Adult-Onset Systemic Chronic Active Epstein-Barr Virus Disease: A Case Report Highlighting Unique Immunophenotype and Novel Molecular Insights in the Context of Chronic HBV Hepatitis
Abstract
1. Introduction
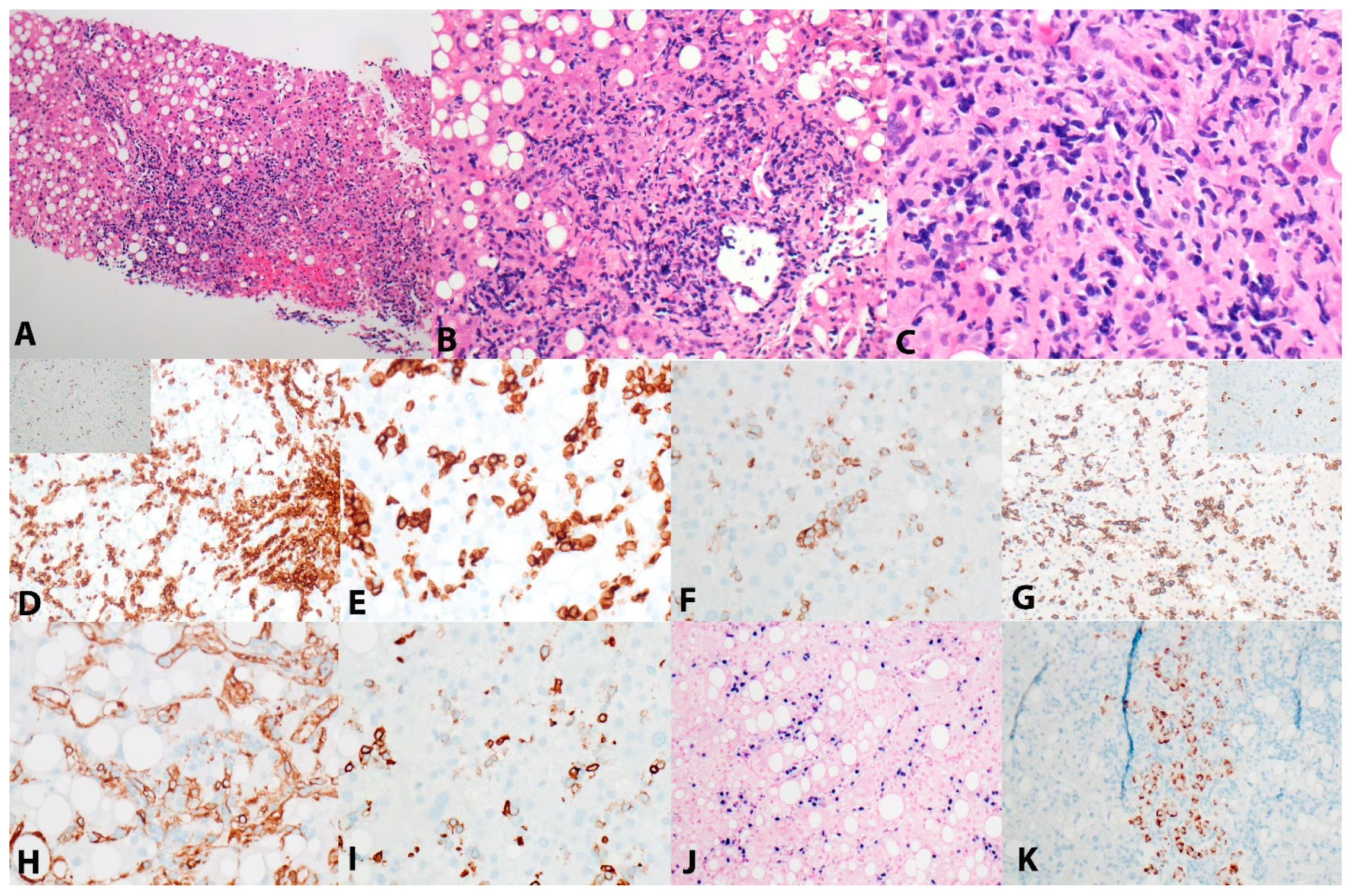
2. Case Presentation
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cohen, J.I.; Kimura, H.; Nakamura, S.; Ko, Y.H.; Jaffe, E.S. Epstein–Barr virus-associated lymphoproliferative disease in non-immunocompromised hosts: A status report and summary of an international meeting, 8–9 September 2008. Ann. Oncol. 2009, 20, 1472–1482. [Google Scholar] [CrossRef]
- Chakravorty, S.; Afzali, B.; Kazemian, M. EBV-associated diseases: Current therapeutics and emerging technologies. Front. Immunol. 2022, 13, 1059133. [Google Scholar] [CrossRef]
- Montes-Mojarro, I.A.; Kim, W.Y.; Fend, F.; Quintanilla-Martinez, L. Epstein–Barr virus positive T and NK-cell lymphoproliferations: Morphological features and differential diagnosis. Semin. Diagn. Pathol. 2020, 37, 32–46. [Google Scholar] [CrossRef]
- Quintanilla-Martinez, L.; Swerdlow, S.H.; Tousseyn, T.; Barrionuevo, C.; Nakamura, S.; Jaffe, E.S. New concepts in EBV-associated B, T, and NK cell lymphoproliferative disorders. Virchows Arch. 2023, 482, 227–244. [Google Scholar] [CrossRef]
- Kawada, J.I.; Ito, Y.; Ohshima, K.; Yamada, M.; Kataoka, S.; Muramatsu, H.; Sawada, A.; Wada, T.; Imadome, K.I.; Arai, A.; et al. Updated guidelines for chronic active Epstein-Barr virus disease. Int. J. Hematol. 2023, 118, 568–576. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J. (Eds.) WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed.; IARC: Lyon, France, 2017. [Google Scholar]
- Kimura, H.; Ito, Y.; Kawabe, S.; Gotoh, K.; Takahashi, Y.; Kojima, S.; Naoe, T.; Esaki, S.; Kikuta, A.; Sawada, A.; et al. EBV-associated T/NK-cell lymphoproliferative diseases in nonimmunocompromised hosts: Prospective analysis of 108 cases. Blood 2012, 119, 673–686. [Google Scholar] [CrossRef]
- Campo, E.; Jaffe, E.S.; Cook, J.R.; Quintanilla-Martinez, L.; Swerdlow, S.H.; Anderson, K.C.; Brousset, P.; Cerroni, L.; de Leval, L.; Dirnhofer, S.; et al. The International Consensus Classification of Mature Lymphoid Neoplasms: A report from the Clinical Advisory Committee. Blood 2022, 140, 1229–1253. [Google Scholar] [CrossRef]
- Liu, P.; Pan, X.; Chen, C.; Niu, T.; Shuai, X.; Wang, J.; Chen, X.; Liu, J.; Guo, Y.; Xie, L.; et al. Nivolumab treatment of relapsed/refractory Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis in adults. Blood 2020, 135, 826–833. [Google Scholar] [CrossRef]
- Ioannidis, N.M.; Rothstein, J.H.; Pejaver, V.; Middha, S.; McDonnell, S.K.; Baheti, S.; Musolf, A.; Li, Q.; Holzinger, E.; Karyadi, D.; et al. REVEL: An Ensemble Method for Predicting the Pathogenicity of Rare Missense Variants. Am. J. Hum. Genet. 2016, 99, 877–885. [Google Scholar] [CrossRef]
