Topical Oxygen Therapy (blue®m) for Post-Surgical Care Protocols to Promote Wound Healing in Periodontology and Dental Implants: A Case-Based Literature Review
Abstract
1. Introduction
Necessity of Surgical Wound Aftercare
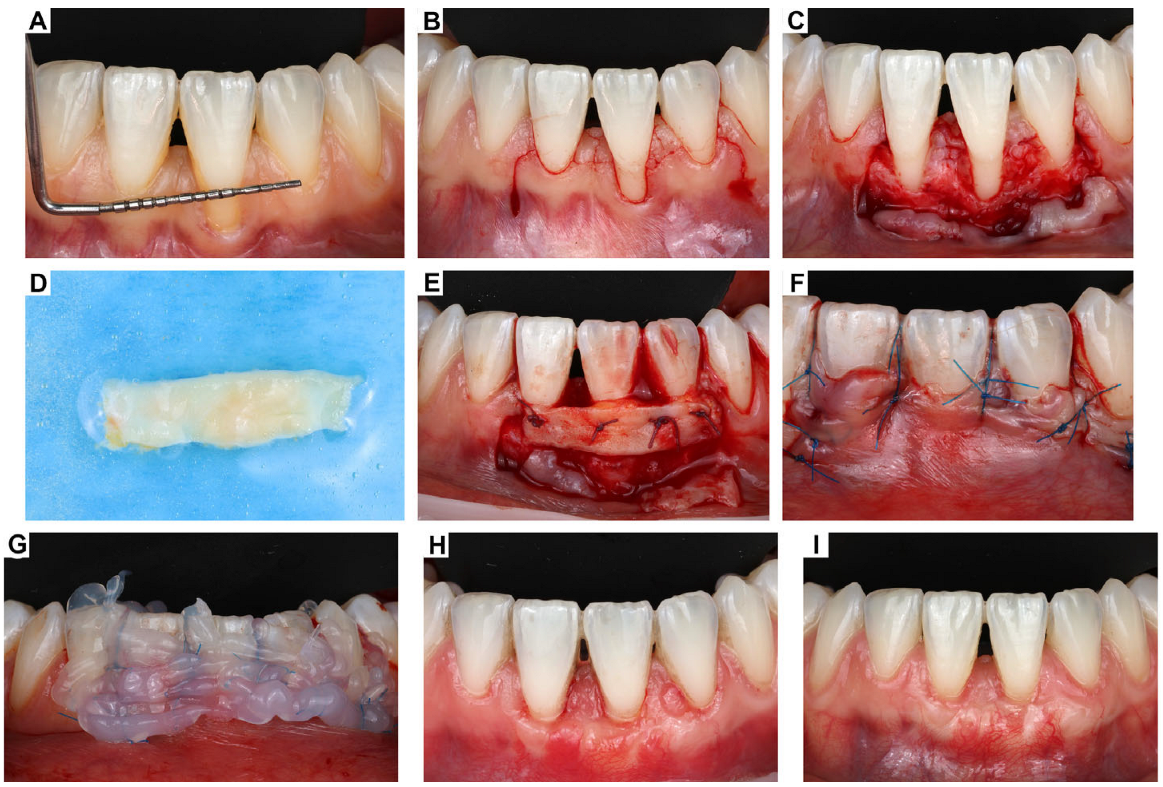
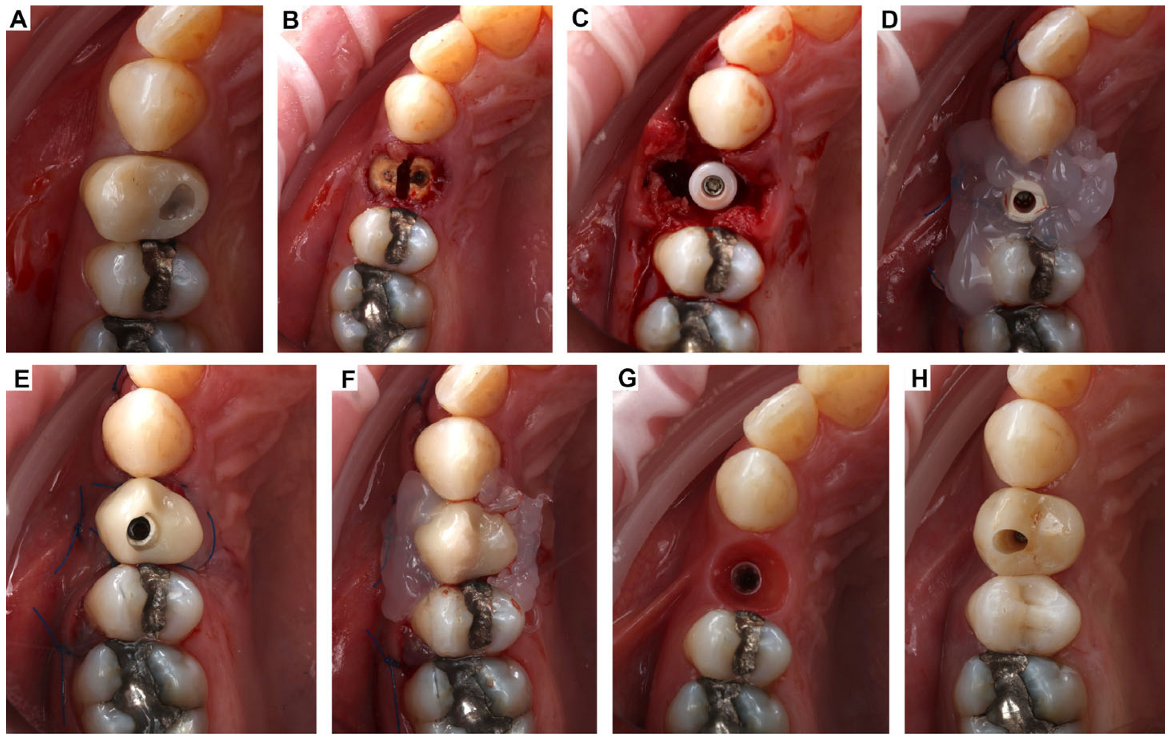
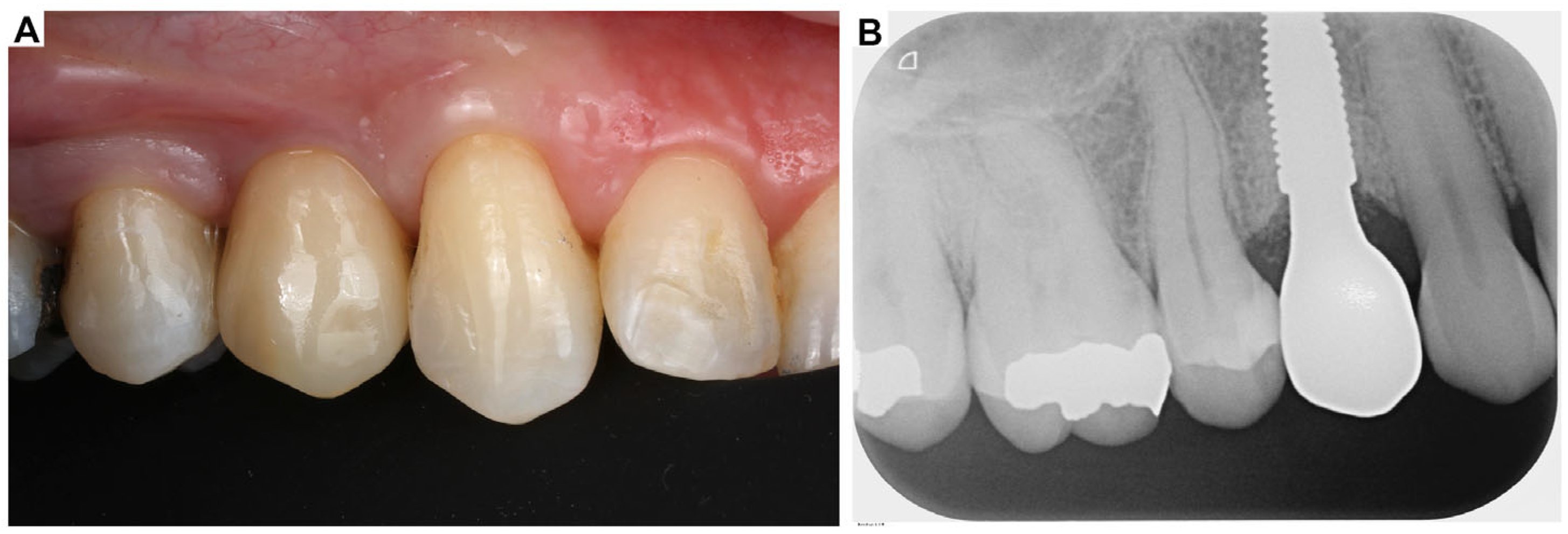
2. Clinical Case Presentation of Complex Periodontal Surgery
Case Report-1
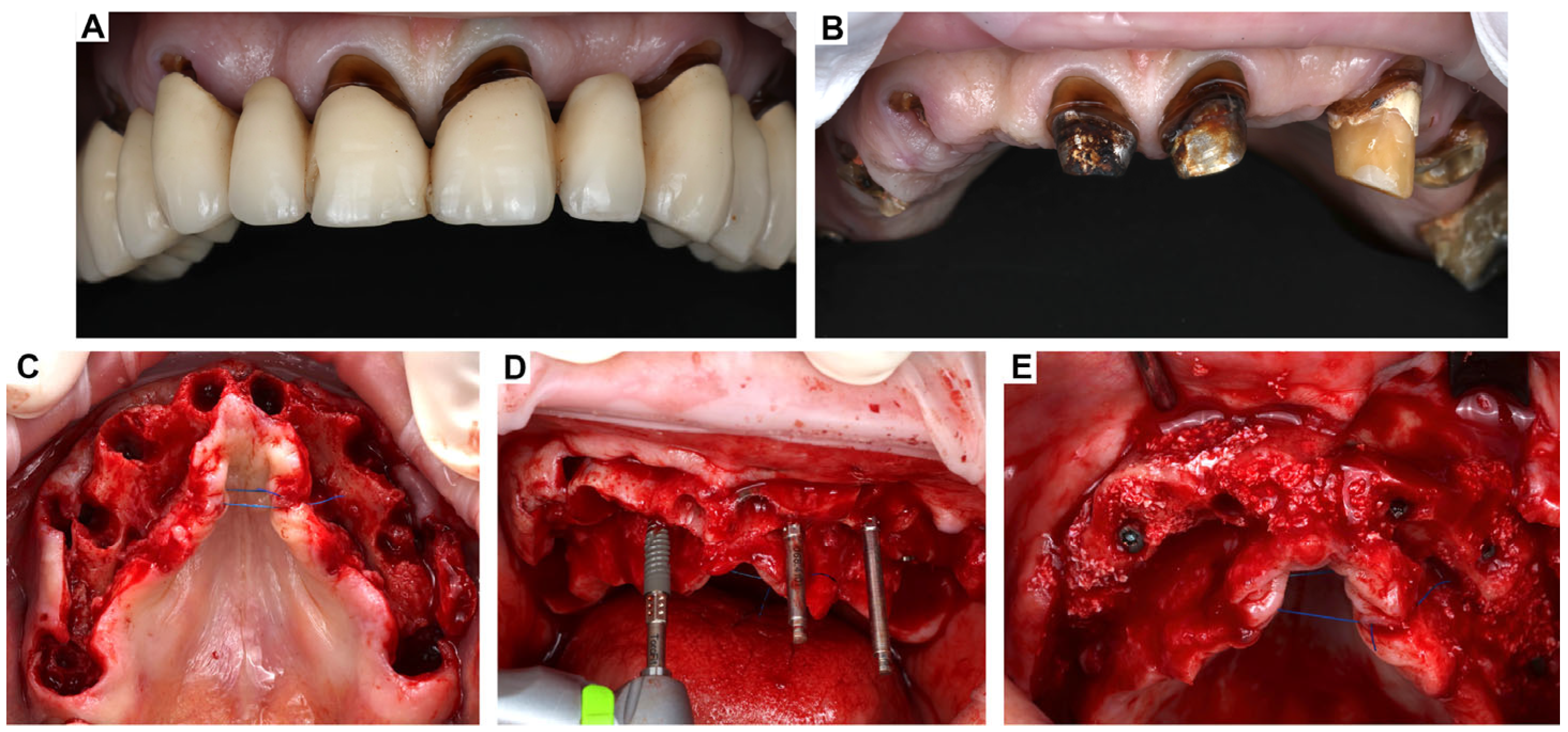
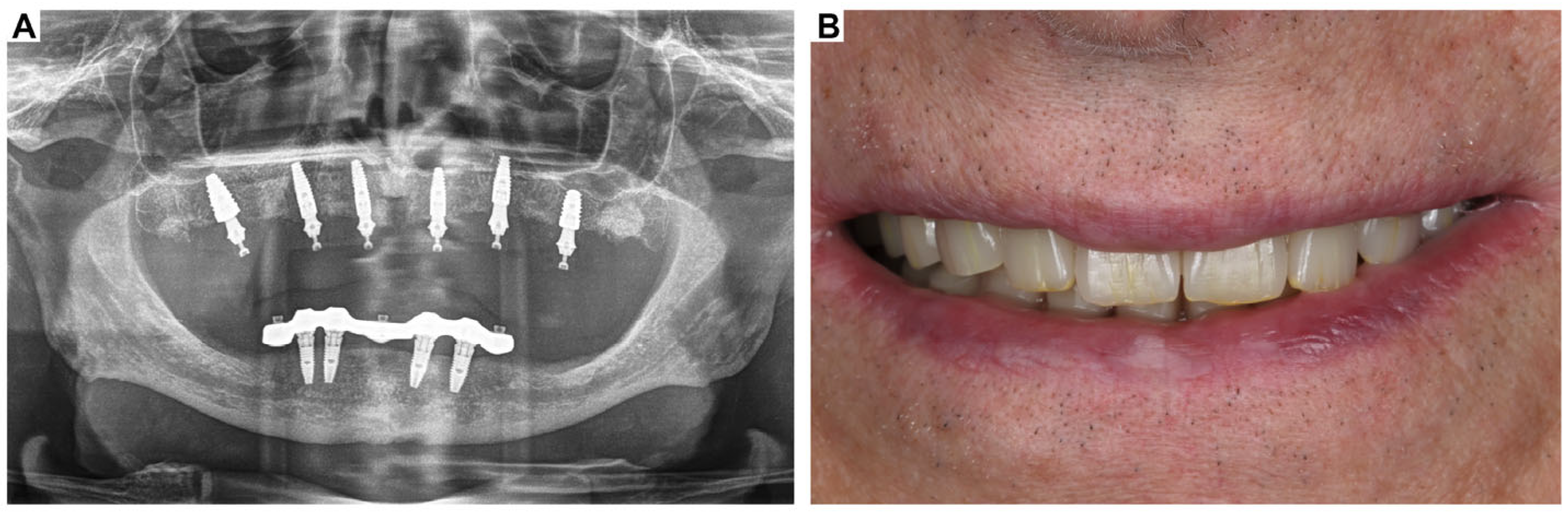
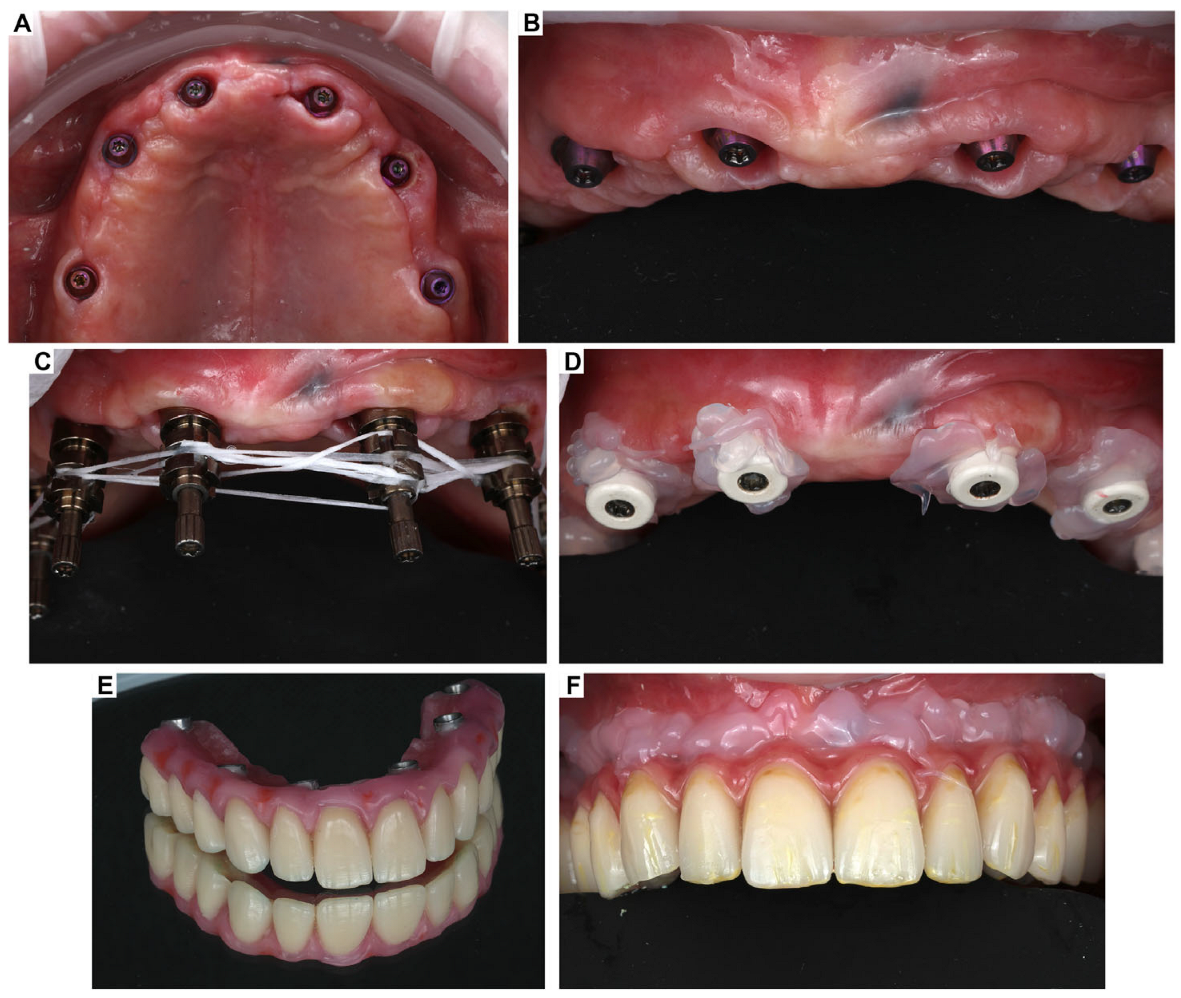
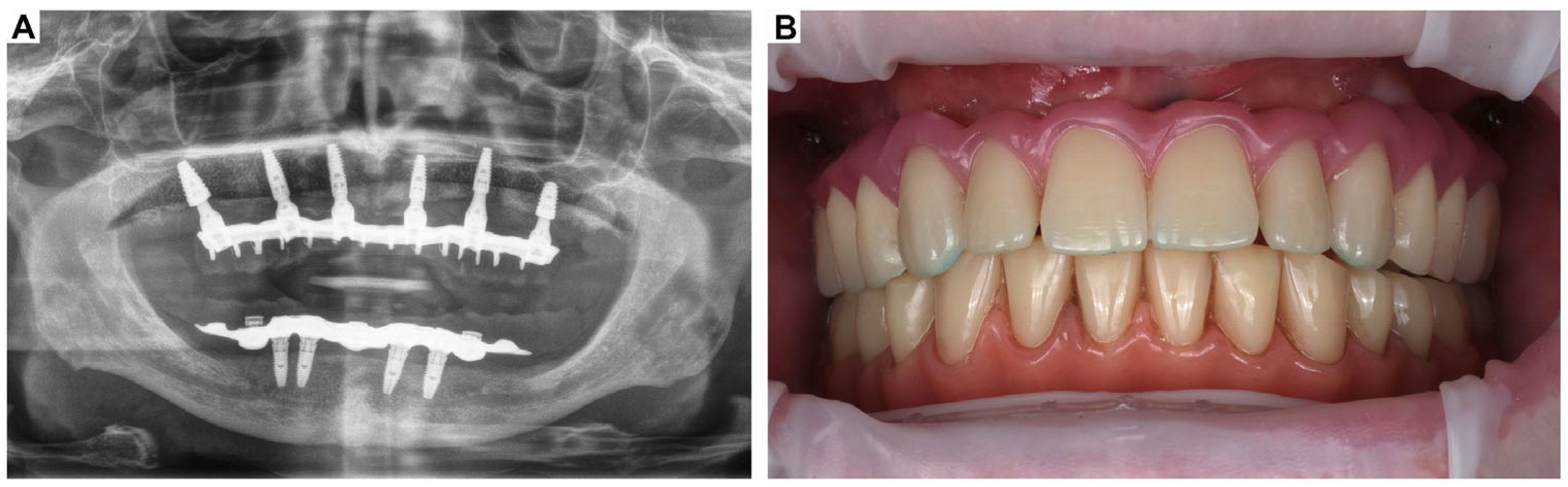
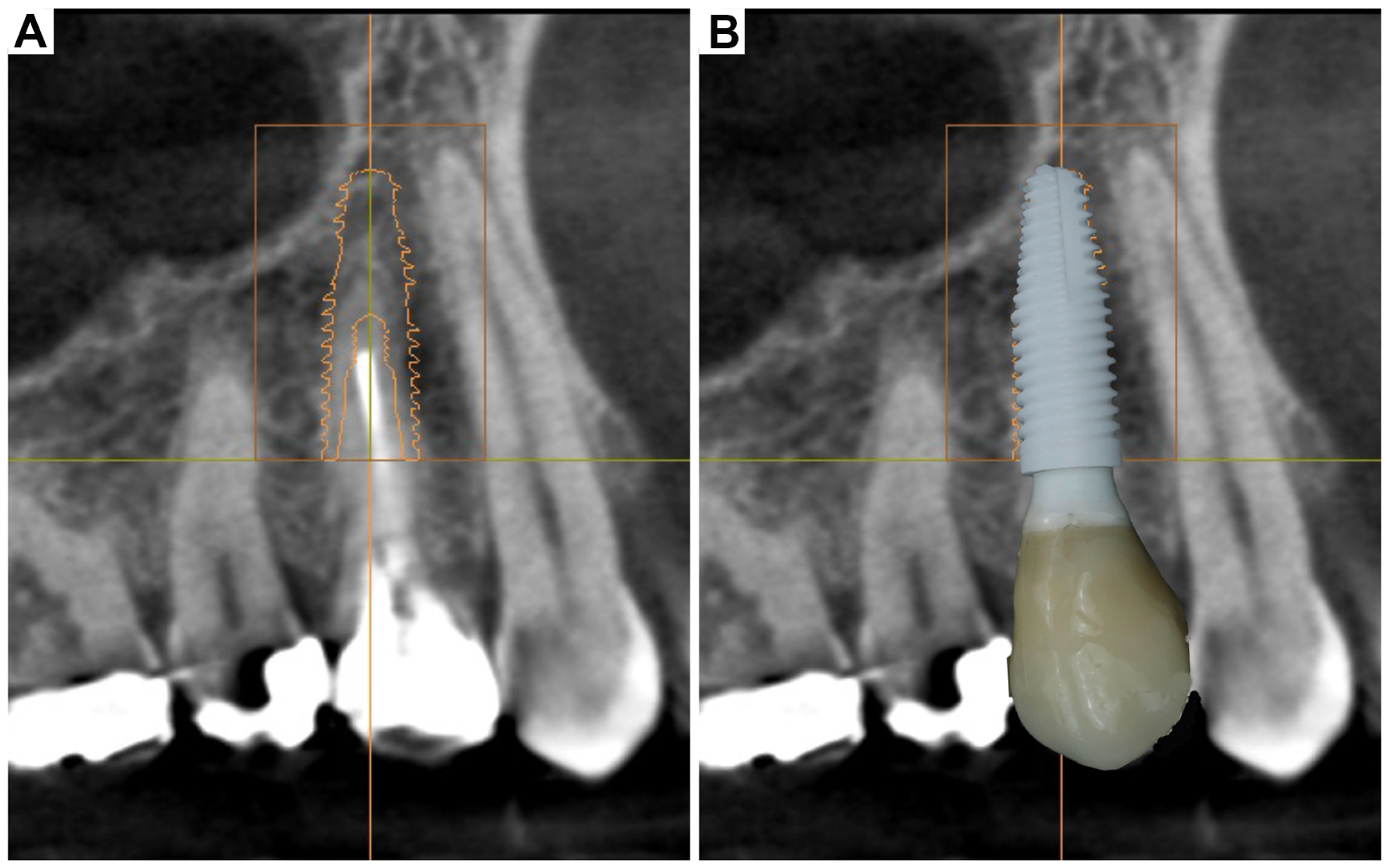
3. Clinical Cases Presentation of Advanced Implant Surgeries
3.1. Case Report-1
3.2. Case Report-2
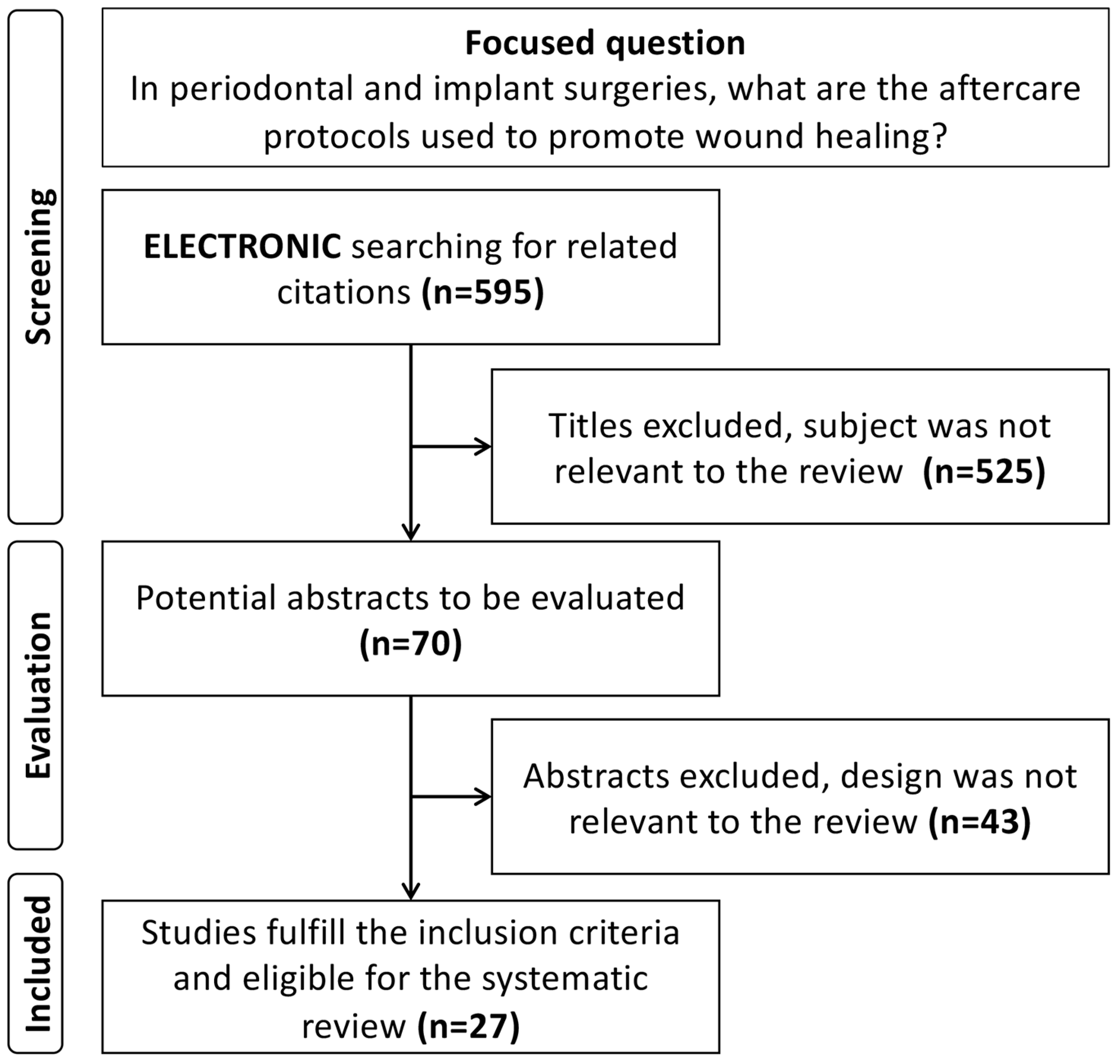
4. Systematic Review of the Literature: Methodology and Results
5. Discussion
Clinical Implications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mani, A.; Mani, S.; Sachdeva, S.; Sodhi, J.K.; Vora, H.R.; Gholap, S. Post-surgical care in surgical periodontics. Int. J. Periodontol. Implantol. 2021, 6, 74–78. [Google Scholar]
- Sanz, M.; Newman, M.G.; Anderson, L.; Matoska, W.; Otomo—Corgel, J.; Saltini, C. Clinical Enhancement of Post-Periodontal Surgical Therapy by a 0.12% Chlorhexidine Gluconate Mouthrinse. J. Periodontol. 1989, 60, 570–576. [Google Scholar] [CrossRef]
- Pippi, R. Post-surgical clinical monitoring of soft tissue wound healing in periodontal and implant surgery. Int. J. Med. Sci. 2017, 14, 721. [Google Scholar] [CrossRef] [PubMed]
- Powell, C.A.; Mealey, B.L.; Deas, D.E.; McDonnell, H.T.; Moritz, A.J. Post-surgical infections: Prevalence associated with various periodontal surgical procedures. J. Periodontol. 2005, 76, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-D.; Kim, K.-H.; Lee, Y.-M.; Ku, Y.; Seol, Y.-J. Periodontal wound healing and tissue regeneration: A narrative review. Pharmaceuticals 2021, 14, 456. [Google Scholar] [CrossRef] [PubMed]
- Brand, H.S.; Ligtenberg, A.; Veerman, E. Saliva and wound healing. Monogr. Oral Sci. 2014, 24, 52–60. [Google Scholar]
- Byrne, M.; Aly, A. The surgical suture. Aesthetic Surg. J. 2019, 39, S67–S72. [Google Scholar] [CrossRef]
- Leventis, M.; Deliberador, T.; Alshehri, F.; Alghamdi, H. Topical oxygen therapy as a novel strategy to promote wound healing and control the bacteria in implantology, oral surgery and periodontology: A review. Saudi Dent. J. 2024, 36, 841–854. [Google Scholar] [CrossRef]
- Shaheen, M.Y.; Abas, I.; Basudan, A.M.; Alghamdi, H.S. Local Oxygen-Based Therapy (blue® m) for Treatment of Peri-Implant Disease: Clinical Case Presentation and Review of Literature about Conventional Local Adjunct Therapies. Medicina 2024, 60, 447. [Google Scholar] [CrossRef]
