Antimicrobial Activity of Oregano, Thyme, and Lavender Oils Against Oral Pathogens: Perspectives for AI-Supported Research
Abstract
1. Introduction
- Thymus vulgaris (thyme): Known for its high thymol and p-cymene content, thyme oil exerts strong antimicrobial and anti-inflammatory actions, particularly against oral streptococci and Candida species [17].
- Lavandula angustifolia (lavender): Traditionally used for its calming and anti-inflammatory properties, lavender EO contains linalool and linalyl acetate, which possess mild antibacterial and antifungal effects. While not as potent as oregano or thyme, its use in oral formulations is often linked to its soothing effects on mucosa [1].
2. Materials and Methods
2.1. Study Design and Bacterial Strains
2.2. EOs Used
2.3. GC-MS
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Study Limitations
4.2. Clinical Implications
4.3. Future Directions: AI in EO-Based Oral Medicine
4.4. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosner, O.; Livne, S.; Bsharat, M.; Dviker, S.; Jeffet, U.; Matalon, S.; Sterer, N. Lavandula angustifolia Essential Oil Inhibits the Ability of Fusobacterium nucleatum to Produce Volatile Sulfide Compounds, a Key Components in Oral Malodor. Molecules 2024, 29, 2982. [Google Scholar] [CrossRef]
- Rajasekaran, J.J.; Krishnamurthy, H.K.; Bosco, J.; Jayaraman, V.; Krishna, K.; Wang, T.; Bei, K. Oral Microbiome: A Review of Its Impact on Oral and Systemic Health. Microorganisms 2024, 12, 1797. [Google Scholar] [CrossRef]
- Quintana Soares Lopes, L.; Fortes Guerim, P.H.; Maldonado, M.E.; Wagner, R.; Hadlich Xavier, A.C.; Gutknecht da Silva, J.L.; Bittencourt da Rosa Leal, D.; de Freitas Daudt, N.; Christ Vianna Santos, R.; Kolling Marquezan, P. Chemical Composition, Cytotoxicity, Antimicrobial, Antibiofilm, and Anti-Quorum Sensing Potential of Mentha piperita Essential Oil against the Oral Pathogen Streptococcus mutans. J. Toxicol. Environ. Health A 2024, 87, 824–835. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Setzer, W.N. Chemical Composition and Biological Activities of Essential Oils of Curcuma Species. Nutrients 2018, 10, 1196. [Google Scholar] [CrossRef] [PubMed]
- Al-Nasser, L.; Lamster, I.B. Prevention and Management of Periodontal Diseases and Dental Caries in the Older Adults. Periodontol. 2000 2020, 84, 69–83. [Google Scholar] [CrossRef]
