Abstract
Background: This review aims to provide an overview of the potential impact of fasting and diet on cancer, and in particular, on chronic lymphocytic leukemia (CLL), which is the most frequent form of leukemia in the Western world. Methods: Experimental and clinical studies have provided evidence of the crucial role of fasting in enhancing cancer treatment and improving outcomes for oncological patients, particularly at the early stages of the disease. Results: Emerging evidence highlights that fasting creates a differential stress response under critical conditions by fostering the survival of normal cells while limiting the survival and growth of cancer cells. Pivotal studies on CLL have highlighted the potential of fasting and dietary components to influence the stromal microenvironment and certain metabolic pathways, thereby affecting cancer cell apoptosis and immune response. In addition, explorative and initial clinical studies suggest that fasting and specific diets can mitigate the toxicity of chemotherapy. Conclusions: Clinical trials are needed to evaluate the efficacy and safety of nutritional and fasting approaches in cancer and CLL. Future investigations could provide new insights into the potential role of diet and fasting in the prevention and treatment of cancer, potentially leading to more effective and personalized therapeutic strategies.
1. Introduction
Fasting has gained significant popularity in recent years due to its potential health benefits across various physiological systems and its promising role in enhancing the effectiveness of cancer treatments [1,2]. Fasting can indeed be categorized into different types, with the most common prolonged fasting periods and intermittent fasting (IF). Prolonged fasting lasts more than 24 h, whereas intermittent fasting is characterized by an alternation between fasting and eating lasting no more than 24 h [3,4]. Fasting plays beneficial roles mainly on obesity and diabetes, the cardiovascular system, the renin–angiotensin system, the immune system, and the nervous system [1,2]. In addition, the fasting-mimicking diet (FMD) is a dietary intervention that replicates the physiological effects of fasting while allowing limited food intake. This diet typically involves a reduced calorie intake over a specific period, ranging from 3 to 7 days, and aims to induce the same metabolic and cellular responses as prolonged fasting without the need for complete food abstinence. FMD has shown promise in cancer treatment by protecting normal cells from chemotherapy toxicity and enhancing the sensitivity of cancer cells to treatment [5,6,7].
In recent years, several studies have provided evidence to demonstrate that intermittent fasting plays a crucial role in promoting beneficial effects in cancer treatment and increasing positive outcomes in oncological patients [2]. Li Sucholeiki et al. investigated the clinical and biological impact of IF in patients with cancer. Some promising clinical studies have highlighted that IF may reduce gastrointestinal toxicities in some cancer patients undergoing treatment, potentially improving their overall quality of life [8,9,10]. IF may also help manage blood sugar levels, reducing hyperglycemia in oncological patients [8]. In addition, IF improves the efficacy and safety of therapies by decreasing off-target DNA damage and inducing favorable cellular-level immune remodeling [11,12]. To evaluate the potential benefits of intermittent fasting as an adjunct intervention in cancer care, larger controlled studies are indeed necessary. These studies should focus on several key endpoints and broader aspects to determine the effectiveness and overall impact of IF in terms of long-term survival rate, immune function, metabolic rate, and quality of life. Tiwari et al. demonstrated that prolonged fasting in certain patients with cancer is safe and limits adverse events as well as the survival and growth of cancer cells [13]. The authors suggested that combining prolonged periodic fasting with standard conventional therapeutic approaches is an emerging strategy aimed at enhancing cancer treatment efficacy, promoting cancer-free survival, and reducing side effects.
2. Fasting, Diet and Metabolism in Cancer
A very recent study conducted by Xiao et al. showed the potential applications of various types of diets as adjunct therapies in the management of certain diseases and cancers [14]. Changes in diet composition influence not only the availability of nutrients within cancer cells, but also the surrounding microenvironment, thus offering potential opportunities to inhibit tumor growth. Fasting can cause several metabolic changes, including alterations in the systemic levels of hormones and growth factors such as insulin, glucagon, growth hormone, IGF-1 (insulin-like growth factor 1), glucocorticoids, or adrenaline [14,15]. In response to these changes, normal cells activate protective mechanisms against stress and toxic substances, thereby reducing their metabolic demands and cell division rate. On the other hand, since fasting reduces nutrients and factors that promote tumor growth, cancer cells struggle to cope with the deprivation of metabolites and thus develop increased sensitivity to anti-tumor therapies [14,16]. Interestingly, cancer cells utilize glucose as their main source of energy. The Warburg effect is a metabolic phenomenon observed in cancer cells where they preferentially produce energy (ATP) through glycolysis, even in the presence of sufficient oxygen (aerobic conditions) [17]. This is in contrast to normal cells, which typically generate energy through oxidative phosphorylation (OXPHOS) in the mitochondria under aerobic conditions. Fasting can also cause an “anti-Warburg effect” by reducing aerobic glycolysis and glutaminolysis while increasing OXPHOS uncoupled from ATP synthesis. In cancer cells, the increase in OXPHOS boosts reactive oxygen species (ROS) production, leading to oxidative stress, activation of p53 signaling, and DNA damage, especially when combined with chemotherapy or other anti-tumor therapies [14].
Diet can also influence the gut microbiome with a potential anti-tumor effect during treatments in cancer patients. The gut microbiome includes the genetic composition of all species present in the gut, such as bacteria, viruses, yeasts, protozoa, fungi, and archaea. It can be influenced by various endogenous and exogenous factors.
The composition of the gut microbiota can impact the health status of certain patients with oncological diseases, as its interactions with the host’s immune system can affect tumor development and carcinogenesis [14].
Deligiorgi et al. suggested that in response to nutrient restriction, healthy cells often enter a “maintenance mode” to adapt to the reduced availability of resources. This condition is characterized by the activation of catabolic processes and repair mechanisms to preserve genome and proteome integrity, prioritizing these functions over cell proliferation and growth [16].
Tumor cells are deprived of this shield (differential stress resistance), thus they become vulnerable to fasting or the combination of fasting with chemotherapy (differential stress sensitization). Supporting the hypothesis of differential stress resistance, it has been shown that fasting combined with chemotherapy protects normal cells but not tumor cells from chemotherapy toxicity [16]. The differential protective effect of fasting against chemotherapy can be attributed to various biological mechanisms, including the fasting-induced reduction in IGF-1 and glucose levels. The reduction in the levels of IGF-1 probably leads to the downregulation of downstream effectors such as the Ras/MAPK and PI3K/Akt pathways in normal cells. However, cancer cells, due to oncogene-driven constitutive activation of these pathways, do not experience the same protective effects. The authors suggest that prescribing fasting as anticancer medicine may not be a long way ahead, provided that large randomized clinical trials consolidate its efficacy, safety, and feasibility [16].
Recent studies have explored the relationship between fasting, dietary patterns, and the development of solid tumors, particularly breast and colorectal cancers. Although these findings may not directly apply to CLL, they provide valuable insights that could guide future research in CLL.
Breast cancer (BC) has been linked to inflammatory, insulin, and estrogenic pathways. Greater adherence to an anti-diabetic and anti-inflammatory diet before diagnosis is associated with lower overall mortality in BC survivors. Long-term commitment to these dietary patterns may improve the prognosis and outcomes for BC survivors [18].
There is increasing interest in the role of diet and physical activity in preventing and treating BC. Evidence suggests that the Mediterranean diet (MedDiet) and regular physical activity can reduce BC risk and, for those already diagnosed, may lower the chances of tumor recurrence and improve quality of life. Additionally, dietary interventions like fasting, calorie restriction, ketogenic diets, and plant-based diets show potential in enhancing BC therapy outcomes [19].