- Benlidayi, I.C.; Gupta, L. CAse-BAsed REview sTandards (CABARET): Considerations for Authors, Reviewers, and Editors. J. Korean Med. Sci. 2024, 39, 225. [Google Scholar] [CrossRef]
- Maffei, S.H.; Fernandes, G.V.O.; Fernandes, J.C.H.; Orth, C.; Joly, J.C. Clinical and histomorphometric soft tissue assessment comparing free gingival graft and a collagen matrix as alveolar-sealer materials: A randomized controlled pilot clinical trial. Quintessence Int. 2023, 54, 756–769. [Google Scholar] [CrossRef]
- Palled, V.; Rao, J.; Singh, R.D.; Tripathi, S.; Singh, K.; Radav, R.; Verma, U.; Chand, P. Assessment of the Healing of Dental Implant Surgical Site Following Low-Level Laser Therapy Using Bioclinical Parameters: An Exploratory Study. J. Oral Implantol. 2021, 47, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Hagenaars, S.; Louwerse, P.H.; Timmerman, M.F.; Van der Velden, U.; Van der Weijden, G.A. Soft-tissue wound healing following periodontal surgery and Emdogain application. J. Clin. Periodontol. 2004, 31, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Vela, O.C.; Boariu, M.; Rusu, D.; Iorio-Siciliano, V.; Ramaglia, L.; Boia, S.; Radulescu, V.; Ilyes, I.; Stratul, S.I. Healing of Periodontal Suprabony Defects following Treatment with Open Flap Debridement with or without Hyaluronic Acid (HA) Application. Medicina 2024, 60, 829. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, E.; Uslu, M. Evaluation of the effects of platelet-rich fibrin, concentrated growth factors, and autologous fibrin glue application on wound healing following gingivectomy and gingivoplasty operations: A randomized controlled clinical trial. Quintessence Int. 2022, 53, 328–341. [Google Scholar] [CrossRef]
- Masse, J.F.; Landry, R.G.; Rochette, C.; Dufour, L.; Morency, R.; D’Aoust, P. Effectiveness of soft laser treatment in periodontal surgery. Int. Dent. J. 1993, 43, 121–127. [Google Scholar]
- Watanabe, H.; Ishikawa, I.; Suzuki, M.; Hasegawa, K. Clinical assessments of the erbium: YAG laser for soft tissue surgery and scaling. J. Clin. Laser Med. Surg. 1996, 14, 67–75. [Google Scholar] [CrossRef]
- Mutallibli, A.; Sağlam, M. Comparison of the effect of A-PRF and L-PRF application to palatal donor sites on quality of life and wound healing after free gingival graft surgery. Quintessence Int. 2024, 55, 472–481. [Google Scholar] [CrossRef]
- Heitz, F.; Heitz-Mayfield, L.J.; Lang, N.P. Effects of post-surgical cleansing protocols on early plaque control in periodontal and/or periimplant wound healing. J. Clin. Periodontol. 2004, 31, 1012–1018. [Google Scholar] [CrossRef]
- de Araújo Nobre, M.; Cintra, N.; Maló, P. Peri-implant maintenance of immediate function implants: A pilot study comparing hyaluronic acid and chlorhexidine. Int. J. Dent. Hyg. 2007, 5, 87–94. [Google Scholar] [CrossRef]
