Abstract
Background/Objectives: Angiotensin-converting enzyme (ACE) inhibitors are commonly prescribed anti-hypertensive agents. However, one of their effects is reducing the breakdown of a number of pro-inflammatory mediators, including bradykinin and substance P. Given the role of inflammation in periodontal disease, the aim of this study was to see whether ACE inhibitors may have an influence on the severity of periodontal disease, as assessed by clinical attachment loss. Methods: A case–control retrospective study was undertaken through analysis of patient records from a specialist periodontic practice. Data regarding the loss of clinical attachment was collected from patients who were non-smokers and grouped according to patients prescribed ACE inhibitors, those taking other antihypertensive medication, and those taking no antihypertensive medication. Results: No statistically significant difference was observed between the three treatment groups in terms of mild to moderate loss of attachment (1–3 mm; 4–5 mm). However, a significantly higher incidence of severe attachment loss (>6 mm) was observed in patients prescribed ACE inhibitors, as compared to another antihypertensive or no antihypertensive medication. Conclusions: The incidence of severe loss of clinical attachment in this study was highest in those patients being prescribed ACE inhibitors. This effect would appear to be independent of the effects of the medication on blood pressure, since this was not observed with other antihypertensive medications, and hence may potentially relate to the known pro-inflammatory action of ACE inhibitors.
1. Introduction
Periodontal disease is characterised by a bacterially induced chronic inflammatory response and an associated loss of tooth support. The presence of periodontopathic bacteria triggers an initial, innate immune response following the recognition of pathogen-derived molecules by Toll-like receptors [1,2,3]. The innate response is coordinated by an array of pro-inflammatory cytokines and anti-inflammatory cytokines, as well as other inflammatory mediators [4,5]. The function of the innate inflammatory response is to control the initiating infection. However, there must be an appropriate balance between pro-inflammatory and anti-inflammatory mediators in order to prevent excessive tissue destruction, as well as to allow for the resolution of inflammation [6,7,8]. Excessive or prolonged activation of the inflammatory response can lead to chronic inflammation and an immune-mediated destruction of the periodiodontal tissues [4,5].
For periodontal tissue destruction to occur, there are two main permissive factors. Firstly, there must be a sufficiently high concentration of the pro-inflammatory factors in the gingival tissue to stimulate osteoclastogenesis, and secondly, the inflammatory front must be in the proximity of the alveolar bone [9,10]. In terms of the pro-inflammatory mediators associated with osteoclastogenesis, there has rightly been a significant focus on interleukin-1 (IL-1), interleukin-6 (IL-6) and tumour necrosis factor-alpha (TNFα) [9,10]. One of the key actions of these pro-inflammatory mediators is to increase the expression of a protein, receptor activator of nuclear factor-kappa B ligand (RANKL), the endogenous ligand for the receptor activator of nuclear factor-kappa B (RANK) [10,11]. Increased expression of RANKL, and the associated activation of the RANK receptor on osteoclast progenerator cells, may lead to excessive formation and activation of osteoclasts, leading to pathologic bone reabsorption. In particular, a relative imbalance caused by increased expression of RANKL, and decreased expression of its decoy receptor, osteoprotegerin (OPG), appears to be central to periodontitis [12,13,14].
There are other pro-inflammatory mediators known to influence the innate immune response in periodontal tissues. These include the peptide mediators, bradykinin and substance P [15,16]. Bradykinin has a number of pro-inflammatory actions, including vasodilation and increased vascular permeability. It is also is a potent pain-producing substance [17]. Substance P is a sensory neuropeptide with a wide range of biological activities. In peripheral tissues, it is primarily associated with nociceptive sensory nerves and is involved in the neurogenic component of acute inflammation [18]. Release of substance P, and stimulation of its specific receptor, the NK1 receptor, can lead to activation of the nuclear factor-kappa-B (NF-κB) pathway and release of pro-inflammatory cytokines, including IL-1 and TNFα [19]. Substance P also has pro-inflammatory effects on the vasculature, increasing vascular permeability, leading to the formation of oedema [20]. In addition, substance P is also involved in regulating the activity and expression of vascular adhesion molecules, such as ICAM-1 and VCAM-1, that facilitate leukocyte infiltration of the inflamed tissue [21,22].
There is evidence that both substance P and bradykinin may enhance inflammatory-mediated bone reabsorption. In vitro studies have shown that bradykinin may stimulate bone reabsorption, and there may be the potential to synergistically enhance the osteoclast-stimulating activity of IL-1 and TNFα through enhanced RANKL expression [23]. The presence of substance P in gingival tissues has been correlated with the degree of periodontal inflammation [24]. Substance P has also been shown to be capable of stimulating bone reabsorption [25,26]. As mentioned previously, one action of substance P is activating the NF-κB pathway. It has also been shown to be capable of upregulating the expression of RANKL, whilst simultaneously downregulating the expression of OPG [27,28]. Hence, along with other pro-inflammatory mediators, substance P and bradykinin may play a role in inflammatory-mediated alveolar bone loss (Figure 1).
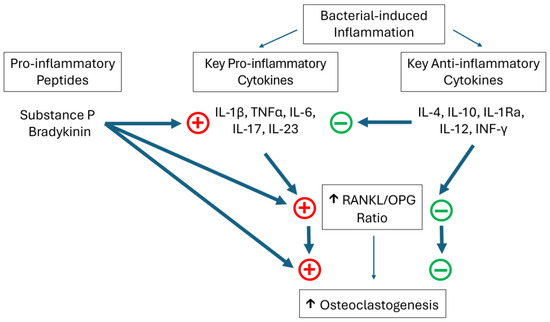
Figure 1.
A simplified representation of some of the key mediators considered to play a role in inflammatory-driven bone loss in periodontal disease. + represents an increase in activity or expression, whilst − represents decreased activity or expression.
