The Impact of HIV Infection and Aging on Periodontitis
Abstract
1. Introduction
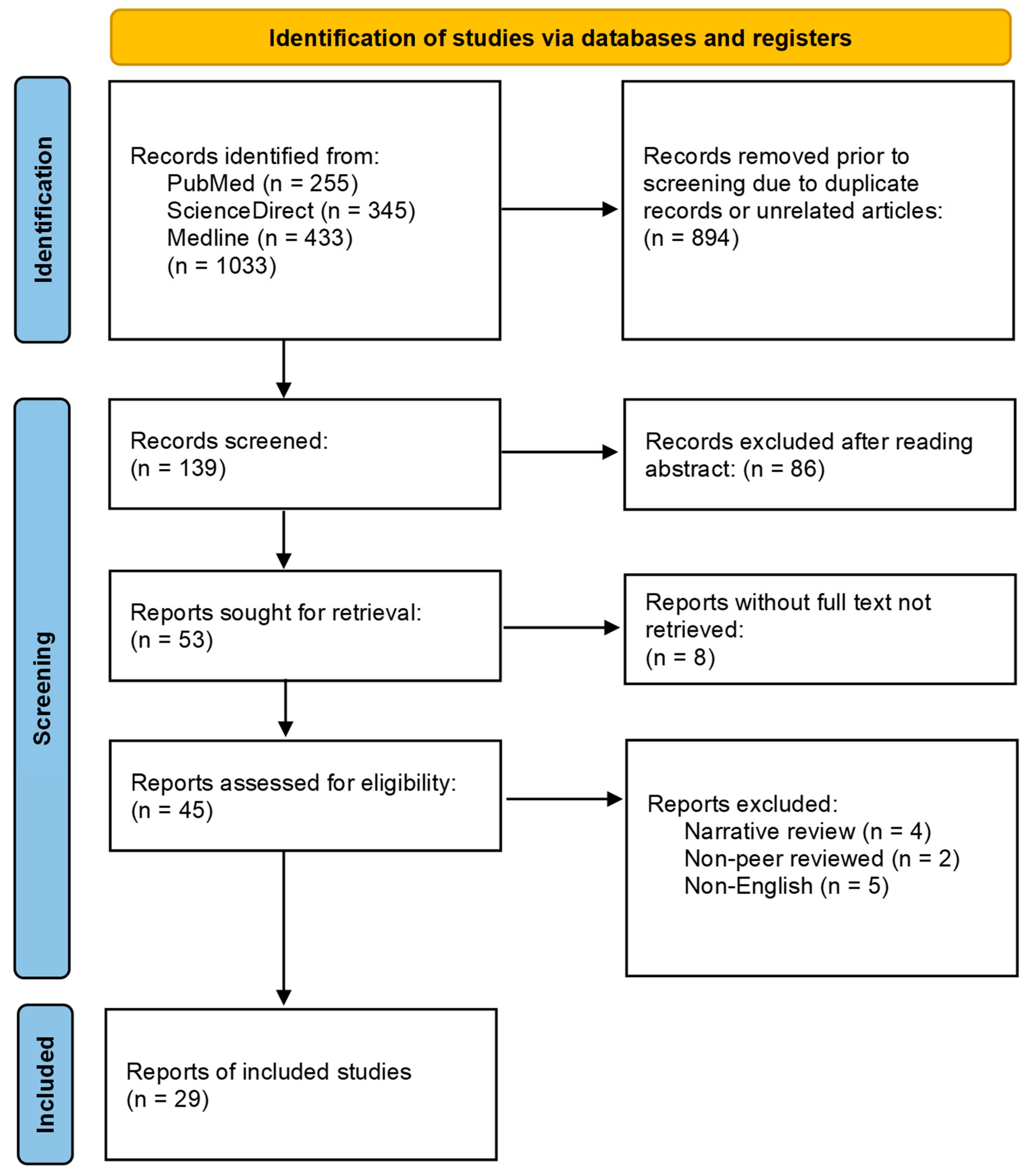
2. Methods
- Categorization: The selected studies were categorized into four main topics: periodontitis and aging, periodontitis and HIV, aging and HIV, and periodontitis, aging, and HIV. This classification allowed for a structured analysis through key associations amongst the three main topics;
- Thematic analysis: Amongst each of the four main topics, themes such as host immune dysfunction, microbial dysbiosis, inflammatory pathways, osteoporosis, and HAART/ART therapy stood out as key points;
- Clinical relevance: The clinical relevance of the literature reviewed from various studies was also evaluated for future clinicians to be aware of when treating these patients affected by periodontitis, HIV, and/or aging;
- Quality assessment: The quality of evidence presented in each study was assessed, considering factors such as study design, sample size, and methodology.
