.; Castro, M.A.; Slebe, J.C.; et al. Muscle glycogen synthase isoform is responsible for testicular glycogen synthesis: Glycogen overproduction induces apoptosis in male germ cells. J. Cell. Biochem. 2013, 114, 1653–1664. [Google Scholar] [CrossRef]
- Halse, R.; Fryer, L.G.D.; McCormack, J.G.; Carling, D.Y.S. Regulation of Glycogen Synthase by Glucose and Glycogen: A Possible Role for Amp-Activated Protein Kinase. Diabetes 2003, 52, 9–15. [Google Scholar] [CrossRef]
- Nuttall, F.Q.; Gannon, M.C. Allosteric Regulation of Glycogen Synthase in Liver. J. Biol. Chem. 1993, 268, 13286–13290. [Google Scholar] [CrossRef]
- Palm, D.C.; Rohwer, J.M.; Hofmeyr, J.-H.S. Regulation of glycogen synthase from mammalian skeletal muscle—A unifying view of allosteric and covalent regulation. FEBS J. 2012, 280, 2–27. [Google Scholar] [CrossRef]
- Jensen, J.; Jebens, E.; Brennesvik, E.O.; Ruzzin, J.; Soos, M.A.; Engebretsen, E.M.L.; O’Rahilly, S.; Whitehead, J.P. Muscle glycogen inharmoniously regulates glycogen synthase activity, glucose uptake, and proximal insulin signaling. Am. J. Physiol. Metab. 2006, 290, E154–E162. [Google Scholar] [CrossRef]
- Kotoulas, O.B.; Kalamidas, S.A.; Kondomerkos, D.J. Glycogen autophagy in glucose homeostasis. Pathol. Res. Pract. 2006, 202, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Wisselaar, H.; Kroos, M.; Hermans, M.; van Beeumen, J.; Reuser, A. Structural and functional changes of lysosomal acid alpha-glucosidase during intracellular transport and maturation. J. Biol. Chem. 1993, 268, 2223–2231. [Google Scholar] [CrossRef]
- Chen, Y.T.; He, J.K.; Ding, J.H.; Brown, B.I. Glycogen debranching enzyme: Purification, antibody characterization, and immunoblot analyses of type III glycogen storage disease. Am. J. Hum. Genet. 1987, 41, 1002–1015. [Google Scholar]
- Burwinkel, B.; Bakker, H.D.; Herschkovitz, E.; Moses, S.W.; Shin, Y.S.; Kilimann, M.W. Mutations in the Liver Glycogen Phosphorylase Gene (PYGL) Underlying Glycogenosis Type VI (Hers Disease). Am. J. Hum. Genet. 1998, 62, 785–791. [Google Scholar] [CrossRef]
- Kato, K.; Shimizu, A.; Kurobe, N.; Takashi, M.; Koshikawa, T. Human Brain-Type Glycogen Phosphorylase: Quantitative Localization in Human Tissues Determined with an Immunoassay System. J. Neurochem. 1989, 52, 1425–1432. [Google Scholar] [CrossRef]
- Rath, V.L.; Ammirati, M.; LeMotte, P.K.; Fennell, K.F.; Mansour, M.N.; Danley, D.E.; Hynes, T.R.; Schulte, G.K.; Wasilko, D.J.; Pandit, J. Activation of Human Liver Glycogen Phosphorylase by Alteration of the Secondary Structure and Packing of the Catalytic Core. Mol. Cell 2000, 6, 139–148. [Google Scholar] [CrossRef]
- Munger, R.; Temler, E.; Jallut, D.; Haesler, E.; Felber, J.-P. Correlations of glycogen synthase and phosphorylase activities with glycogen concentration in human muscle biopsies. Evidence for a double-feedback mechanism regulating glycogen synthesis and breakdown. Metabolism 1993, 42, 36–43. [Google Scholar] [CrossRef]