- Chapple, I.L.C.; Bouchard, P.; Cagetti, M.G.; Campus, G.; Carra, M.C.; Cocco, F.; Nibali, L.; Hujoel, P.; Laine, M.L.; Lingstrom, P.; et al. Interaction of Lifestyle, Behaviour or Systemic Diseases with Dental Caries and Periodontal Diseases: Consensus Report of Group 2 of the Joint EFP/ORCA Workshop on the Boundaries between Caries and Periodontal Diseases. J. Clin. Periodontol. 2017, 44, S39–S51. [Google Scholar] [CrossRef]
- Thosar, N.; Basak, S.; Bahadure, R.N.; Rajurkar, M. Antimicrobial Efficacy of Five Essential Oils against Oral Pathogens: An In Vitro Study. Eur. J. Dent. 2013, 7, S071–S077. [Google Scholar] [CrossRef]
- Lynch, M.C.; Cortelli, S.C.; McGuire, J.A.; Zhang, J.; Ricci-Nittel, D.; Mordas, C.J.; Aquino, D.R.; Cortelli, J.R. The Effects of Essential Oil Mouthrinses with or without Alcohol on Plaque and Gingivitis: A Randomized Controlled Clinical Study. BMC Oral Health 2018, 18, 6. [Google Scholar] [CrossRef]
- Yuan, Y.; Hui, X.; Liu, Z.; Sun, J.; Raka, R.N.; Xiao, J.; Zhang, Z.; Wu, H. Investigation of Differential Multi-Mode Antibacterial Mechanisms of Essential Oils of Satureja montana L. and Leptospermum scoparium J.R.Forst. & G.Forst. Against Porphyromonas gingivalis. BMC Complement. Med. Ther. 2025, 25, 283. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Setzer, W.N. Maternal Reproductive Toxicity of Some Essential Oils and Their Constituents. Int. J. Mol. Sci. 2021, 22, 2380. [Google Scholar] [CrossRef] [PubMed]
- Alrashdan, M.S.; Al-Shorman, H.; Al-Dwairi, A.; Qutieshat, A.; Al-Omiri, M.K. Salivary Oxidative Stress Biomarkers in Periodontitis-Free Smokers: A Cross Sectional Study. Minerva Dent. Oral Sci. 2024, 73, 209–216. [Google Scholar] [CrossRef]
- Aziz, M.I.; Hasan, M.M.; Ullah, R.; Bari, A.; Khan, M.A.; Hasnain, S.Z.U.; Baloch, R.; Akram, M.; Obaid, A.; Ullah, A.; et al. Potential Role of Citrus bergamia Flower Essential Oil against Oral Pathogens. BMC Complement. Med. Ther. 2024, 24, 157. [Google Scholar] [CrossRef]