Many dental patients may have systemic conditions, as well as associated medications, which may impact their oral health. Hypertension is a common disorder that is routinely controlled by a variety of antihypertensive agents. The Australian Bureau of Statistics estimates that approximately one third of the adult population may be prescribed antihypertensive medication (https://www.abs.gov.au/ausstats/abs@.nsf/Lookup/by%20Subject/4364.0.55.002~2014-15~Main%20Features~Hypertension~10007; published 2017, accessed on 25 August 2025). Whilst there are number of classes of antihypertensive medication, one commonly prescribed agent is an angiotensin-converting enzyme (ACE) inhibitor [29]. ACE inhibitors are very effective antihypertensive agents and are widely prescribed for patients with hypertension, as well as being used in the treatment of cardiac failure. Their primary mode of action is on the renin-angiotensin system, where they have an inhibitory effect the enzyme, ACE, thereby reducing the conversion of angiotensin I to angiotensin II [30]. This may lead to a significant fall in blood pressure in hypertensive patients. However, in addition to being involved in the conversion of angiotensin I to II, the ACE also plays a role in degrading a number of peptide mediators [31]. As a result, another action of ACE inhibitors is they decrease the ACE-dependent degradation of substance P and bradykinin, leading to increased levels of these mediators [32,33]. This action is considered to account for some of the beneficial cardiovascular effects of ACE inhibitors but also some of their potential side effects [34,35].
The aim of the present study was to evaluate whether ACE inhibitors may have a potential influence on the severity of periodontal disease-related attachment loss. It is recognised that in periodontal disease, the inflammatory-mediated bone reabsorption results from an imbalance between pro- and anti-inflammatory mediators [6]. Whilst a range of inflammatory mediators are considered to play a role in this, two peptide mediators, substance P and bradykinin, have been identified as having a potential pro-inflammatory role [28,36]. Given that ACE inhibitors are known to increase the levels of substance P and bradykinin, as a result of reduced metabolism, we hypothesised that ACE inhibitors may have the potential to exacerbate periodontal disease [37].
2. Materials and Methods
The study was undertaken as an observational, cross-sectional comparative study performed using patient records from a specialist periodontic practice. Ethical approval for the study was obtained from the James Cook University Human Ethics Committee (H4560), 2 July,2012. The study complied with the National Health and Medical Research Council (NHMRC) “National Statement on Ethical Conduct in Human Research” (2007). Patient confidentiality was maintained at all times during the study, and no information was recorded that could enable the identification of an individual patient.
The initial cohort selection was undertaken by examining the clinical history and records of patients who had attended the specialist periodontist clinic in the preceding 10 years (2003–2012), and who had received a positive diagnosis of periodontal disease. Diagnosis was completed following comprehensive periodontal examination including clinical examination, periodontal charting, radiographic examination and assessment of medical history. A positive diagnosis was based on the American Academy of Periodontology Consensus Guidelines [38]. Whilst the diagnostic labelling nomenclature has changed, the diagnostic procedures and processes have not [39]. Initial identification of the study cohort was done on the basis of identifying patients who had been diagnosed with periodontitis and were on any prescribed medication. Any patient records, which indicated that the patient was a current or previous smoker, were automatically excluded from the study. In addition, patients with a diagnosed systemic medical condition known to have a significant impact on periodontal health, such as diabetes mellitus, were also excluded from the study. Patient data was sorted into three groups according to prescribed antihypertensive medication: patients being prescribed ACE inhibitors to manage hypertension, patients being prescribed another class of anti-hypertensive medication, and patients being prescribed no antihypertensive medication. For the purposes of the study, the patients taking other antihypertensive medications were viewed as a positive control for the potential effects of medication-induced xerostomia [40].
The data recorded for analysis in this study was patient age, gender, the total number of teeth, the number of sites that exhibited bleeding upon probing, and the clinical attachment level (CAL). The severity of periodontal disease was assessed from the CAL measurements, with the loss of attachment being grouped according to CAL 1–3 mm, CAL 4–5 mm and CAL > 6 mm. All periodontal measurements were taken on six surfaces per tooth, and all measurements were performed by a single periodontist.
Prior to analysis, the Shapiro–Wilk test was performed on the recorded data to test for a normal distribution (p values of <0.05). Analysis of the data was performed using GraphPad Prism version 6.01 for Windows (GraphPad Software, La Jolla, CA, USA). A two-way analysis of variance was performed to assess whether there was any association between the extent of clinical attachment loss (CAL 1–3 mm; CAL 4–5 mm; CAL > 6 mm) and the patient’s antihypertensive medication (no antihypertensive medication; ACE inhibitor; other antihypertensive medication). The two-way ANOVA indicated that medication had a significant effect on results (p < 0.0001). A comparison between the three medication groups was performed using a one-way analysis of variance followed by post-test analysis using Newman-Keuls Multiple Comparison Test. A p value of <0.05 was taken as being statistically significant.
3. Results
Following the inclusion and exclusion criteria outlined, the data from 72 patients was included in this study. This comprised of 19 patients being prescribed ACE inhibitors to manage hypertension, 19 patients being prescribed other antihypertensive medication, and 34 patients being prescribed no antihypertensive medication. The patient profiles for each group are shown in Table 1. The average age of each patient group was 63.1 ± 2.0 years (mean ± SEM) for those prescribed ACE inhibitors, 56.2 ± 2.8 years for those prescribed other antihypertensives, and 61.9 ± 1.4 years for those not being prescribed antihypertensive medication. Patient dentition was recorded, with the average number of teeth in each patient group being 24 ± 1.4 (mean ± SEM) for those prescribed ACE inhibitors, 25.9 ± 1 for those prescribed other antihypertensives, and 23.3 ± 1.4 for those not being prescribed antihypertensive medication. Each patient group was assessed to see whether there was a correlation between age and number of teeth, but no direct correlation was observed.

