A Novel FACS-Based Workflow for Simultaneous Assessment of RedOx Status, Cellular Phenotype, and Mitochondrial Genome Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. MUTZ-3 Cell Culture and CD34+ Immunomagnetic Separation
2.2. Peripheral Blood Mononuclear Cell Culture
2.3. FACS Assay Reagents and Antibody Labeling
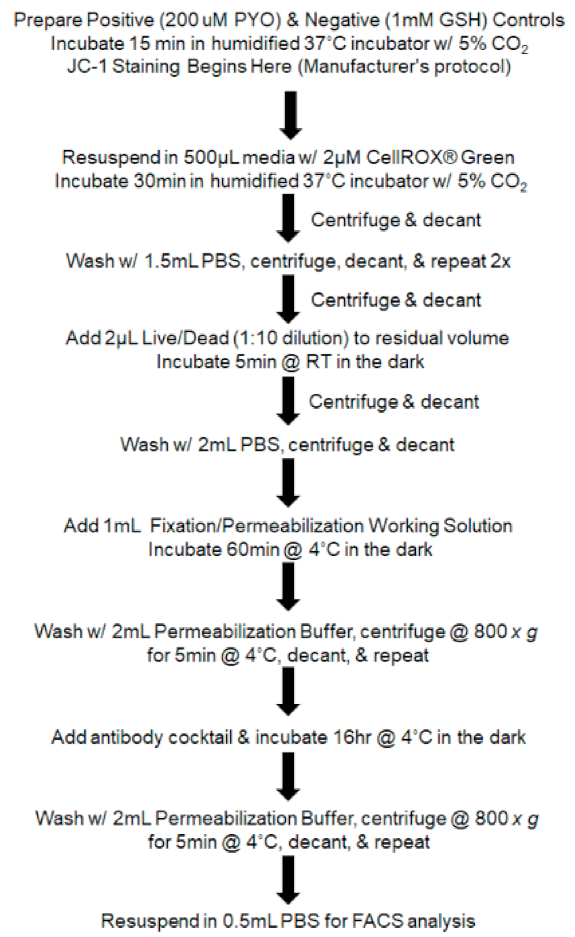
2.4. Fluorescence Activated Cell Sorting (FACS) and Analysis of Cell Phenotype, RedOx Parameters, and Mitochondrial Genome Stability
2.5. JC-1 Staining
2.6. Quantitative PCR for Mitochondrial DNA Enumeration
2.7. QuantiGene Plex Assay for mRNA Quantification
2.8. Animals, Gastric Catheter Implantation, and Alcohol Administration Protocol
2.9. Bone Marrow Mononuclear Cell Isolation and MSC Culture and Enrichment
2.10. Statistical Analysis
3. Results
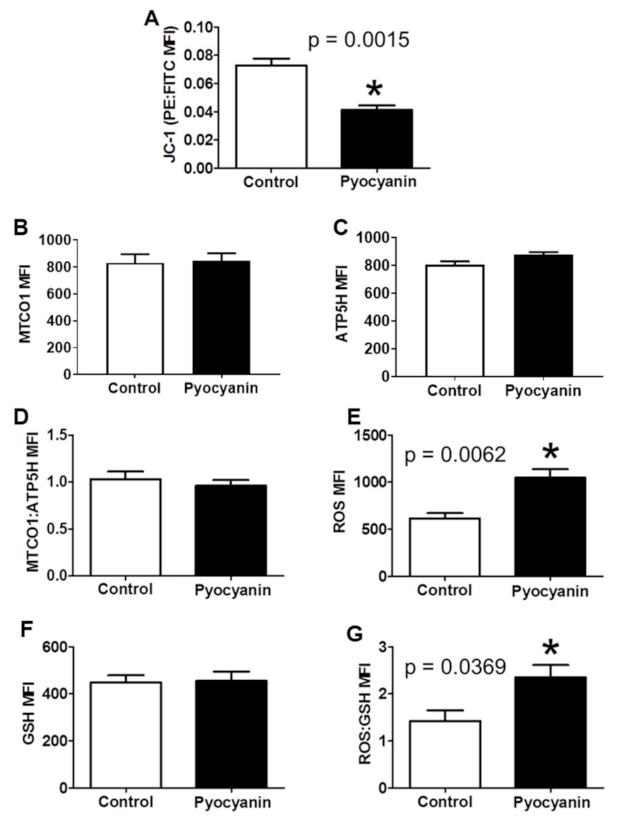
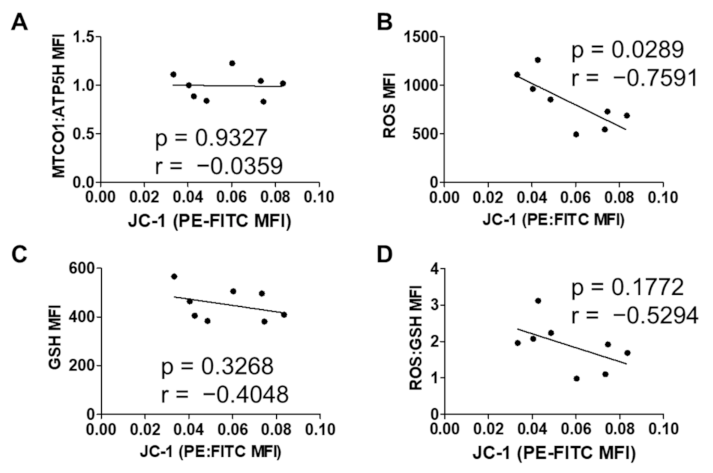
3.1. Short-Term In Vitro Oxidative Stress in MUTZ-3 Hematopoietic Progenitor Cells
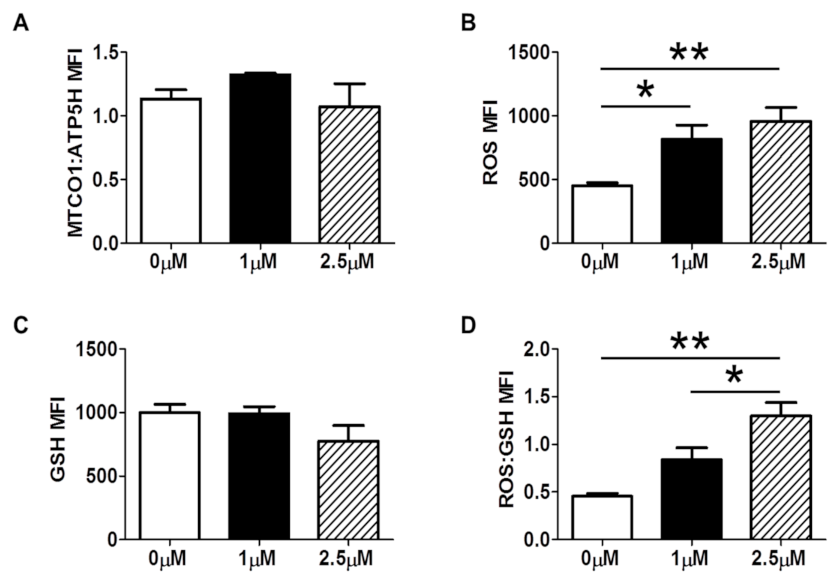
3.2. Long-Term In Vitro Oxidative Stress in MUTZ-3 Hematopoietic Progenitor Cells
3.3. T Cell Antioxidant Status, Mitochondrial Genomic Stability, mtDNA Quantification and Gene Expression Analysis
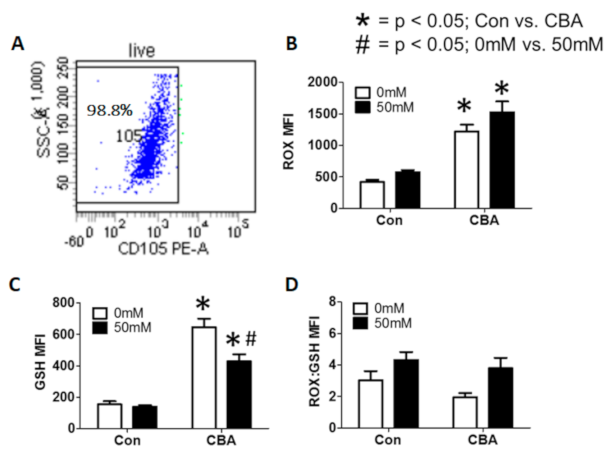
3.4. Effect of CBA and In Vitro Alcohol on MSCs and Oxidative Burden
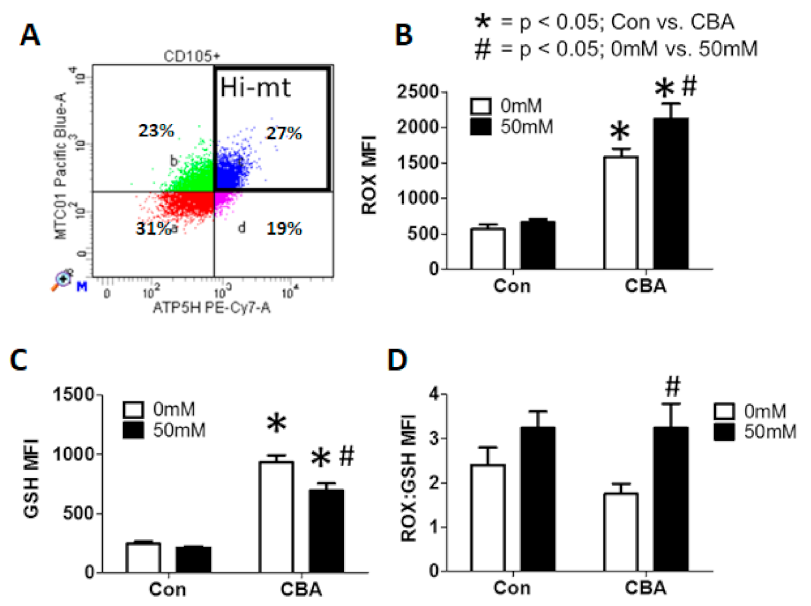
3.5. Mitochondrial DNA Quantification and Indication of Mitochondrial Genomic Stability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bigarella, C.L.; Liang, R.; Ghaffari, S. Stem cells and the impact of ROS signaling. Development 2014, 141, 4206–4218. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.; Wang, Y.; Kim, H.-S.; Lalli, M.A.; Kosik, K.S. Nrf2, a Regulator of the Proteasome, Controls Self-Renewal and Pluripotency in Human Embryonic Stem Cells. STEM Cells 2014, 32, 2616–2625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, K.; Suda, T. Metabolic requirements for the maintenance of self-renewing stem cells. Nat. Rev. Mol. Cell Biol. 2014, 15, 243–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.-H.; Sloan, D.D.; Dang, C.H.; Wagner, T.; Cabrera, A.J.E.; Tobin, N.H.; Frenkel, L.M.; Jerome, K.R. Assessment of mitochondrial toxicity by analysis of mitochondrial protein expression in mononuclear cells. Cytom. Part. B Clin. Cytom. 2009, 76, 181–190. [Google Scholar] [CrossRef]