- Keppens, S.; Vandekerckhove, A.; Moshage, H.; Yap, S.H.; Aerts, R.; de Wulf, H. Regulation of glycogen phosphorylase activity in isolated human hepatocytes. Hepatology 1993, 17, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Zhang, M.; Gentry, M.S.; Worby, C.A.; Dixon, J.E.; Saltiel, A.R. A role for AGL ubiquitination in the glycogen storage disorders of Lafora and Cori’s disease. Genes Dev. 2007, 21, 2399–2409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griswold, M.D. The central role of Sertoli cells in spermatogenesis. Semin. Cell Dev. Biol. 1998, 9, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Jutte, N.H.P.M.; Grootegoed, J.A.; Rommerts, F.F.G.; Van Der Molen, H.J. Exogenous lactate is essential for metabolic activities in isolated rat spermatocytes and spermatids. Reproduction 1981, 62, 399–405. [Google Scholar] [CrossRef]
- Boussouar, F.; Benahmed, M. Lactate and energy metabolism in male germ cells. Trends Endocrinol. Metab. 2004, 15, 345–350. [Google Scholar] [CrossRef]
- Wong, C.; Cheng, C.Y. The Blood-Testis Barrier: Its Biology, Regulation, and Physiological Role in Spermatogenesis. Curr. Top. Dev. Biol. 2005, 71, 263–296. [Google Scholar] [CrossRef]
- Mital, P.; Hinton, B.T.; Dufour, J.M. The Blood-Testis and Blood-Epididymis Barriers Are More than Just Their Tight Junctions. Biol. Reprod. 2011, 84, 851–858. [Google Scholar] [CrossRef]
- Xiong, W.; Wang, H.; Wu, H.; Chen, Y.; Han, D. Apoptic Spermatogenic Cells Can Be Energy Sources for Sertoli Cells. Reproduction 2009, 137, 469–479. [Google Scholar] [CrossRef]
- Erkkila, K.; Aito, H.; Aalto, K.; Pentikäinen, V.; Dunkel, L. Lactate inhibits germ cell apoptosis in the human testis. Mol. Hum. Reprod. 2002, 8, 109–117. [Google Scholar] [CrossRef]
- Oliveira, P.F.; Martins, A.D.; Moreira, A.C.; Cheng, C.Y.; Alves, M.G. The Warburg Effect Revisited-Lesson from the Sertoli Cell. Med. Res. Rev. 2014, 35, 126–151. [Google Scholar] [CrossRef]
- Kokk, K.; Veräjänkorva, E.; Wu, X.-K.; Tapfer, H.; Põldoja, E.; Pöllänen, P. Immunohistochemical detection of glucose transporters class I subfamily in the mouse, rat and human testis. Medicina 2004, 40, 156–160. [Google Scholar] [PubMed]
- Galardo, M.N.; Riera, M.F.; Pellizzari, E.H.; Chemes, H.E.; Venara, M.C.; Cigorraga, S.B.; Meroni, S.B. Regulation of expression of Sertoli cell glucose transporters 1 and 3 by FSH, IL1β, and bFGF at two different time-points in pubertal development. Cell Tissue Res. 2008, 334, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Carosa, E.; Radico, C.; Giansante, N.; Rossi, S.; D’Adamo, F.; Di Stasi, S.M.; Lenzi, A.; Jannini, E.A. Ontogenetic profile and thyroid hormone regulation of type-1 and type-8 glucose transporters in rat Sertoli cells. Int. J. Androl. 2005, 28, 99–106. [Google Scholar] [CrossRef]
- Ulisse, S.; Jannini, E.A.; Pepe, M.; De Matteis, S.; D’Armiento, M. Thyroid Hormone Stimulates Glucose Transport and GLUT1 MRNA in Rat Sertoli Cells. Mol. Cell. Endocrinol. 1992, 87, 131–137. [Google Scholar] [CrossRef]
- Xu, B.; Chen, M.; Ji, X.; Yao, M.; Mao, Z.; Zhou, K.; Xia, Y.; Han, X.; Tang, W. Metabolomic profiles reveal key metabolic changes in heat stress-treated mouse Sertoli cells. Toxicol. Vitr. 2015, 29, 1745–1752. [Google Scholar] [CrossRef]
- Gualtieri, A.F.; Mazzone, G.L.; Rey, R.A.; Schteingart, H.F. FSH and bFGF stimulate the production of glutathione in cultured rat Sertoli cells. Int. J. Androl. 2009, 32, 218–225. [Google Scholar] [CrossRef]
