Abstract
Background: Serum albumin is crucial for critically ill patients. To date, several reports have focused on the influence of lower albumin levels on poorer prognosis and disease outcome in different subsets of critical clinical conditions varying from sepsis, to cirrhosis, renal failure, and cancer. In the last few years, investigators reported the role of serum albumin levels in predicting the thrombotic risk in patients with nephrotic syndrome, and, in particular, the degree of hypoalbuminemia seemed to influence the risk of thromboembolism. Decreased serum albumin has been associated with the risk of venous thromboembolism and mortality in adult cancer patients after ending chemotherapy for different malignancies. Aims: We aimed to investigate the role of serum albumin in a cohort of children diagnosed as having VTE (venous thromboembolism) during their treatment for acute lymphoblastic leukemia (ALL) compared to ALL children who did not experience VTE. Methods: A nested case-control study was conducted at the Pediatric Oncology and Hematology Department, University Hospital of Bari. A total of 167 patients were diagnosed as having ALL and treated according to AIEOP-BFM ALL 2000-R2006 protocol. Among these, 12 cases of VTE were recorded and matched to 31 controls, for a total of 43 ALL patients (30 males, aged 1.2–16.6 years) enrolled in the present study. Serum albumin level was collected at diagnosis—before the start of any treatment—(time point 0) and at the moment of the VTE or corresponding time point of the protocol (time point 1). Information on inherited thrombophilia genotype were also recorded. Results: Patients presenting VTE showed a marked reduction of average albumin levels as compared to the control children: t0–t1 1.1 IC (95%) = (0.55, 1.65) vs. 0.31 IC (95%) = (0.08, 0.55); p < 0.005. Conclusions: The reduction of serum albumin levels in our cohort might be an expression of altered vascular and endothelial homeostasis, likely predisposing to VTE. This important clinical observation warrants further larger studies.
1. Introduction
Serum albumin is crucial for critically ill patients [1]. To date, several reports have focused on the influence of lower albumin levels on poorer prognosis and disease outcome in different subsets of critical clinical conditions varying from sepsis [2], to cirrhosis [3,4], renal failure [5], and cancer in adult [6] and pediatric patients [7].
In the last few years, investigators reported the role of serum albumin levels in predicting the thrombotic risk in patients with nephrotic syndrome [8], and, in particular, the degree of hypoalbuminemia seemed to influence the risk of thromboembolism [9].
The hypothesis that low serum albumin could be associated with venous thromboembolism (VTE) has been tested in a large population-based cohort, as well [10]. The study concluded low serum albumin being a modest marker of increased VTE risk. A large metanalysis confirmed the role of hypoalbuminemia as a risk factor for VTE in both medical and surgical patients irrespective of cancer coexistence. According to the authors of [11], serum albumin analysis may represent a simple and cheap tool to identify patients at VTE risk.
Decreased serum albumin has been associated with the risk of venous thromboembolism and mortality in adult cancer patients after ending chemotherapy for different malignancies [12]. Moreover, more recently, others reported the predictive role of hypoalbuminemia on VTE in gastric and pancreatic cancer [13,14].
However, it is still a major debate as to why and how albumin decrease might influence the occurrence of VTE, and the majority of authors conclude that albumin decline is a marker of increased risk, rather than a direct cause, of VTE.
In children treated for acute lymphoblastic leukemia (ALL), hypoalbuminemia is often present likely as a side effect of therapy, in particular, hepatic toxicity with reduced protein synthesis. Asparaginase (ASP), used in the treatment of ALL since the 1960s, causes depletion of L-asparagine, reducing hepatic protein synthesis [15].
Moreover children with ALL have an increased risk of VTE due to a multifactorial etiology, such as the unavoidable presence of a central venous catheter, chemotherapy protocols affecting the endothelium and hemostatic system, and inherited risk factors, such as thrombophilia [16]. Derangement of the hemostatic system, hepatic toxicity, and VTE are amongst major side effects of ASP during treatment for ALL, which are to be taken into account with hypoalbuminemia considering the cumulative risk of VTE.
We aimed to investigate the role of serum albumin in a cohort of children diagnosed with VTE during their treatment for ALL compared to ALL children who did not experience VTE. The hypothesis is that adequate levels of albumin would represent a protective factor for the endothelium and for the endothelial homeostasis, acting positively on the inflammatory state caused mainly by chemotherapy [17]. The reduction of albumin could favor the occurrence of thromboembolic events.