- Kesarwani, P.; Murali, A.K.; Al-Khami, A.A.; Mehrotra, S. Redox Regulation of T-Cell Function: From Molecular Mechanisms to Significance in Human Health and Disease. Antioxid. Redox Signal. 2013, 18, 1497–1534. [Google Scholar] [CrossRef] [Green Version]
- Cottet-Rousselle, C.; Ronot, X.; Leverve, X.; Mayol, J.-F. Cytometric assessment of mitochondria using fluorescent probes. Cytom. Part. A 2011, 79, 405–425. [Google Scholar] [CrossRef]
- Eruslanov, E.; Kusmartsev, S. Identification of ROS Using Oxidized DCFDA and Flow-Cytometry. Methods Mol. Biol. 2010, 594, 57–72. [Google Scholar] [CrossRef]
- Li, R.; Jen, N.; Yu, F.; Hsiai, T.K. Assessing Mitochondrial Redox Status by Flow Cytometric Methods: Vascular Response to Fluid Shear Stress. Curr. Protoc. Cytom. 2011, 58, 9.37.1–9.37.14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dikalov, S.I.; Harrison, D.G. Methods for Detection of Mitochondrial and Cellular Reactive Oxygen Species. Antioxid. Redox Signal. 2014, 20, 372–382. [Google Scholar] [CrossRef] [Green Version]
- King, M.A.; Eddaoudi, A.; Davies, D.C. A comparison of three flow cytometry methods for evaluating mitochondrial damage during staurosporine-induced apoptosis in Jurkat cells. Cytom. Part. A 2007, 71, 668–674. [Google Scholar] [CrossRef]
- Rottenberg, H.; Wu, S. Quantitative assay by flow cytometry of the mitochondrial membrane potential in in-tact cells. Biochim. Biophys. Acta 1998, 1404, 393–404. [Google Scholar] [CrossRef] [Green Version]
- Mukhopadhyay, P.; Rajesh, M.; Hasko, G.; Hawkins, B.J.; Madesh, M.; Pacher, P. Simultaneous detection of apop-tosis and mitochondrial superoxide production in live cells by flow cytometry and confocal microscopy. Nat. Protoc. 2007, 2, 2295–2301. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.D.; Nicholls, D.G. Assessing mitochondrial dysfunction in cells. Biochem. J. 2011, 435, 297–312. [Google Scholar] [CrossRef] [Green Version]