- Pioli, P.A.; Hamilton, B.J.; Connolly, J.E.; Brewer, G.; Rigby, W.F. Lactate Dehydrogenase Is an AU-rich Element-binding Protein That Directly Interacts with AUF1. J. Biol. Chem. 2002, 277, 35738–35745. [Google Scholar] [CrossRef]
- Galardo, M.N.; Riera, M.F.; Pellizzari, E.H.; Cigorraga, S.B.; Meroni, S.B. The AMP-activated protein kinase activator, 5-aminoimidazole-4-carboxamide-1-b-d-ribonucleoside, regulates lactate production in rat Sertoli cells. J. Mol. Endocrinol. 2007, 39, 279–288. [Google Scholar] [CrossRef]
- Bonen, A. The expression of lactate transporters (MCT1 and MCT4) in heart and muscle. Eur. J. Appl. Physiol. 2001, 86, 6–11. [Google Scholar] [CrossRef]
- Robinson, R.F.I. Metabolsim of Glucose by Sertoli in Culture. Biol. Reprod. 1981, 24, 1032–1041. [Google Scholar] [CrossRef]
- Leiderman, B.; Mancini, R.E. Glycogen Content in the Rat Testis from Postnatal to Adult Ages. Endocrinology 1969, 85, 607–609. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, G.R.; Means, A.R. Follicle-Stimulating Hormone Activation of Glycogen Phosphorylase in the Sertoli Cell-Enriched Rat Testis. Endocrinology 1983, 113, 1476–1485. [Google Scholar] [CrossRef] [PubMed]
- Matoba, S.; Hiramatsu, R.; Kanai-Azuma, M.; Tsunekawa, N.; Harikae, K.; Kawakami, H.; Kurohmaru, M.; Kanai, Y. Establishment of testis-specific SOX9 activation requires high-glucose metabolism in mouse sex differentiation. Dev. Biol. 2008, 324, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, R.; Mancilla, H.; Villarroel-Espíndola, F.; Slebe, F.; Slebe, J.C.; Méndez, R.; Guinovart, J.J.; Concha, I.I. Glycogen Synthase in Sertoli Cells: More Than Glycogenesis? J. Cell Biochem. 2016, 117, 2597–2607. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.B.; Chan, K.C.; Hakovirta, H.; Xiao, Y.; Toppari, J.; Mitchell, A.P.; Salameh, W.A. Evidence for a Role of Glycogen Synthase Kinase-3β in Rodent Spermatogenesis. J. Androl. 2003, 24, 332–342. [Google Scholar] [CrossRef]
- Singh, T.J.; Huang, K.-P. Glycogen synthase (casein) kinase-1: Tissue distribution and subcellular localization. FEBS Lett. 1985, 190, 84–88. [Google Scholar] [CrossRef]
- Fuchs, G.; Diges, C.; Kohlstaedt, L.A.; Wehner, K.A.; Sarnow, P. Proteomic Analysis of Ribosomes: Translational Control of mRNA Populations by Glycogen Synthase GYS1. J. Mol. Biol. 2011, 410, 118–130. [Google Scholar] [CrossRef]
- Vilchez, D.; Ros, S.; Cifuentes, D.; Pujadas, L.; Vallès, J.; García-Fojeda, B.; Criado-García, O.; Sanchez, M.E.F.; Fernandez, I.M.; Domínguez, J.; et al. Mechanism suppressing glycogen synthesis in neurons and its demise in progressive myoclonus epilepsy. Nat. Neurosci. 2007, 10, 1407–1413. [Google Scholar] [CrossRef]
- Mruk, D.D.; Cheng, C.Y. Tight junctions in the testis: New perspectives. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 1621–1635. [Google Scholar] [CrossRef]
- Yazama, F.; Esaki, M.; Sawada, H. Immunocytochemistry of extracellular matrix components in the rat seminiferous tubule: Electron microscopic localization with improved methodology. Anat. Rec. 1997, 248, 51–62. [Google Scholar] [CrossRef]
- Thakur, S.C.; Thakur, S.S.; Chaube, S.K.; Singh, S.P. Subchronic Supplementation of Lithium Carbonate Induces Reproductive System Toxicity in Male Rat. Reprod. Toxicol. 2003, 17, 683–690. [Google Scholar] [CrossRef]