Table 1.
Summary of patient profiles for the cohorts of patients being prescribed ACE inhibitors for hypertension, another anti-hypertensive medication, or no anti-hypertensive. Patient gender, age and number of teeth are presented, with the range being indicated, and means being expressed to two decimal places.
The percentage of total sites that exhibited bleeding upon probing was compared (Figure 2). Whilst the percentage of bleeding sites was lowest in the patient group not prescribed antihypertensive medication (16.1% ± 3.35; mean ± SEM), and highest in the group prescribed ACE inhibitors (23% ± 5.75), these differences were not statistically significant.
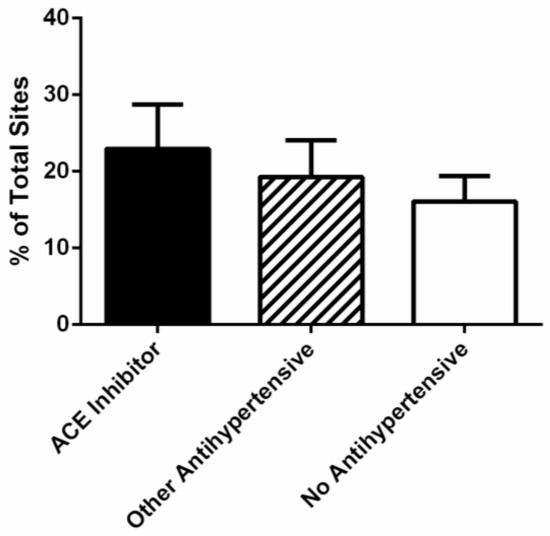
Figure 2.
Percentage of bleeding sites in periodontal patients being prescribed an ACE inhibitor (n = 19), an antihypertensive medication other than an ACE inhibitor (n = 19), or no antihypertensive medication (n = 34). No statistically significant difference was observed between the different medication groups.
The severity of periodontal disease was assessed by the clinical loss of attachment, with the percentage of sites being categorised as CAL 1–3 mm, CAL 4–5 mm and CAL > 6 mm (Table 2). Initial analysis of the data was performed using a two-way ANOVA to determine whether a patient’s medication had any significant effect upon their CAL measurements. This initial analysis indicated that patient medication had a significant effect on CAL measurements (p < 0.0001).

Table 2.
Percentage of sites showing the degree of loss of clinical attachment in relation to a patient’s antihypertensive medication (mean ± SEM; results are rounded to one decimal place). A statistically significant difference was observed between patients taking no antihypertensive medication and those being prescribed ACE inhibitors, as well as between those taking an antihypertensive other than an ACE inhibitor and those being prescribed ACE inhibitors (Newman-Keuls Multiple Comparison Test, p < 0.05).
Subsequent analysis was performed to compare the three medication groups in relation to the severity of periodontal disease. An interesting trend was observed between the degree of CAL, and the type of antihypertensive medication used. Patients taking an antihypertensive medication other than an ACE inhibitor, had the highest percentage of sites with minimal loss of attachment (CAL 1–3 mm; 71.7% ± 5.5; mean ± SEM), whilst patients prescribed ACE inhibitors had the lowest percentage of sites with minimal attachment loss (55.2% ± 7.1). In terms of the percentage of sites with CAL 4–5 mm, the picture started to change. Patients taking an antihypertensive medication other than an ACE inhibitor, had the lowest percentage of sites with moderate loss of attachment (CAL 4–5 mm; 23.4% ± 3.6; mean ± SEM), whilst patients prescribed ACE inhibitors had the highest percentage of sites with a CAL of 4–5 mm (31.0% ± 4.8). Whilst a trend is observable, it is not statistically significant. However, for more severe attachment loss (CAL > 6 mm), a significant difference was observed between medication groups (Figure 3). The percentage of sites with more severe loss of attachment was significantly higher (p < 0.05) for patients taking ACE inhibitors (13.8 ± 4.2; mean ± SEM) as compared to patients taking another antihypertensive (4.9 ± 1.1) or no antihypertensive medication (7.0 ± 1.7).
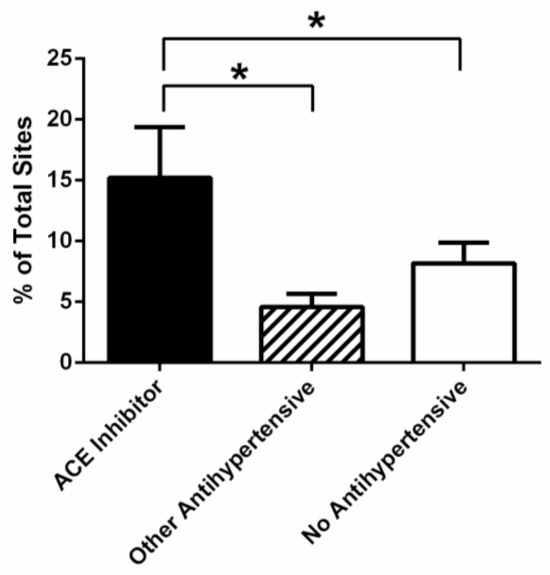
Figure 3.
Percentage of sites in periodontal patients, with a clinical loss of attachment >6 mm, being prescribed an ACE inhibitor (n = 19), an antihypertensive medication other than an ACE inhibitor (n = 19), or no antihypertensive medication (n = 34). A statistically significant difference was observed between patients prescribed an ACE inhibitor relative to other antihypertensive medications, as well as between those being prescribed an ACE inhibitor and those receiving no antihypertensive medication (p < 0.05) *.