- Schiller, J.T.; Lowy, D.R. Virus infection and human cancer: An overview. Recent Results Cancer Res. 2014, 193, 1–10. [Google Scholar] [CrossRef]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Morishima, T.; Kanegane, H.; Ohga, S.; Hoshino, Y.; Maeda, A.; Imai, S.; Okano, M.; Morio, T.; Yokota, S.; et al. Prognostic factors for chronic active Epstein-Barr virus infection. J. Infect. Dis. 2003, 187, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Yonese, I.; Sakashita, C.; Imadome, K.I.; Kobayashi, T.; Yamamoto, M.; Sawada, A.; Ito, Y.; Fukuhara, N.; Hirose, A.; Takeda, Y.; et al. Nationwide survey of systemic chronic active EBV infection in Japan in accordance with the new WHO classification. Blood Adv. 2020, 4, 2918–2926. [Google Scholar] [CrossRef]
- Okuno, Y.; Murata, T.; Sato, Y.; Muramatsu, H.; Ito, Y.; Watanabe, T.; Okuno, T.; Murakami, N.; Yoshida, K.; Sawada, A.; et al. Defective Epstein–Barr virus in chronic active infection and haematological malignancy. Nat. Microbiol. 2019, 4, 404–413. [Google Scholar] [CrossRef]
- Murata, T.; Okuno, Y.; Sato, Y.; Watanabe, T.; Kimura, H. Oncogenesis of CAEBV revealed: Intragenic deletions in the viral genome and leaky expression of lytic genes. Rev. Med. Virol. 2020, 30, e2095. [Google Scholar] [CrossRef]
- Jiang, L.; Gu, Z.H.; Yan, Z.X.; Zhao, X.; Xie, Y.Y.; Zhang, Z.G.; Pan, C.M.; Hu, Y.; Cai, C.P.; Dong, Y.; et al. Exome sequencing identifies somatic mutations of DDX3X in natural killer/T-cell lymphoma. Nat. Genet. 2015, 47, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.Z.L.; Yokomori, R.; Wong, R.W.J.; Tan, T.K.; Ueda, R.; Ishida, T.; Iida, S.; Sanda, T. Requirement for TP73 and genetic alterations originating from its intragenic super-enhancer in adult T-cell leukemia. Leukemia 2022, 36, 2293–2305. [Google Scholar] [CrossRef]
- Hassan, H.M.; Dave, B.J.; Singh, R.K. TP73, an under-appreciated player in non-Hodgkin lymphoma pathogenesis and management. Curr. Mol. Med. 2014, 14, 432–439. [Google Scholar] [CrossRef]
- Rosenberg, M.; Poluch, M.; Thomas, C.; Sindaco, P.; Khoo, A.; Porcu, P. Hepatitis B Virus and B-cell lymphoma: Evidence, unmet need, clinical impact, and opportunities. Front. Oncol. 2023, 13, 1275800. [Google Scholar] [CrossRef]
- Wang, K.; Yang, H.; He, W.; Xia, Y.; Xia, Z.; Li, S.; Huang, H.; Li, Z.; Liu, P.; Jiang, W. Association between extranodal natural killer/T-cell lymphoma and hepatitis B viral infection: A case-control study. J. Cancer 2017, 8, 2676–2683. [Google Scholar] [CrossRef]