- MacAulay, K.; Woodgett, J.R. Targeting glycogen synthase kinase-3 (GSK-3) in the treatment of Type 2 diabetes. Expert Opin. Ther. Targets 2008, 12, 1265–1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leydig, F. Zur Anatomie der Männlichen Geschlechtsorgane und Analdrüsen der Säugethiere. Z. Wiss. Zool. 1850, 2, 1–57. [Google Scholar]
- Mendis-Handagama, S.M.L.C.; Ariyaratne, H.B.S. Differentiation of the Adult Leydig Cell Population in the Postnatal Testis. Biol. Reprod. 2001, 65, 660–671. [Google Scholar] [CrossRef]
- Shima, Y.; Miyabayashi, K.; Haraguchi, S.; Arakawa, T.; Otake, H.; Baba, T.; Matsuzaki, S.; Shishido, Y.; Akiyama, H.; Tachibana, T.; et al. Contribution of Leydig and Sertoli Cells to Testosterone Production in Mouse Fetal Testes. Mol. Endocrinol. 2013, 27, 63–73. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, F.; Ye, L.; Zirkin, B.; Chen, H. Steroidogenesis in Leydig cells: Effects of aging and environmental factors. Reproduction 2017, 154, R111–R122. [Google Scholar] [CrossRef]
- O’Donnell, L. Testosterone Promotes the Conversion of Round Spermatids between Stages VII and VIII of the Rat Spermatogenic Cycle. Endocrinology 1994, 135, 2608–2614. [Google Scholar] [CrossRef]
- O’Donnell, L.; McLachlan, R.L.; Wreford, N.G.; de Kretser, D.M.; Robertson, D.M. Testosterone Withdrawal Promotes Stage-Specific Detachment of Round Spermatids from the Rat Seminiferous Epithelium. Biol. Reprod. 1996, 55, 895–901. [Google Scholar] [CrossRef]
- Bartlett, J.M.; Kerr, J.B.; Sharpe, R.M. The Effect of Selective Destruction and Regeneration of Rat Leydig Cells on the Intratesticular Distribution of Testosterone and Morphology of the Seminiferous Epithelium. J. Androl. 1986, 7, 240–253. [Google Scholar] [CrossRef]
- Holdcraft, R.W.; Braun, R.E. Androgen receptor function is required in Sertoli cells for the terminal differentiation of haploid spermatids. Development 2004, 131, 459–467. [Google Scholar] [CrossRef]
- O’Donnell, L.; Pratis, K.; Wagenfeld, A.; Gottwald, U.; Müller, J.; Leder, G.; McLachlan, R.I.; Stanton, P. Transcriptional Profiling of the Hormone-Responsive Stages of Spermatogenesis Reveals Cell-, Stage-, and Hormone-Specific Events. Endocrinology 2009, 150, 5074–5084. [Google Scholar] [CrossRef]
- Pelletier, R.-M. The blood-testis barrier: The junctional permeability, the proteins and the lipids. Prog. Histochem. Cytochem. 2011, 46, 49–127. [Google Scholar] [CrossRef] [PubMed]
- Kopera, I.A.; Bilinska, B.; Cheng, C.Y.; Mruk, D.D. Sertoli-Germ Cell Junctions in the Testis: A Review of Recent Data. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 1593–1605. [Google Scholar] [CrossRef] [Green Version]
- Midzak, A.; Akula, N.; Lecanu, L.; Papadopoulos, V. Novel Androstenetriol Interacts with the Mitochondrial Translocator Protein and Controls Steroidogenesis. J. Biol. Chem. 2011, 286, 9875–9887. [Google Scholar] [CrossRef] [PubMed]
- Midzak, A.; Zirkin, B.; Papadopoulos, V. Translocator protein: Pharmacology and steroidogenesis. Biochem. Soc. Trans. 2015, 43, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Aghazadeh, Y.; Zirkin, B.R.; Papadopoulos, V. Pharmacological Regulation of the Cholesterol Transport Machinery in Steroidogenic Cells of the Testis. Vitam. Horm. 2015, 98, 189–227. [Google Scholar] [CrossRef]