- Kumar, M.B.V.; Narayanan, V.; Jalaluddin, M.; Almalki, S.A.; Dey, S.M.; Sathe, S. Assessment of Clinical Efficacy of Different Periodontal Dressing Materials on Wound Healing: A Comparative Study. J. Contemp. Dent. Pract. 2019, 20, 896–900. [Google Scholar]
- Gatti, F.; Iorio-Siciliano, V.; Scaramuzza, E.; Tallarico, M.; Vaia, E.; Ramaglia, L.; Chiapasco, M. Patient-reported outcome measures of leucocyte- and platelet-rich fibrin (L-PRF) or hemostatic agent application at palatal donor sites after free gingival graft harvesting: A randomized controlled clinical trial. Quintessence Int. 2023, 54, 408–417. [Google Scholar] [CrossRef]
- Sander, L.; Frandsen, E.V.; Arnbjerg, D.; Warrer, K.; Karring, T. Effect of local metronidazole application on periodontal healing following guided tissue regeneration. Clinical findings. J. Periodontol. 1994, 65, 914–920. [Google Scholar] [CrossRef]
- Uslu, M.; Akgül, S. Evaluation of the effects of photobiomodulation therapy and ozone applications after gingivectomy and gingivoplasty on postoperative pain and patients’ oral health-related quality of life. Lasers Med. Sci. 2020, 35, 1637–1647. [Google Scholar] [CrossRef]
- Trombelli, L.; Scabbia, A.; Scapoli, C.; Calura, G. Clinical effect of tetracycline demineralization and fibrin-fibronectin sealing system application on healing response following flap debridement surgery. J. Periodontol. 1996, 67, 688–693. [Google Scholar] [CrossRef]
- Yaghobee, S.; Rouzmeh, N.; Taheri, M.; Aslroosta, H.; Mahmoodi, S.; Mohammadnejad Hardoroodi, M.; Soleimanzadeh Azar, P.; Khorsand, A. Evaluation of topical erythropoietin application on the healing outcome of gingival graft recipient site; A randomized controlled clinical trial. BMC Oral Health 2021, 21, 578. [Google Scholar] [CrossRef]
- Langebaek, J.; Bay, L. The effect of chlorhexidine mouthrinse on healing after gingivectomy. Eur. J. Oral Sci. 1976, 84, 224–228. [Google Scholar] [CrossRef]
- Frandsen, E.V.; Sander, L.; Arnbjerg, D.; Theilade, E. Effect of local metronidazole application on periodontal healing following guided tissue regeneration. Microbiological findings. J. Periodontol. 1994, 65, 921–928. [Google Scholar] [CrossRef]
- Palombo, D.; Dobos, A.; Duran, M.L.; Esporrin, J.S.; Sanz, M. Harvest of epithelialized gingival grafts without application of hemostatic sutures: A randomized clinical trial using laser speckle contrast imaging. J. Periodontol. 2024, 95, 1160–1170. [Google Scholar] [CrossRef]
- Ibrahim, S.S.A.; Mandil, I.A.; Ezzatt, O.M. Injectable platelet rich fibrin effect on laser depigmented gingiva: A clinical randomized controlled split mouth trial with histological assessment. J. Appl. Oral Sci. 2024, 32, e20230307. [Google Scholar] [CrossRef]