Interestingly, Boden et al. examined both data-driven and hypothesis-driven dietary patterns to explore their relationship with plasma metabolite profiles and colorectal cancer (CRC) risk in 680 CRC cases and matched controls. Dietary patterns were identified using exploratory/confirmatory factor analysis. The study found that the associations between certain dietary patterns such as meat, fast-food, and fruit soup/rice and CRC risk varied based on tumor location in women. Alcohol and fruit/vegetable intake were linked to specific metabolite profiles. In addition, one alcohol-related metabolite was associated with increased CRC risk, while three metabolites related to fiber, whole grains, and fruit/vegetables were linked to decreased CRC risk [20].
3. Fasting in Hematology
About 15,000 studies regarding fasting have been published, focusing primarily on its potential impact on oncologic cancers. On the other hand, there is less scientific evidence on the effects of fasting and dietary interventions in hematological malignancies such as leukemias and lymphomas. Studies in the hematological field are still limited and require further research to understand the therapeutic potential of dietary modifications. As mentioned before, fasting in the context of solid tumors has demonstrated potential benefits in altering the metabolic environment, affecting cancer cell viability and enhancing treatment efficacy [1,2,13]. Similar principles can be applied to hematologic disorders, where fasting may also influence the metabolic environment and impact cancer cell behavior. In this regard, Di Biase et al. suggested that immune-based interventions, or immunotherapies, represent a promising strategy for achieving long-term cancer-free survival. These therapies harness and enhance the body’s immune system to recognize and eradicate cancer cells. The combination of chemotherapy and a fasting-mimicking diet appeared to protect normal cells, including hematopoietic stem and immune cells, from the toxic side-effects of chemotherapy, and simultaneously sensitized tumor cells to the treatment. In particular, the combination of chemotherapy and FMD increases the levels of common lymphoid progenitor cells (CLPs) in the bone marrow. These progenitor cells are crucial for the development of lymphocytes, including T cells and B cells, which play vital roles in immune responses against tumors. FMD combined with chemotherapy significantly boosts the levels of CD8+ tumor-infiltrating lymphocytes (TILs) within tumor tissues. These cytotoxic T cells are essential for the targeted killing of cancer cells, contributing to improving anti-tumor immunity. Thus, the generation of more lymphoid progenitor cells can develop into various immune cells, which are essential for mounting effective anti-tumor responses [21].
4. Chronic Lymphocytic Leukemia (CLL)
Within hematological disorders, chronic lymphocytic leukemia (CLL) is indeed the most frequent form of leukemia in the western world [22]. According to statistics from the Surveillance, Epidemiology, and End Results (SEER) database, it is estimated that approximately 4.9 new cases of CLL per 100,000 individuals occur annually in the United States and Europe [23]. The median age at diagnosis is around 70 years, although a significant percentage of patients (around 10%) are diagnosed with CLL before the age of 45 [23,24]. In Europe, the annual incidence rate is 5.87 cases per 100,000 male individuals, while among female individuals, the rate is approximately 4 cases per 100,000 individuals [25]. CLL is characterized by the gradual accumulation of immature lymphocytes in the blood, bone marrow, lymph nodes, and spleen [23]. In most cases, CLL diagnosis is established in the absence of clinically evident symptoms, typically through complete blood count tests conducted as part of routine analyses. A distinctive hematological feature of CLL is a significant increase in absolute lymphocyte count (lymphocytosis) in peripheral blood, with average values ranging from 35 to 50 × 109/L. Indeed, the diagnosis of CLL requires the presence of ≥5 × 109 B lymphocytes in the peripheral blood for at least 3 months. CLL cells are characteristically mature lymphocytes [26]. There are two staging systems to determine the progression status of CLL: Rai and Binet methods. These are established through a simple physical examination and blood count, based on the presence of lymphadenopathy, splenomegaly, anemia, and thrombocytopenia [27,28]. Flow cytometry of peripheral blood is the diagnostic test of choice to confirm the presence of circulating clonal B lymphocytes expressing CD5⁺, CD19⁺, CD20⁺, and CD23⁺ antigen expression [23,29]. Subsequently, a physical examination and computerized tomography scan can reveal lymphadenopathy, splenomegaly, and hepatomegaly.
Most patients diagnosed with CLL do not require immediate treatment and are placed on active observation with regular monitoring every 3–6 months. Treatment begins when specific criteria are met, as established by the International Workshop on Chronic Lymphocytic Leukemia (iwCLL) [26]. In the early stages of CLL, most patients are asymptomatic. As the disease progresses, they may experience B symptoms such as fever, night sweats, weight loss, and worsening fatigue. Common reasons for initiating therapy include progressive marrow failure (hemoglobin < 10 mg/dL and platelet count < 100 × 10⁹/L), large or symptomatic lymphadenopathy or splenomegaly, significant symptoms impacting quality of life, symptomatic extranodal involvement, and autoimmune anemia or thrombocytopenia unresponsive to steroids [26].
Patients with CLL may develop autoimmune complications, such as autoimmune hemolytic anemia and immune thrombocytopenia. Regular, age-appropriate cancer screening is advised for all CLL patients [30].
In early-stage CLL, several prognostic factors can help predict the progression of the disease and guide treatment decisions. While early-stage CLL (Rai stage 0 or Binet stage A) often requires a “watch and wait” approach, these factors are essential in determining which patients may develop more aggressive forms of the disease [31].
Beta-2 Microglobulin (B2M) is a protein that plays a significant role as a prognostic marker in CLL. High levels of B2M are associated with a poor prognosis as shown in Figure 1. Patients with more aggressive disease (Rai stage III/IV or Binet stage B/C) tend to have higher B2M levels [32]. Elevated B2M is linked with faster disease progression and a greater likelihood of requiring earlier treatment. B2M is often used as part of a broader set of prognostic markers (e.g., IGHV mutation status, cytogenetic abnormalities, CD38, and ZAP-70) to stratify patients by risk.
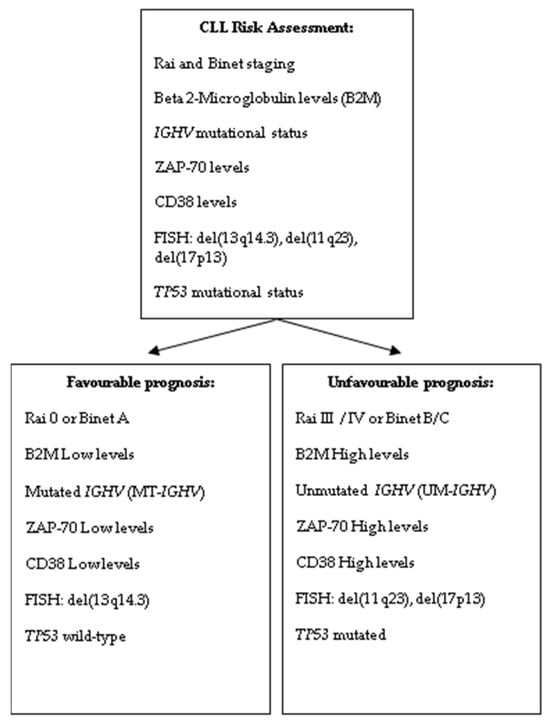
Figure 1.
Risk assessment in CLL patients at diagnosis.