4. Discussion
The recognition of the role that inflammation plays in periodontal disease is helping to shape clinical intervention. Importantly, it may help guide novel clinical interventions that address, not just the infectious component of periodontal disease, but also the inflammatory component [6,41]. However, another important factor is that it promotes an awareness of the factors that may influence and impact upon periodontal disease, allowing for a more timely and vigorous intervention, as now occurs for patients with diabetes mellitus [42]. The aim of this study was to see whether there was any evidence to support the hypothesis that the use of ACE inhibitors may have any impact on the severity of periodontal disease. This hypothesis is based on the recognition the ACE inhibitors may result in decreased degradation of some peptide pro-inflammatory mediators, especially bradykinin and substance P [31,33,34,35,43]. Both bradykinin and substance P are thought to contribute to periodontal inflammatory responses [15,16,24,25,26,27,28,36,44].
The present study was conducted as an observational, cross-sectional comparative study. Having obtained the data for the different treatment groups, it was observed that there was a mean age difference, albeit non-significant, between those patients being prescribed ACE inhibitors, and those prescribed other antihypertensive medication. A small, but again non-significant, difference was also observed between the mean number of teeth per patient, with the highest retention being seen in the younger group being prescribed “other antihypertensive” medications. In order to assess whether the patient age may be having a significant influence here, we looked to see whether there was a correlation between the patient age and number of existing teeth in these two groups. However, there was no significant correlation between patient age and number of teeth between the two “treated” patient groups. Hence, it appears that other patient factors may have a more significant influence.
Periodontal disease is a complex, multifactorial process, and its development and progression is influenced by a fine balance between the levels of pro- and anti-inflammatory mediators [6]. The aim of the present study was to evaluate whether the prescribing of ACE inhibitors may have an influence on the severity of periodontal disease as a result of their known inhibitory effect in relation to the breakdown of peptide pro-inflammatory mediators, particularly bradykinin and substance P [33]. The results of this study have revealed a significantly higher percentage of sites with Cal > 6 mm in those patients prescribed ACE inhibitors, as compared to those prescribed another antihypertensive, or no antihypertensive medication, suggesting that there may be a negative influence.
One of the potential side effects of antihypertensive medication is xerostomia, with its’ associated impacts on oral health [45]. Reports of xerostomia are less common in patients taking ACE inhibitors as compared to other antihypertensive medication [45], possibly as a result of the vasodilator component of their action. However, in order to try and control for any potential effects of xerostomia, patients prescribed antihypertensive medications other than ACE inhibitors served as a positive control. The incidence of sites with significant loss of attachment (CAL > 6 mm) was significantly higher in the patients prescribed ACE inhibitors versus other antihypertensive medication (13.8% versus 4.9%). This would suggest that any negative effects associated with ACE inhibitors are more likely due to their specific mode of action rather than their antihypertensive effects. Whilst there was a significant difference in the percentage of sites showing clinical loss of attachment, the same distinction was not observed in relation to the percentage of bleeding sites (23% versus 19.3%).
The normal processes that control alveolar bone reabsorption and deposition are finely balanced, a control that is achieved by maintaining a balance of the regulating mediators, including RANKL and OPG [11]. Net bone reabsorption may occur when that normal balance in disturbed. Inflammation, and associated pro-inflammatory mediators, may have this effect. ACE inhibitors, by decreasing ACE-dependant metabolism of bradykinin and substance P, may have the potential to exacerbate the process of bone loss, and that is congruent with the results from this study.
Inflammation is a natural, protective mechanism. However, if inflammation does not resolve, and acute inflammation progressives to chronic, then the inflammatory process starts to become harmful rather than protective. As mentioned previously, Toll-like receptors (TLR), such as TLR2, play a key part in the recognition of pathogenic organisms. Studies using TLR2 knockout mice have shown that animals lacking the receptor cannot mount a normal immune response to periapical infections, leading to greater tissue destruction [46]. However, one mechanism that may enable the resolution of acute inflammation, and prevent the development of chronic inflammation, is the capacity for TLR2 receptors to desensitise [47]. Using an animal model of periodontal disease, Sun and colleagues have shown that the ability to develop tolerance to bacterial toxins declined with age, providing one explanation for an increased susceptibility to periodontal disease in ageing patients [47].
In terms of the mediators influenced by ACE inhibitors, bradykinin has been shown to increase the expression of TLR2 receptors in human gingival fibroblasts [48], which could have a potentiating effect on periodontal disease. Bradykinin also has the potential to synergistically enhance the osteoclast-stimulating activity of IL-1 and TNFα through enhanced RANKL expression [23]. Substance P has also been shown to be capable of stimulating bone reabsorption [25]. One action of substance P is to activate the NF-κB pathway, promoting the expression of IL-1 and TNFα. It has also been shown to be capable of upregulating the expression of RANKL, whilst simultaneously downregulating the expression of OPG [27]. The presence of substance P in gingival tissues has also been correlated with the degree of periodontal inflammation [24]. In addition, there is evidence that substance P levels may be raised in patients with poorly controlled diabetes [49]. Ozturk and colleagues have shown that increased levels of substance P in gingival crevicular fluid in these patients correlated significantly with other clinical periodontal findings [49].
ACE inhibitors represent a key agent in cardiovascular medicine, not just in the management of hypertension, but also cardiac failure, post-myocardial infarction, and in renal disease, including diabetic neuropathy. As such, they are effective agents that are widely prescribed. Whilst their primary mode of action is to prevent the conversion of angiotensin I to angiotensin II, their ability to affect the levels of other mediators, such as bradykinin, may have both clinically beneficial effects [33], as well as contribute to their side effect profile. Overall, the clinical benefits conferred by these agents far outweigh their potential side effects. In terms of periodontal disease, these agents are not causative, but our results are suggestive that in patients with existing, impaired periodontal health, they may have the potential to exacerbate the condition. There is now evidence appearing in the literature to suggest that ACE inhibitors, as opposed to other antihypertensive agents, may have the potential to exacerbate periodontal inflammation [50,51]. As such, an awareness of this element in a patient’s medical history may be valuable in the clinical decision-making process.
