Low-Grade Myofibroblastic Sarcoma in the Oral Cavity: Case Report and Clinical Insights
Abstract
1. Introduction
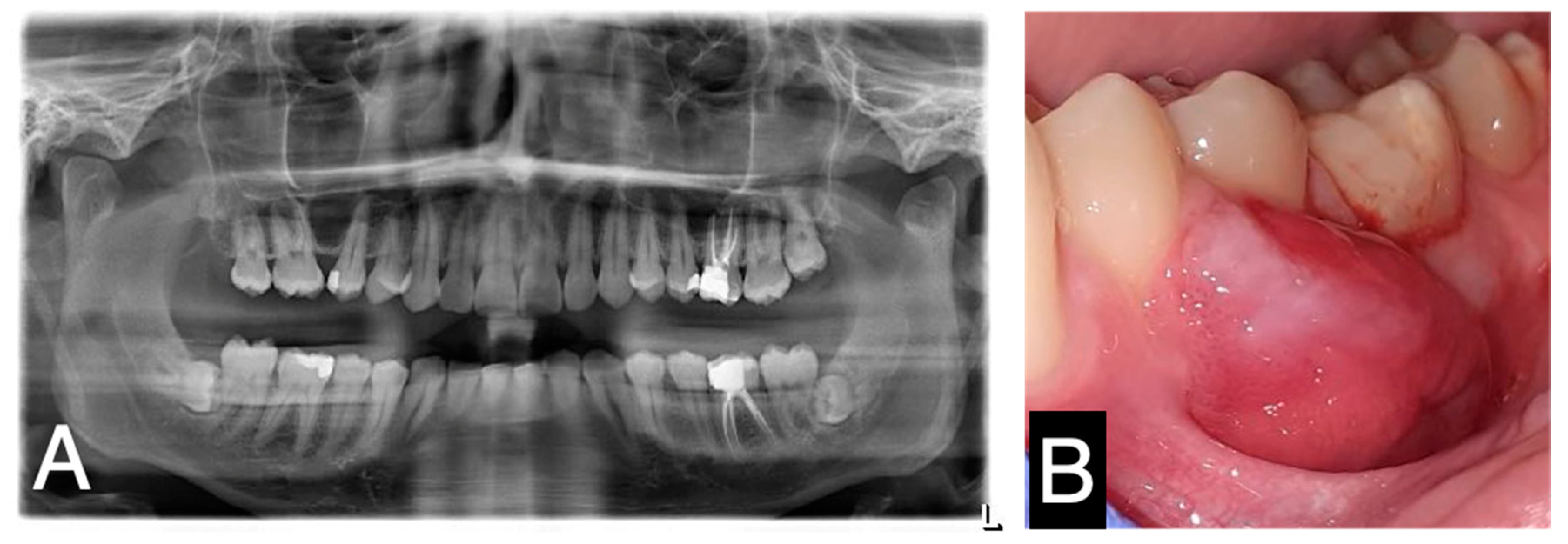
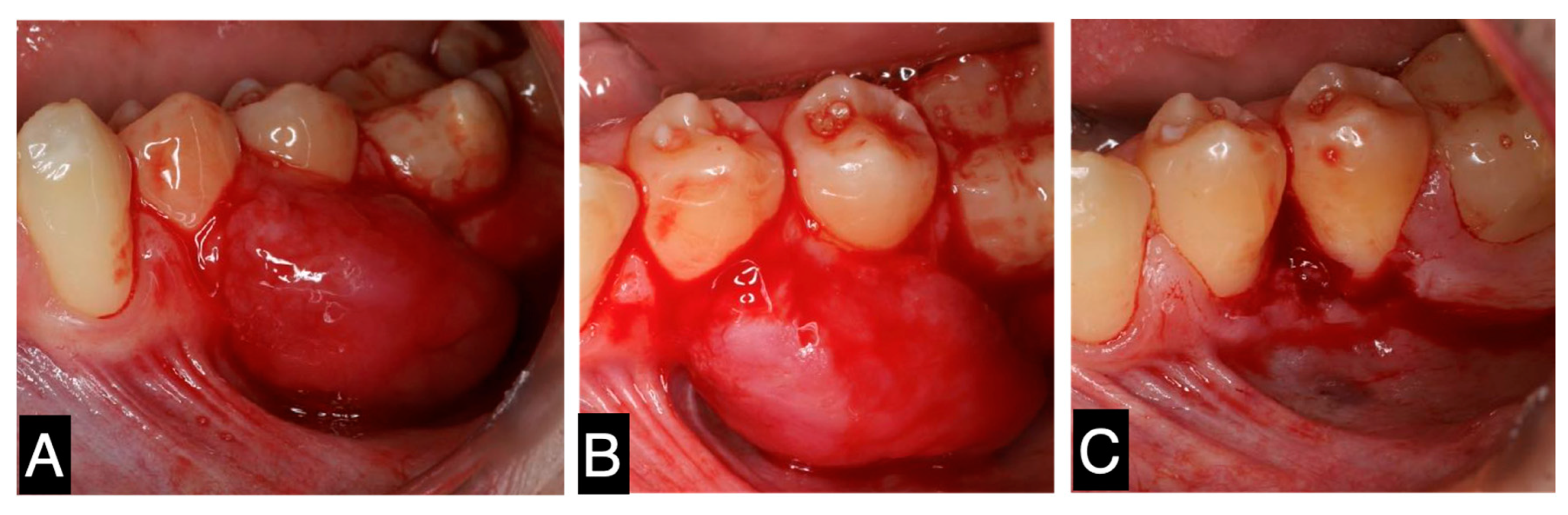
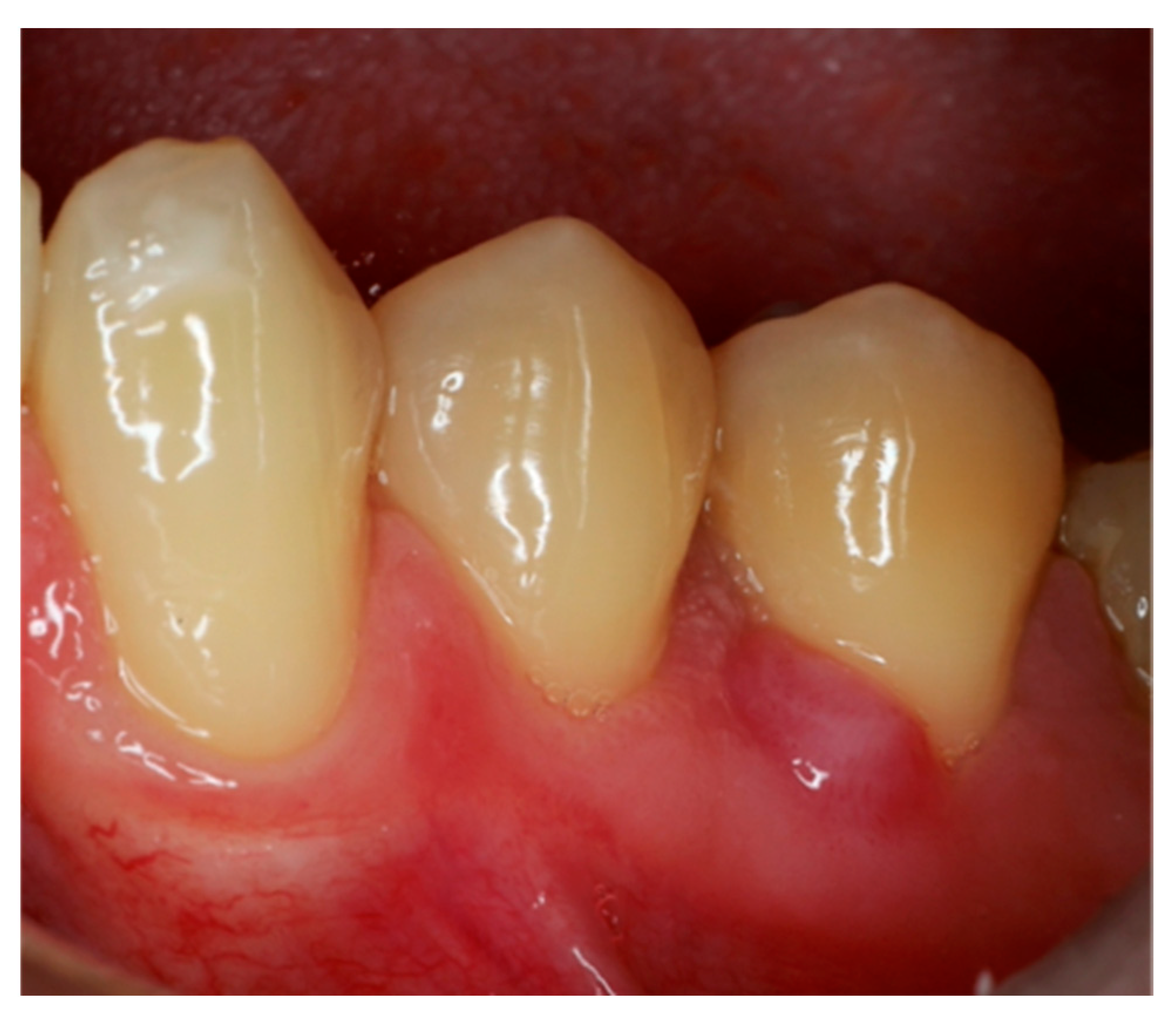
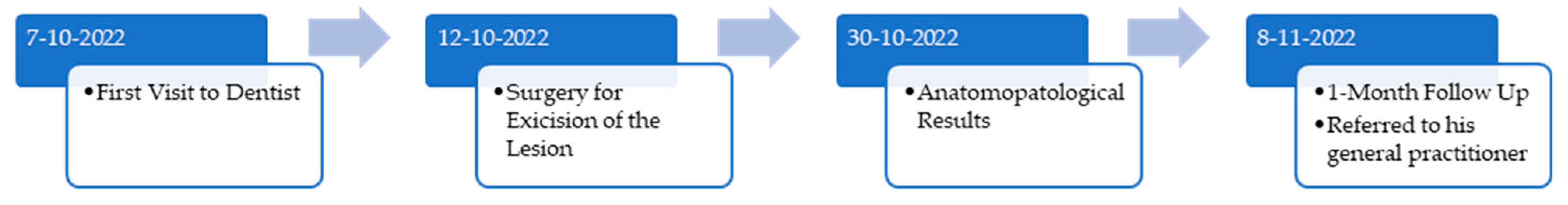
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gómez-Oliveira, G.; Arribas-García, I.; Serrano Alvarez-Buylla, A.; Sánchez-Burgos, R.; Martínez-Pérez, F.; Alvarez-Flores, M. Low-grade myofibroblastic sarcoma. Two rare tumors in two rare locations. Rev. Española Cirugía Oral Maxilofacial 2015, 37, 108–112. [Google Scholar] [CrossRef]
- Wang, L.; Li, L.X.; Chen, D.Q.; Yang, L.; Li, S.K.; Cheng, C. Low-grade Myofibroblastic sarcoma: Clinical and imaging findings. BMC Med. Imaging 2019, 19, 1471–2342. [Google Scholar] [CrossRef] [PubMed]
- Jayasooriya, P.R.; Athukorala, C.; Attygalla, M.; Mendis, B.; Lombardi, T. Low-Grade Myofibroblastic Sarcoma of the Oral Cavity: A Report of Three Cases Illustrating an Emerging Disease in Children. Dermatopathology 2021, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, E.; Goldblum, J.R.; Fisher, C. Myofibrosarcoma: A clinicopathologic study. Am. J. Surg. Pathol. 2001, 25, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Dehner, L.P.; El-Mofty, S.K. In myofibroblastic sarcomas of the head and neck, mitotic activity and necrosis define grade: A case study and literature review. Virchows Arch. 2013, 463, 827–836. [Google Scholar] [CrossRef]