- Antonio-Cabrera, E.; Paredes, R.G. Effects of chronic estradiol or testosterone treatment upon sexual behavior in sexually sluggish male rats. Pharmacol. Biochem. Behav. 2012, 101, 336–341. [Google Scholar] [CrossRef]
- O’Shaughnessy, P.J.; Verhoeven, G.; De Gendt, K.; Monteiro, A.; Abel, M.H. Direct Action through the Sertoli Cells Is Essential for Androgen Stimulation of Spermatogenesis. Endocrinology 2010, 151, 2343–2348. [Google Scholar] [CrossRef]
- Haider, S.G. Cell Biology of Leydig Cells in the Testis. Int. Rev. Cytol. 2004, 233, 181–241. [Google Scholar] [CrossRef]
- Svechnikov, K.; Landreh, L.; Weisser, J.; Izzo, G.; Colón, E.; Svechnikova, I.; Söder, O. Origin, Development and Regulation of Human Leydig Cells. Horm. Res. Paediatr. 2010, 73, 93–101. [Google Scholar] [CrossRef]
- King, S.R.; Stocco, D.M. ATP and A mitochondrial electrochemical gradient are required for functional activity of the steroidogenic acute regulatory (star) protein in isolated mitochondria. Endocr. Res. 1996, 22, 505–514. [Google Scholar] [CrossRef]
- Murono, E.P.; Lin, T.; Osterman, J.; Nankin, H.R. Relationship between Inhibition of Interstitial Cell Testosterone Synthesis by Cytochalasin B and Glucose. Biochem. Biophys. Res. Commun. 1982, 104, 299–306. [Google Scholar] [CrossRef]
- Chen, Y.; Nagpal, M.L.; Lin, T. Expression and regulation of glucose transporter 8 in rat Leydig cells. J. Endocrinol. 2003, 179, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, A.; Anuradha; Mukherjee, K.; Krishna, A. Testicular glucose and its transporter GLUT 8 as a marker of age-dependent variation and its role in steroidogenesis in mice. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2014, 321, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.W.; Gang, G.T.; Tadi, S.; Nedumaran, B.; Kim, Y.D.; Park, J.H.; Kweon, G.R.; Koo, S.H.; Lee, K.; Ahn, R.S.; et al. Phosphoenolpyruvate Carboxykinaseandglucose-6-Phosphatase Are Required for Steroidogenesis in Testicular Leydig Cells. J. Biol. Chem. 2012, 287, 41875–41887. [Google Scholar] [CrossRef]
- Russo, J. Glycogen content during the postnatal differentiation of the Leydig cell in the mouse testis. Z. Zellforsch. Mikrosk. Anat. 1970, 104, 14–18. [Google Scholar] [CrossRef]
- Prince, F.P. Ultrastructure of immature leydig cells in the human prepubertal testis. Anat. Rec. 1984, 209, 165–176. [Google Scholar] [CrossRef]
- Khalaf, G.; Fahmy, H.F.; Saleh, H.A. Effect of a zinc-free diet from weaning through puberty on the structure of the testis in albino rats, with a special focus on the Leydig cells. Egypt. J. Histol. 2014, 37, 304–315. [Google Scholar] [CrossRef]
- Kerr, J.; Knell, C. The fate of fetal Leydig cells during the development of the fetal and postnatal rat testis. Development 1988, 103, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Hess, R.; de Franca, L. Molecular Mechanisms in Spermatogenesis; Cheng, C.Y., Ed.; Landes Bioscience: Austin, TX, USA; Springer Science: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Cheng, C.Y.; Mruk, D.D. An intracellular trafficking pathway in the seminiferous epithelium regulating spermatogenesis: A biochemical and molecular perspective. Crit. Rev. Biochem. Mol. Biol. 2009, 44, 245–263. [Google Scholar] [CrossRef]