There is a strong interest in the relationship between periodontal disease and systemic disease, particularly in relation to cardiovascular disease, diabetes and poor outcomes in pregnancy [52,53]. Obviously, an important aspect here is the potential for bacteraemia, as well as the common inflammatory processes [54,55]. Perhaps ironically, given the current focus on the potential negative aspects of ACE inhibitors on periodontal disease, there is preclinical (mouse model) evidence that an ACE inhibitor may protect the heart from Porphyromonas gingivalis LPS-induced cardiac dysfunction [56]. Either way, there is no question that ACE inhibitors will remain an important class of medication in cardiovascular and renal medicine [57,58].
Both cardiovascular disease and periodontal disease are complex, multifactorial conditions [59,60]. There are also a number of common risk factors that these conditions share. Whilst in the current study we have excluded patients with a history of cigarette smoking and diabetes, there are still a range of common risk factors that remain, including diet and oral hygiene practices. Poor oral health status is known increase the risk of cardiovascular disease [61]. The results of a recent systematic review and meta-analysis suggest that there may be a bidirectional relationship between tooth loss and hypertension [62]. Given the two-way nature of the relationship, the authors recommend that both hypertensive patients and patients with partial edentulism require careful monitoring and care [62]. The quality of a patient’s diet is also known to play an important role in oral and systemic health. A direct relationship between poor diet quality and poor oral health has been demonstrated, with poor diet quality being associated with an increase in tooth loss and accelerated ageing [63].
In terms of the current study, there are a number of limitations associated with the scale and retrospective nature of the study. The size of the patient cohort is relatively small, and this was partly due to the exclusion criteria (non-smoker; non-diabetic) that were applied. Whilst the study did demonstrate a significant difference between medication groups for patients with CAL > 6 mm, it is possible that a more subtle differences may be detected if the study was performed using a larger cohort. The study was undertaken using the clinical data obtained by a specialist practitioner. On one hand, this avoids inter-clinician variability, which may be useful given the scale of the study, but it does mean that the study is based on a single professional perspective. However, the retrospective nature of the study would limit any bias this could introduce.
The retrospective, observational, cross-sectional, and comparative nature of the current study enabled a comparison between patients receiving different, or no, antihypertensive medications and their degree of clinical attachment loss. However, the nature of the study means that one cannot establish any cause-and-effect relationship. Thus, whilst the data does indicate that there may be a relationship between the use of ACE inhibitors and an increased loss of clinical attachment there are a range of variables are unknown, including the dose of medication, the duration of treatment, and whether the medication is providing effective control of their hypertension. Hence, undertaking a larger, prospective study could allow for the measurement and control of these factors, as well as other patient-related factors. Given the potential for antihypertensives to cause xerostomia, both subjective assessment and objective measurement of salivary flow could provide valuable additional information. In terms of the potential for ACE inhibitors to influence periodontal inflammation, the hypothesis has been based upon a known action of this medication [31,33,35]. However, in order to fully establish a direct relationship between action of ACE inhibitors and an enhanced pro-inflammatory effect in periodontal tissues, a prospective study could directly monitor the levels of the key inflammatory mediators in gingival crevicular fluid [44]. The pro-inflammatory effects of ACE inhibitors have been demonstrated in animal models of disease that involve inflammation [43], hence, there could also be value in studying their effects in an animal model of periodontal disease.
5. Conclusions
In conclusion, our findings, together with those of other researchers, indicate that ACE inhibitors may have the potential to exacerbate clinical attachment loss in periodontal disease, presumably through their known ability to decrease the metabolism of pro-inflammatory peptides [31,51]. Given the clinical value of ACE inhibitors in cardiovascular and renal medicine, they are like to remain in common clinical use. Hence a better understanding and awareness of their potential effects on periodontal inflammation may help guide clinical intervention, possibly through increased, timely preventive care.
Author Contributions
The study described was undertaken as a Year Four research project in the BDS programme at James Cook University. Conceptualization and supervision of the study was provided by A.N. and B.J. The data collection, analysis, and original draft preparation was undertaken equally between K.C., M.B., K.D.S., K.J., A.J. and A.N. was responsible for the final review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical approval for the study was obtained from the James Cook University Human Ethics Committee (H4560). The study complied with the National Health and Medical Research Council (NHMRC) “National Statement on Ethical Conduct in Human Research” (2007). Patient confidentiality was maintained at all times during the study, and no information was recorded that could enable the identification of an individual patient.
Informed Consent Statement
The study was undertaken as a case–control retrospective analysis of patient records from a specialist periodontic practice. Patient consent was not required due to patient confidentiality being maintained at all times during the study, and no information was recorded that could enable the identification of an individual patient.
Data Availability Statement
The original patient records that were analysed for this study is unavailable due to patient confidentiality considerations. However, a copy of the relevant extracted and de-identified data may be obtained upon request to the corresponding author.