- Giraldo-Roldan, D.; Louredo, B.V.R.; Penafort, P.V.M.; Pontes, H.A.R.; Alves, A.P.; Lima, F.C.A.; Fonseca, T.C.; Abrahão, A.C.; Romañach, M.J.; Fonseca, F.P.; et al. Low-Grade Myofibroblastic Sarcoma of the Oral and Maxillofacial Region: An International Clinicopathologic Study of 13 Cases and Literature Review. Head Neck Pathol. 2023, 17, 832–850. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Bandopadhyay, A.; Sarkar, R. Low-grade myofibroblastic sarcoma of maxillary sinus and buccal mucosa: Two rare cases and review of the literature. Indian J. Pathol. Microbiol. 2019, 62, 119–121. [Google Scholar] [CrossRef]
- Demarosi, F.; Bay, A.; Moneghini, L.; Carrassi, A. Low-grade myofibroblastic sarcoma of the oral cavity. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009, 108, 248–254. [Google Scholar] [CrossRef]
- Schenker, A.; Gutjahr, E.A.-O.; Lehner, B.; Mechtersheimer, G.; Wardelmann, E.A.-O.; Klotz, R.A.-O.X.; Kalkum, E.; Schiltenwolf, M.; Harhaus, L.; Renkawitz, T.; et al. A Systematic Review and Illustrative Case Presentation of Low-Grade Myofibroblastic Sarcoma (LGMS) of the Extremities. J. Clin. Med. 2023, 12, 7027. [Google Scholar] [CrossRef]
- Feng, Y.; Du, Y.; Li, M.; Jia, G.; Gong, L.; Li, L. Low-Grade malignant myofibroblastic sarcoma of the larynx: A case report. J. Int. Med. Res. 2023, 51, 3000605231193929. [Google Scholar] [CrossRef] [PubMed]