- Bajpai, M.; Gupta, G.; Setty, B.S. Changes in carbohydrate metabolism of testicular germ cells during meiosis in the rat. Eur. J. Endocrinol. 1998, 138, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Setchell, B.P. Hormones: What the Testis Really Sees. Reprod. Fertil. Dev. 2004, 6, 535–545. [Google Scholar] [CrossRef]
- Wenger, R.H.; Katschinski, D.M. The hypoxic testis and post-meiotic expression of PAS domain proteins. Semin. Cell Dev. Biol. 2005, 16, 547–553. [Google Scholar] [CrossRef] [Green Version]
- Williams, A.C.; Ford, W.C. The role of glucose in supporting motility and capacitation in human spermatozoa. J. Androl. 2001, 22, 680–695. [Google Scholar]
- Carrageta, D.F.; Guerra-Carvalho, B.; Sousa, M.; Barros, A.; Oliveira, P.F.; Monteiro, M.P.; Alves, M.G. Mitochondrial Activation and Reactive Oxygen-Species Overproduction during Sperm Capacitation are Independent of Glucose Stimuli. Antioxidants 2020, 9, 750. [Google Scholar] [CrossRef] [PubMed]
- Burant, C.F.; Takedatll, J.; Brot, E.; Bellstii, G.I.; Davidsons, N. Fructose Transporter in Human Spermatozoa and Small Intestine Is GLUT5. J. Biol. Chem. 1992, 267, 14523–14526. [Google Scholar] [CrossRef]
- Angulo, C.; Rauch, C.; Droppelmann, A.; Reyes, A.M.; Slebe, J.C.; Delgado-lo, F.; Guaiquil, V.H.; Vera, J.C. Hexose Transporter Expression and Function in Mammalian Spermatozoa: Cellular Localization and Transport of Hexoses and Vitamin C. J. Cell Biochem. 1998, 71, 189–203. [Google Scholar] [CrossRef]
- Gómez, O.; Romero, A.; Terrado, J.; Mesonero, J.E.; Romero-Picó, A. Differential expression of glucose transporter GLUT8 during mouse spermatogenesis. Reproduction 2006, 131, 63–70. [Google Scholar] [CrossRef]
- Roth, R.; Ibberson, M.; Riederer, B.M.; Uldry, M.; Guhl, B.; Thorens, B.; Toxicology, M.I.; Biology, C.; Morphology, B.M.R. Immunolocalization of GLUTX1 in the Testis and to Specific Brain Areas and Vasopressin—Containing Neurons. Endocrinology 2002, 143, 276–284. [Google Scholar]
- Haber, R.S.; Weinstein, S.P.; O’Boyle, E.; Morgello, S. Tissue distribution of the human GLUT3 glucose transporter. Endocrinology 1993, 132, 2538–2543. [Google Scholar] [CrossRef] [PubMed]
- Rauch, M.C.; Ocampo, M.E.; Bohle, J.; Amthauer, R.; Yáñez, A.J.; Rodríguez-Gil, J.E.; Slebe, J.C.; Re Yes, J.G.; Concha, I.I. Hexose Transporters GLUT1 and GLUT3 Are Colocalized with Hexokinase I in Caveolae Microdomains of Rat Spermatogenic Cells. J. Cell. Physiol. 2006, 207, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Rato, L.; Alves, M.G.; Socorro, S.; Duarte, A.I.; Cavaco, J.E.; Oliveira, P.F. Metabolic regulation is important for spermatogenesis. Nat. Rev. Urol. 2012, 9, 330–338. [Google Scholar] [CrossRef]
- Mita, M.; Hall, P.F. Metabolism of Round Spermatids from Rats: Lactate as the Preferred Substrate. Biol. Reprod. 1982, 26, 445–448. [Google Scholar] [CrossRef]
- Mueckler, M.; Thorens, B. The SLC2 (GLUT) family of membrane transporters. Mol. Asp. Med. 2013, 34, 121–138. [Google Scholar] [CrossRef]