Acknowledgments
We would like to gratefully acknowledge the support provided to this project by Cairns Specialist Dental, as well as acknowledge the support received from the College of Medicine and Dentistry, James Cook University.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ACE | Angiotensin-converting enzyme |
| IL-1 | Interleukin-1 |
| IL-6 | Interleukin-6 |
| TNFα | Tumour necrosis factor-alpha |
| RANKL | Receptor activator of nuclear factor-kappa B ligand |
| RANK | Receptor activator of nuclear factor-kappa B |
| OPG | Osteoprotegerin |
| CAL | Clinical attachment level |
| SEM | Standard error of the mean |
| TLR | Toll-like receptor |
References
- Medzhitov, R.; Preston-Hurlburt, P.; Janeway, C.A., Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 1997, 388, 394–397. [Google Scholar] [CrossRef]
- Wara-Aswapati, N.; Chayasadom, A.; Surarit, R.; Pitiphat, W.; Boch, J.A.; Nagasawa, T.; Ishikawa, I.; Izumi, Y. Induction of Toll-Like Receptor Expression by Porphyromonas Gingivalis. J. Periodontol. 2012, 84, 1010–1018. [Google Scholar] [CrossRef]
- Song, B.; Zhang, Y.L.; Chen, L.J.; Zhou, T.; Huang, W.K.; Zhou, X.; Shao, L.Q. The role of Toll-like receptors in periodontitis. Oral. Dis. 2017, 23, 168–180. [Google Scholar] [CrossRef]
- Graves, D.T.; Li, J.; Cochran, D.L. Inflammation and uncoupling as mechanisms of periodontal bone loss. J. Dent. Res. 2011, 90, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Ruiz, J.S.; Guerrero-Velázquez, C.; Martínez-Esquivias, F.; Martínez-Pérez, L.A.; Guzmán-Flores, J.M. Innate and adaptive immunity of periodontal disease. From etiology to alveolar bone loss. Oral Dis. 2022, 28, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Ivashkiv, L.B.; Zhao, B.; Park-Min, K.H.; Takami, M. Feedback inhibition of osteoclastogenesis during inflammation by IL-10, M-CSF receptor shedding, and induction of IRF8. Ann. N. Y. Acad. Sci. 2011, 1237, 88–94. [Google Scholar] [CrossRef]
- Murray, P.J.; Smale, S.T. Restraint of inflammatory signaling by interdependent strata of negative regulatory pathways. Nat. Immunol. 2012, 13, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Mo, K.; Wang, Y.; Lu, C.; Li, Z. Insight into the role of macrophages in periodontitis restoration and development. Virulence 2024, 15, 2427234. [Google Scholar] [CrossRef]
- Graves, D.T.; Cochran, D. The contribution of interleukin-1 and tumor necrosis factor to periodontal tissue destruction. J. Periodontol. 2003, 74, 391–401. [Google Scholar] [CrossRef]
- Usui, M.; Onizuka, S.; Sato, T.; Kokabu, S.; Ariyoshi, W.; Nakashima, K. Mechanism of alveolar bone destruction in periodontitis—Periodontal bacteria and inflammation. Jpn. Dent. Sci. Rev. 2021, 57, 201–208. [Google Scholar] [CrossRef]
- Cochran, D.L. Inflammation and bone loss in periodontal disease. J. Periodontol. 2008, 79, 1569–1576. [Google Scholar] [CrossRef]
- Bostanci, N.; Ilgenli, T.; Emingil, G.; Afacan, B.; Han, B.; Toz, H.; Berdeli, A.; Atilla, G.; McKay, I.J.; Hughes, F.J.; et al. Differential expression of receptor activator of nuclear factor-kappaB ligand and osteoprotegerin mRNA in periodontal diseases. J. Periodontal Res. 2007, 42, 287–293. [Google Scholar] [CrossRef]
- Bostanci, N.; Ilgenli, T.; Emingil, G.; Afacan, B.; Han, B.; Toz, H.; Atilla, G.; Hughes, F.J.; Belibasakis, G.N. Gingival crevicular fluid levels of RANKL and OPG in periodontal diseases: Implications of their relative ratio. J. Clin. Periodontol. 2007, 34, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Cirelli, J.A.; Park, C.H.; Sugai, J.V.; Taba, M., Jr.; Kostenuik, P.J.; Giannobile, W.V. RANKL inhibition through osteoprotegerin blocks bone loss in experimental periodontitis. J. Periodontol. 2007, 78, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Lundy, F.T.; Linden, G.J. Neuropeptides and Neurogenic Mechanisms in Oral and Periodontal Inflammation. Crit. Rev. Oral Biol. Med. Off. Publ. Am. Assoc. Oral Biol. 2004, 15, 82–98. [Google Scholar] [CrossRef] [PubMed]
- Jayanthi, A.; Tiwari, D.; Puzhankara, L. Substance P-A neuropeptide regulator of periodontal disease pathogenesis and potential novel therapeutic entity: A narrative review. J. Indian Soc. Periodontol. 2024, 28, 284–289. [Google Scholar] [CrossRef]
- Dray, A.; Perkins, M. Bradykinin and inflammatory pain. Trends Neurosci. 1993, 16, 99–104. [Google Scholar] [CrossRef]
- Bhatia, M.; Saluja, A.K.; Hofbauer, B.; Frossard, J.L.; Lee, H.S.; Castagliuolo, I.; Wang, C.C.; Gerard, N.; Pothoulakis, C.; Steer, M.L. Role of substance P and the neurokinin 1 receptor in acute pancreatitis and pancreatitis-associated lung injury. Proc. Natl. Acad. Sci. USA 1998, 95, 4760–4765. [Google Scholar] [CrossRef]
- Douglas, S.D.; Leeman, S.E. Neurokinin-1 receptor: Functional significance in the immune system in reference to selected infections and inflammation. Ann. N. Y. Acad. Sci. 2011, 1217, 83–95. [Google Scholar] [CrossRef]