2. Methods
A case-control study was conducted at the Pediatric Oncology and Hematology Department, University Hospital of Bari. The study regarded patients being treated for ALL enrolled in the AIEOP-BFM ALL 2000-R2006 protocol (which is an Italian front-line protocol for the treatment of acute lymphoblastic leukemia in children and adolescent <18 years of age) [18] based on polychemotherapy, including corticosteroids and asparaginase.
We retrospectively collected data from patients treated at our Institution, notified as having a VTE in the specific database of the AIEOP-BFM ALL 2000-R2006 protocol, which evaluates the adverse events occurring during protocol application.
In the present study, symptomatic cerebral venous thromboses were included. A symptomatic VTE was defined as any VTE diagnosed by appropriate instrumental testing after the appearance of clinical symptoms typically suggesting VTE.
Each VTE case was matched to a set of two to three controls extracted from the cohort of AIEOP-BFM ALL 2000-R2006 patients treated at our Pediatric Oncologic AIEOP Center. Cases and controls were matched according to sex, age, the ALL immunophenotype (T-ALL, pB-ALL), the steroid used (DXM, PDN), the phase of therapy, the outcome (dead or alive and in complete remission, when applicable) at the same time of scheduled chemotherapy with respect to the case patient, as previously reported [19].
Exclusion criteria were: (1) acute infection, sepsis, or septic shock within the preceding 2 weeks, (2) dehydration, (3) liver or renal insufficiency, and (4) congenital malformations.
2.1. Patients
At our AIEOP Center, 167 patients were diagnosed as having ALL and treated according to AIEOP-BFM ALL 2000-R2006 protocol. Among these, 12 cases of VTE were recorded and matched to 31 controls, for a total of 43 ALL patients (30 males, aged 1.2–16.6 years) enrolled in the present study.
2.2. Laboratory Data Collection
Data collection regarded white blood cell count (WBC), platelet count, C-reactive protein (CRP), liver transaminase, clotting test in terms of levels of ATIII, FBG, the presence of a central venous catheter at the moment of VTE in cases or at the same time of scheduled chemotherapy in controls. Serum albumin level was collected at diagnosis—before the start of any treatment—(time point 0, t0) and at the moment of the VTE or corresponding time point of the protocol (time point 1, t1).
Inherited thrombophilia genotype was also recorded; in particular, data about the presence of factor V Leiden, the presence of a G20210A mutation in the prothrombin gene, and the presence of C677T and A1298C polymorphism of the methylene tetrahydrofolate reductase (MTHFR) gene.
The genetic data were extracted from a database enrolling approximately 200 ALL patients. Among these, 30 out of 43 ALL patients participating in the present study had genetic data available.
The AIEOP-BFM ALL 2000-R2006 multicenter study was conducted in accordance with the ethical standards stated in the Declaration of Helsinki and approved by the local ethical committee; informed consent was obtained from parents or guardians.
2.3. Statistical Analysis
The comparison between groups was conducted by means of the two-tailed Student’s t-test for comparison between two averages.
Pearson′s correlation coefficient was used to analyze the correlation between albumin levels at time point 0 and time point 1.
A logistic regression model was estimated to assess the influence of polymorphisms for thrombophilia and reductions in albumin levels on event probability.
All analyses and graphs were obtained by means of the statistical language R version 4.3.3.
Relative change in the albumin levels was computed as (alb1–alb0)/alb0, so negative values mean a reduction from time 0 to time 1.
3. Results
Forty-three patients were recruited, including 12 patients with thrombosis and 31 controls, and 30 were male and 13 were female. All patients were diagnosed with ALL with a mean age of onset of 6.2 years. The leukemic immunophenotype was found to be B-lineage in 37/43 patients, T-lineage 6/43 patients. According to the treatment protocol, 25/43 patients were treated with prednisone (PDN), 18/43 were treated with dexamethasone (DXM).
Ten out of twelve patients with VTE presented B-lineage leukemia, 2/12 T-lineage immunophenotype. The analysis of leukemic immunophenotype showed no difference between cases and controls. Eight out of 12 patients presented the VTE during the induction phase of therapy (between day 16 and day 42 of the induction phase), while 4/12 during the reinduction phase (between day 16 and day 22 of the reinduction). In all patients VTE occurred after ASP administration.