- Çankaya, Z.T.; Gürbüz, S.; Bakirarar, B.; Kurtiş, B. Evaluation of the Effect of Hyaluronic Acid Application on the Vascularization of Free Gingival Graft for Both Donor and Recipient Sites with Laser Doppler Flowmetry: A Randomized, Examiner-Blinded, Controlled Clinical Trial. Int. J. Periodontics Restor. Dent. 2020, 40, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Peñarrocha-Diago, M.; Menéndez-Nieto, I.; Cervera-Ballester, J.; Maestre-Ferrín, L.; Blaya-Tárraga, J.A.; Peñarrocha-Oltra, D. Influence of Hemostatic Agents in the Prognosis of Periapical Surgery: A Randomized Study of Epinephrine versus Aluminum Chloride. J. Endod. 2018, 44, 1205–1209. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, M.P.; Fernandes-Dias, S.B.; Araújo, C.F.; Lucas da Silva Neves, F.; Mathias, I.F.; Rebelato Bechara Andere, N.M.; Neves Jardini, M.A. 2-Year Assessment of Tissue Biostimulation With Low-Level Laser on the Outcomes of Connective Tissue Graft in the Treatment of Single Gingival Recession: A Randomized Clinical Trial. J. Periodontol. 2017, 88, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Monea, A.; Beresescu, G.; Boeriu, S.; Tibor, M.; Popsor, S.; Antonescu, D.M. Bone healing after low-level laser application in extraction sockets grafted with allograft material and covered with a resorbable collagen dressing: A pilot histological evaluation. BMC Oral Health 2015, 15, 134. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.V.; Kumar, S.; Vidya, G.D.; Patel, A.; Holmes, J.C.; Kumar, V. Cytological assessment of healing palatal donor site wounds and grafted gingival wounds after application of ozonated oil: An eighteen-month randomized controlled clinical trial. Acta Cytol. 2012, 56, 277–284. [Google Scholar] [CrossRef]
- Bokor, M. The effect of hexetidine spray on dental plaque following periodontal surgery. J. Clin. Periodontol. 1996, 23, 1080–1083. [Google Scholar] [CrossRef]
- Trombelli, L.; Scabbia, A.; Wikesjö, U.M.; Calura, G. Fibrin glue application in conjunction with tetracycline root conditioning and coronally positioned flap procedure in the treatment of human gingival recession defects. J. Clin. Periodontol. 1996, 23, 861–867. [Google Scholar] [CrossRef]
- Menchini-Fabris, G.-B.; Cosola, S.; Toti, P.; Hwan Hwang, M.; Crespi, R.; Covani, U. Immediate Implant and Customized Healing Abutment for a Periodontally Compromised Socket: 1-Year Follow-Up Retrospective Evaluation. J. Clin. Med. 2023, 12, 2783. [Google Scholar] [CrossRef]
- Blinder, D.; Rotenberg, L.; Peleg, M.; Taicher, S. Patient compliance to instructions after oral surgical procedures. Int. J. Oral Maxillofac. Surg. 2001, 30, 216–219. [Google Scholar] [CrossRef]
- Pilloni, A.; Ceccarelli, S.; Bosco, D.; Gerini, G.; Marchese, C.; Marini, L.; Rojas, M.A. Effect of chlorhexidine digluconate in early wound healing of human gingival tissues. A histological, immunohistochemical and biomolecular analysis. Antibiotics 2021, 10, 1192. [Google Scholar] [CrossRef]