- Leino, V.; Airaksinen, R.; Viluksela, M.; Vähäkangas, K. Toxicity of Colloidal Silver Products and Their Marketing Claims in Finland. Toxicol. Rep. 2021, 8, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.S.; Paula, M.S.A.; Cardoso, M.M.; Silva, N.P.; Tavares, L.C.D.; Gomes, T.V.; Porto, D.L.; Aragão, C.F.S.; Fabri, R.L.; Tavares, G.D.; et al. Exploring the Antimicrobial Efficacy of Tea Tree Essential Oil and Chitosan against Oral Pathogens to Overcome Antimicrobial Resistance. Microb. Pathog. 2024, 196, 107006. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Sun, J.; Song, Y.; Raka, R.N.; Xiang, J.; Wu, H.; Xiao, J.; Jin, J.; Hui, X. Antibacterial Activity of Oregano Essential Oils against Streptococcus mutans In Vitro and Analysis of Active Components. BMC Complement. Med. Ther. 2023, 23, 61. [Google Scholar] [CrossRef] [PubMed]
- Muresan, S.M.C.; Dreanca, A.; Repciuc, C.; Dejescu, C.; Rotar, O.; Pop, R.A.; Pantea, S.; Pall, E.; Ciotlaus, I.; Sarosi, C.; et al. Dental Hydrogels with Essential Oils with Potential Activity in Periodontitis. Appl. Sci. 2023, 13, 1787. [Google Scholar] [CrossRef]
- Vassiliou, E.; Awoleye, O.; Davis, A.; Mishra, S. Anti-Inflammatory and Antimicrobial Properties of Thyme Oil and Its Main Constituents. Int. J. Mol. Sci. 2023, 24, 6936. [Google Scholar] [CrossRef]
- Cuevas-Gonzalez, M.V.; Cuevas-Gonzalez, J.C.; Espinosa-Cristóbal, L.F.; Donohue-Cornejo, A.; Reyes López, S.Y.; Saucedo Acuña, R.A.; García Calderón, A.G.; Guzmán Gastelum, D.A. Use or Abuse of Antibiotics as Prophylactic Therapy in Oral Surgery: A Systematic Review. Medicine 2023, 102, e35011. [Google Scholar] [CrossRef]
- Park, S.-Y.; Raka, R.N.; Hui, X.-L.; Song, Y.; Sun, J.-L.; Xiang, J.; Wang, J.; Jin, J.-M.; Li, X.-K.; Xiao, J.-S.; et al. Six Spain Thymus Essential Oils Composition Analysis and Their In Vitro and in Silico Study against Streptococcus mutans. BMC Complement. Med. Ther. 2023, 23, 106. [Google Scholar] [CrossRef]
- Moynihan, P.; Petersen, P.E. Diet, Nutrition and the Prevention of Dental Diseases. Public Health Nutr. 2004, 7, 201–226. [Google Scholar] [CrossRef]
- Ikeda, N.Y.; Ambrosio, C.M.S.; Miano, A.C.; Rosalen, P.L.; Gloria, E.M.; Alencar, S.M. Essential Oils Extracted from Organic Propolis Residues: An Exploratory Analysis of Their Antibacterial and Antioxidant Properties and Volatile Profile. Molecules 2021, 26, 4694. [Google Scholar] [CrossRef]