In 21/43 patients a central venous catheter (CVC) was present, while in 22 it was not. Three out of twelve patients with VTE had a CVC at the moment of the event.
The 12 patients were treated with low molecular weight heparin (LMWH), with complete regression of the thrombosis, and are alive. One patient is dead due to the progression of the leukemia.
3.1. Inherited Thrombophilia
Information about inherited thrombophilia were available in 31/43 patients: one patient was a carrier of the gene for factor V Leiden in heterozygosis, and 4 patients carried the G20210A mutation of the prothrombin gene in heterozygosis. Regarding the clinically less relevant MTHFR gene polymorphism, 14 patients had the C677T polymorphism of the MTHFR gene in heterozygosis, 4 of whom were homozygosis; 14 patients carried the A1298C polymorphism of the MTHFR gene in heterozygosis, 4 of whom were homozygosis. The remaining patients did not show any genetic mutations.
Nine out of twelve patients who presented VTE had information for inherited thrombophilia: 2 patients carried the G20210A mutation of the prothrombin gene in heterozygosis, 5 patients were carriers of the C677T polymorphism of the MTHFR gene in heterozygosis, 1 of whom was homozygosis, 4 patients carried the A1298C polymorphism of the MTHFR gene in heterozygosis.
The results from the analysis of genotypes for hereditary thrombophilia show that there is no statistically significant difference between the control group and the group of thrombotic patients in terms of genetic predisposition to thrombotic events.
3.2. Albumin Levels
The average serum albumin at onset in patients with thrombosis was 4.16 g/dl and 3.55 g/dl in control cases. On the day of the event, albumin averaged 3.11 g/dl in patients with thrombosis and 3.19 g/dl in control cases. In patients with VTE, there was a 24.84% reduction in mean serum albumin levels, whereas in the controls the reduction in albumin averaged 7.83%.
The clinical characteristics of patients are shown in Table 1.

Table 1.
Clinical characteristics of patients.
According to the statistical analysis results, there were no statistically significant differences between the two groups with regard to age, gender, antithrombin III, fibrinogen, glutamic oxaloacetic transaminase (GOT), glutamic pyruvate transaminase (GPT), C-reactive protein (CRP), presence of CVC at the day of the event, and albumin levels at the day of the event.
Albumin levels at diagnosis and the change (expressed as a percentage) in albumin values between the onset of the disease and the day of the thrombotic event were significantly different (Table 2). Beyond these values, platelets were also significantly different (Table 1).

Table 2.
Albumin levels at the onset of leukemia (time point 0) and at the time of the event (time point 1).
Figure 1 shows the distribution of albumin levels in relation to the percentage of reduction for patients with and without VTE.
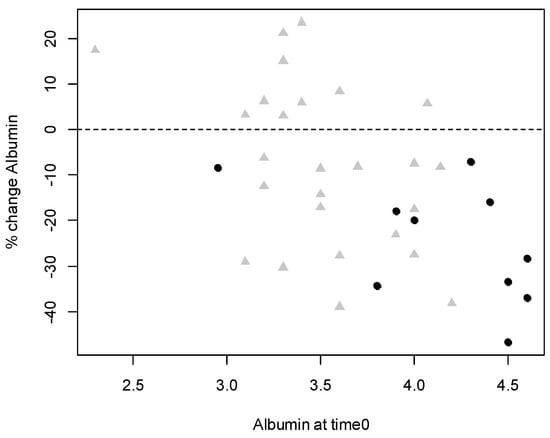
Figure 1.
Scatterplot of albumin levels at time 0 and relative change. Black dots refer to patients who presented venous thromboembolism and grey triangles refer to patients who did not.
The relative change in albumin levels was calculated as (alb1–alb0)/alb0, such that negative values indicate a reduction in albumin from time 0 (date of onset) to time 1 (date of event).
Patients with VTE showed a more pronounced mean reduction in albumin levels than patients without a thromboembolic event: mean t0–t1 1.1 IC (95%) = (0.55, 1.65) vs. 0.31 IC (95%) = (0.08, 0.55); p < 0.0050.
Changes in albumin levels (t0–t1) in the group of patients with thrombosis were strongly correlated with albumin level at time 0 (r = −0.56, p = 0.0002) indicating that higher albumin levels at the onset of leukemia (time 0) corresponded to a greater reduction in albumin levels detected on the day of the thrombotic event (time 1) (Figure 1).