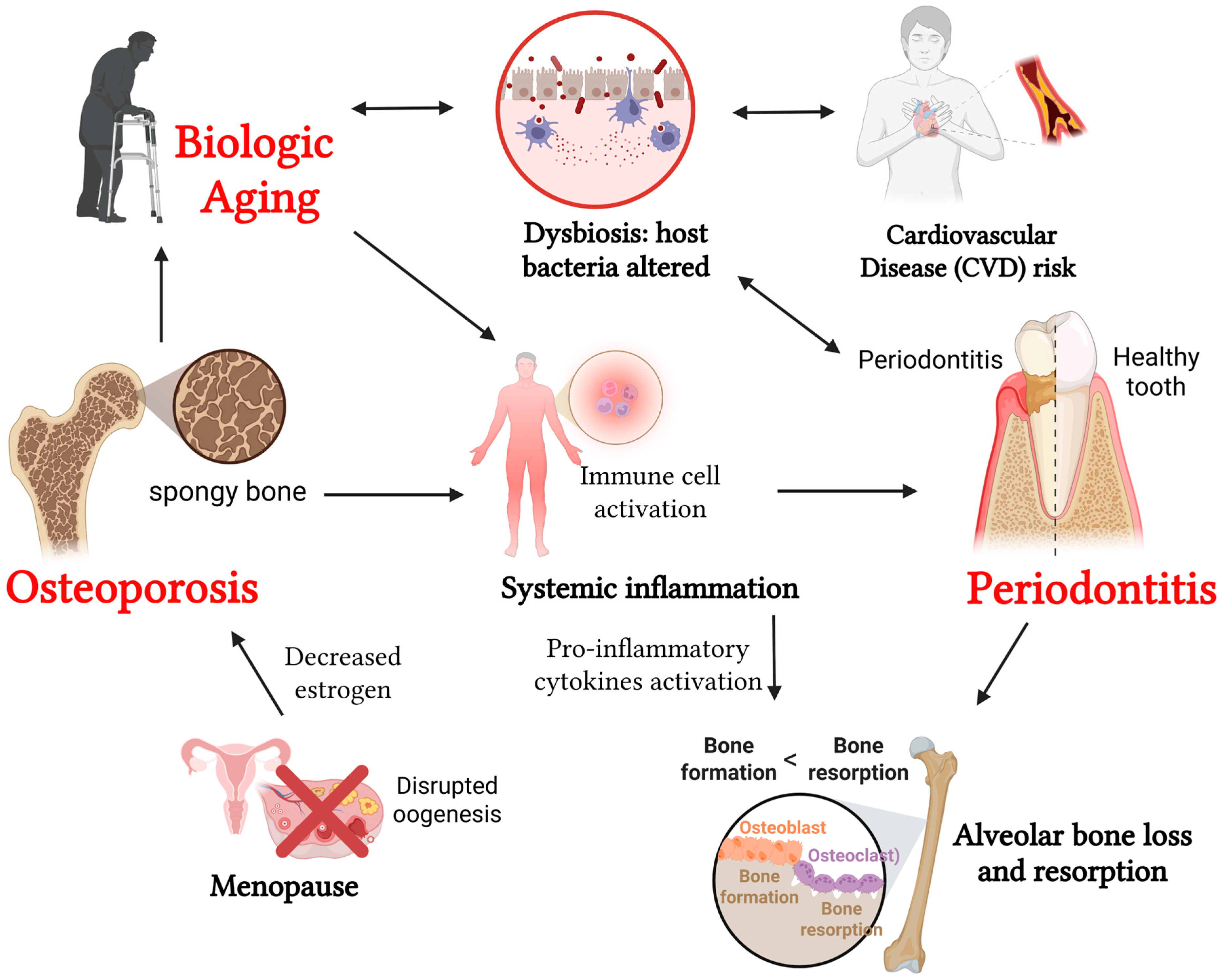
3. Periodontitis, Aging, and Osteoporosis
3.1. Periodontitis and Aging
3.2. Periodontitis and Osteoporosis
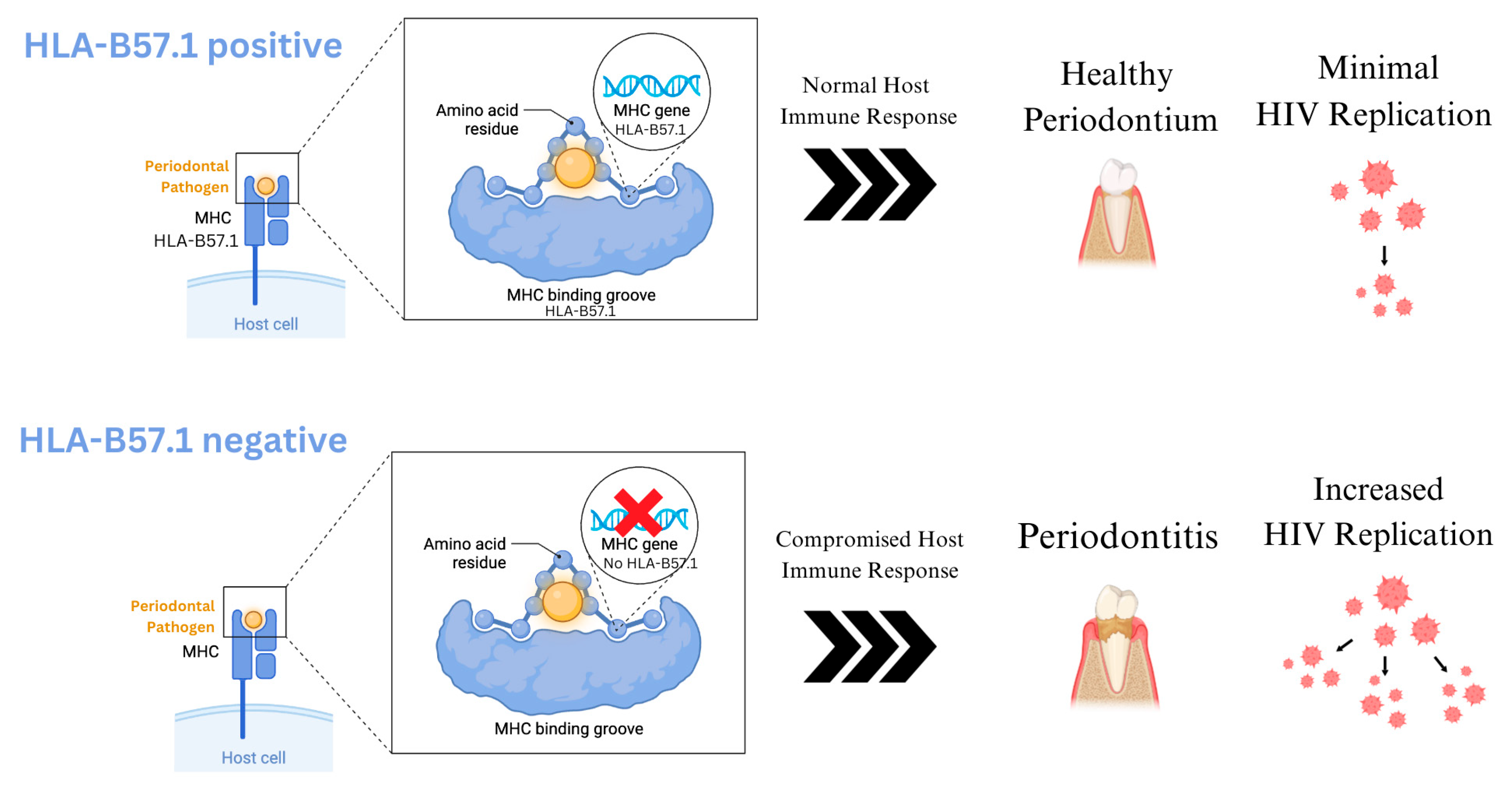
4. Periodontitis and HIV
4.1. HIV Infection and ART Therapy
4.2. Impact of HIV on Periodontitis
5. HIV and Aging
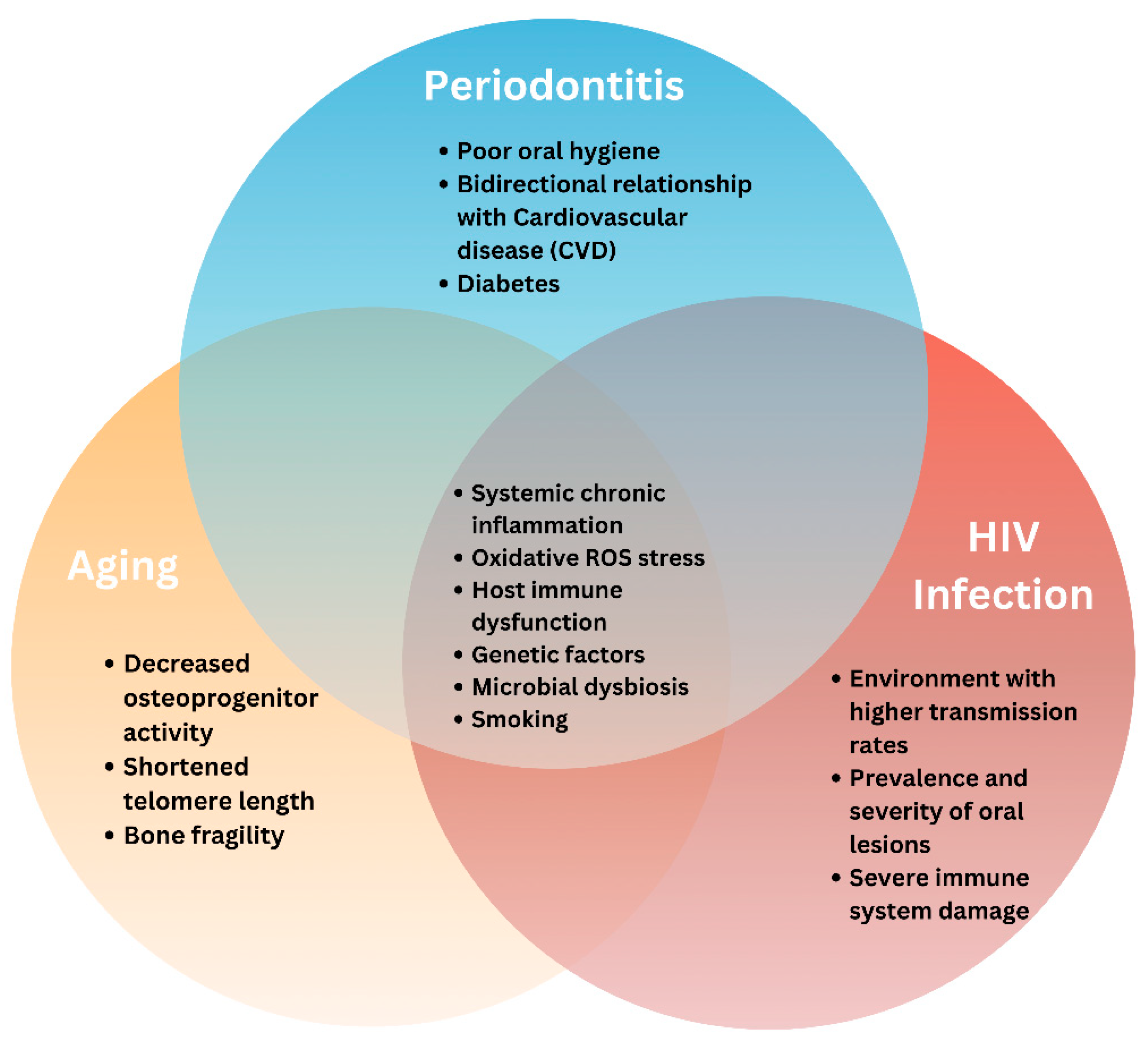
6. Interactions Between Periodontitis, Early Aging, and HIV Infection
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABL | Alveolar Bone Loss |
| AIDS | Acquired Immunodeficiency Syndrome |
| ART | Antiretroviral Therapy |
| BMI | Body Mass Index |
| BOP | Bleeding on Probing |
| BMD | Bone Mineral Density |
| CAL | Clinical Attachment Loss |
| cART | Combined Antiretroviral Therapy |
| CD4+ | Cluster of Differentiation 4 (Helper T-Cell Protein) |
| COPD | Chronic Obstructive Pulmonary Diseases |
| CiMT | Carotid Intima-Media Thickness |
| CVD | Cardiovascular Disease |
| DPSI | Dutch Periodontal Screening Index |
| DXA | Dual-energy X-ray Absorptiometry |
| HAART | Highly Active Antiretroviral Treatment |
| HIV | Human Immunodeficiency Virus |
| HLA | Human Leukocyte Antigen |
| HPV | Human Papillomavirus |
| HRQoL | Health-Related Quality of Life |
| MHC | Major Histocompatibility Complex |
| MSM | Methylsulfonylmethane |
| NNRTI | Non-Nucleoside Reverse-Transcriptase Inhibitors |
| OHI | Oral Hygiene Index |
| PDs | Pocket Depths |
| PI | Plaque Index |
| PLWH | People Living With HIV |
| PPD | Probing Pocket Depth |
| RANKL | Receptor Activator of Nuclear Factor-κB Ligand |
| RBL | Radiographic Bone Loss |
| ROS | Reactive Oxygen Species |
| rDNA | Ribosomal Deoxyribonucleic Acid |
| SRP | Scaling and Root Planing |
| TNF | Tumor Necrosis Factor |
| WHO | World Health Organization |
References
- Yu, B.; Wang, C.Y. Osteoporosis and periodontal diseases—An update on their association and mechanistic links. Periodontology 2000 2022, 89, 99–113. [Google Scholar] [CrossRef]
- Nunes, G.P.; de Oliveira Alves, R.; Ragghianti, M.H.F.; Martins, T.P.; Dos Reis Prado, A.H.; Nunes, L.P.; Poli, M.C.F.; Silva, A.N.A.; Benetti, F. Antimicrobial photodynamic therapy in the nonsurgical treatment of periodontitis in patients with HIV infection: A systematic review and meta-analysis. Lasers Med. Sci. 2024, 39, 155. [Google Scholar] [CrossRef] [PubMed]
- Valian, N.K.; Houshmand, B.; Ardakani, M.T.; Mahmoudi, S. Microbiological study of periodontal disease in populations with HIV: A systematic review and meta-analysis. Clin. Lab. 2023, 69, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ponce, P.N.O.; Chaves, L.B.; Perce-da-Silva, D.S.; Carneiro-Alencar, A.L.; Rodolphi, C.M.; Soares, I.F.; Rodrigues-da-Silva, R.N.; Alves-da-Silva, A.C.; Marques, F.V.; Peres, R.V.; et al. Periodontal health in individuals living with HIV: An exploratory and descriptive molecular approach of microbial interspecific and intraspecific diversity in brazilian patients. Microorganisms 2025, 13, 867. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, S.; Cai, Q.; Chen, Y.; Zhu, P.; Du, M.; Visser, A.; Li, A. Does periodontitis affect the association of biological aging with mortality? J. Dent. Res. 2023, 102, 909–918. [Google Scholar] [CrossRef]
- Gholami, M.; Asadinejad, S.M.; Kakavand, D.; Jafari Doudaran, P.; Fathi, A.H. Association of periodontitis and aging-related diseases: A review of mechanistic studies. J. Res. Dent. Maxillofac. Sci. 2023, 8, 62–70. [Google Scholar] [CrossRef]
- Mashalkar, V.N.; Suragimath, G.; Zope, S.A.; Varma, S.A. A cross-sectional study to assess and correlate osteoporosis and periodontitis among postmenopausal women: A dual energy X-ray absorptiometry study. J. Midlife Health 2018, 9, 2–7. [Google Scholar] [CrossRef]
- Clark, D.; Kotronia, E.; Ramsay, S.E. Frailty, aging, and periodontal disease: Basic biologic considerations. Periodontology 2000 2021, 87, 143–156. [Google Scholar] [CrossRef]
- Juluri, R.; Prashanth, E.; Gopalakrishnan, D.; Kathariya, R.; Devanoorkar, A.; Viswanathan, V.; Romanos, G.E. Association of postmenopausal osteoporosis and periodontal disease: A double-blind case-control study. J. Int. Oral Health 2015, 7, 119–123. [Google Scholar]
- Wang, C.-W.; McCauley, L.K. Osteoporosis and periodontitis. Curr. Osteoporos. Rep. 2016, 14, 284–291. [Google Scholar] [CrossRef]