- Lohr, J.G.; Stojanov, P.; Lawrence, M.S.; Auclair, D.; Chapuy, B.; Sougnez, C.; Cruz-Gordillo, P.; Knoechel, B.; Asmann, Y.W.; Slager, S.L.; et al. Discovery and prioritization of somatic mutations in diffuse large B-cell lymphoma (DLBCL) by whole-exome sequencing. Proc. Natl. Acad. Sci. USA 2012, 109, 3879–3884. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.N.; Scuoppo, C.; Vlasevska, S.; Bal, E.; Holmes, A.B.; Holloman, M.; Garcia-Ibanez, L.; Nataraj, S.; Duval, R.; Vantrimpont, T.; et al. Unique and Shared Epigenetic Programs of the CREBBP and EP300 Acetyltransferases in Germinal Center B Cells Reveal Targetable Dependencies in Lymphoma. Immunity 2019, 51, 535–547. [Google Scholar] [CrossRef] [PubMed]
- da Silva Almeida, A.C.; Abate, F.; Khiabanian, H.; Martinez-Escala, E.; Guitart, J.; Tensen, C.P.; Vermeer, M.H.; Rabadan, R.; Ferrando, A.; Palomero, T. The mutational landscape of cutaneous T-cell lymphoma and Sezary syndrome. Nat. Genet. 2015, 47, 1465–1470. [Google Scholar] [CrossRef]
- Schatz, J.H.; Horwitz, S.M.; Teruya-Feldstein, J.; Lunning, M.A.; Viale, A.; Huberman, K.; Socci, N.D.; Lailler, N.; Heguy, A.; Dolgalev, I.; et al. Targeted mutational profiling of peripheral T-cell lymphoma not otherwise specified highlights new mechanisms in a heterogeneous pathogenesis. Leukemia 2015, 29, 237–241. [Google Scholar] [CrossRef]
- Hathuc, V.; Kreisel, F. Genetic Landscape of Peripheral T-Cell Lymphoma. Life 2022, 12, 410. [Google Scholar] [CrossRef] [PubMed]
- Shah, U.A.; Chung, E.Y.; Giricz, O.; Pradhan, K.; Kataoka, K.; Gordon-Mitchell, S.; Bhagat, T.D.; Mai, Y.; Wei, Y.; Ishida, E.; et al. North American ATLL has a distinct mutational and transcriptional profile and responds to epigenetic therapies. Blood 2018, 132, 1507–1518. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Z.; Li, Y.; Peng, H.; Liu, J.; Zhang, J.; Xiao, X. The Role of CREBBP/EP300 and Its Therapeutic Implications in Hematological Malignancies. Cancers 2023, 15, 1219. [Google Scholar] [CrossRef]
- Veazey, K.J.; Cheng, D.; Lin, K.; Villarreal, O.D.; Gao, G.; Perez-Oquendo, M.; Van, H.T.; Stratton, S.A.; Green, M.; Xu, H.; et al. CARM1 inhibition reduces histone acetyltransferase activity causing synthetic lethality in CREBBP/EP300-mutated lymphomas. Leukemia 2020, 34, 3269–3285. [Google Scholar] [CrossRef]
- Hartert, K.T.; Wenzl, K.; Krull, J.E.; Manske, M.; Sarangi, V.; Asmann, Y.; Larson, M.C.; Maurer, M.J.; Slager, S.; Macon, W.R.; et al. Targeting of inflammatory pathways with R2CHOP in high-risk DLBCL. Leukemia 2021, 35, 522–533. [Google Scholar] [CrossRef]
- Ariyoshi, M.; Schwabe, J.W. A conserved structural motif reveals the essential transcriptional repression function of Spen proteins and their role in developmental signaling. Genes Dev. 2003, 17, 1909–1920. [Google Scholar] [CrossRef]
- Quinquenel, A.; Fornecker, L.M.; Letestu, R.; Ysebaert, L.; Fleury, C.; Lazarian, G.; Dilhuydy, M.S.; Nollet, D.; Guieze, R.; Feugier, P.; et al. Prevalence of BTK and PLCG2 mutations in a real-life CLL cohort still on ibrutinib after 3 years: A FILO group study. Blood 2019, 134, 641–644. [Google Scholar] [CrossRef] [PubMed]