- Krutzik, P.O.; Clutter, M.R.; Trejo, A.; Nolan, G.P. Fluorescent Cell Barcoding for Multiplex Flow Cytometry. Curr. Protoc. Cytom. 2011, 55, 6.31.1–6.31.15. [Google Scholar] [CrossRef] [PubMed]
- Schmid, I.; Lambert, C.; Ambrozak, D.; Marti, G.E.; Moss, D.M.; Perfetto, S.P. International Society for Analytical Cytology Biosafety Standard for Sorting of Unfixed Cells. Cytom. Part. A 2007, 71, 414–437. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Duan, C.; Kuang, Z.; Hao, Y.; Jeffries, J.L.; Lau, G.W. Pseudomonas aeruginosa Pyocyanin Activates NRF2-ARE-Mediated Transcriptional Response via the ROS-EGFR-PI3K-AKT/MEK-ERK MAP Kinase Signaling in Pulmonary Epithelial Cells. PLoS ONE 2013, 8, e72528. [Google Scholar] [CrossRef]
- Duplanty, A.A.; Simon, L.; Molina, P.E. Chronic Binge Alcohol-Induced Dysregulation of Mitochondrial-Related Genes in Skeletal Muscle of Simian Immunodeficiency Virus-Infected Rhesus Macaques at End-Stage Disease. Alcohol Alcohol. 2017, 52, 298–304. [Google Scholar] [CrossRef] [Green Version]
- Yun, J.; Finkel, T. Mitohormesis. Cell Metab. 2014, 19, 757–766. [Google Scholar] [CrossRef] [Green Version]
- Van Der Windt, G.J.W.; Everts, B.; Chang, C.-H.; Curtis, J.D.; Freitas, T.C.; Amiel, E.; Pearce, E.J.; Pearce, E.L. Mitochondrial Respiratory Capacity Is a Critical Regulator of CD8+ T Cell Memory Development. Immunity 2012, 36, 68–78. [Google Scholar] [CrossRef] [Green Version]
- Phillips, N.R.; Sprouse, M.L.; Roby, R.K. Simultaneous quantification of mitochondrial DNA copy number and deletion ratio: A multiplex real-time PCR assay. Sci. Rep. 2015, 4, 3887. [Google Scholar] [CrossRef] [Green Version]
- Bagby, G.J.; Zhang, P.; Purcell, J.E.; Didier, P.J.; Nelson, S. Chronic Binge Ethanol Consumption Accelerates Progression of Simian Immunodeficiency Virus Disease. Alcohol. Clin. Exp. Res. 2006, 30, 1781–1790. [Google Scholar] [CrossRef]
- Izadpanah, R.; Joswig, T.; Tsien, F.; Dufour, J.; Kirijan, J.C.; Bunnell, B.A.; Bunnell, B.A. Characterization of Multipotent Mesenchymal Stem Cells from the Bone Marrow of Rhesus Macaques. Stem Cells Dev. 2005, 14, 440–451. [Google Scholar] [CrossRef]