- Pereira, L.L.; Veiga Siqueira Amorim, D.; Brito Sampaio, W.; Almeida Cruz Azevêdo, T.; Bispo Pereira Cardoso, V.; Barreto Lemos, F.; Silva Chang, A.; Machado, F.; Pereira Lima, F.; Sampaio Neves, F.; et al. Factors associated with periodontitis in patients with and without HIV. Int. J. Dent. 2023, 2023, 9929835. [Google Scholar] [CrossRef] [PubMed]
- Ryder, M.I.; Shiboski, C.; Yao, T.J.; Moscicki, A.B. Current trends and new developments in HIV research and periodontal diseases. Periodontology 2000 2020, 82, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Bloch, M.; John, M.; Smith, D.; Rasmussen, T.A.; Wright, E. Managing HIV-associated inflammation and ageing in the era of modern ART. HIV Med. 2020, 21, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Schünemann, H.J.; Oxman, A.D.; Brozek, J.; Glasziou, P.; Jaeschke, R.; Vist, G.E.; Williams, J.W., Jr.; Kunz, R.; Craig, J.; Montori, V.M.; et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ 2008, 336, 1106–1110. [Google Scholar] [CrossRef]
- Eke, P.I.; Thornton-Evans, G.O.; Wei, L.; Borgnakke, W.S.; Dye, B.A.; Genco, R.J. Periodontitis in US adults: National health and nutrition examination survey 2009–2014. J. Am. Dent. Assoc. 2018, 149, 576–588.e576. [Google Scholar] [CrossRef]
- Janorkar, D.A.; Long, D.M.; Weber, K.M.; Sharma, A.; Lin, G.H.; D’Souza, G.; Edmonds, A.; Kassaye, S.; Lahiri, C.D.; Konkle-Parker, D. Association between BMI and periodontitis in women living with or at risk for HIV. Spec. Care Dent. 2022, 42, 486–493. [Google Scholar] [CrossRef]
- Silva, N.; Abusleme, L.; Bravo, D.; Dutzan, N.; Garcia-Sesnich, J.; Vernal, R.; Hernández, M.; Gamonal, J. Host response mechanisms in periodontal diseases. J. Appl. Oral Sci. 2015, 23, 329–355. [Google Scholar] [CrossRef]
- Nilsson, H.; Sanmartin Berglund, J.; Renvert, S. Longitudinal evaluation of periodontitis and tooth loss among older adults. J. Clin. Periodontol. 2019, 46, 1041–1049. [Google Scholar] [CrossRef]
- Schuettfort, G.; de Leuw, P.; Haberl, A.; Herrmann, E.; Park, K.H.; Wolf, T.; Stephan, C. HLA-B57.01 shields people living with HIV for significantly better periodontal health. J. Periodontol. 2018, 89, 966–972. [Google Scholar] [CrossRef]
- Hamczyk, M.R.; Nevado, R.M.; Barettino, A.; Fuster, V.; Andrés, V. Biological versus chronological aging. J. Am. Coll. Cardiol. 2020, 75, 919–930. [Google Scholar] [CrossRef]
- Song, L.; Wang, Y.; Zheng, Q.; Li, W. Periodontitis prevalence and acceleration of biological aging: Insights from NHANES 2009-2014 and Mendelian randomization study. J. Periodontal. Res. 2024, 60, 350–360. [Google Scholar] [CrossRef]
- Biguetti, C.C.; Lakkasetter Chandrashekar, B.; Simionato, G.B.; Momesso, N.R.; Duarte, M.A.H.; Rodrigues, D.C.; Matsumoto, M.A. Influence of age and gender on alveolar bone healing post tooth extraction in 129 Sv mice: A microtomographic, histological, and biochemical characterization. Clin. Oral Investig. 2023, 27, 4605–4616. [Google Scholar] [CrossRef]
- Razi, H.; Birkhold, A.I.; Weinkamer, R.; Duda, G.N.; Willie, B.M.; Checa, S. Aging leads to a dysregulation in mechanically driven bone formation and resorption. J. Bone Miner. Res. 2015, 30, 1864–1873. [Google Scholar] [CrossRef] [PubMed]
- Infante, A.; Rodríguez, C.I. Osteogenesis and aging: Lessons from mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 244. [Google Scholar] [CrossRef] [PubMed]
- Baima, G.; Romandini, M.; Citterio, F.; Romano, F.; Aimetti, M. Periodontitis and accelerated biological aging: A geroscience approach. J. Dent. Res. 2022, 101, 125–132. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, X.; Gao, S.; Li, A.; Deng, K.; Yang, K.; Liu, W.; Du, M. Biological aging mediates the association between periodontitis and cardiovascular disease: Results from a national population study and Mendelian randomization analysis. Clin. Epigenetics 2024, 16, 116. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Marco Del Castillo, A.; Jepsen, S.; Gonzalez-Juanatey, J.R.; D’Aiuto, F.; Bouchard, P.; Chapple, I.; Dietrich, T.; Gotsman, I.; Graziani, F.; et al. Periodontitis and cardiovascular diseases: Consensus report. J. Clin. Periodontol. 2020, 47, 268–288. [Google Scholar] [CrossRef]