In the box-and-whisker diagram (boxplot) in Figure 2, the sharp reduction in albumin detected in the thrombosis cases compared to the control group can be directly observed. The patients with thrombosis suffered a sharp decline in albumin levels.
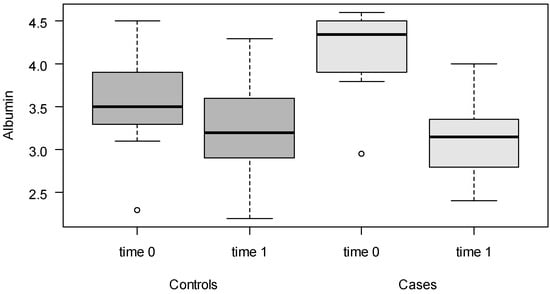
Figure 2.
Boxplots of albumin levels for cases and controls, at time point 0 (before any treatment) and time point 1 (event).
4. Discussion
In the present study, we found a reduction in serum albumin levels in patients who experienced thromboembolic events during ALL treatment, which was statistically significantly greater than in patients who had not experienced thromboembolic complications.
Venous thromboembolism during nephrotic syndrome, associated with albuminuria and hypoalbuminemia, has been reported to be associated to significant morbidity and mortality. Retrospective studies have concluded that the degree of hypoalbuminemia increases the risk of VTE in renal disease. Every 1 g/dl decrease in albumin concentration is associated with a 2.13-fold increase in the risk of VTE. Thrombosis and the risk of VTE has been correlated with hypoalbuminemia, which could be as a marker of VTE [8,9].
In addition to patients with nephrotic syndrome, an increased risk of thromboembolism, including both deep vein thrombosis and pulmonary embolism, has also been reported for adult patients with cancer. According to a cohort study of 1070 patients, albumin depletion in cancer patients correlated with thromboembolic events, and, in particular, with related morbidity and mortality, and could be an expression of tumor cachexia [12]. Although this study was unable to establish a causal relationship between albumin levels and the occurrence of VTE, it was able to show that in cancer patients, albumin levels are associated with an increased risk of VTE independently of renal function, inflammatory markers, and liver synthesis capacity.
The correlation between reduced serum albumin levels and venous thromboembolism has been reported in pediatric patients as well, in particular, for catheter-related thrombosis [20].
Recently different published evidences have underlined the role of albumin levels on thrombotic risk in large adult populations [11,21,22] or, in particular, clinical conditions such as vasculitis [23], community-acquired pneumonia [24], patients being treated with checkpoint inhibitors [25], or hospitalized acutely ill patients [26].
Interestingly, the protective role of albumin on risk of thrombosis has been recently reported in patients with COVID-19 [27,28]
Such findings have not been reported before in pediatric patients under treatment for ALL. However, it is important because VTE in patients with ALL might be a serious complication during chemotherapy due to the known multifactorial and cumulative thrombotic risk in such patients [16]. In our pediatric case series, the pathogenetic mechanism remains debated. First of all, ASP, a key and old drug in the treatment of ALL, might be responsible for hypoalbuminemia due to impaired hepatic synthesis. Furthermore, a possible hypothesis could start from the consideration of endothelial damage due to chemotherapy observed in pediatric patients with ALL [15]. Drugs such as asparaginase, dexamethasone, and anthracyclines have toxic effects on the endothelium through destabilizing coagulation homeostasis.
In view of the thromboembolic risk present in patients with ALL undergoing chemotherapy, in this study, we evaluated not only albumin values but also antithrombin III, fibrinogen, GOT, GPT, CRP, and platelet values, factors with a potential influence on thrombotic risk. No statistically significant difference was found between the two groups of patients in these parameters, except for the platelet value. It was plausible for us to expect this difference in platelet count values, venous thromboembolism being an event that, in itself, causes platelet consumption.
Furthermore, low albumin levels have been found in critically ill patients [1], and the correlation between inflammatory state and hypoalbuminemia has been shown.
For all the above considerations, it could be assumed that albumin may prove to be a useful marker in ALL patients, hypoalbuminemia may indirectly be a risk factor for VTE and may affect coagulative homeostasis due to the numerous metabolic interactions of albumin and its biochemical role.