Based on the gene of the variable region of the heavy chain of immunoglobulin (IGHV), patients with CLL can be clinically divided into two distinct groups (Figure 1). The unmutated IGHV (UM-IGHV) usually presents with a more aggressive form of CLL, characterized by faster disease progression and a higher likelihood of requiring earlier treatment. Patients with UM-IGHV tend to respond less favorably to standard therapies and may need closer monitoring. In contrast, the mutated IGHV (MT-IGHV) tend to have a more favorable prognosis [33]. Their disease typically progresses more slowly, and they often remain stable for longer periods without requiring treatment. As a result, MT-IGHV patients usually benefit from a “watch and wait” approach, with a lower risk of early intervention.
ZAP-70 (zeta-chain-associated protein kinase 70) is an important prognostic marker in early-stage CLL (Figure 1). Its expression can provide insights into the disease’s aggressiveness and help guide management, even in patients at an early stage.
High levels of ZAP-70 expression in CLL cells are associated with a more aggressive disease course. Patients with elevated ZAP-70 tend to have a faster disease progression, shorter time to treatment, and an overall poorer prognosis compared to those with low ZAP-70 expression. ZAP-70 expression is strongly correlated with UM-IGHV status, which is itself a marker of poor prognosis. Patients with high ZAP-70 levels are often found to have UM-IGHV, indicating a more aggressive form of CLL. Conversely, low ZAP-70 expression is more common in patients with MT-IGHV, which is associated with a more favorable prognosis [34].
CD38 is another important prognostic marker in early-stage CLL, similar to ZAP-70 (Figure 1). Higher levels of CD38 expression on the surface of CLL cells are associated with more aggressive disease. Similar to ZAP-70, high CD38 expression is often correlated with UM-IGHV, which is a marker of more aggressive CLL. This means patients with high CD38 levels tend to have more rapidly progressing disease, whereas low CD38 expression is more frequently seen in patients with MT-IGHV, indicating a more indolent disease course [34,35].
A complex karyotype, defined as having three or more chromosomal abnormalities, is indeed associated with a poor prognosis in CLL. This is due to the increased genomic instability, which can lead to more rapid disease progression, higher rates of treatment resistance, and shorter overall survival [36].
In CLL, there are four common chromosomal abnormalities present in approximately 80% of patients as follows: del(13q14.3) found in 55% of patients with a favorable prognosis, especially if not associated with other aberrations, trisomy 12 present in 10–20% of cases with intermediate risk when occurring alone, del(11q23) found in 18% of patients with unfavorable prognosis. In addition, del(17p13) is associated with high risk in fewer than 10% of patients at the time of diagnosis. Deletions or mutations of the TP53 gene, which is crucial for DNA repair and apoptosis, provide valuable insights into disease progression and have a significant influence on clinical decision-making as illustrated in Figure 1 [23,25,28,29]. The presence of TP53 abnormalities is associated with a more aggressive disease course, poor response to standard chemotherapy, and shorter overall survival. As a result, these genetic changes influence both prognostic and therapeutic evaluations, guiding clinicians toward alternative treatment strategies, such as targeted therapies (e.g., BTK inhibitors or BCL-2 inhibitors), which may be more effective in patients with TP53 abnormalities [37,38].
In addition to TP53 mutations, other gene mutations with prognostic significance in CLL include ATM, NOTCH1, SF3B1, and BIRC3. These mutations occur in 4–15% of newly diagnosed CLL patients, rising to 15–25% in fludarabine-refractory cases. ATM, SF3B1, and NOTCH1 mutations predict shorter time to first treatment (TTFT), independent of IGHV mutation status. TP53 and NOTCH1 mutations, along with IGHV unmutated status, predict shorter overall survival (OS). An integrated prognostic model classifies CLL patients into four risk groups based on genetic abnormalities: (1) high-risk: TP53 and/or BIRC3 abnormalities; (2) intermediate-risk: NOTCH1 and/or SF3B1 mutations, and/or del(11q); (3) low-risk: trisomy 12, wild-type genes; and (4) very low-risk: del(13q) only (National Comprehensive Cancer Network (NCNN) Guidelines 1.2025).
Several studies identified biological pathways associated with common mutations in CLL. Molecular mechanisms are responsible for genomic alterations and disruptions in the cell cycle (TP53, ATM, and POT1), chromatin alterations (HIST1H1E, HIST1H1B, CHD2, ZMYM3, BAZ2A, ASXL1, SYNE1, ARID1, KMT2D, and SETD2), as well as mRNA variations and ribosomal processes (SF3B1, XPO1, RPS15, DOX3X, ZNF292, MED12, CNOT3, U1, FUBP1, DDX3X, and NXF1) [25,39]. Certain pathways play a crucial role in CLL: the WNT signaling pathway associated with the MYC oncogene (MGA and PTPN11), the Notch signaling pathway (NOTCH1 and FBXW7), and the inflammatory cascade (MYD88, NFKBIE, BIRC3, TRAF3, and SAMHD1) [25,39]. Additionally, mutations in B-cell receptor (BCR) signaling pathway (EGR2, PAX5, BCOR, IRF4, and IKZF3), genetic variants in the MAPK-ERK pathway involving PTPN11, BRAF, KRAS, MAP2K1, and NRAS are common in CLL [25,39,40].
Studies on gene expression profiling deepened the understanding of the pathophysiology of leukemic cells and provided valuable insights into the biomolecular mechanisms that regulate the growth and survival of tumor cells, as well as predicting early progression in CLL patients [41].
Several studies have shown the potential role of miRNAs as molecules playing a crucial role in the prognosis of different cancers, including CLL, and their epigenetic alterations can predict disease progression and response to treatment.
CLL showed a notable discrepancy in incidence between Asian and Western populations, suggesting the importance of genetic alterations in its pathogenesis [42]. A recent study identified potential mediators of certain differences in microRNAs (miRNAs) by microarray analysis among B lymphocytes from Asian CLL patients, Western CLL patients and healthy individuals. In particular, miR-4485 acted through the suppression of the TGR5 receptor (G-protein coupled bile acid receptor 1), which plays a crucial role in regulating metabolism and tumor suppression. Interestingly, miR-4485 was significantly overexpressed in tumor cells of Asian and Western patients compared to normal lymphocytes in their respective populations.
On the contrary, miR-138, miR-181a, miR-181c, miR-181d, and miR-363 appeared to promote tumor-suppressive functions [42]. These findings indicated miRNAs as potential therapeutic targets for developing personalized treatment strategies based on genetic differences between Asian and Western populations [42,43].
Trojani et al. conducted a microarray study on 112 CLL patients divided into two groups based on the mutational status of IGHV and the expression of ZAP-70. The comparison between the two groups (MTZAP70- and UMZAP70+) highlighted significant gene expression alterations in various enzymes involved in lipid metabolism, such as CHPT1, ARSD, LPL, AGPAT2, MBOAT1, AGPAT4, PLD1, and APP [44]. Some authors confirmed the crucial role of LPL and ZAP-70 in CLL, as previously described [45,46]. Indeed, LPL expression showed a strong correlation with IGHV mutational status and overall survival, underscoring the importance of LPL as a prognostic marker in CLL [45].