- Symes, E.; Wang, P.; Lager, A.M.; Bishop, M.R.; Aqil, B.; Venkataraman, G. TP53/PLCG2-mutated diffuse large B-cell lymphoma richter transformation (DLBCL-RT) of CLL with unusual CD2 and PD-1 expression. Leuk. Lymphoma 2022, 63, 2735–2738. [Google Scholar] [CrossRef]
- Manso, R.; Rodriguez-Pinilla, S.M.; Gonzalez-Rincon, J.; Gomez, S.; Monsalvo, S.; Llamas, P.; Rojo, F.; Perez-Callejo, D.; Cereceda, L.; Limeres, M.A.; et al. Recurrent presence of the PLCG1 S345F mutation in nodal peripheral T-cell lymphomas. Haematologica 2015, 100, e25–e27. [Google Scholar] [CrossRef] [PubMed]
- Vaque, J.P.; Gomez-Lopez, G.; Monsalvez, V.; Varela, I.; Martinez, N.; Perez, C.; Dominguez, O.; Grana, O.; Rodriguez-Peralto, J.L.; Rodriguez-Pinilla, S.M.; et al. PLCG1 mutations in cutaneous T-cell lymphomas. Blood 2014, 123, 2034–2043. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Ye, X.; Su, H.; Li, W.; Liu, D.; Pirmoradian, M.; Wang, X.; Zhang, B.; Zhang, Q.; Chen, L.; et al. Genetic landscape of hepatitis B virus-associated diffuse large B-cell lymphoma. Blood 2018, 131, 2670–2681. [Google Scholar] [CrossRef]
| Gene | Transcript | cDNA | Protein | VAF |
|---|---|---|---|---|
| CREBBP | NM_004380.2 | c.2447A>T | p.Gln816Leu | 9.6% |
| PLCG2 | NM_002661.3 | c.202A>T | p.Met68Leu | 48.5% |
| SPEN | NM_015001.2 | c.2318G>A | p.Arg773Lys | 47.6% |
| TP73 | NM_005427.3 | c.958G>A | p.Ala320Thr | 10.4% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geevar, T.; Sabatini, P.J.B.; Zhang, T.; Sakhdari, A. Adult-Onset Systemic Chronic Active Epstein-Barr Virus Disease: A Case Report Highlighting Unique Immunophenotype and Novel Molecular Insights in the Context of Chronic HBV Hepatitis. Hemato 2024, 5, 251-257. https://doi.org/10.3390/hemato5030020
Geevar T, Sabatini PJB, Zhang T, Sakhdari A. Adult-Onset Systemic Chronic Active Epstein-Barr Virus Disease: A Case Report Highlighting Unique Immunophenotype and Novel Molecular Insights in the Context of Chronic HBV Hepatitis. Hemato. 2024; 5(3):251-257. https://doi.org/10.3390/hemato5030020
Chicago/Turabian StyleGeevar, Tulasi, Peter J. B. Sabatini, Tong Zhang, and Ali Sakhdari. 2024. "Adult-Onset Systemic Chronic Active Epstein-Barr Virus Disease: A Case Report Highlighting Unique Immunophenotype and Novel Molecular Insights in the Context of Chronic HBV Hepatitis" Hemato 5, no. 3: 251-257. https://doi.org/10.3390/hemato5030020
APA StyleGeevar, T., Sabatini, P. J. B., Zhang, T., & Sakhdari, A. (2024). Adult-Onset Systemic Chronic Active Epstein-Barr Virus Disease: A Case Report Highlighting Unique Immunophenotype and Novel Molecular Insights in the Context of Chronic HBV Hepatitis. Hemato, 5(3), 251-257. https://doi.org/10.3390/hemato5030020