- Siggins, R.W.; Bagby, G.J.; Molina, P.; Dufour, J.; Nelson, S.; Zhang, P. Alcohol Exposure Impairs Myeloid Dendritic Cell Function in Rhesus Macaques. Alcohol. Clin. Exp. Res. 2009, 33, 1524–1531. [Google Scholar] [CrossRef] [Green Version]
- Desjardins, P.; Frost, E.; Morais, R. Ethidium bromide-induced loss of mitochondrial DNA from primary chicken embryo fibroblasts. Mol. Cell Biol. 1985, 5, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Bess, A.S.; Crocker, T.L.; Ryde, I.T.; Meyer, J.N. Mitochondrial dynamics and autophagy aid in removal of persistent mitochondrial DNA damage in Caenorhabditis elegans. Nucleic Acids Res. 2012, 40, 7916–7931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steele, M.L.; Fuller, S.; Patel, M.; Kersaitis, C.; Ooi, L.; Münch, G. Effect of Nrf2 activators on release of glutathione, cysteinylglycine and homocysteine by human U373 astroglial cells. Redox Biol. 2013, 1, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Hodes, R.J.; Hathcock, K.S.; Weng, N.-P. Telomeres in T and B cells. Nat. Rev. Immunol. 2002, 2, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Effros, R.B.; Allsopp, R.; Chiu, C.P.; Hausner, M.A.; Hirji, K.; Wang, L.; Harley, C.B.; Villeponteau, B.; West, M.D.; Giorgi, J.V. Shortened telomeres in the expanded CD28-CD8+ cell subset in HIV disease implicate replicative senescence in HIV pathogenesis. AIDS 1996, 10, F17–F22. [Google Scholar] [CrossRef]
- Monteiro, J.; Batliwalla, F.; Ostrer, H.; Gregersen, P.K. Shortened telomeres in clonally expanded CD28-CD8+ T cells imply a replicative history that is distinct from their CD28+CD8+ counterparts. J. Immunol. 1996, 156, 3587–3590. [Google Scholar] [PubMed]
- Goronzy, J.J.; Fang, F.; Cavanagh, M.M.; Fengqin, F.; Weyand, C.M. Naive T Cell Maintenance and Function in Human Aging. J. Immunol. 2015, 194, 4073–4080. [Google Scholar] [CrossRef]
- Ushio-Fukai, M.; Rehman, J. Redox and Metabolic Regulation of Stem/Progenitor Cells and Their Niche. Antioxid. Redox Signal. 2014, 21, 1587–1590. [Google Scholar] [CrossRef] [Green Version]
- Urao, N.; Ushio-Fukai, M. Redox regulation of stem/progenitor cells and bone marrow niche. Free Radic. Biol. Med. 2013, 54, 26–39. [Google Scholar] [CrossRef] [Green Version]