- Padmawar, N.S.; Bhadange, S.; Mustilwar, R.G.; Mopagar, V.P.; Vadvadgi, V.H.; Joshi, S.R. Aberrant Location of Low-Grade Myofibroblastic Sarcoma of the Gingiva in Posterior Maxilla. Int. J. Clin. Pediatr. Dent. 2021, 14, 816–819. [Google Scholar] [CrossRef] [PubMed]
- Coyne, J.D. Low-grade myofibroblastic sarcoma of the piriform fossa: A case report with a literature review of a tumour with a predilection for the head and neck. Br. J. Oral Maxillofac. Surg. 2007, 45, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Mentzel, T.; Dry, S.; Katenkamp, D.; Christopher, F. Low-grade myofibroblastic sarcoma: Analysis of 18 cases in the spectrum of myofibroblastic tumors. Am. J. Surg. Pathol. 1998, 22, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C. Myofibrosarcoma. Virchows Arch. 2004, 445, 215–223. [Google Scholar] [CrossRef]
- Magro, G. Differential Diagnosis of Benign Spindle Cell Lesions. Surg. Pathol. Clin. 2018, 11, 91–121. [Google Scholar] [CrossRef]
- Christopher, D.M.; Fletcher, K.K.U.; Mertens, F. Pathology and Genetics of Tumours of Soft Tissue and Bone; IARC Press: Lyon, France, 2002; pp. 94–95. [Google Scholar]
- Velez Torres, J.M.; Martinez Duarte, E.; Diaz-Perez, J.A.; Leibowitz, J.; Weed, D.T.; Thomas, G.; Civantos, F.J.; Arnold, D.J.; Gomez-Fernandez, C.; Rosenberg, A.E. Primary Sarcomas of the Larynx: A Clinicopathologic Study of 27 Cases. Head Neck Pathol. 2021, 15, 905–916. [Google Scholar] [CrossRef]
- Jay, A.; Piper, K.; Farthing, P.M.; Carter, J.; Diwakar, A. Low-grade myofibroblastic sarcoma of the tongue. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2007, 104, e52–e58. [Google Scholar] [CrossRef] [PubMed]