Novel targeted agents in CLL have significantly advanced treatment by focusing on specific pathways involved in the disease. Ibrutinib is the first Bruton tyrosine kinase inhibitor (BTKi) approved for CLL which inhibits B-cell receptor signaling, a key driver of CLL cell survival. Acalabrutinib and Zanubrutinib are newer-generation BTKis with improved safety profiles and reduced side effects compared to Ibrutinib. Venetoclax targets BCL-2, a protein that prevents cancer cells from undergoing apoptosis. Venetoclax is often combined with anti-CD20 antibodies like Obinutuzumab for time-limited treatment regimens. Idelalisib and Duvelisib block the PI3K pathway, which plays a role in CLL cell growth and survival. They are typically used in patients who are refractory to other therapies or have relapsed. Rituximab, Obinutuzumab, and Ofatumumab target CD20 on the surface of B cells, marking them for destruction by the immune system. They are often used in combination with other agents like Venetoclax to enhance efficacy while maintaining manageable safety profiles [47].
5. Diet and Fasting in CLL
Nutritional experts from the World Cancer Research Fund concluded that there is currently no convincing epidemiological evidence that fruits and vegetables play a role in cancer etiology with the exception of colorectal cancer and fiber intake [48]. Vitamin C, also known as ascorbic acid, is an essential nutrient found in various fruits and vegetables.
Darwiche et al. conducted an in vitro study examining the effects of 250 μM of ascorbic acid (AA), an orally achievable dose, on primary CLL B-cells and two CLL cell lines, focusing on cell death and the underlying mechanisms involved. The authors noticed the cytotoxic effects of AA from the pro-oxidant damage due to the production of reactive oxygen species in both the extracellular media and CLL cells, leading to caspase-dependent apoptosis. Additionally, AA was shown to enhance the cytotoxicity of targeted therapies for CLL. These preclinical findings suggest that ascorbic acid could serve as an effective adjuvant therapy, potentially enhancing CLL treatments when used in combination with targeted therapies [49]. Further clinical trials are crucial to fully understand the potential benefits and risks of high-dose vitamin C as a complementary treatment for cancer patients [50]. In this regard, Casabonne et al. conducted research on the role of certain genetic variants in the vitamin C transporter gene (SLC23A2) and its interaction with fruit intake in patients with CLL. The authors demonstrated that both environmental and genetic factors affect CLL independently. The association between the genetic variants SLC23A2 and CLL need to be investigated in larger prospective studies with nutritional information including SLC23A1 expression, as well as tissue and circulating (blood and urine) levels of ascorbic acid [51].
Vitamin D is a vital nutrient involved in many biochemical processes and plays a significant role in various diseases. It is essential for cancer prevention and serves as a complementary treatment for cancer through both direct and indirect biochemical pathways. Sobhi et al. highlighted that while vitamin D is thought to have a positive impact on breast, colorectal, hepatocellular cancers, and leukemia, the relationship between vitamin D and prostate cancer, as well as melanoma, remains inconclusive. Current research is insufficient for drawing definitive conclusions, and further targeted studies are needed to clarify how vitamin D affects cancer cells [52].
Vitamin D has been investigated in relation to CLL, and certain studies suggest that it could play a role in disease progression and treatment outcomes [53,54,55]. Tadmor et al. suggested that low levels of vitamin D are associated with a shorter TTFT and inferior overall survival in patients with CLL. Indeed, the authors demonstrated that the administration of vitamin D to patients with CLL in a watch and wait active surveillance is significantly associated with a longer treatment free survival, and a longer time to first treatment among young patients (age ≤ 65) [56].
In addition, Vitamin D deficiency has been associated with worse outcomes in various hematological malignancies, including diffuse large B cell lymphoma (DLBCL) [57]. Lower vitamin D levels may impact immune function and the body’s ability to respond effectively to cancer. There is a growing body of research exploring the relationship between vitamin D levels and sarcopenia (loss of muscle mass and strength), particularly as vitamin D plays a critical role in muscle health and function [58]. The study by Nakamura et al. highlighted that the combination of low vitamin D levels and a low skeletal muscle index (SMI) correlates with a worse prognosis in DLBCL patients, providing valuable insights for patient management [57].
An intriguing clinical trial was conducted by Rojas et al. who explored the effects of consuming OC/OL-EVOO, which is composed of extra virgin olive oil (EVOO) rich in bioactive compounds such as oleocanthal (OC) and oleacein (OL), on early-stage CLL patients. The authors observed increased levels of the apoptotic markers ccK18 and Apo1-Fas and the cell cycle negative regulator p21, while decreased levels of the antiapoptotic protein Survivin and the cellular proliferation marker Cyclin D were noticed.
Specifically, they demonstrated that oral administration of a daily dose of 25 mg of OC and OL through the consumption of 40 mL of EVOO, could be beneficial for CLL patients. The study provided evidence that this regimen could induce apoptosis in cancer cells and improve the metabolism of these patients. Further investigations are indeed necessary to fully understand the effects of oleocanthal and/or oleacein to increase life expectancy and stabilize neoplastic blood diseases like CLL [59].
In CLL, the non-hematopoietic stromal microenvironment plays a critical role in promoting tumor cell recruitment, activation, survival, and expansion.
The interaction between dietary components and stromal cells is indeed a critical factor in modulating various biological processes, including the conversion of precursors into active retinoic acid (RA). This interaction can have profound implications for CLL progression, and can significantly influence the effectiveness of dietary interventions aimed at managing this disease. In this regard, Farinello et al. uncovered significant insights into the role of the non-hematopoietic stromal microenvironment in CLL. Leukemic B lymphocytes induced the activation of RA signaling within the stromal microenvironment. RA signaling in stromal cells regulates genes involved in cell adhesion, tissue organization, and chemokine secretion, including the B-cell chemokine CXCL13. The inhibition of RA signaling in stromal cells, either through dietary reduction in retinoic acid precursors or retinoid-antagonist therapy, disrupts the supportive microenvironment. This inhibition results in the deregulation of critical genes, thereby preventing leukemia cell dissemination into lymphoid tissues and prolonging survival. Leukemia cells express higher levels of retinoic acid receptors, specifically Rarγ2 in murine leukemia cells and RXRα in human leukemia cells compared to normal B cells, respectively. This study highlighted the role of retinoids in murine CLL pathogenesis, and provided new therapeutic strategies to target the microenvironment and to control disease progression [60].
Alterations in fatty acid (FA) metabolism can affect CLL cells in several ways, including providing energy, supporting cell membrane synthesis, and influencing certain signaling pathways which are crucial for cell survival and proliferation. FA metabolism is known to contribute to tumorigenesis, progression, and therapy resistance through enhanced lipid synthesis, storage, and catabolism. In this regard, Pan et al. aimed to construct a prognostic model to improve risk stratification in CLL and explore the link between FA metabolism and CLL. The study successfully established a reliable predictive signature based on FA metabolism-related genes and constructed a novel nomogram prognostic model. These findings support the potential preclinical implications of FA metabolism in CLL research, offering new avenues for risk stratification and targeted therapies in CLL [61]. Table 1 summarized the possible dietary intervention for the management of CLL.

Table 1.
Dietary interventions in management of CLL.
6. Case Reports in CLL
In this review, we aim to include three case reports suggesting the potential impact of diet on patients with CLL. The first case study involved a 56-year-old woman diagnosed with CLL at Rai stage 2 and Binet stage A, who successfully managed CLL for over 15 years without conventional chemotherapy [62]. The patient’s white blood cells (WBC) count reached 175.3 × 103/μL at diagnosis. The patient began a physician-assisted regimen of alternative dietary supplements that included omega-3, EGCG (epigallocatechin-3-gallate green tea extract), meriva-500 (curcumin phytosome), and vitamin D3. After high-dose EGCG and supplements regimen were added to her diet, the WBC count stabilized and later decreased slightly, plateauing at approximately 130 to 140 × 103/μL (Table 2).