- Hoshi, K.; Tsukikawa, S.; Sato, A. Importance of Ca2+, K+ and Glucose in the Medium for Sperm Penetration through the Human Zona Pellucida. Tohoku J. Exp. Med. 1991, 165, 99–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, M.; Fujiwara, A.; Yasumasu, I.; Okinaga, S.; Arai, K. Regulation of glucose metabolism by adenine nucleotides in round spermatids from rat testes. J. Biol. Chem. 1982, 257, 13945–13950. [Google Scholar] [CrossRef]
- Yáñez, A.J.; Bustamante, X.; Bertinat, R.; Werner, E.; Rauch, M.C.; Concha, I.I.; Reyes, J.G.; Slebe, J.C. Expression of key substrate cycle enzymes in rat spermatogenic cells: Fructose 1,6 bisphosphatase and 6 phosphofructose 1-kinase. J. Cell. Physiol. 2007, 212, 807–816. [Google Scholar] [CrossRef]
- Jones, A.R.; Chantrill, L.A.; Cokinakis, A. Metabolism of glycerol by mature boar spermatozoa. J. Reprod. Fertil. 1992, 94, 129–134. [Google Scholar] [CrossRef]
- Bustamante-Marín, X.; Quiroga, C.; Lavandero, S.; Reyes, J.G.; Moreno, R.D. Apoptosis, necrosis and autophagy are influenced by metabolic energy sources in cultured rat spermatocytes. Apoptosis 2012, 17, 539–550. [Google Scholar] [CrossRef]
- Herrera, E.; Salas, K.; Lagos, N.; Benos, D.J.; Reyes, J.G. Energy metabolism and its linkage to intracellular Ca2+ and pH regulation in rat spermatogenic cells. Biol. Cell 2000, 92, 429–440. [Google Scholar] [CrossRef]
- Gómez, M.; Navarro-Sabaté, A.; Manzano, A.; Duran, J.; Obach, M.; Bartrons, R. Switches in 6-phosphofructo-2-kinase isoenzyme expression during rat sperm maturation. Biochem. Biophys. Res. Commun. 2009, 387, 330–335. [Google Scholar] [CrossRef]
- Li, S.S.-L.; O’Brien, D.A.; Hou, E.W.; Versola, J.; Rockett, D.L.; Eddy, E.M. Differential Activity and Synthesis of Lactate Dehydrogenase Isozymes A (Muscle), B (Heart), and C (Testis) in Mouse Spermatogenic Cells. Biol. Reprod. 1989, 40, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, E.; Eddy, E.M.; Duan, C.; Odet, F. LDHC: The Ultimate Testis-Specific Gene. J. Androl. 2010, 31, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Alcivar, A.A.; Trasler, J.M.; Hake, L.E.; Salehi-Ashtiani, K.; Goldberg, E.; Hecht, N.B. DNA Methylation and Expression of the Genes Coding for Lactate Dehydrogenases A and C during Rodent Spermatogenesis. Biol. Reprod. 1991, 44, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Odet, F.; Duan, C.; Willis, W.D.; Goulding, E.H.; Kung, A.; Eddy, E.M.; Goldberg, E. Expression of the Gene for Mouse Lactate Dehydrogenase C (Ldhc) Is Required for Male Fertility. Biol. Reprod. 2008, 79, 26–34. [Google Scholar] [CrossRef] [Green Version]
- Welch, J.E.; Brown, P.L.; O’Brien, D.A.; Magyar, P.L.; Bunch, D.O.; Mori, C.; Eddy, E.M. Human Glyceraldehyde 3-Phosphate Dehydrogenase-2 Gene Is Expressed Specifically in Spermatogenic Cells. J. Androl. 2000, 21, 328–338. [Google Scholar] [CrossRef]
- Boer, P.H.; Adra, C.N.; Lau, Y.M.M. The Testis-Specific Phosphoglycerate Kinase Gene Pgk-2 Is a Recruited Retroposon. Mol. Cell Biol. 1987, 7, 3107–3112. [Google Scholar]
- McCarrey, J.R.; Thomas, K. Human testis-specific PGK gene lacks introns and possesses characteristics of a processed gene. Nature 1987, 326, 501–505. [Google Scholar] [CrossRef]