- Teixeira, D.D.S.; Figueiredo, M.A.Z.; Cherubini, K.; de Oliveira, S.D.; Salum, F.G. The topical effect of chlorhexidine and povidone-iodine in the repair of oral wounds. A review. Stomatologija 2019, 21, 35–41. [Google Scholar]
- Alghamdi, H.; Leventis, M.; Deliberador, T. Management of Infected Tissues Around Dental Implants: A Short Narrative Review. Braz. Dent. J. 2024, 35, e246160. [Google Scholar] [CrossRef] [PubMed]
- Shibli, J.A.; Rocha, T.F.; Coelho, F.; de Oliveira Capote, T.S.; Saska, S.; Melo, M.A.; Pingueiro, J.M.S.; de Faveri, M.; Bueno-Silva, B. Metabolic activity of hydro-carbon-oxo-borate on a multispecies subgingival periodontal biofilm: A short communication. Clin. Oral Investig. 2021, 25, 5945–5953. [Google Scholar] [CrossRef] [PubMed]
- Grover, M.; Gupta, A.; Dubey, T.; Dua, P.; Arora, G.K. New age oxygen therapy for enhanced recovery: BLUE® M oral gel in post-extraction socket healing. J. Dent. Spec. 2025, 13, 131–137. [Google Scholar] [CrossRef]
- Basudan, A.M.; Abas, I.; Shaheen, M.Y.; Alghamdi, H.S. Effectiveness of Topical Oxygen Therapy in Gingivitis and Periodontitis: Clinical Case Reports and Review of the Literature. J. Clin. Med. 2024, 13, 1451. [Google Scholar] [CrossRef]
- Yakout, B.K.; Kamel, F.R.; Khadr, M.A.E.-A.A.; Heikal, L.A.H.; El-Kimary, G.I. Efficacy of hyaluronic acid gel and photobiomodulation therapy on wound healing after surgical gingivectomy: A randomized controlled clinical trial. BMC Oral Health 2023, 23, 805. [Google Scholar] [CrossRef]
- Sadeghian, A.; Rohani, B.; Salehi-Marzijarani, M.; Fekrazad, R. Radiographical impact of photobiomodulation therapy on bone regeneration in clinical studies: A systematic review. Lasers Med. Sci. 2025, 40, 23. [Google Scholar] [CrossRef]
- Damante, C.A.; Greghi, S.L.; Sant’Ana, A.C.; Passanezi, E.; Taga, R. Histomorphometric study of the healing of human oral mucosa after gingivoplasty and low-level laser therapy. Lasers Surg. Med. 2004, 35, 377–384. [Google Scholar] [CrossRef]

| Study | Intervention | Post-Surgical Care | Follow-Up | Assessment | Outcome |
|---|---|---|---|---|---|
| Maffei et al. [11] | Socket preservation | Alveolar sealing: free gingival graft (FGG) vs. porcine collagen membrane (MS) | 3, 7, 15, 30, 60, 90, and 120 days | Visual analog scale | The result was superior and more significant for the MS group, with faster wound healing and lower discomfort. |
| Palled et al. [12] | Implant surgical site | Low-level laser therapy | 2 weeks, 6 weeks, and 3 months | Implant stability, probing index, bleeding index, and osteoprotegerin level | The healing of peri-implant soft tissues may be enhanced with the use of low-level laser therapy. |
| Hagenaars et al. [13] | Periodontal flap surgery | Emdogain | 1, 4, and 8 weeks | Gingival index | The early wound-healing using Emdogain exhibited higher gingival swelling. |