- Donkin, J.J.; Nimmo, A.J.; Cernak, I.; Blumbergs, P.C.; Vink, R. Substance P is associated with the development of brain edema and functional deficits after traumatic brain injury. J. Cereb. Blood Flow Metab. 2009, 29, 1388–1398. [Google Scholar] [CrossRef]
- Lau, H.Y.; Bhatia, M. Effect of CP-96,345 on the expression of adhesion molecules in acute pancreatitis in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G1283–G1292. [Google Scholar] [CrossRef]
- Nessler, S.; Stadelmann, C.; Bittner, A.; Schlegel, K.; Gronen, F.; Brueck, W.; Hemmer, B.; Sommer, N. Suppression of autoimmune encephalomyelitis by a neurokinin-1 receptor antagonist—A putative role for substance P in CNS inflammation. J. Neuroimmunol. 2006, 179, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Brechter, A.B.; Persson, E.; Lundgren, I.; Lerner, U.H. Kinin B1 and B2 receptor expression in osteoblasts and fibroblasts is enhanced by interleukin-1 and tumour necrosis factor-alpha. Effects dependent on activation of NF-kappaB and MAP kinases. Bone 2008, 43, 72–83. [Google Scholar] [CrossRef]
- Lundy, F.T.; Mullally, B.H.; Burden, D.J.; Lamey, P.J.; Shaw, C.; Linden, G.J. Changes in substance P and neurokinin A in gingival crevicular fluid in response to periodontal treatment. J. Clin. Periodontol. 2000, 27, 526–530. [Google Scholar] [CrossRef]
- Mori, T.; Ogata, T.; Okumura, H.; Shibata, T.; Nakamura, Y.; Kataoka, K. Substance P regulates the function of rabbit cultured osteoclast; increase of intracellular free calcium concentration and enhancement of bone resorption. Biochem. Biophys. Res. Commun. 1999, 262, 418–422. [Google Scholar] [CrossRef]
- Niedermair, T.; Schirner, S.; Seebröker, R.; Straub, R.H.; Grässel, S. Substance P modulates bone remodeling properties of murine osteoblasts and osteoclasts. Sci. Rep. 2018, 8, 9199. [Google Scholar] [CrossRef]
- Lee, H.J.; Jeong, G.S.; Pi, S.H.; Lee, S.I.; Bae, W.J.; Kim, S.J.; Lee, S.K.; Kim, E.C. Heme oxygenase-1 protects human periodontal ligament cells against substance P-induced RANKL expression. J. Periodontal Res. 2010, 45, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Lin, Q.; Tang, K.; Liu, S.; Du, Y.; Yu, X.; Li, S. Substance P participates in periodontitis by upregulating HIF-1α and RANKL/OPG ratio. BMC Oral Health 2020, 20, 27. [Google Scholar] [CrossRef] [PubMed]
- Gabb, G.M.; Mangoni, A.A.; Anderson, C.S.; Cowley, D.; Dowden, J.S.; Golledge, J.; Hankey, G.J.; Howes, F.S.; Leckie, L.; Perkovic, V.; et al. Guideline for the diagnosis and management of hypertension in adults—2016. Med. J. Aust. 2016, 205, 85–89. [Google Scholar] [CrossRef]
- Lang, C.C.; Struthers, A.D. Targeting the renin-angiotensin-aldosterone system in heart failure. Nat. Rev. Cardiol. 2013, 10, 125–134. [Google Scholar] [CrossRef]
- Shen, X.Z.; Bernstein, K.E. The peptide network regulated by angiotensin converting enzyme (ACE) in hematopoiesis. Cell Cycle 2011, 10, 1363–1369. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Williams, T.A.; Hooper, N.M.; Turner, A.J. Characterization of neuronal and endothelial forms of angiotensin converting enzyme in pig brain. J. Neurochem. 1991, 57, 193–199. [Google Scholar] [CrossRef]
- Sulpizio, A.C.; Pullen, M.A.; Edwards, R.M.; Brooks, D.P. The effect of acute angiotensin-converting enzyme and neutral endopeptidase 24.11 inhibition on plasma extravasation in the rat. J. Pharmacol. Exp. Ther. 2004, 309, 1141–1147. [Google Scholar] [CrossRef]
- Borsook, D.; Sava, S. Pain: Do ACE inhibitors exacerbate complex regional pain syndrome? Nat. Rev. Neurol. 2009, 5, 306–308. [Google Scholar] [CrossRef]
- Volans, A.; Ferguson, R. Using a bradykinin blocker in ACE inhibitor-associated angioedema in the emergency department. BMJ Case Rep. 2013, 2013, bcr2012008295. [Google Scholar] [CrossRef]
- Lu, X.; Liu, J.; Wei, T.; Zhou, X. Elevated salivary activity of mast cell chymase of periodontitis patients, and a new bradykinin generation cascade, mediating the cross-talks between mast cell and gingival fibroblast. Int. Immunopharmacol. 2021, 101, 108269. [Google Scholar] [CrossRef]
- Byrd, J.B.; Touzin, K.; Sile, S.; Gainer, J.V.; Yu, C.; Nadeau, J.; Adam, A.; Brown, N.J. Dipeptidyl peptidase IV in angiotensin-converting enzyme inhibitor associated angioedema. Hypertension 2008, 51, 141–147. [Google Scholar] [CrossRef]
- Lindhe, J.; Ranney, R.; Lamster, I.; Charles, A.; Chung, C.P.; Flemmig, T.; Kinane, D.; Listgarten, M.; Löe, H.; Schoor, R.; et al. Consensus Report: Chronic Periodontitis. Ann. Periodontol. 1999, 4, 38. [Google Scholar] [CrossRef]
- American Academy of Periodontology Task Force Report on the Update to the 1999 Classification of Periodontal Diseases and Conditions. J. Periodontol. 2015, 86, 835–838. [CrossRef] [PubMed]
- Ramírez Martínez-Acitores, L.; Hernández Ruiz de Azcárate, F.; Casañas, E.; Serrano, J.; Hernández, G.; López-Pintor, R.M. Xerostomia and Aalivary Flow in Patients Taking Antihypertensive Drugs. Int. J. Environ. Res. Public Health 2020, 17, 2478. [Google Scholar] [CrossRef] [PubMed]