From a metabolic point of view, albumin has the ability to mobilize polyunsaturated fatty acids (PUFAs) from the liver and other tissues so as to increase the formation of cytoprotective bioactive lipids, lipoxins, resolvins, and protectins that reduce leukocyte infiltration, suppress inflammation, increase endothelial nitric oxide, protect endothelial cells, and rebalance hemostasis.
From a biochemical point of view, albumin is very important as it plays a key role in the detoxification of reactive oxygen species that can disrupt endothelial cells. Human serum albumin (HSA) holds about 80 per cent of the thiol groups in blood. Albumin is considered to be an important intravascular scavenger for radicals in plasma due to its intrinsic production by the liver and its constant high concentration (0.5 mM); therefore, the reduction of this antioxidant power could favor endothelial damage and consequently the onset of VTE [29,30].
In this study, for the first time, a reduction in hypoalbuminemia has been related to the thromboembolic event during chemotherapy for ALL. In particular, our findings refer to a sharp reduction of albumin levels, indicating that a rapid and consistent reduction deranged homeostatic system. The concept of the “magnitude of decline in serum albumin” as a significant predictor of worse outcomes has also been reported in different settings, such as graft vs. host disease (GVHD) in transplanted patients [31,32].
This finding needs further confirmation with larger studies; however, it should be considered that the number of 12 patients with VTE found in our study population of the AIEOP LAL 2000-R2006 protocol represent 25% of the patients with VTE recorded in this protocol in the entire national territory (48 cases) [19]. Therefore, the reduced sample size should be attributed to the rarity of the event in a pediatric population.
Following our observation and as reported for patients with COVID-19, in which the albumin supplementation has been used to dampen hypercoagulability, in our experience, albumin supplementation during treatment for ALL is being used to prevent critical clinical conditions. Albumin 20% is being administered intravenously depending on albumin level drop at the daily dose of 1 mL per kilogram of body weight.
5. Conclusions
The reduction in albumin levels we observed could be an expression of an imbalance in vascular and endothelial homeostasis, probably concurrent in the determinism of thromboembolic complications. In the context of children being treated for ALL, hypoalbuminemia might indicate those patients at a higher risk of VTE. This consideration may be useful for therapeutic interventions to prevent or correct the reduction in albuminemia in order to prevent severe toxic complications during chemotherapy for ALL.
Further studies are needed to confirm this, in our opinion, important, observation.
Author Contributions
Conceptualization, P.M., M.G. and T.P.; methodology, P.M. and J.F.; validation, P.M. and T.P.; formal analysis, V.M.R.M.; investigation, C.R.; resources, P.M. and C.R.; data curation, V.M.R.M.; original draft preparation, P.M.; review and editing, C.R.; supervision, N.S. and M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was performed according to the Declaration of Helsinki. Ethical review and approval were waived for this study due to the retrospective nature of the study.
Informed Consent Statement
Written consent was obtained from all participants and their parents prior to the treatment protocol. The need for individual consent was waived by the committee due to the retrospective nature of the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author, due to privacy.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Das, U.N. Albumin infusion for the critically ill—Is it beneficial and, if so, why and how? Crit. Care. 2015, 19, 156. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, C.; Liu, Z.; Tian, M.; Xu, P.; Li, B.; Yang, Q.; Yang, Y. Relationship Between Serum Albumin Levels and Infections in Newborn Late Preterm Infants. Med. Sci. Monit. 2016, 22, 92–98. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caraceni, P.; Tufoni, M.; Zaccherini, G.; Riggio, O.; Angeli, P.; Alessandria, C.; Neri, S.; Foschi, F.G.; Levantesi, F.; Airoldi, A.; et al. On-treatment serum albumin level can guide long-term treatment in patients with cirrhosis and uncomplicated ascites. J. Hepatol. 2021, 74, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Chen, H.; Ma, C.; Sun, Q.; Yang, M.; Wang, H.; Peng, Q.; Wang, J.; Zhang, C.; Huang, W.; et al. Identification of indications for albumin administration in septic patients with liver cirrhosis. Crit. Care. 2023, 27, 300. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheng, T.; Wang, X.; Han, Y.; Hao, J.; Hu, H.; Hao, L. The level of serum albumin is associated with renal prognosis and renal function decline in patients with chronic kidney disease. BMC Nephrol. 2023, 24, 57. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gupta, D.; Lis, C.G. Pretreatment serum albumin as a predictor of cancer survival: A systematic review of the epidemiological literature. Nutr. J. 2010, 9, 69. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McLean, T.W.; Stewart, R.M.; Curley, T.P.; Dewsnup, M.Y.; Thomas, S.G.; Russell, T.B.; Tooze, J.A. Hypoalbuminemia in children with cancer treated with chemotherapy. Pediatr. Blood Cancer. 2020, 67, e28065. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reynolds, M.L.; Nachman, P.H.; Mooberry, M.J.; Crona, D.J.; Derebail, V.K. Recurrent venous thromboembolism in primary membranous nephropathy despite direct Xa inhibitor therapy. J. Nephrol. 2019, 32, 669–672. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gyamlani, G.; Molnar, M.Z.; Lu, J.L.; Sumida, K.; Kalantar-Zadeh, K.; Kovesdy, C.P. Association of serum albumin level and venous thromboembolic events in a large cohort of patients with nephrotic syndrome. Nephrol. Dial. Transplant. 2017, 32, 157–164. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Folsom, A.R.; Lutsey, P.L.; Heckbert, S.R.; Cushman, M. Serum albumin and risk of venous thromboembolism. J. Thromb. Haemost. 2010, 104, 100–104. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Valeriani, E.; Pannunzio, A.; Palumbo, I.M.; Bartimoccia, S.; Cammisotto, V.; Castellani, V.; Porfidia, A.; Pignatelli, P.; Violi, F. Risk of venous thromboembolism and arterial events in patients with hypoalbuminemia: A comprehensive meta-analysis of more than 2 million patients. J. Thromb. Haemost. 2024, 22, 2823–2833. [Google Scholar] [CrossRef] [PubMed]
- Königsbrügge, O.; Posch, F.; Riedl, J.; Reitter, E.M.; Zielinski, C.; Pabinger, I.; Ay, C. Association Between Decreased Serum Albumin with Risk of Venous Thromboembolism and Mortality in Cancer Patients. Oncologist 2016, 21, 252–257. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Takayoshi, K.; Kusaba, H.; Aikawa, T.; Koreishi, S.; Sagara, K.; Nakano, M.; Komoda, M.; Kono, M.; Fukata, M.; Arita, T.; et al. Hypoalbuminemia for the prediction of venous thromboembolism and treatment of direct oral anticoagulants in metastatic gastric cancer patients. Gastric Cancer 2019, 22, 988–998. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, H.; Li, B.; Cui, S.; Lyu, S.; Lang, R. A nomogram model to predict the portal vein thrombosis risk after surgery in patients with pancreatic cancer. Front. Surg. 2023, 10, 1293004. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Christensen, S.R.; Jensen, C.F.; Heldrup, J.; Taylor, Z.; Ramsey, L.B.; Rosthøj, S. Hypoalbuminemia in children with acute lymphoblastic leukemia: Relation to asparaginase therapy and impact on high dose methotrexate elimination. Cancer Chemother. Pharmacol. 2024. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Levy-Mendelovich, S.; Barg, A.A.; Kenet, G. Thrombosis in pediatric patients with leukemia. Thromb. Res. 2018, 164, S94–S97. [Google Scholar] [CrossRef]
- Giordano, P.; Muggeo, P.; Delvecchio, M.; Carbonara, S.; Romano, A.; Altomare, M.; Ricci, G.; Valente, F.; Zito, A.; Scicchitano, P.; et al. Endothelial dysfunction and cardiovascular risk factors in childhood acute lymphoblastic leukemia survivors. Int. J. Cardiol. 2017, 228, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Möricke, A.; Zimmermann, M.; Valsecchi, M.G.; Stanulla, M.; Biondi, A.; Mann, G.; Locatelli, F.; Cazzaniga, G.; Niggli, F.; Aricò, M.; et al. Dexamethasone vs. prednisone in induction treatment of pediatric ALL: Results of the randomized trial AIEOP-BFM ALL 2000. Blood 2016, 127, 2101–2112. [Google Scholar] [CrossRef] [PubMed]