| Vela et al. [14] | Suprabony periodontal defects | Hyaluronic acid (HA) | baseline and 12 months | Probing pocket depth (PPD), gingival recession (GR), full-mouth plaque score (FMPS), and full-mouth bleeding score (FMBS) | Wound healing could benefit from the additional application of HA. |
| Bozkurt et al. [15] | Gingivectomy and gingivoplasty | Platelet-rich fibrin (PRF), concentrated growth factors (CGF), and autologous fibrin glue (AFG) | days 0, 7, 14, and 28 | Wound healing was evaluated with H2O2 test, visual analog scale for pain, and Landry, Turnbull, and Howley (LTH) index | PRF, CGF, and AFG application were found to have positive effects on wound healing. |
| Masse et al. [16] | Mucogingival procedures | Soft-laser treatment | days 7 and 14 | Modified McGill pain scale, inflammatory index, and Landry, Turnbull, Howley (LTH) Index | Soft laser (As-Ga and He-Ne) not a useful aftercare treatment for wound healing. |
| Watanabe et al. [17] | Soft tissue surgery | Erbium:YAG laser | weeks 2, 4, and 6 | Pain, redness, and swelling of the gingiva and the subjective patient comfort parameters | Er:YAG laser is useful for soft tissue surgery and scaling. |
| Mutallibli and Sağlam, [18] | Palatal donor wounds | Leukocyte platelet-rich fibrin (L-PRF) and advanced platelet-rich fibrin (A-PRF) | days 7 and 14 | H2O2 test, visual analog scale, and Oral Health Impact Profile-14 (OHIP-14) score | Both PRF procedures have similar effects on palatal wound healing and quality of life. |
| Heitz et al. [19] | Periodontal flap surgery | 0.1% of chlorhexidine (CHX) | 1, 2, and 4 weeks | Gingival crevicular fluid (GCF) flow rate, probing depth, probing attachment level, presence of bleeding on probing, and full-mouth plaque score | The use of post-surgical cleansing protocols may be recommended. |
| de Araújo Nobre et al. [20] | Dental implant surgery | Hyaluronic acid (HA) vs. chlorhexidine (CHX) gels | 10th day, 2 months, 4 months, and 6 months post-surgery | Plaque and bleeding index | The findings point out the importance of a maintenance protocol in immediate function implants. |
| Kumar et al. [21] | Periodontal flap surgery | Collagen dressing, light-cure dressing, and non-eugenol-based dressing | days 7 and 14 | Plaque index, vertical probing depth, pain, gingival index, patient satisfaction, and visual analog scale (VAS) | The periodontal wound covered with a collagen dressing provide significant symptomatic relief and better healing. |
| Gatti et al. [22] | Palatal donor wounds | Leucocyte- and platelet-rich fibrin (L-PRF) membranes or a hemostatic agent with oxidized and regenerated cellulose | 1 week | Postoperative pain, postoperative discomfort, inability to chew, postoperative stress, surgical chair time, and thickness of the palatal fibro-mucosa | The application of L-PRF membrane at palatal donor sites after FGG harvesting did not produce significant advantages for the patients. |