- Taubman, M.A.; Kawai, T.; Han, X. The new concept of periodontal disease pathogenesis requires new and novel therapeutic strategies. J. Clin. Periodontol. 2007, 34, 367–369. [Google Scholar] [CrossRef] [PubMed]
- Azarpazhooh, A.; Tenenbaum, H.C. Separating fact from fiction: Use of high-level evidence from research syntheses to identify diseases and disorders associated with periodontal disease. J. Can. Dent. Assoc. 2012, 78, c25. [Google Scholar] [PubMed]
- Harford-Wright, E.; Thornton, E.; Vink, R. Angiotensin-converting enzyme (ACE) inhibitors exacerbate histological damage and motor deficits after experimental traumatic brain injury. Neurosci. Lett. 2010, 481, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, A.R.; Raj, S.; Aruna, G.; Chowdhry, S. Gingival crevicular fluid and plasma levels of neuropeptide Substance-P in periodontal health, disease and after nonsurgical therapy. J. Periodontal Res. 2009, 44, 232–237. [Google Scholar] [CrossRef]
- Habbab, K.M.; Moles, D.R.; Porter, S.R. Potential oral manifestations of cardiovascular drugs. Oral Dis. 2010, 16, 769–773. [Google Scholar] [CrossRef]
- da Silva, R.A.; Ferreira, P.D.; De Rossi, A.; Nelson-Filho, P.; Silva, L.A. Toll-like receptor 2 knockout mice showed increased periapical lesion size and osteoclast number. J. Endod. 2012, 38, 803–813. [Google Scholar] [CrossRef]
- Sun, Y.; Li, H.; Yang, M.F.; Shu, W.; Sun, M.J.; Xu, Y. Effects of aging on endotoxin tolerance induced by lipopolysaccharides derived from Porphyromonas gingivalis and Escherichia coli. PLoS ONE 2012, 7, e39224. [Google Scholar] [CrossRef]
- Gutierrez-Venegas, G.; Arreguin-Cano, J.A. Bradykinin promotes TLR2 expression in human gingival fibroblasts. Int. Immunopharmacol. 2011, 11, 2079–2085. [Google Scholar] [CrossRef]
- Ozturk, A.; Bilgici, B.; Odyakmaz, S.; Konas, E. The relationship of periodontal disease severity to serum and GCF substance P levels in diabetics. Quintessence Int. 2012, 43, 587–596. [Google Scholar]
- Zhou, Y.; Sun, L.; Hu, J.; Liu, X.; Ma, Y. Association of antihypertensive drugs with periodontitis: A comprehensive drug-target Mendelian randomization study. Quintessence Int. 2024, 55, 814–823. [Google Scholar] [CrossRef]
- Rodrigues, M.; Barbirato, D.; Luiz, R.R.; Scharfstein, J.; Salles, G.F.; Feres-Filho, E.J. Effect of antihypertensive therapy with angiotensin-converting enzyme inhibitors on chronic periodontitis: A case-control study. Oral Dis. 2016, 22, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.; Janu, U.; Chiou, L.L.; Gandhi, K.K.; Palomo, L.; John, V. Periodontal Health and Systemic Conditions. Dent. J. 2020, 8, 130. [Google Scholar] [CrossRef]
- Craig, R.G.; Pernat, A.M.; Pecoits-Filho, R.; Levin, N.W.; Kotanko, P. Periodontal diseases and systemic inflammation. Semin. Dial. 2013, 26, 23–28. [Google Scholar] [CrossRef]
- Parahitiyawa, N.B.; Jin, L.J.; Leung, W.K.; Yam, W.C.; Samaranayake, L.P. Microbiology of odontogenic bacteremia: Beyond endocarditis. Clin. Microbiol. Rev. 2009, 22, 46–64. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Kong, C.; Yuan, L.; Liu, L.; Zhao, K.; Lü, J.; Wang, X. The bidirectional relationship between periodontitis and diabetes: New prospects for stem cell-derived exosomes. Biomed. Pharmacother. 2023, 165, 115219. [Google Scholar] [CrossRef] [PubMed]
- Kiyomoto, K.; Matsuo, I.; Suita, K.; Ohnuki, Y.; Ishikawa, M.; Ito, A.; Mototani, Y.; Tsunoda, M.; Morii, A.; Nariyama, M.; et al. Oral angiotensin-converting enzyme inhibitor captopril protects the heart from Porphyromonas gingivalis LPS-induced cardiac dysfunction in mice. PLoS ONE 2023, 18, e0292624. [Google Scholar] [CrossRef]
- Strauss, M.H.; Hall, A.S.; Narkiewicz, K. The Combination of Beta-Blockers and ACE Inhibitors Across the Spectrum of Cardiovascular Diseases. Cardiovasc. Drugs Ther. 2023, 37, 757–770. [Google Scholar] [CrossRef]
- Khalil, M.E.; Basher, A.W.; Brown, E.J., Jr.; Alhaddad, I.A. A remarkable medical story: Benefits of angiotensin-converting enzyme inhibitors in cardiac patients. J. Am. Coll. Cardiol. 2001, 37, 1757–1764. [Google Scholar] [CrossRef]
- Kwon, T.; Lamster, I.B.; Levin, L. Current Concepts in the Management of Periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef]
- Poulter, N. Coronary heart disease is a multifactorial disease. Am. J. Hypertens. 1999, 12, 92S–95S. [Google Scholar] [CrossRef]
- Etta, I.; Kambham, S.; Girigosavi, K.B.; Panjiyar, B.K. Mouth-Heart Connection: A Systematic Review on the Impact of Periodontal Disease on Cardiovascular Health. Cureus 2023, 15, e46585. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Yu, W.; Li, Y.; Li, Y.; Wan, Q.; Chen, L.; Dong, Y.; Tay, F.R.; Niu, L. Association between tooth loss and hypertension: A systematic review and meta-analysis. J. Dent. 2022, 123, 104178. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.H.; Li, L.; Jia, S.L.; Li, Q.; Hao, J.X.; Ma, S.; He, Z.K.; Wan, Q.Q.; Cai, Y.F.; Li, Z.T.; et al. Association of Tooth Loss and Diet Quality with Acceleration of Aging: Evidence from NHANES. Am. J. Med. 2023, 136, 773–779. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).