- Shetty, B.; Fazal, I.; Khan, S.F.; Nambiar, M.; Irfana, I.D.; Prasad, R.; Raj, A. Association between cardiovascular diseases and periodontal disease: More than what meets the eye. Drug Target Insights 2023, 17, 31–38. [Google Scholar] [CrossRef]
- Rydén, L.; Buhlin, K.; Ekstrand, E.; de Faire, U.; Gustafsson, A.; Holmer, J.; Kjellström, B.; Lindahl, B.; Norhammar, A.; Nygren, Å.; et al. Periodontitis increases the risk of a first myocardial infarction. Circulation 2016, 133, 576–583. [Google Scholar] [CrossRef]
- Aljohani, H.; Senbanjo, L.T.; Al Qranei, M.; Stains, J.P.; Chellaiah, M.A. Methylsulfonylmethane increases the alveolar bone density of mandibles in aging female mice. Front. Physiol. 2021, 12, 708905. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, R.K.; Siwach, R.C.; Tewari, S.; Narula, S.C. Association of bone mineral density with periodontal status in postmenopausal women. J. Investig. Clin. Dent. 2014, 5, 275–282. [Google Scholar] [CrossRef]
- Passos-Soares, J.S.; Vianna, M.I.P.; Gomes-Filho, I.S.; Cruz, S.S.; Barreto, M.L.; Adan, L.F.; Rösing, C.K.; Trindade, S.C.; Cerqueira, E.M.M.; Scannapieco, F.A. Association between osteoporosis treatment and severe periodontitis in postmenopausal women. Menopause 2017, 24, 789–795. [Google Scholar] [CrossRef]
- Ioannidou, E. The sex and gender intersection in chronic periodontitis. Front. Public Health 2017, 5, 189. [Google Scholar] [CrossRef]
- Briot, K.; Geusens, P.; Em Bultink, I.; Lems, W.F.; Roux, C. Inflammatory diseases and bone fragility. Osteoporos. Int. 2017, 28, 3301–3314. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; LaMonte, M.J.; Hovey, K.M.; Mai, X.; Tezal, M.; Millen, A.E.; Ochs-Balcom, H.M.; Genco, R.J.; Barnabei, V.M.; Wactawski-Wende, J. Association of serum 17β-Estradiol concentration, hormone therapy, and alveolar crest height in postmenopausal women. J. Periodontol. 2015, 86, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Lobo, J.D.; Sundermann, E.; Baker, D.J.; Tracy, R.P.; Kuchel, G.A.; Stephenson, K.E.; Letendre, S.L.; Brew, B.; Cysique, L.A.; et al. Current challenges and solutions for clinical management and care of people with HIV: Findings from the 12th annual international HIV and aging workshop. AIDS Res. Hum. Retroviruses 2023, 39, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bernal, M.; Elenkova, M.; Evensky, J.; Stein, S.H. Periodontal disease and osteoporosis-shared risk factors and potentiation of pathogenic mechanisms. Curr. Oral Health Rep. 2018, 5, 26–32. [Google Scholar] [CrossRef]
- Mazur, I.; Dilbarkhanov, B.; Kuracha, X.; Novoshytskyy, V.; Suprunovych, I.; Zhakipbekov, K. Periodontal status and bone metabolism in women in reproductive and postmenopausal periods. Horm. Mol. Biol. Clin. Investig. 2020, 41, 20200011. [Google Scholar] [CrossRef]
- Tounta, T.S. Diagnosis of osteoporosis in dental patients. J. Frailty Sarcopenia Falls 2017, 2, 21–27. [Google Scholar] [CrossRef]
- Ishtiaq, S.; Fogelman, I.; Hampson, G. Treatment of post-menopausal osteoporosis: Beyond bisphosphonates. J. Endocrinol. Investig. 2015, 38, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Annavajhala, M.K.; Khan, S.D.; Sullivan, S.B.; Shah, J.; Pass, L.; Kister, K.; Kunen, H.; Chiang, V.; Monnot, G.C.; Ricupero, C.L.; et al. Oral and gut microbial diversity and immune regulation in patients with HIV on antiretroviral therapy. mSphere 2020, 5, e00798-19. [Google Scholar] [CrossRef]
- Fokam, J.; Geh, B.K.N.; Sosso, S.M.; Takou, D.; Ngufack, E.S.; Nka, A.D.; Bissek, A.Z.; Eko, D.M.; Ndjolo, A. Determinants of periodontitis according to the immunological and virological profiles of HIV-infected patients in Yaoundé, Cameroon. BMC Oral Health 2020, 20, 359. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, L.S.; de Carvalho Ferreira, D.; Vidal, F.; Souza, R.C.; Gonçalves, C.; Pavan, P.; Carrouel, F.; Bourgeois, D.; Seymour, G.J. Stage II and stage III periodontitis clinical burdens of HIV-1 undergoing antiretroviral therapy. Clin. Oral Investig. 2022, 26, 2187–2195. [Google Scholar] [CrossRef] [PubMed]
- Massanella, M.; Ignacio, R.A.B.; Lama, J.R.; Pagliuzza, A.; Dasgupta, S.; Alfaro, R.; Rios, J.; Ganoza, C.; Pinto-Santini, D.; Gilada, T.; et al. Long-term effects of early antiretroviral initiation on HIV reservoir markers: A longitudinal analysis of the MERLIN clinical study. Lancet Microbe 2021, 2, 198–209. [Google Scholar] [CrossRef]