- Santoro, N.; Colombini, A.; Silvestri, D.; Grassi, M.; Giordano, P.; Parasole, R.; Barisone, E.; Caruso, R.; Conter, V.; Valsecchi, M.G.; et al. Screening for coagulopathy and identification of children with acute lymphoblastic leukemia at a higher risk of symptomatic venous thrombosis: An AIEOP experience. J. Pediatr. Hematol. Oncol. 2013, 35, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, N.; Roe, D.J.; Saboda, K.; Journeycake, J.; Moreno, V.; Lentz, S.R. Association of low serum albumin with venous thrombosis in pediatric patients. Thromb. Res. 2022, 218, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Kunutsor, S.K.; Seidu, S.; Katechia, D.T.; Laukkanen, J.A. Inverse association between serum albumin and future risk of venous thromboembolism: Interrelationship with high sensitivity C-reactive protein. Ann. Med. 2018, 50, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Deng, J.; Ding, Y.; Luo, S.; Li, S.; Guan, Y.; Cao, X.; Hao, X.; Hu, Y. Serum albumin, genetic susceptibility, and risk of venous thromboembolism. Res. Pract. Thromb. Haemost. 2024, 8, 102509. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Isaacs, B.; Gapud, E.J.; Antiochos, B.; Seo, P.; Geetha, D. Venous Thrombotic Events in ANCA-Associated Vasculitis: Incidence and Risk Factors. Kidney360 2020, 1, 258–262. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Valeriani, E.; Cangemi, R.; Carnevale, R.; Romiti, G.F.; Pannunzio, A.; Pignatelli, P.; Violi, F. Hypoalbuminemia as predictor of thrombotic events in patients with community-acquired pneumonia. Int. J. Cardiol. 2024, 404, 131942. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Cánovas, M.; Fernández Garay, D.; Adoamnei, E.; Guirao García, E.; López Robles, J.; Cacho Lavin, D.; Martínez de Castro, E.; Campos Balea, B.; Garrido Fernández, A.; Fernández Pérez, I.; et al. Immune checkpoint inhibitor-associated thrombosis in patients with bladder and kidney cancer: A study of the Spanish Society of Medical Oncology (SEOM) thrombosis and cancer group. Clin. Transl. Oncol. 2023, 25, 3021–3031. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chi, G.; Gibson, C.M.; Liu, Y.; Hernandez, A.F.; Hull, R.D.; Cohen, A.T.; Harrington, R.A.; Goldhaber, S.Z. Inverse relationship of serum albumin to the risk of venous thromboembolism among acutely ill hospitalized patients: Analysis from the APEX trial. Am. J. Hematol. 2019, 94, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Violi, F.; Ceccarelli, G.; Loffredo, L.; Alessandri, F.; Cipollone, F.; D’ardes, D.; D’Ettorre, G.; Pignatelli, P.; Venditti, M.; Mastroianni, C.M.; et al. Albumin Supplementation Dampens Hypercoagulability in COVID-19: A Preliminary Report. J. Thromb. Haemost. 2021, 121, 102–105. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kheir, M.; Saleem, F.; Wang, C.; Mann, A.; Chua, J. Higher albumin levels on admission predict better prognosis in patients with confirmed COVID-19. PLoS ONE 2021, 16, e0248358. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anraku, M.; Chuang, V.T.; Maruyama, T.; Otagiri, M. Redox properties of serum albumin. Biochim. Biophys. Acta. 2013, 1830, 5465–5472. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martinez, R.; Andreola, F.; Mehta, G.; Poulton, K.; Oria, M.; Jover, M.; Soeda, J.; Macnaughtan, J.; De Chiara, F.; Habtesion, A.; et al. Immunomodulatory and antioxidant function of albumin stabilises the endothelium and improves survival in a rodent model of chronic liver failure. J. Hepatol. 2015, 62, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, A.; DiPersio, J.F.; Westervelt, P.; Abboud, C.N.; Schroeder, M.A.; Cashen, A.F.; Pusic, I.; Romee, R. Peritransplant Serum Albumin Decline Predicts Subsequent Severe Acute Graft-versus-Host Disease after Mucotoxic Myeloablative Conditioning. Biol. Blood Marrow Transplant. 2016, 22, 1137–1141. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Mochizuki, K.; Sano, H.; Kobayashi, S.; Ohara, Y.; Ikeda, K.; Ohto, H.; Kikuta, A. Decline of serum albumin precedes severe acute GVHD after haploidentical HSCT. Pediatr. Int. 2021, 63, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).