- Bejarano-García, J.A.; Millán-Uclés, Á.; Rosado, I.V.; Sánchez-Abarca, L.I.; Caballero-Velázquez, T.; Durán-Galván, M.J.; Pérez-Simón, J.A.; Piruat, J.I. Sensitivity of hematopoietic stem cells to mitochondrial dysfunction by SdhD gene deletion. Cell Death Dis. 2016, 7, e2516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribas, V.; García-Ruiz, C.; Fernández-Checa, J.C. Mitochondria, cholesterol and cancer cell metabolism. Clin. Transl. Med. 2016, 5, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Detmer, S.A.; Ewald, A.J.; Griffin, E.E.; Fraser, S.E.; Chan, D.C. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J. Cell Biol. 2003, 160, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Rouzier, C.; Bannwarth, S.; Chaussenot, A.; Chevrollier, A.; Verschueren, A.; Bonello-Palot, N.; Fragaki, K.; Cano, A.; Pouget, J.; Pellissier, J.-F.; et al. The MFN2 gene is responsible for mitochondrial DNA instability and optic atrophy ‘plus’ phenotype. Brain 2012, 135, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Bin-Umer, M.A.; McLaughlin, J.E.; Butterly, M.S.; McCormick, S.; Tumer, N.E. Elimination of damaged mitochondria through mitophagy reduces mitochondrial oxidative stress and increases tolerance to trichothecenes. Proc. Natl. Acad. Sci. USA 2014, 111, 11798–11803. [Google Scholar] [CrossRef] [Green Version]
- Larson-Casey, J.L.; Deshane, J.S.; Ryan, A.J.; Thannickal, V.J.; Carter, A.B. Macrophage Akt1 Kinase-Mediated Mitophagy Modulates Apoptosis Resistance and Pulmonary Fibrosis. Immunity 2016, 44, 582–596. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2021, 284, 13291–13295. [Google Scholar] [CrossRef]
- Révész, D.; Milaneschi, Y.; Verhoeven, J.E.; Penninx, B.W.J.H. Telomere Length as a Marker of Cellular Aging Is Associated with Prevalence and Progression of Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2014, 99, 4607–4615. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Cederbaum, A.I. Alcohol, oxidative stress, and free radical damage. Alcohol. Res. Health 2003, 27, 277–284. [Google Scholar]