- Ntolou, P.; Pani, P.; Panis, V.; Madianos, P.; Vassilopoulos, S. The effect of antiretroviral therapyon the periodontal conditions of patients with HIV infection: A systematic review and meta-analysis. J. Clin. Periodontol. 2023, 50, 170–182. [Google Scholar] [CrossRef]
- Sufiawati, I.; Amalia, T.; Dewi, T.S.; Wisaksana, R. The association between oral mucosal lesions and oral health-related quality of life using the validated indonesian version of OHIP-14 among people living with HIV/AIDS. Res. Palliat. Care 2024, 16, 9–16. [Google Scholar] [CrossRef]
- Blignaut, E.; Rossouw, T.M.; Becker, P.J.; Mavuso, D.S.; Feucht, U.D. Gingival recession and localized aggressive periodontitis among HIV-infected children and adolescents receiving antiretroviral therapy. Pediatr. Infect. Dis. J. 2019, 38, 112–115. [Google Scholar] [CrossRef]
- Parish, C.L.; Feaster, D.J.; Pereyra, M.R.; Alcaide, M.L.; Weber, K.M.; Cohen, M.; Levin, S.; Gustafson, D.; Merenstein, D.; Aouizerat, B.E.; et al. Oral health-related quality of life and unmet dental needs among women living with HIV. J. Am. Dent. Assoc. 2020, 151, 527–535. [Google Scholar] [CrossRef]
- Tappuni, A.R. The global changing pattern of the oral manifestations of HIV. Oral Dis. 2020, 26, 22–27. [Google Scholar] [CrossRef]
- Syrjänen, S. Oral manifestations of human papillomavirus infections. Eur. J. Oral Sci. 2018, 126, 49–66. [Google Scholar] [CrossRef] [PubMed]
- Wulandari, E.A.T.; Wijaya, I.P.; Karim, B.; Ariyanto, I.; Tanudjaja, S.A.; Lee, S.; Price, P. Periodontitis and cytomegalovirus associate with atherosclerosis among HIV patients after 5 years on ART. J. Acquir. Immune Defic. Syndr. 2020, 85, 195–200. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.K.; de Carvalho, A.C.G.; Alves, E.H.P.; da Silva, F.R.P.; Pessoa, L.d.S.; Vasconcelos, D.F.P. Genetic factors and the risk of periodontitis development: Findings from a systematic review composed of 13 studies of meta-analysis with 71,531 participants. Int. J. Dent. 2017, 2017, 1914073. [Google Scholar] [CrossRef] [PubMed]
- Weyand, C.M.; Goronzy, J.J. Aging of the immune system. mechanisms and therapeutic targets. Ann. Am. Thorac. Soc. 2016, 13, s422–s428. [Google Scholar] [CrossRef]
- Leng, S.X.; Margolick, J.B. Aging, sex, inflammation, frailty, and CMV and HIV infections. Cell. Immunol. 2020, 348, 104024. [Google Scholar] [CrossRef]
- Trickey, A.; Sabin, C.A.; Burkholder, G.; Crane, H.; d’Arminio Monforte, A.; Egger, M.; Gill, M.J.; Grabar, S.; Guest, J.L.; Jarrin, I.; et al. Life expectancy after 2015 of adults with HIV on long-term antiretroviral therapy in Europe and North America: A collaborative analysis of cohort studies. Lancet HIV 2023, 10, e295–e307. [Google Scholar] [CrossRef]
- Kokorelias, K.M.; Grosse, A.; Zhabokritsky, A.; Sirisegaram, L. Understanding geriatric models of care for older adults living with HIV: A scoping review and qualitative analysis. BMC Geriatr. 2023, 23, 417. [Google Scholar] [CrossRef]
- Wilson, M.P.; Jankowski, C.M.; Cook, P.F.; Kulik, G.L.; Iriarte, E.; SantaBarbara, N.J.; Fourman, L.T.; Erlandson, K.M. Effect of a supervised exercise program on exercise self-efficacy in aging adults with and without HIV: A secondary analysis of the exercise for healthy aging study. AIDS Behav. 2025, 29, 535–545. [Google Scholar] [CrossRef]
- Legarth, R.A.; Ahlström, M.G.; Kronborg, G.; Larsen, C.S.; Pedersen, C.; Pedersen, G.; Mohey, R.; Gerstoft, J.; Obel, N. Long-term mortality in HIV-infected individuals 50 years or older: A nationwide, population-based cohort study. J. Acquir. Immune Defic. Syndr. 2016, 71, 213–218. [Google Scholar] [CrossRef]
- Guaraldi, G.; Malagoli, A.; Calcagno, A.; Mussi, C.; Celesia, B.M.; Carli, F.; Piconi, S.; De Socio, G.V.; Cattelan, A.M.; Orofino, G.; et al. The increasing burden and complexity of multi-morbidity and polypharmacy in geriatric HIV patients: A cross sectional study of people aged 65–74 years and more than 75 years. BMC Geriatr. 2018, 18, 99. [Google Scholar] [CrossRef]