- Benzaid, C.; Belmadani, A.; Tichati, L.; Djeribi, R.; Rouabhia, M. Effect of Citrus aurantium L. Essential Oil on Streptococcus mutans Growth, Biofilm Formation and Virulent Genes Expression. Antibiotics 2021, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Castagliuolo, G.; Di Napoli, M.; Vaglica, A.; Badalamenti, N.; Antonini, D.; Varcamonti, M.; Bruno, M.; Zanfardino, A.; Bazan, G. Thymus richardii Subsp. nitidus (Guss.) Jalas Essential Oil: An Ally against Oral Pathogens and Mouth Health. Molecules 2023, 28, 4803. [Google Scholar] [CrossRef]
- Hammer, K.A.; Carson, C.F.; Riley, T. V Antimicrobial Activity of Essential Oils and Other Plant Extracts. J. Appl. Microbiol. 1999, 86, 985–990. [Google Scholar] [CrossRef]
- Salam, M.A.; Al-Amin, M.Y.; Pawar, J.S.; Akhter, N.; Lucy, I.B. Conventional Methods and Future Trends in Antimicrobial Susceptibility Testing. Saudi J. Biol. Sci. 2023, 30, 103582. [Google Scholar] [CrossRef]
- Maynard, R.; Manuel, C.; Simpkins, S.; Haro, M.; Ledeboer, N.; Simner, P.J.; Fisher, M.; Humphries, R. Development of Disk Diffusion Susceptibility Test Methods for Aerococcus spp. and Updates to Clinical and Laboratory Standards Institute MIC Breakpoints. J. Clin. Microbiol. 2025, 63, e0011525. [Google Scholar] [CrossRef]
- Mendoza, R.; Alvitez-Temoche, D.; Chiong, L.; Silva, H.; Mauricio, F.; Mayta-Tovalino, F. Antibacterial Efficacy of Matricaria recutita Essential Oil against Porphyromonas gingivalis and Prevotella intermedia: In Vitro Study. J. Contemp. Dent. Pract. 2023, 24, 551–555. [Google Scholar] [CrossRef]
- Nagy-Bota, M.C.; Man, A.; Santacroce, L.; Brinzaniuc, K.; Pap, Z.; Pacurar, M.; Pribac, M.; Ciurea, C.N.; Pintea-Simon, I.A.; Kovacs, M. Essential Oils as Alternatives for Root-Canal Treatment and Infection Control against Enterococcus faecalis—A Preliminary Study. Appl. Sci. 2021, 11, 1422. [Google Scholar] [CrossRef]
- Hans, V.M.; Grover, H.S.; Deswal, H.; Agarwal, P. Antimicrobial Efficacy of Various Essential Oils at Varying Concentrations against Periopathogen Porphyromonas Gingivalis. J. Clin. Diagn. Res. 2016, 10, ZC16–ZC19. [Google Scholar] [CrossRef] [PubMed]
- Trindade, L.A.; de Araújo Oliveira, J.; de Castro, R.D.; de Oliveira Lima, E. Inhibition of Adherence of C. Albicans to Dental Implants and Cover Screws by Cymbopogon nardus Essential Oil and Citronellal. Clin. Oral Investig. 2015, 19, 2223–2231. [Google Scholar] [CrossRef]