- Okolicsanyi, R.K.; Camilleri, E.T.; Oikari, L.E.; Yu, C.; Cool, S.M.; Van Wijnen, A.J.; Griffiths, L.R.; Haupt, L.M. Human Mesenchymal Stem Cells Retain Multilineage Differentiation Capacity Including Neural Marker Expression after Extended In Vitro Expansion. PLoS ONE 2015, 10, e0137255. [Google Scholar] [CrossRef] [PubMed]
- Soro-Arnaiz, I.; Li, Q.O.Y.; Torres-Capelli, M.; Meléndez-Rodríguez, F.; Veiga, S.; Veys, K.; Sebastian, D.; Elorza, A.; Tello, D.; Hernansanz-Agustín, P.; et al. Role of Mitochondrial Complex IV in Age-Dependent Obesity. Cell Rep. 2019, 16, 2991–3002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.S.; Sharp, R.; Hofferek, C.; Kuai, L.; Dorn, G.W.; Wang, J.; Chen, M. NIX-Mediated Mitophagy Promotes Effector Memory Formation in Antigen-Specific CD8+ T Cells. Cell Rep. 2019, 29, 1862–1877.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Panel for MSCs | |
| ATP5H | PE_Cy7-ATP5H |
| MTCO1 | Qd655-MTCO1 |
| GSH | APC-GSH |
| CD105 | PE-CD105 |
| Panel for HSPCs | |
| ATP5H | PE_Cy7-ATP5H |
| MTCO1 | Qd655-MTCO1 |
| GSH | APC-GSH |
| CD34 | PE-CD34 |
| Panel for T Cells | |
| ATP5H | PE_Cy7-ATP5H |
| MTCO1 | Qd655-MTCO1 |
| GSH | APC-GSH |
| CD3 | PerCP-CD3 |
| CD4 | PE-CD4 |
| CD8 | AF700-CD8 |
| Primer Pair & Probe 1 | |
| mtMin Forward | CTAAATAGCCCACACGTTCCC |
| mtMin Reverse | AGAGCTCCCGTGAGTGGTTA |
| mtMin Probe | 6FAM/CATCACGATGGATCACAGGT/MGBNFQ |
| Primer Pair & Probe 2 | |
| mtMaj Forward | CTGTTCCCCAACCTTTTCCT |
| mtMaj Reverse | CCATGATTGTGAGGGGTAGG |
| mtMaj Probe | VIC/GACCCCCTAACAACCCCC/MGBNFQ |
| Housekeeping Gene | |
| RNaseP Forward | AGATTTGGACCTGCGAGCG |
| RNaseP Reverse | GAGCGGCTGTCTCCACAAGT |
| RNaseP Probe | 5Cy5/TTCTGACCTTAOGAAGGCTCTGCGCG/3IAbRQSp |
| Gene Block Standards | |
| mtMin | TCCTACTTCAGGGTCATAAAGCCTAAATAGCCCACACGTTCCCCTTAAATAA GACATCACGATGGATCACAGGTCTATCACCCTATTAACCACTCACGGGAGCT CTCCATGCATTTGGTATTTTCGTCT |
| mtMaj | AATCAACAACAACCTATTTAGCTGTTCCCCAACCTTTTCCTCCGACCCCCTAA CAACCCCCCTCCTAATACTAACTACCTGACTCCTACCCCTCACAATCATGGCA AGCCAACGCCACTTATCCAGTGA |
| RNaseP | ATGGCGGTGTTTGCAGATTTGGACCTGCGAGCGGGTTCTGACCTGAAGGCTCT GCGCGGACTTGTGGAGACAGCCGCTCACCGTGAGTTGCCCCGGCTTCGCGCC TGGCCAACCTCATGCCACCC |
| Primer Pair & Probe 1 | |
| mtDNA Forward | GTGAACTTGCCCTCGTAGTATAA |
| mtDNA Reverse | GGTGGTGGAGTTAGGTACTTT |
| Housekeeping Gene | |