- Woldesemayat, E.M. Chronic diseases multimorbidity among adult people living with HIV at Hawassa university comprehensive specialized hospital, Southern Ethiopia. Int. J. Chronic. Dis. 2020, 2020, 2190395. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Rodés, B.; Cadiñanos, J.; Esteban-Cantos, A.; Rodríguez-Centeno, J.; Arribas, J.R. Ageing with HIV: Challenges and biomarkers. eBioMedicine 2022, 77, 103896. [Google Scholar] [CrossRef]
- Kamin Mukaz, D.; Gergi, M.; Koh, I.; Zakai, N.A.; Judd, S.E.; Sholzberg, M.; Baumann Kreuziger, L.; Freeman, K.; Colovos, C.; Olson, N.C.; et al. Thrombo-inflammatory biomarkers and D-dimer in a biracial cohort study. Res. Pract. Thromb. Haemost. 2021, 5, e12632. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, M.; Sauce, D. Mechanisms of immune aging in HIV. Clin. Sci. 2022, 136, 61–80. [Google Scholar] [CrossRef] [PubMed]
- Akusjärvi, S.S.; Neogi, U. Biological aging in people living with HIV on successful antiretroviral therapy: Do they age faster? Curr. HIV/AIDS Rep. 2023, 20, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.A.; Legault, V.; Fülöp, T. What if there’s no such thing as “aging”? Mech. Ageing Dev. 2020, 192, 111344. [Google Scholar] [CrossRef]
- Oliveira, V.H.F.; Willig, A.L.; Horvat Davey, C.; Buford, T.W.; Menezes, P.; Cachay, E.; Crane, H.M.; Burkholder, G.A.; Gripshover, B.M.; Fleming, J.G.; et al. Brief report: Relationship between adiposity and biomarkers of aging and frailty among adults aging with HIV. J. Acquir. Immune Defic. Syndr. 2024, 95, 377–382. [Google Scholar] [CrossRef]
- Haas, D.W.; Tarr, P.E. Perspectives on pharmacogenomics of antiretroviral medications and HIV-associated comorbidities. Curr. Opin. HIV AIDS 2015, 10, 116–122. [Google Scholar] [CrossRef][Green Version]
- Leung, J.M.; Fishbane, N.; Jones, M.; Morin, A.; Xu, S.; Liu, J.C.; MacIsaac, J.; Milloy, M.J.; Hayashi, K.; Montaner, J.; et al. Longitudinal study of surrogate aging measures during human immunodeficiency virus seroconversion. Aging 2017, 9, 687–705. [Google Scholar] [CrossRef]
- Gonzalez-Serna, A.; Ajaykumar, A.; Gadawski, I.; Muñoz-Fernández, M.A.; Hayashi, K.; Harrigan, P.R.; Côté, H.C.F. Rapid decrease in peripheral blood mononucleated cell telomere length after HIV seroconversion, but Not HCV seroconversion. J. Acquir. Immune Defic. Syndr. 2017, 76, 29–32. [Google Scholar] [CrossRef]
- Montejano, R.; Stella-Ascariz, N.; Monge, S.; Bernardino, J.I.; Pérez-Valero, I.; Montes, M.L.; Valencia, E.; Martín-Carbonero, L.; Moreno, V.; González-Garcia, J.; et al. Impact of nucleos(t)ide reverse transcriptase inhibitors on blood telomere length changes in a prospective cohort of aviremic HIV-infected adults. J. Infect. Dis. 2018, 218, 1531–1540. [Google Scholar] [CrossRef]
- Toljić, B.; Trbovich, A.M.; Petrović, S.M.; Kannosh, I.Y.; Dragović, G.; Jevtović, D.; De Luka, S.R.; Ristić-Djurović, J.L.; Milašin, J. Ageing with HIV—A periodontal perspective. New Microbiol. 2018, 41, 61–66. [Google Scholar] [PubMed]
- Lewy, T.; Hong, B.Y.; Weiser, B.; Burger, H.; Tremain, A.; Weinstock, G.; Anastos, K.; George, M.D. Oral microbiome in HIV-infected women: Shifts in the abundance of pathogenic and beneficial bacteria are associated with aging, HIV load, CD4 count, and antiretroviral therapy. AIDS Res. Hum. Retroviruses 2019, 35, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, S.; Finn, T.R.; Kister, K.; Matsumura, S.; Levit, M.; Cantos, A.; Shah, J.; Bohn, B.; Lalla, E.; Grbic, J.T.; et al. Postmenopausal women with HIV have increased tooth loss. BMC Oral Health 2024, 24, 52. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef] [PubMed]
- Groenewegen, H.; Bierman, W.F.W.; Delli, K.; Dijkstra, P.U.; Nesse, W.; Vissink, A.; Spijkervet, F.K.L. Severe periodontitis is more common in HIV-infected patients. J. Infect. 2019, 78, 171–177. [Google Scholar] [CrossRef]
- Rhoades, N.; Mendoza, N.; Jankeel, A.; Sureshchandra, S.; Alvarez, A.D.; Doratt, B.; Heidari, O.; Hagan, R.; Brown, B.; Scheibel, S.; et al. Altered immunity and microbial dysbiosis in aged individuals with long-Term controlled HIV infection. Front. Immunol. 2019, 10, 463. [Google Scholar] [CrossRef]
- Groenewegen, H.; Delli, K.; Vissink, A.; Spijkervet, F.K.L.; Bierman, W.F.W. Immune markers and microbial factors are related with periodontitis severity in people with HIV. Clin. Oral Investig. 2023, 27, 1255–1263. [Google Scholar] [CrossRef]