| Sander et al. [23] | Guided tissue regeneration (GTR) | Metronidazole gel | 4 and 6 weeks | Plaque, bleeding on probing, and inflammation of marginal gingiva | Application of metronidazole gel has a beneficial effect on healing of guided tissue regeneration. |
| Uslu and Akgül [24] | Gingivectomy and gingivoplasty | Photobiomodulation therapy (PBM) and ozone applications | days 3, 7, 14, and 28 | Visual analogue scale (VAS) and Oral Health Impact Profile (OHIP-14) score | The PBM and ozone applications after gingivectomy and gingivoplasty reduce the pain levels. |
| Trombelli et al. [25] | Flap debridement surgery | Tetracycline (TTC) conditioning and fibrin-fibronectin sealing system (FFSS) | 0–6 months | Gingival index, plaque control record, clinical attachment level, probing depth, recession, and bleeding on probing | These results suggest there is no additional benefit with TTC demineralization and topical FFSS application on wound healing. |
| Yaghobee et al. [26] | Free gingival graft (FGG) | Topical erythropoietin | days 7, 14, 21, 28, 60, and 90 | Blinded observers to compare the healing and inflammation of the areas | Topical application of erythropoietin can accelerate the healing of gingival grafts and reduce the inflammation during healing period. |
| Langebaek and Bay [27] | Gingivectomy | 0.2% chlorhexidine (CHX) and Coe-Pak dressing | days 7, 14, and 21 | Plaque index and gingival index | CHX and Coe-Pak dressing maintained low plaque scores and promoted healing. |
| Frandsen et al. [28] | Guided tissue regeneration (GTR) | Local metronidazole gel | 2 weeks | Microbial colonization of the wound area | The influence of metronidazole gel on wound healing appears not significant. |
| Palombo et al. [29] | Palatal epithelialized gingival grafts (EGG) | Hemostatic sponges | 7, 14, and 30 days | Laser speckle contrast imaging (LSCI), postoperative bleeding, pain, discomfort, and analgesic consumption | Hemostatic sutures provide no relevant differences in microvascular, clinical, and patient-related results. |
| Ibrahim et al. [30] | Diode laser gingival depigmentation (DLGD) | Injectable platelet-rich fibrin (i-PRF) | 1 week and 1 and 3 months | Wound Healing Score (WHS), patient satisfaction, and Pigmentation Index (DOPI) | i-PRF demonstrated better clinical and histological healing potential and less patient discomfort. |
| Çankaya et al. [31] | Free gingival graft (FGG) | Topical hyaluronic acid (HA) | 4, 7, 10, 14, and 30 days | Laser doppler flowmetry (LDF) | Application of HA on the recipient bed under the FGG at the first week of healing allows the formation of a well-vascularized layer. |