| RPLP0 Forward | CAGCAAGTGGGAAGGTGTAATCC |
| RPLP0 Reverse | CCCATTCTATCATCAACGGGTACAA |
| Standards | |
| mtDNA | GTGAACTTGCCCTCGTAGTATAAATTAGTACACTGGCCTTGT AAACCAGAAATGAACATCTTCCTAGGGCAGTCAGAAAGAAA GTACCTAACTCCACCACC |
| RPLP0 | ACCCCATTCTATCATCAACGGGTACAAACGAGTCCTGGCCTT GTCTGTGGAGACGGATTACACCTTCCCACTTGCTGAA |
| Gene Symbol | Name |
|---|---|
| Mitochondrial DNA & Protein Maintenance | |
| MFN1 & MFN2 | Mitofusin 1 & 2 |
| TFAM | Transcription Factor A, mitochondrial |
| Regulators of Oxidative Stress | |
| NOS1, NOS2, & NOS3 | Nitric Oxide Synthase 1, 2, & 3 |
| NRF1 & NRF2 | Nuclear Respiratory Factor 1 & 2 |
| PPARGC1A & PPARGC1B | PPAR-γ Co-activator α & β |
| UCP1, UCP2, & UCP3 | Uncoupling Protein 1, 2, & 3 |
| Telomere Maintenance | |
| RTEL1 | Regulator of Telomere Elongation Helicase |
| TERT | Telomerase Reverse Transcriptase |
| House Keeping Genes | |
| B2M | Beta-2-microglobulin |
| HPRT1 | Hypoxanthine Phosphoribosyltransferase 1 |
| RPLP0 | Ribosomal Protein, Large, P0 |
| TBP | TATA-box Binding Protein |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McTernan, P.M.; Katz, P.S.; Porretta, C.; Welsh, D.A.; Siggins, R.W. A Novel FACS-Based Workflow for Simultaneous Assessment of RedOx Status, Cellular Phenotype, and Mitochondrial Genome Stability. BioChem 2021, 1, 1-18. https://doi.org/10.3390/biochem1010001
McTernan PM, Katz PS, Porretta C, Welsh DA, Siggins RW. A Novel FACS-Based Workflow for Simultaneous Assessment of RedOx Status, Cellular Phenotype, and Mitochondrial Genome Stability. BioChem. 2021; 1(1):1-18. https://doi.org/10.3390/biochem1010001
Chicago/Turabian StyleMcTernan, Patrick M., Paige S. Katz, Constance Porretta, David A. Welsh, and Robert W. Siggins. 2021. "A Novel FACS-Based Workflow for Simultaneous Assessment of RedOx Status, Cellular Phenotype, and Mitochondrial Genome Stability" BioChem 1, no. 1: 1-18. https://doi.org/10.3390/biochem1010001
APA StyleMcTernan, P. M., Katz, P. S., Porretta, C., Welsh, D. A., & Siggins, R. W. (2021). A Novel FACS-Based Workflow for Simultaneous Assessment of RedOx Status, Cellular Phenotype, and Mitochondrial Genome Stability. BioChem, 1(1), 1-18. https://doi.org/10.3390/biochem1010001