- Ebersole, J.L.; Nguyen, L.M.; Gonzalez, O.A. Gingival tissue antibody gene utilization in aging and periodontitis. J. Periodontal. Res. 2022, 57, 780–798. [Google Scholar] [CrossRef]
| Subjects and Methods | HAART/ART Effect on Periodontal Health | Authors and Published Year |
|---|---|---|
| Subgingival plaque samples from 24 HIV+ patients were assessed using PCR and amplicon sequencing. | HAART reduced the prevalence of HIV-related oral lesions. | Ponce et al., 2025 [4] |
| OHRQoL of 110 PLWH were assessed using OHIP-14. | There was a significant association between oral mucosal lesions and OHRQoL. 60–90% of PLWH had at least one oral lesion despite ART availability. | Sufiawati et al., 2024 [47] |
| Five databases were searched to identify longitudinal and cross-sectional studies on prevalence of NG, NP and periodontitis among HIV patients with or without HAART treatment. | NG prevalence was significantly reduced in patients with HAART therapy. HAART was linked to the reduction in HIV infection related oral manifestations. | Ntolou et al., 2023 [46] |
| A total of 200 (100 HIV+, 100 HIV−) subjects were examined. Each subject had a clinical periodontal examination, questionnaire on personal data and oral hygiene, and medical records reviewed. | No observed effect of ART on periodontitis. The use of NNRTIs was also associated with moderate and severe periodontitis. | Pereira et al., 2023 [11] |
| 165 (44 ART-naïve and 121 ART-experienced) patients had their periodontal status assessed and a standard-questionnaire were given. | ART-naïve patients had a higher risk of periodontitis. This is indicative of the protective impact ART has on this population. | Fokam et al., 2020 [43] |
| 554 children and adolescents infected with HIV on ART were included in this cross-sectional study to study the gingival recession and localized aggressive periodontitis. | Gingival recession and aggressive periodontitis occurred more in patients that had a significantly shorter duration of ART treatment and suboptimal HIV control. | Blignaut et al., 2019 [48] |
| Subjects and Methods | Main Finding | p-Value and/or Confidence Intervals | Authors and Year |
|---|---|---|---|
| Analyzing subgingival plaque samples from 24 HIV+ patients | 7 species were detected, highlighting the complex oral microbial interactions in PLWH. | Intraclass correlation coefficient of 0.86 for probing depth and 0.80 for clinical attachment loss | Ponce et al., 2025 [4] |
| Cross-sectional study with 9558 participants from the National Health and Nutrition Examination Survey (2009–2014) | Periodontitis was associated with increased biological aging; Subgroup analysis found stronger associations in males for BioAgeAccele and current smokers for PehonAgeAccel. | Periodontitis and biological aging association, with 0.57-year (95% CI: 0.28–0.86, p < 0.001) increases in BioAgeAccel and 0.41-year (95% CI: 0.04–0.78, p = 0.034) increases in PhenoAgeAccel. | Song et al., 2024 [22] |
| 135 self-reported postmenopausal women were recruited (including 59 HIV−, 76 HIV+ on cART) | The mean age of participants was 57.04 ± 6.25 years. Postmenopausal women with HIV had deterioration of the alveolar trabecular bone microarchitecture. | Women with HIV had higher RANKL expression in gingival crevicular fluid (p < 0.001), fewer teeth (p < 0.001), and lower trabecular number (p = 0.004) compared with women without HIV. | Wadhwa et al., 2024 [75] |
| 3269 participants from the National Health and Nutrition Examination Survey (2009–2014) were included | Participants with periodontitis had increased biological aging, which was associated with increased CVD risk. | Reverse MR analysis showed that DNAm Hannum age acceleration could increase the risk of periodontitis (95% CI 1.01–1.11 p = 0.023); and there was a two-way causal relationship between CVD and biological aging (95% CI 0.87–0.99 p = 0.017 and 95% CI 1.01–1.20 p = 0.027). | Zhang et al., 2024 [27] |
| 52 (28 young, 24 aged) 129-Sv mice were used to extract their upper right incisor | Age and gender significantly contributed to slower bone healing in craniofacial bones. | Aged females (6.03 ± 1.03) had significantly reduced mineralized bone content in alveolar sockets compared with young females (12.25 ± 3.09 p < 0.05) and aged males (9.86 ± 2.10 p < 0.05). | Biguetti et al., 2023 [23] |