- Vlad, D.; Albu, S. Low-Grade Myofibroblastic Sarcoma of the Larynx. J. Craniofac. Surg. 2016, 27, e270–e271. [Google Scholar] [CrossRef] [PubMed]
- Kordač, P.; Nikolov, D.H.; Smatanová, K.; Kalfeřt, D. Low-Grade Myofibroblastic Sarcoma of the LARYNX: Case Report and Review of Literature. Acta Medica 2014, 57, 162–164. [Google Scholar] [CrossRef]
- Qiu, J.Y.; Liu, P.; Shi, C.; Han, B. Low-grade myofibroblastic sarcomas of the maxilla. Oncol. Lett. 2015, 9, 619–625. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azevedo, M.; de Araújo, P.; Faria-Almeida, R.; Correia, F. Low-Grade Myofibroblastic Sarcoma in the Oral Cavity: Case Report and Clinical Insights. Oral 2025, 5, 6. https://doi.org/10.3390/oral5010006
Azevedo M, de Araújo P, Faria-Almeida R, Correia F. Low-Grade Myofibroblastic Sarcoma in the Oral Cavity: Case Report and Clinical Insights. Oral. 2025; 5(1):6. https://doi.org/10.3390/oral5010006
Chicago/Turabian StyleAzevedo, Mafalda, Paulo de Araújo, Ricardo Faria-Almeida, and Francisco Correia. 2025. "Low-Grade Myofibroblastic Sarcoma in the Oral Cavity: Case Report and Clinical Insights" Oral 5, no. 1: 6. https://doi.org/10.3390/oral5010006
APA StyleAzevedo, M., de Araújo, P., Faria-Almeida, R., & Correia, F. (2025). Low-Grade Myofibroblastic Sarcoma in the Oral Cavity: Case Report and Clinical Insights. Oral, 5(1), 6. https://doi.org/10.3390/oral5010006