- Ballester, J.; Fernández-Novell, J.M.; Rutllant, J.; García-Rocha, M.; Jesus Palomo, M.; Mogas, T.; Peña, A.; Rigau, T.; Guinovart, J.J.; Rodríguez-Gil, J.E. Evidence for a Functional Glycogen Metabolism in Mature Mammalian Spermatozoa. Mol. Reprod. Dev. 2000, 56, 207–219. [Google Scholar] [CrossRef]
- Marin, S.; Chiang, K.; Bassilian, S.; Lee, W.-N.P.; Boros, L.G.; Fernández-Novell, J.M.; Centelles, J.J.; Medrano, A.; Rodriguez-Gil, J.E.; Cascante, M. Metabolic strategy of boar spermatozoa revealed by a metabolomic characterization. FEBS Lett. 2003, 554, 342–346. [Google Scholar] [CrossRef]
- Albarracín, J.; Fernández-Novell, J.; Ballester, J.; Rauch, M.; Quintero-Moreno, A.; Peña, A.; Mogas, T.; Rigau, T.; Yañez, A.; Guinovart, J.; et al. Gluconeogenesis-Linked Glycogen Metabolism Is Important in the Achievement of In Vitro Capacitation of Dog Spermatozoa in a Medium without Glucose. Biol. Reprod. 2004, 71, 1437–1445. [Google Scholar] [CrossRef] [PubMed]
- Yánez, A.J.; Nualart, F.; Droppelmann, C.; Bertinat, R.; Brito, M.; Concha, I.I.; Slebe, J.C. Broad expression of fructose-1,6-bisphosphatase and phosphoenolpyruvate carboxykinase provide evidence for gluconeogenesis in human tissues other than liver and kidney. J. Cell. Physiol. 2003, 197, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Panse, G.T.; Sheth, A.R. Glycogen Metabolism in Epididymal Spermatozoa of Developing Mice. Ind. J. Exp. Biol. 1981, 19, 183–185. [Google Scholar]
- Datta, K.M.; Dasgupta, J.; Sengupta, T.; De, S. Glycogen metabolism in human fetal testes. J. Biosci. 1988, 13, 117–121. [Google Scholar] [CrossRef]
- Reddy, K.V.; Geethanjali, N.; Reddy, Y.D.; Reddanna, P.; Govindappa, S. Effect of induced bilateral cryptorchidism on the carbohydrate metabolism of reproductive tissues in albino rats. Arch. Int. Physiol. Biochim. 1983, 91, 405–410. [Google Scholar] [CrossRef]
- Kuramori, C.; Hase, Y.; Hoshikawa, K.; Watanabe, K.; Nishi, T.; Hishiki, T.; Soga, T.; Nashimoto, A.; Kabe, Y.; Yamaguchi, Y.; et al. Mono-(2-ethylhexyl) phthalate Targets Glycogen Debranching Enzyme and Affects Glycogen Metabolism in Rat Testis. Toxicol. Sci. 2009, 109, 143–151. [Google Scholar] [CrossRef]
- Arzac, J.P. Glycogen in human testicular biopsy material: Preliminary report. J. Clin. Endocrinol. Metab. 1950, 10, 1465–1470. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, R.; Carrageta, D.F.; Alves, M.G.; Oliveira, P.F. Testicular Glycogen Metabolism: An Overlooked Source of Energy for Spermatogenesis? BioChem 2022, 2, 198-214. https://doi.org/10.3390/biochem2030014
Silva R, Carrageta DF, Alves MG, Oliveira PF. Testicular Glycogen Metabolism: An Overlooked Source of Energy for Spermatogenesis? BioChem. 2022; 2(3):198-214. https://doi.org/10.3390/biochem2030014
Chicago/Turabian StyleSilva, Ricardo, David F. Carrageta, Marco G. Alves, and Pedro F. Oliveira. 2022. "Testicular Glycogen Metabolism: An Overlooked Source of Energy for Spermatogenesis?" BioChem 2, no. 3: 198-214. https://doi.org/10.3390/biochem2030014
APA StyleSilva, R., Carrageta, D. F., Alves, M. G., & Oliveira, P. F. (2022). Testicular Glycogen Metabolism: An Overlooked Source of Energy for Spermatogenesis? BioChem, 2(3), 198-214. https://doi.org/10.3390/biochem2030014