Table 2.
Clinical characteristics of patients with CLL reported in three cases reports.
Interestingly, these supplements were chosen based on their potential role to modulate inflammatory processes by the inhibition of nuclear factor kappa B (NF-κB) signaling pathway, which has been associated with more aggressive tumor growth and resistance to chemotherapy and radiotherapy [65]. As previously mentioned, vitamin D analogues induced apoptosis in primary CLL cells by a p-53 independent mechanism and vitamin D insufficiency is a risk factor for CLL. Conversely, high levels of vitamin D are predictive of a long time to first treatment in CLL [56]. Moreover, clinical trial findings and preclinical research underscored the potential therapeutic benefits of EGCG and curcumin in the context of CLL, particularly in overcoming CLL cells’ resistance to apoptosis and their interaction with vascular endothelial growth factor (VEGF) receptors [65]. In conclusion, the authors highlighted the potential benefits of lifestyle strategies in managing indolent cancers like CLL. While conventional treatments remain crucial, integrating lifestyle approaches can offer complementary benefits, potentially slowing disease progression and improving quality of life for CLL patients [62].
The second case report focused on a 63-year-old man diagnosed with CLL at Rai stage 0, who displayed an elevated lymphocyte count of 27 × 109/L at diagnosis (Table 2). This patient followed two different trials one after the other. During the first trial, he took oral Polyphenon E (antioxidant EGCG), but his lymphocyte and leukocyte counts continued to follow an exponential growth curve. The second trial concerned a change from the regular diet (with a lot of processed food and animal protein) to a flexitarian diet. This diet mainly consisted of vegetarian food, no red meat, twice weekly a little piece of fat fish, hardly any dairy products, no cookies and instead once daily a little piece of dark chocolate (72%), no desserts, no alcohol in the evening but instead fresh fruit juice. In addition, daily vitamin B12 (1000 μg) was taken orally.
Following the flexitarian diet, the patient showed a decrease of approximately 40% in lymphocyte count compared to the first diet trial. In conclusion, the authors suggested that, if the lymphocyte count is still sufficiently low (<100 × 109/L), the first part of the wait-and-see approach for asymptomatic Rai stage 0 classical B-CLL can be used to determine if the lymphocyte counts follow an exponential growth curve. If they do, the whole food, plant-based (WFPB) diet intervention can be started [63].
The third study conducted by Bossi et al. investigated an untreated patient with CLL (#1), who voluntarily opted for a predominantly fruit and raw vegetable-based diet, periodically engaging in prolonged total fasting (ranging from a minimum of 4 days to a maximum of 39 days) during which he consumed only water and herbal tea. This patient was a man aged 50 years with Rai stage 0 and Binet stage A, and displayed an absolute lymphocyte count (ALC) of 11.84 × 109/L at diagnosis (Table 2). The authors observed continuous fluctuations in ALC of this patient alongside a favorable prognosis since diagnosis. Interestingly, the authors noticed that approximately 4 to 6 weeks after the end of fasting the absolute lymphocyte count was reduced by about half. A wide gene expression profiling study was conducted on peripheral blood CD19+ cells from this patient at various time-points (during nutrition and fasting), compared to the same cell counterpart from five other untreated CLL patients following varied diets. Bioinformatic analyses results demonstrated that nine genes (IGLC3, RPS26, CHPT1, PCDH9, IGHV3-43, IGKV3D-20, PLEKHA1, CYBB, and GABRB2) were differently expressed between patient #1 and the other 5 patients with CLL. In addition, clustering analysis confirmed distinct gene expression patterns in patient #1 compared to the other patients. This study suggests that prolonged absolute fasting and a specific gene expression signatures may play a role in the observed favorable prognosis and a slow-growth trend of lymphocytosis in CLL, warranting further investigation into their potential implications for disease management [64].
7. Conclusions
Despite some studies on the effects of diet and fasting in oncology, the potential impact of dietary interventions on cancer treatment is not fully understood. The latest guidelines from the American Society of Clinical Oncology (ASCO) suggest that there is currently insufficient evidence to recommend for or against dietary interventions such as ketogenic or low-carbohydrate diets, low-fat diets, functional foods, or fasting to improve outcomes related to quality of life, treatment toxicity, or cancer control [14].
Without a doubt, recognizing the importance of metabolic reprogramming in disease progression underscores the potential of dietary interventions in cancer management.
By understanding and leveraging these metabolic changes, dietary interventions can be tailored to support traditional therapies and improve patient outcomes.
Fasting and a fasting-mimicking diet has shown promise in cancer treatment by protecting normal cells from chemotherapy toxicity while enhancing the sensitivity of cancer cells to treatment including hematopoietic stem and immune cells, from its toxic side effects [21].
Fasting offers protective effects against chemotherapy toxicity through various biological mechanisms, mainly by reducing IGF-1 and glucose levels, which downregulate protective pathways in normal cells. In contrast, cancer cells, with their constitutive activation of these pathways, do not experience the same benefits [16]. Therefore, integrating fasting as an anticancer treatment could be viable if large randomized clinical trials validate its efficacy, safety, and feasibility.
Recent studies indicate that intermittent fasting plays a crucial role in promoting beneficial effects for cancer patients and improving treatment outcomes [2].
Diet also influences the gut microbiome, which can have an anti-tumor effect during cancer treatment. The gut microbiome’s composition can interact with the immune system, affecting tumor development and carcinogenesis [16].
Interestingly, vitamin D plays a multifaceted role in hematological diseases, affecting immune response, cell function, and disease outcomes. Deficiency can lead to sarcopenia, which is particularly concerning in cancer patients. Recent research indicates that low vitamin D levels and low skeletal muscle index (SMI) can worsen prognosis in hematological malignancies. Some studies have shown that vitamin D deficiency is associated with poorer outcomes in various hematological malignancies, such as CLL and DLBCL [53,57,66]. In addition, low vitamin D levels may correlate with advanced disease stages and reduced survival rates [57].
Vitamin C has gained attention in research related to CLL due to its potential therapeutic effects. An in vitro study on primary CLL B-cells and CLL cell lines indicated that ascorbic acid induces cell death through caspase-dependent apoptosis. Furthermore, ascorbic acid enhances the cytotoxicity of targeted CLL therapies, suggesting its potential as an adjuvant therapy when used in combination with these treatments [49].
While fasting has shown promising potential as an adjunct therapy in solid-tumor treatment, its application in hematologic cancers is still underexplored. The recent surge of interest in IF stems from its preclinical benefits in cancer management. In CLL, novel agents like Venetoclax and BTKis are commonly used as frontline treatments. However, these therapies raise concerns about tumor lysis syndrome (TLS) and gastrointestinal bleeding (GIB) in patients. In fluid-restricted intermittent fasting, there is an increased risk of TLS with Venetoclax due to dehydration, whereas fluid-liberal intermittent fasting may reduce its absorption. Additionally, fasting can elevate gastric acid levels, increasing the risk of GIB in patients taking BTKis. Further research is needed to determine the safety of IF for CLL patients undergoing treatment with novel agents [67].
In summary, although preclinical studies and some epidemiological research support the hypothesis that dietary practices and fasting may benefit CLL, further research is necessary to confirm these findings and determine their clinical applicability [49,67,68,69].