| 200 (100 HIV+, 100 HIV−) subjects were examined, 81 (40.3%) had periodontitis | PLWH and those older than 43 were more likely to develop moderate and severe periodontitis, suggesting an association between HIV, advanced age, and periodontitis. | Individuals who were over 43 years old (OR = 1.557; CI = 0.882−2.747) and were HIV+ (OR = 3.064; CI = 1.698–5.529) were more likely to develop periodontitis. | Pereira et al., 2023 [11] |
| 351 WLWH and 52 WRH participants had pocket depths and clinical periodontal attachment loss assessments | There was no association between BMI and periodontitis among women with or without HIV infection | aOR of mild, moderate, and severe periodontitis in obese women were: 1.14 (95% CI: 0.51–2.52), 1.02 (95% CI: 0.46–2.29), and 0.24 (95% CI: 0.06–1.07), respectively. | Janorkar et al., 2022 [17] |
| 205 patients (74 HIV+ and 131 HIV−) were tested for PPD, CAL, BOP, and VSB. | HIV patients on HAART had direct association of HIV-1 infection with BOP, suggesting that monitoring gingival bleeding would be beneficial in the periodontitis prevention in HIV-1 patients on HAART. | HIV-1 infection (OR = 5.53, p < 0.0001, 95% CI: 2.45–13.64) and age (compared to young (18–35 years old), 35–50 years old: OR = 5.73, p < 0.0001, 95% CI: 2.49–13.20, >50 years old: OR = 6.29, p = 0.002, 95% CI: 1.94–20.42) had significant association with BOP outcome. | Gonçalves et al., 2022 [44] |
| Periodontal status was assessed in 65 patients (44 ART-naïve and 121 ART-experienced) by measuring CAL, PPD, plaques index. | ART-naïve patients had a higher risk, indicating the protective role of ART; severely immune-compromised patients and men were vulnerable to periodontitis. | Periodontitis risk of the (a) ART-naïve population versus the ART-experienced population was doubled (OR 2.06, p = 0.03), (b) ART-naïve, CD4 < 200 cells versus those with higher CD4-values was threefold higher (OR 3.21, p = 0.06). | Fokam et al., 2020 [43] |
| 82 HIV+ adults with <200 CD4 T-cells/μL were examined at 0 and 3 months after ART; 32 patients were reassessed after 5 years. | 5 years after ART, periodontitis was higher with greater age and poor oral hygiene, while smoking, oral candidiasis, or low CD4 T-cell counts showed no effect. | Periodontitis was potentiated by older age (p = 0.03) and poor oral hygiene (p = 0.05). | Wulandari et al., 2020 [52] |
| Prevalence and severity of periodontitis were assessed in 258 HIV+ patients and 539 controls | Severe periodontitis (DPSI 4) was higher in HIV+ patients than in controls. Periodontitis prevalence and severity were increased in HIV+ patients compared with controls, particularly in older males. | HIV-infection (95% CI: 1.13–2.34, p = 0.008), increasing age (95% CI: 1.16–1.35, p < 0.001), and male sex (95% CI: 1.18–2.3, p = 0.003) were significant risk factors of severe periodontitis with logistic regression analysis. | Groenewegen et al., 2019 [77] |
| 94 postmenopausal women in the range of 45–65 years old | Periodontitis severity and osteoporosis among postmenopausal women were correlated. | There was a significant correlation between periodontitis and osteoporosis (x2 = 9.76, p = 0.045). | Mashalkar et al., 2018 [7] |
| 60 HIV+ male, including 30 younger (≤35 years) and 30 older (≥50 years) patients | All periodontal parameters were higher in older HIV+ patients. HIV-associated gingivitis and periodontitis were more frequent in patients over 50 years. | HIV-associated gingivitis (p = 0.007) and HIV-associated periodontitis (p = 0.010) were higher in older than younger HIV+ patients. | Toljić et al., 2018 [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
DeVore, S.; Seleem, D.; Zhou, M. The Impact of HIV Infection and Aging on Periodontitis. Oral 2025, 5, 64. https://doi.org/10.3390/oral5030064
DeVore S, Seleem D, Zhou M. The Impact of HIV Infection and Aging on Periodontitis. Oral. 2025; 5(3):64. https://doi.org/10.3390/oral5030064
Chicago/Turabian StyleDeVore, Sophia, Dalia Seleem, and Miou Zhou. 2025. "The Impact of HIV Infection and Aging on Periodontitis" Oral 5, no. 3: 64. https://doi.org/10.3390/oral5030064
APA StyleDeVore, S., Seleem, D., & Zhou, M. (2025). The Impact of HIV Infection and Aging on Periodontitis. Oral, 5(3), 64. https://doi.org/10.3390/oral5030064