| Peñarrocha-Diago et al. [32] | Periapical surgery | Hemostatic agents: epinephrine or aluminum chloride | 12 months | Hemorrhage control index | The hemostatic agents showed no relationship with the healing outcome. |
| Santamaria et al. [33] | Periodontal plastic surgery | Low-level laser therapy (LLLT) | 0, 2, 4, 6, 7, 10, 12, and 14 days and 2 years | Clinical and esthetic evaluations | LLLT showed no additional benefit in wound healing. |
| Monea et al. [34] | Extraction/socket graft and implant placement | Low-level laser therapy (LLLT) | 2 and 3 months | Biopsy analysis | LLLT photobiomodulation can reduce the healing time after grafting the extraction socket. |
| Patel et al. [35] | Free gingival graft (FGG) | Topical ozonated oil | days 1, 3, 7, 14, and 21; months 2, 3, 8, and 18 | Cytological analysis: keratinization and superficial cell indices | Aftercare showed significant improvement in epithelial healing and gingival health. |
| Bokor [36] | Periodontal surgery | 0.2% hexetidine spray | days 0, 7, 14, 21, and 28 | Turesky modification of Quigley–Hein index, Löe–Silness, and the papilla bleeding index | Significant reduction in plaque accumulation and an improvement in wound healing were demonstrated for the test spray. |
| Trombelli et al. [37] | Coronally positioned flap (CPF) | Fibrin glue (FG) and tetracycline HCI (TTC) | 6 months | Recession depth reduction and attachment gain | There were no clinically significant effects of fibrin glue on wound healing. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scognamiglio, C.; Perucchi, A.; Sundar, C.; Deliberador, T.M.; Alghamdi, H. Topical Oxygen Therapy (blue®m) for Post-Surgical Care Protocols to Promote Wound Healing in Periodontology and Dental Implants: A Case-Based Literature Review. Oral 2025, 5, 53. https://doi.org/10.3390/oral5030053
Scognamiglio C, Perucchi A, Sundar C, Deliberador TM, Alghamdi H. Topical Oxygen Therapy (blue®m) for Post-Surgical Care Protocols to Promote Wound Healing in Periodontology and Dental Implants: A Case-Based Literature Review. Oral. 2025; 5(3):53. https://doi.org/10.3390/oral5030053
Chicago/Turabian StyleScognamiglio, Cristian, Alessandro Perucchi, Chalini Sundar, Tatiana Miranda Deliberador, and Hamdan Alghamdi. 2025. "Topical Oxygen Therapy (blue®m) for Post-Surgical Care Protocols to Promote Wound Healing in Periodontology and Dental Implants: A Case-Based Literature Review" Oral 5, no. 3: 53. https://doi.org/10.3390/oral5030053
APA StyleScognamiglio, C., Perucchi, A., Sundar, C., Deliberador, T. M., & Alghamdi, H. (2025). Topical Oxygen Therapy (blue®m) for Post-Surgical Care Protocols to Promote Wound Healing in Periodontology and Dental Implants: A Case-Based Literature Review. Oral, 5(3), 53. https://doi.org/10.3390/oral5030053