Thus, controlled and randomized clinical trials are necessary to evaluate the efficacy and safety of these nutritional and fasting approaches in CLL, as well as to better understand the biomolecular mechanisms involved in their beneficial effects. Future studies could provide new insights into the potential of diet and fasting as personalized medicine, offering new avenues for the prevention and treatment of cancer.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable in this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mackieh, R.; Al-Bakkar, N.; Kfoury, M.; Okdeh, N.; Pietra, H.; Roufayel, R.; Legros, C.; Fajloun, Z.; Sabatier, J.M. Unlocking the Benefits of Fasting: A Review of its Impact on Various Biological Systems and Human Health. Curr. Med. Chem. 2024, 31, 1781–1803. [Google Scholar] [CrossRef] [PubMed]
- Li Sucholeiki, R.; Propst, C.L.; Hong, D.S.; George, G.C. Intermittent fasting and its impact on toxicities, symptoms and quality of life in patients on active cancer treatment. Cancer Treat. Rev. 2024, 126, 102725. [Google Scholar] [CrossRef] [PubMed]
- Attinà, A.; Leggeri, C.; Paroni, R.; Pivari, F.; Dei Cas, M.; Mingione, A.; Dri, M.; Marchetti, M.; Di Renzo, L. Fasting: How to Guide. Nutrients 2021, 13, 1570. [Google Scholar] [CrossRef] [PubMed]
- Clifton, K.K.; Ma, C.X.; Fontana, L.; Peterson, L.L. Intermittent fasting in the prevention and treatment of cancer. CA Cancer J. Clin. 2021, 71, 527–546. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.W.; Adams, G.B.; Perin, L.; Wei, M.; Zhou, X.; Lam, B.S.; Da Sacco, S.; Mirisola, M.; Quinn, D.I.; Dorff, T.B.; et al. Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression. Cell Stem Cell 2014, 14, 810–823. [Google Scholar] [CrossRef]
- Brandhorst, S.; Levine, M.E.; Wei, M.; Shelehchi, M.; Morgan, T.E.; Nayak, K.S.; Dorff, T.; Hong, K.; Crimmins, E.M.; Cohen, P.; et al. Fasting-mimicking diet causes hepatic and blood markers changes indicating reduced biological age and disease risk. Nat. Commun. 2024, 15, 1309. [Google Scholar] [CrossRef]
- Lin, X.; Gao, Y. A bibliometric analysis of the Fasting-Mimicking Diet. Front. Nutr. 2024, 11, 1328450. [Google Scholar] [CrossRef]
- Omar, E.M.; Omran, G.A.; Mustafa, M.F.; El-Khodary, N.M. Intermittent fasting during adjuvant chemotherapy may promote differential stress resistance in breast cancer patients. J. Egypt Natl. Cancer Inst. 2022, 34, 38. [Google Scholar] [CrossRef]
- Zorn, S.; Ehret, J.; Schäuble, R.; Rautenberg, B.; Ihorst, G.; Bertz, H.; Urbain, P.; Raynor, A. Impact of modified short-term fasting and its combination with a fasting supportive diet during chemotherapy on the incidence and severity of chemotherapy-induced toxicities in cancer patients—A controlled cross-over pilot study. BMC Cancer 2020, 20, 578. [Google Scholar] [CrossRef]
- Tsuda, M.; Ishiguro, H.; Toriguchi, N.; Masuda, N.; Bando, H.; Ohgami, M.; Homma, M.; Morita, S.; Yamamoto, N.; Kuroi, K.; et al. Overnight fasting before lapatinib administration to breast cancer patients leads to reduced toxicity compared with nighttime dosing: A retrospective cohort study from a randomized clinical trial. Cancer Med. 2020, 9, 9246–9255. [Google Scholar] [CrossRef]
- de Groot, S.; Vreeswijk, M.P.; Welters, M.J.; Gravesteijn, G.; Boei, J.J.; Jochems, A.; Houtsma, D.; Putter, H.; van der Hoeven, J.J.; Nortier, J.W.; et al. The effects of short-term fasting on tolerance to (neo) adjuvant chemotherapy in HER2-negative breast cancer patients: A randomized pilot study. BMC Cancer 2015, 15, 652. [Google Scholar] [CrossRef] [PubMed]
- Dorff, T.B.; Groshen, S.; Garcia, A.; Shah, M.; Tsao-Wei, D.; Pham, H.; Cheng, C.W.; Brandhorst, S.; Cohen, P.; Wei, M.; et al. Safety and feasibility of fasting in combination with platinum-based chemotherapy. BMC Cancer 2016, 16, 360. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Sapkota, N.; Han, Z. Effect of fasting on cancer: A narrative review of scientific evidence. Cancer Sci. 2022, 113, 3291–3302. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.L.; Gong, Y.; Qi, Y.J.; Shao, Z.M.; Jiang, Y.Z. Effects of dietary intervention on human diseases: Molecular mechanisms and therapeutic potential. Signal Transduct. Target. Ther. 2024, 9, 59. [Google Scholar] [CrossRef]
- Martínez-Garay, C.; Djouder, N. Dietary interventions and precision nutrition in cancer therapy. Trends Mol. Med. 2023, 29, 489–511. [Google Scholar] [CrossRef]
- Deligiorgi, M.V.; Liapi, C.; Trafalis, D.T. How Far Are We from Prescribing Fasting as Anticancer Medicine? Int. J. Mol. Sci. 2020, 21, 9175. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Castro-Espin, C.; Bonet, C.; Crous-Bou, M.; Nadal-Zaragoza, N.; Tjønneland, A.; Mellemkjær, L.; Hajji-Louati, M.; Truong, T.; Katzke, V.; Le Cornet, C.; et al. Association of Mediterranean diet with survival after breast cancer diagnosis in women from nine European countries: Results from the EPIC cohort study. BMC Med. 2023, 21, 225. [Google Scholar] [CrossRef]
- Khalifa, A.; Guijarro, A.; Nencioni, A. Advances in Diet and Physical Activity in Breast Cancer Prevention and Treatment. Nutrients 2024, 16, 2262. [Google Scholar] [CrossRef]
- Bodén, S.; Zheng, R.; Ribbenstedt, A.; Landberg, R.; Harlid, S.; Vidman, L.; Gunter, M.J.; Winkvist, A.; Johansson, I.; Van Guelpen, B.; et al. Dietary patterns, untargeted metabolite profiles and their association with colorectal cancer risk. Sci. Rep. 2024, 14, 2244. [Google Scholar] [CrossRef]
- Di Biase, S.; Lee, C.; Brandhorst, S.; Manes, B.; Buono, R.; Cheng, C.W.; Cacciottolo, M.; Martin-Montalvo, A.; de Cabo, R.; Wei, M.; et al. Fasting-Mimicking Diet Reduces HO-1 to Promote T Cell-Mediated Tumor Cytotoxicity. Cancer Cell 2016, 30, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Moia, R.; Gaidano, G. Prognostication in chronic lymphocytic leukemia. Semin. Hematol. 2024, 61, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Hallek, M.; Al-Sawaf, O. Chronic lymphocytic leukemia: 2022 update on diagnostic and therapeutic procedures. Am. J. Hematol. 2021, 96, 1679–1705. [Google Scholar] [CrossRef] [PubMed]