- Bogdan, M.A.; Bungau, S.; Tit, D.M.; Zaha, D.C.; Nechifor, A.C.; Behl, T.; Chambre, D.; Lupitu, A.I.; Copolovici, L.; Copolovici, D.M. Chemical Profile, Antioxidant Capacity, and Antimicrobial Activity of Essential Oils Extracted from Three Different Varieties (Moldoveanca 4, Vis Magic 10, and Alba 7) of Lavandula angustifolia. Molecules 2021, 26, 4381. [Google Scholar] [CrossRef]
- Seabold, S.; Perktold, J. Statsmodels: Econometric and Statistical Modeling with Python. In Proceedings of the the 9th Python in Science Conference, Austin, TX, USA, 28 June–3 July 2010; pp. 92–96. [Google Scholar]
- Rodríguez, J.A.L.; Casana, S.T.V.; Gómez, P.A.M. Effectiveness of Chlorhexidine and Essential Oils Associated with Scaling and Root Planing in the Treatment of Chronic Periodontitis. Rev. Cienc. Salud 2020, 18, 1–11. [Google Scholar] [CrossRef]
- Marinković, J.; Ćulafić, D.M.; Nikolić, B.; Đukanović, S.; Marković, T.; Tasić, G.; Ćirić, A.; Marković, D. Antimicrobial Potential of Irrigants Based on Essential Oils of Cymbopogon Martinii and Thymus Zygis towards In Vitro Multispecies Biofilm Cultured in Ex Vivo Root Canals. Arch. Oral Biol. 2020, 117, 104842. [Google Scholar] [CrossRef]
- Sherief, D.I.; Fathi, M.S.; Abou El Fadl, R.K. Antimicrobial Properties, Compressive Strength and Fluoride Release Capacity of Essential Oil-Modified Glass Ionomer Cements—An In Vitro Study. Clin. Oral Investig. 2021, 25, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Aebisher, D.; Cichonski, J.; Szpyrka, E.; Masjonis, S.; Chrzanowski, G. Essential Oils of Seven Lamiaceae Plants and Their Antioxidant Capacity. Molecules 2021, 26, 3793. [Google Scholar] [CrossRef]
- Karami, F.; Dastan, D.; Fallah, M.; Matini, M. In Vitro Antitrichomonal Activity of Satureja Khuzestanica and Main Essential Oil Components Carvacrol, Thymol, and Eugenol. J. Infect. Dev. Ctries. 2023, 17, 80–85. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Speranza, B.; Perricone, M.; Sinigaglia, M.; Corbo, M.R. Bioactivity of Essential Oils Towards Fungi and Bacteria. In Essential Oils in Food Processing; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 231–246. ISBN 9781119149392. [Google Scholar]