- Kipps, T.J.; Stevenson, F.K.; Wu, C.J.; Croce, C.M.; Packham, G.; Wierda, W.G.; O’Brien, S.; Gribben, J.; Rai, K. Chronic lymphocytic leukaemia. Nat. Rev. Dis. Primers 2017, 3, 16096. [Google Scholar] [CrossRef]
- Montague, A.M.; Pathak, S. Chronic Lymphocytic Leukemia with Variant Genetics. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Hallek, M.; Cheson, B.D.; Catovsky, D.; Caligaris-Cappio, F.; Dighiero, G.; Döhner, H.; Hillmen, P.; Keating, M.; Montserrat, E.; Chiorazzi, N.; et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood 2018, 131, 2745–2760. [Google Scholar] [CrossRef]
- Schroers, R.; Griesinger, F.; Trümper, L.; Haase, D.; Kulle, B.; Klein-Hitpass, L.; Sellmann, L.; Dührsen, U.; Dürig, J. Combined analysis of ZAP-70 and CD38 expression as a predictor of disease progression in B-cell chronic lymphocytic leukemia. Leukemia 2005, 19, 750–758. [Google Scholar] [CrossRef]
- Binet, J.L.; Auquier, A.; Dighiero, G.; Chastang, C.; Piguet, H.; Goasguen, J.; Vaugier, G.; Potron, G.; Colona, P.; Oberling, F.; et al. A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysis. Cancer 1981, 48, 198–206. [Google Scholar] [CrossRef]
- Kay, N.E.; Hampel, P.J.; Van Dyke, D.L.; Parikh, S.A. CLL update 2022: A continuing evolution in care. Blood Rev. 2022, 54, 100930. [Google Scholar] [CrossRef]
- Jain, N.; Wierda, W.G.; O’Brien, S. Chronic lymphocytic leukaemia. Lancet 2024, 404, 694–706. [Google Scholar] [CrossRef]
- Arguello-Tomas, M.; Albiol, N.; Moreno, C. Frontline Therapy in Chronic Lymphocytic Leukemia. Acta Haematol. 2024, 147, 47–59. [Google Scholar] [CrossRef]
- Moreno, C.; Hodgson, K.E.; Rovira, M.; Esteve, J.; Martinez, C.; Fernandez, F.; Ferrer, G.; Gel, B.; Carreras, E.; Bosch, F.; et al. Beta-2 Microglobulin Is a Strong Prognostic Marker in Patients with Chronic Lymphocytic Leukemia Submitted to Allogeneic Stem Cell Transplantation. Blood 2009, 114, 1244. [Google Scholar] [CrossRef]
- Hampel, P.J.; Parikh, S.A. Chronic lymphocytic leukemia treatment algorithm 2022. Blood Cancer J. 2022, 12, 172. [Google Scholar] [CrossRef] [PubMed]
- Malavasi, F.; Deaglio, S.; Damle, R.; Cutrona, G.; Ferrarini, M.; Chiorazzi, N. CD38 and chronic lymphocytic leukemia: A decade later. Blood 2011, 118, 3470–3478. [Google Scholar] [CrossRef] [PubMed]
- Tepper, J.E.; Niederhuber, J.E. Chronic Lymphocytic Leukemia. In Abeloff’s Clinical Oncology, 6th ed.; Elsevier-Health Sciences Division: Amsterdam, The Netherlands, 2019; p. 1850. [Google Scholar]
- Yun, X.; Zhang, Y.; Wang, X. Recent progress of prognostic biomarkers and risk scoring systems in chronic lymphocytic leukemia. Biomark. Res. 2020, 8, 40. [Google Scholar] [CrossRef]
- Campo, E.; Cymbalista, F.; Ghia, P.; Jäger, U.; Pospisilova, S.; Rosenquist, R.; Schuh, A.; Stilgenbauer, S. TP53 aberrations in chronic lymphocytic leukemia: An overview of the clinical implications of improved diagnostics. Haematologica 2018, 103, 1956–1968. [Google Scholar] [CrossRef]
- Stefaniuk, P.; Onyszczuk, J.; Szymczyk, A.; Podhorecka, M. Therapeutic Options for Patients with TP53 Deficient Chronic Lymphocytic Leukemia: Narrative Review. Cancer Manag. Res. 2021, 13, 1459–1476. [Google Scholar] [CrossRef]
- Chiorazzi, N.; Chen, S.S.; Rai, K.R. Chronic Lymphocytic Leukemia. Cold Spring Harb. Perspect. Med. 2021, 11, a035220. [Google Scholar] [CrossRef]
- Mansouri, L.; Thorvaldsdottir, B.; Sutton, L.A.; Karakatsoulis, G.; Meggendorfer, M.; Parker, H.; Nadeu, F.; Brieghel, C.; Laidou, S.; Moia, R.; et al. Different prognostic impact of recurrent gene mutations in chronic lymphocytic leukemia depending on IGHV gene somatic hypermutation status: A study by ERIC in HARMONY. Leukemia 2023, 37, 339–347. [Google Scholar] [CrossRef]
- Abrisqueta, P.; Medina, D.; Villacampa, G.; Lu, J.; Alcoceba, M.; Carabia, J.; Boix, J.; Tazón-Vega, B.; Iacoboni, G.; Bobillo, S.; et al. A gene expression assay based on chronic lymphocytic leukemia activation in the microenvironment to predict progression. Blood Adv. 2022, 6, 5763–5773. [Google Scholar] [CrossRef]
- Liu, P.; Wang, K.; Li, J.; Ogasawara, M.A.; Xia, Z.; Wierda, W.G.; Keating, M.J.; Li, Y.; Huang, P. Global miRNA profiling reveals key molecules that contribute to different chronic lymphocytic leukemia incidences in Asian and Western populations. Haematologica 2024, 109, 479–492. [Google Scholar] [CrossRef]
- Ali, A.; Mahla, S.B.; Reza, V.; Hossein, A.; Bahareh, K.; Mohammad, H.; Fatemeh, S.; Mostafa, A.B.; Leili, R. MicroRNAs: Potential prognostic and theranostic biomarkers in chronic lymphocytic leukemia. eJHaem 2024, 5, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Trojani, A.; Di Camillo, B.; Tedeschi, A.; Lodola, M.; Montesano, S.; Ricci, F.; Vismara, E.; Greco, A.; Veronese, S.; Orlacchio, A.; et al. Gene expression profiling identifies ARSD as a new marker of disease progression and the sphingolipid metabolism as a potential novel metabolism in chronic lymphocytic leukemia. Cancer Biomark. 2012, 11, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Bilous, N.; Abramenko, I.; Chumak, A.; Dyagil, I.; Martina, Z. Analysis of LPL gene expression in patients with chronic lymphocytic leukemia. Exp. Oncol. 2019, 41, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sathiaseelan, V.; Moore, A.; Tan, S.; Chilamakuri, C.S.R.; Roamio Franklin, V.N.; Shahsavari, A.; Jakwerth, C.A.; Hake, S.B.; Warren, A.J.; et al. ZAP-70 constitutively regulates gene expression and protein synthesis in chronic lymphocytic leukemia. Blood 2021, 137, 3629–3640. [Google Scholar] [CrossRef]
- Coombs, C.C. Frontline Therapy of CLL-Changing Treatment Paradigms. Curr. Hematol. Malig. Rep. 2024, 19, 65–74. [Google Scholar] [CrossRef]