- Hashim, N.T.; Babiker, R.; Priya, S.P.; Mohammed, R.; Chaitanya, N.C.; Padmanabhan, V.; El Bahra, S.; Rahman, M.M.; Gismalla, B.G. Microbial Dynamics in Periodontal Regeneration: Understanding Microbiome Shifts and the Role of Antifouling and Bactericidal Materials: A Narrative Review. Curr. Issues Mol. Biol. 2024, 46, 12196–12213. [Google Scholar] [CrossRef] [PubMed]
- El-Demerdash, F.M.; El-Sayed, R.A.; Abdel-Daim, M.M. Rosmarinus officinalis Essential Oil Modulates Renal Toxicity and Oxidative Stress Induced by Potassium Dichromate in Rats. J. Trace Elem. Med. Biol. 2021, 67, 126791. [Google Scholar] [CrossRef] [PubMed]

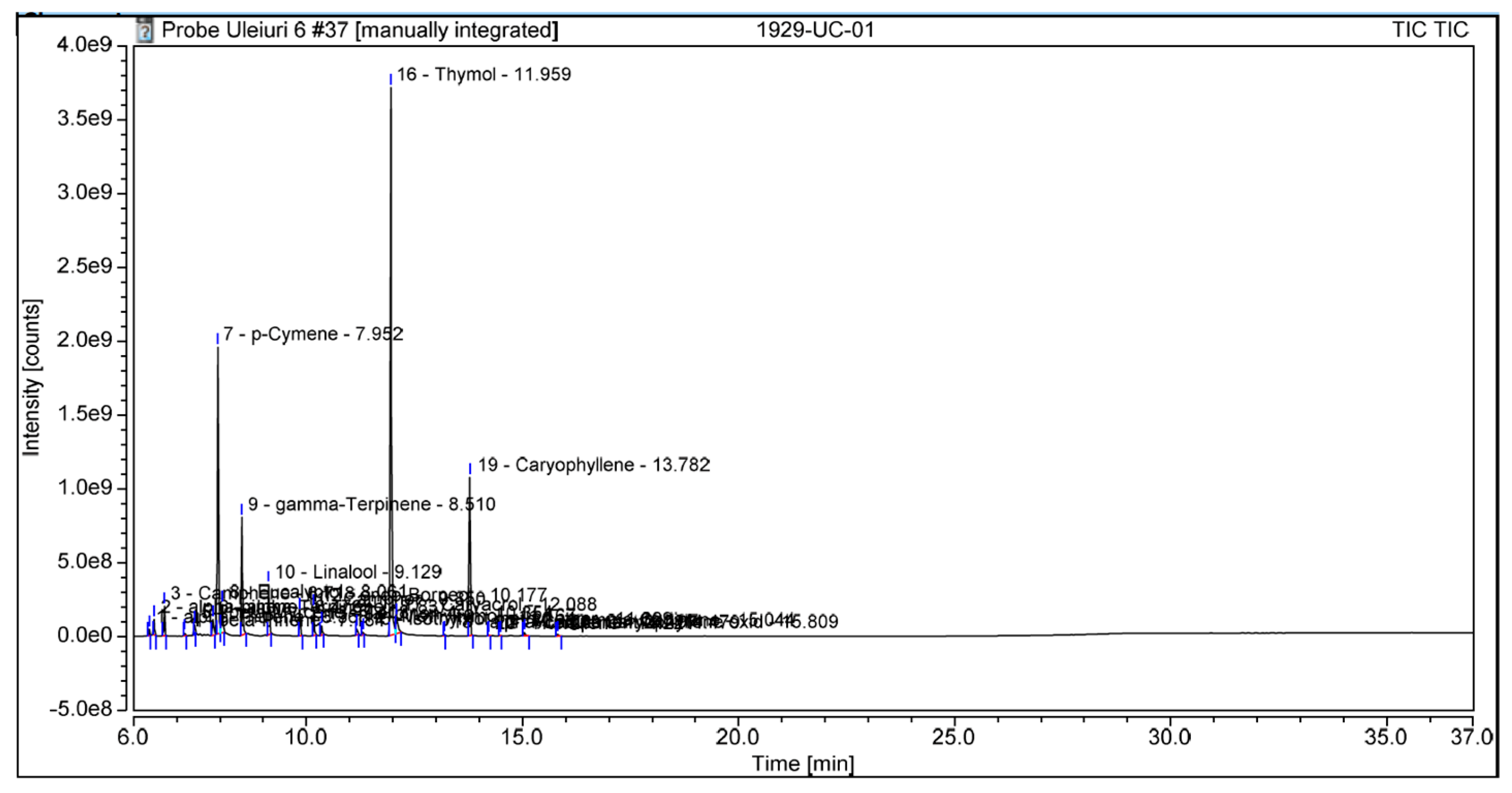

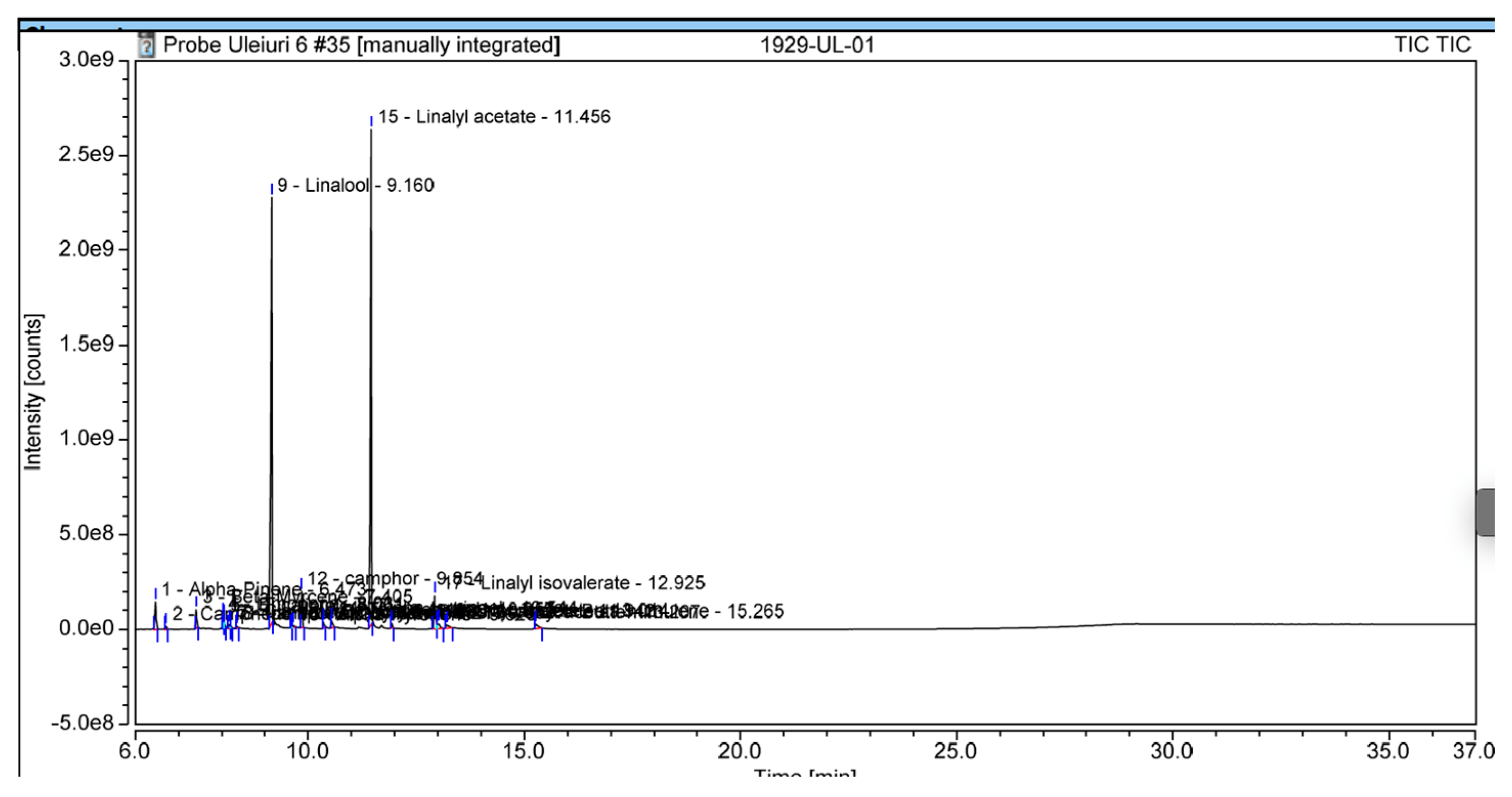
| Samples 1 µL/disc | Staphylococcus aureus (mm) | Enterococcus faecalis (mm) | Escherichia coli (mm) | Candida albicans (mm) |
|---|---|---|---|---|
| P1 (Thymus vulgaris oil) | 12.8 ± 0.6 | 8.5 ± 0.4 | 13.0 ± 0.5 | 17.2 ± 0.7 |
| P2 (Origanum vulgare oil) | 12.4 ± 0.7 | 7.8 ± 0.3 | 12.3 ± 0.6 | 18.4 ± 0.5 |
| P3 (Lavandula angustifolia oil) | 6.0 † | 6.0 † | 6.0 † | 7.2 ± 0.4 |
| Clindamycin | 27.0 ± 0.5 | - | - | - |
| Gentamicin | - | 12.0 ± 0.5 | 18.0 ± 0.6 | 0.0 |
| Nystatin | - | - | - | 20.0 ± 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radu, C.-M.; Radu, C.C.; Venter, I.-D.; Bogdan, M.A.; Zaha, D.C. Antimicrobial Activity of Oregano, Thyme, and Lavender Oils Against Oral Pathogens: Perspectives for AI-Supported Research. Oral 2025, 5, 87. https://doi.org/10.3390/oral5040087
Radu C-M, Radu CC, Venter I-D, Bogdan MA, Zaha DC. Antimicrobial Activity of Oregano, Thyme, and Lavender Oils Against Oral Pathogens: Perspectives for AI-Supported Research. Oral. 2025; 5(4):87. https://doi.org/10.3390/oral5040087
Chicago/Turabian StyleRadu, Casandra-Maria, Carmen Corina Radu, Ionut-Daniel Venter, Mihaela Alexandra Bogdan, and Dana Carmen Zaha. 2025. "Antimicrobial Activity of Oregano, Thyme, and Lavender Oils Against Oral Pathogens: Perspectives for AI-Supported Research" Oral 5, no. 4: 87. https://doi.org/10.3390/oral5040087
APA StyleRadu, C.-M., Radu, C. C., Venter, I.-D., Bogdan, M. A., & Zaha, D. C. (2025). Antimicrobial Activity of Oregano, Thyme, and Lavender Oils Against Oral Pathogens: Perspectives for AI-Supported Research. Oral, 5(4), 87. https://doi.org/10.3390/oral5040087