- Norat, T.; Aune, D.; Chan, D.; Romaguera, D. Fruits and vegetables: Updating the epidemiologic evidence for the WCRF/AICR lifestyle recommendations for cancer prevention. Cancer Treat. Res. 2014, 159, 35–50. [Google Scholar]
- Darwiche, W.; Gomila, C.; Ouled-Haddou, H.; Naudot, M.; Doualle, C.; Morel, P.; Nguyen-Khac, F.; Garçon, L.; Marolleau, J.P.; Ghamlouch, H. Ascorbic acid (vitamin C) synergistically enhances the therapeutic effect of targeted therapy in chronic lymphocytic leukemia. J. Exp. Clin. Cancer Res. 2020, 39, 228. [Google Scholar] [CrossRef]
- Abiri, B.; Vafa, M. Vitamin C and Cancer: The Role of Vitamin C in Disease Progression and Quality of Life in Cancer Patients. Nutr. Cancer 2021, 73, 1282–1292. [Google Scholar] [CrossRef]
- Casabonne, D.; Gracia, E.; Espinosa, A.; Bustamante, M.; Benavente, Y.; Robles, C.; Costas, L.; Alonso, E.; Gonzalez-Barca, E.; Tardón, A.; et al. Fruit and vegetable intake and vitamin C transporter gene (SLC23A2) polymorphisms in chronic lymphocytic leukaemia. Eur. J. Nutr. 2017, 56, 1123–1133. [Google Scholar] [CrossRef]
- Sobhi, P.; Bahrami, M.; Mahdizadeh, F.; Fazaeli, A.; Babaei, G.; Rezagholizadeh, L. Vitamin D and potential effects on cancers: A review. Mol. Biol. Rep. 2024, 51, 190. [Google Scholar] [CrossRef]
- Ito, Y.; Honda, A.; Kurokawa, M. Impact of vitamin D level at diagnosis and transplantation on the prognosis of hematological malignancy: A meta-analysis. Blood Adv. 2022, 6, 1499–1511. [Google Scholar] [CrossRef] [PubMed]
- Gerousi, M.; Psomopoulos, F.; Kotta, K.; Tsagiopoulou, M.; Stavroyianni, N.; Anagnostopoulos, A.; Anastasiadis, A.; Gkanidou, M.; Kotsianidis, I.; Ntoufa, S.; et al. The Calcitriol/Vitamin D Receptor System Regulates Key Immune Signaling Pathways in Chronic Lymphocytic Leukemia. Cancers 2021, 13, 285. [Google Scholar] [CrossRef] [PubMed]
- Kubeczko, M.; Nowara, E.; Spychałowicz, W.; Wdowiak, K.; Bednarek, A.; Karwasiecka, D.; Chudek, J.; Wojnar, J. Efficacy and safety of vitamin D supplementation in patients with chronic lymphocytic leukemia. Postępy Hig. Med. Doświadczalnej 2016, 70, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Tadmor, T.; Melamed, G.; Alapi, H.; Gazit, S.; Patalon, T.; Rokach, L. Supplement of Vitamin D for early-stage Chronic Lymphocytic Leukemia Patients is Associated with a Longer Time to first Treatment. Blood Adv. 2024, 3, 3840–3846. [Google Scholar] [CrossRef]
- Nakamura, N.; Kanemura, N.; Matsumoto, T.; Nakamura, H.; Shibata, Y.; Yamaguchi, K.; Kitagawa, J.; Ikoma, Y.; Suzaki, T.; Kaneda, Y.; et al. Effect of Vitamin D and Skeletal Muscle Mass on Prognosis of Patients with Diffuse Large B-Cell Lymphoma. Nutrients 2024, 16, 2653. [Google Scholar] [CrossRef]
- Remelli, F.; Vitali, A.; Zurlo, A.; Volpato, S. Vitamin D Deficiency and Sarcopenia in Older Persons. Nutrients 2019, 11, 2861. [Google Scholar] [CrossRef]
- Rojas Gil, A.P.; Kodonis, I.; Ioannidis, A.; Nomikos, T.; Dimopoulos, I.; Kosmidis, G.; Katsa, M.E.; Melliou, E.; Magiatis, P. The Effect of Dietary Intervention with High-Oleocanthal and Oleacein Olive Oil in Patients with Early-Stage Chronic Lymphocytic Leukemia: A Pilot Randomized Trial. Front. Oncol. 2022, 11, 810249. [Google Scholar] [CrossRef]
- Farinello, D.; Wozińska, M.; Lenti, E.; Genovese, L.; Bianchessi, S.; Migliori, E.; Sacchetti, N.; di Lillo, A.; Bertilaccio, M.T.S.; de Lalla, C.; et al. A retinoic acid-dependent stroma-leukemia crosstalk promotes chronic lymphocytic leukemia progression. Nat. Commun. 2018, 9, 1787. [Google Scholar] [CrossRef]
- Pan, B.; Xu, Z.; Du, K.; Gao, R.; Zhang, J.; Yin, H.; Shen, H.; Liang, J.; Li, Y.; Wang, L.; et al. Investigation of fatty acid metabolism in chronic lymphocytic leukemia to guide clinical outcome and therapy. Ann. Hematol. 2024, 103, 1241–1254. [Google Scholar] [CrossRef]
- Haskin, G.; Kogan, M. Case Report of Unexpectedly Long Survival of Patient with Chronic Lymphocytic Leukemia: Why Integrative Methods Matter. Integr. Med. A Clin. J. 2018, 17, 51–56. [Google Scholar]
- Plooij, F.X.; Raemaekers, J. Stable improvement in classical B-cell chronic lymphocytic leukemia with dietary interventions: A personal experience. Clin. Case Rep. 2020, 8, 2948–2954. [Google Scholar] [CrossRef] [PubMed]
- Bossi, L.E.; Palumbo, C.; Trojani, A.; Melluso, A.; Di Camillo, B.; Beghini, A.; Sarnataro, L.M.; Cairoli, R. A Nine-Gene Expression Signature Distinguished a Patient with Chronic Lymphocytic Leukemia Who Underwent Prolonged Periodic Fasting. Medicina 2023, 59, 1405. [Google Scholar] [CrossRef] [PubMed]
- Forester, S.C.; Lambert, J.D. The role of antioxidant versus pro-oxidant effects of green tea polyphenols in cancer prevention. Mol. Nutr. Food Res. 2011, 55, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Potre, C.; Borsi, E.; Potre, O.; Ionita, I.; Samfireag, M.; Costachescu, D.; Secosan, C.; Lazar, S.; Ristescu, A.I. A Systematic Review Assessing the Impact of Vitamin D Levels on Adult Patients with Lymphoid Malignancies. Curr. Oncol. 2023, 30, 4351–4364. [Google Scholar] [CrossRef]
- Benkhadra, M.; Fituri, N.; Aboukhalaf, S.; Ghasoub, R.; Mattar, M.; Alfarsi, K.; Alshemmari, S.; Yassin, M.A. The Safety of Novel Therapies in Chronic Lymphocytic Leukemia in the Era of Intermittent Fasting: A Pharmacology-Based Review. Cancers 2024, 16, 2079. [Google Scholar] [CrossRef]
- Raucci, F.; Vernieri, C.; Di Tano, M.; Ligorio, F.; Blaževitš, O.; Lazzeri, S.; Shmahala, A.; Fragale, G.; Salvadori, G.; Varano, G.; et al. Cyclic Fasting-Mimicking Diet Plus Bortezomib and Rituximab Is an Effective Treatment for Chronic Lymphocytic Leukemia. Cancer Res. 2024, 84, 1133–1148. [Google Scholar] [CrossRef]
- de Jong, J.; Sukbuntherng, J.; Skee, D.; Murphy, J.; O’Brien, S.; Byrd, J.C.; James, D.; Hellemans, P.; Loury, D.J.; Jiao, J.; et al. The effect of food on the pharmacokinetics of oral ibrutinib in healthy participants and patients with chronic lymphocytic leukemia. Cancer Chemother. Pharmacol. 2015, 75, 907–916. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).