Abstract
Hematopoietic stem cell transplantation (HSCT) results in profound immunosuppression for the first few months after the procedure, requiring patients to be revaccinated against childhood vaccine-preventable infectious diseases. Patients who undergo allo-HSCT are at high risk of bacterial, fungal, and viral infections, with infectious complications responsible for at least one third of deaths. Even before the COVID-19 pandemic, respiratory virus infections were known to be more severe in HSCT recipients. The pandemic has highlighted the vulnerability of HSCT recipients, who experience an increased risk of morbidity and mortality after COVID-19 compared with healthy populations due to their severe immunodeficiency status. However, the current pandemic has also provided an exceptional scenario to better understand the immune response to SARS-CoV-2 cases and mRNA vaccines in HSCT recipients, including those receiving CD19-directed chimeric antigen receptor T cell (CAR-T) therapy. Researchers have focused on the role of the immune system in protecting against severe SARS-CoV-2 in patients with hematologic malignancies, including HSCT recipients. Insights gained during the pandemic will likely soon be used to improve preventive strategies in this population against viral infections in the near future. This narrative review summarizes the current knowledge on SARS-CoV-2 immunity in HSCT and cell therapy recipients following SARS-CoV-2 cases or vaccination.
1. Introduction
Recipients of hematopoietic stem cell transplantation (HSCT) experience profound cellular and humoral immunosuppression during the first few months after the procedure due to a reset of their immune systems caused by administrating a conditioning regimen, naïve hematopoietic stem cell infusion, and immunosuppressant drugs. For this reason, these patients need to be revaccinated against most childhood vaccine-preventable infectious diseases, usually starting three months after autologous stem cell transplantation (ASCT), six months after allogeneic stem cell transplantation (allo-HSCT) [1], and even later in the case of moderate-to-severe graft-versus-host disease (GvHD). Allo-HSCT is considered one of the most immunosuppressive procedures in modern medicine, with bacterial, fungal, and viral infectious complications responsible for at least one third of deaths [2]. Indeed, even before the ongoing pandemic, we knew that community-acquired respiratory virus infections are more severe in these patients, particularly in older patients, those who recently underwent a transplant, those with hypogammaglobulinemia or lymphopenia, or those taking immunosuppressant drugs to prevent or treat moderate-to-severe GvHD [3,4]. The outbreak of the severe acute respiratory syndrome coronavirus (SARS-CoV-2) disease (COVID-19) pandemic has confirmed the vulnerability of these patients in terms of increased morbidity and mortality compared with patients with solid tumors or healthy populations, which is obviously linked to their severe immunodeficiency status [5,6]. However, the enduring COVID-19 pandemic has provided an exceptional opportunity to better understand the humoral and cellular immune response in HSCT recipients. Researchers have focused on investigating the effectiveness of the immune system in protecting against SARS-CoV-2 in patients with hematologic malignancies, including those receiving allo-HSCT, ASCT, and CD19-directed chimeric antigen receptor T cell (CAR-T) therapy. These patients are particularly vulnerable due to their profound immune dysfunction and prolonged timeline for immune reconstitution. The insights gained during the pandemic are likely soon to be used to improve preventive strategies in this population. In this review, we outline the state of the art (summarized in Table 1) on SARS-CoV-2 immunity in HSCT recipients following contraction of the virus and vaccination.

Table 1.
Summary of immunosuppressive conditions influencing seroconversion, antibody waning, specific T-cell response, and breakthrough SARS-CoV-2 cases.
2. Methods
For this narrative review, the PubMed, WHO COVID-19 repository, and Google Scholar databases were searched for publications available through 15 January 2022. The search terms included the following: “allogeneic stem cell transplantation* or autologous stem cell transplantation*”, “COVID-19 or SARS-CoV-2”, “Specific T cell response*”, “SARS-CoV-2 immunity”, “Breakthrough COVID-19” “humoral immunity”, “vaccine*”, “cellular immunity”, and “mRNA vaccine”. Additional studies were identified through review of the reference lists of the included studies. Both authors participated in study identification, screening, and data extraction, and all included studies were reviewed by the three authors. Studies reporting exclusively on children were excluded.
3. Cellular and Humoral Immune Response after COVID-19
3.1. Humoral Response
Immunocompromised patients, particularly HSCT recipients, have been well documented to experience higher mortality after COVID-19, ranging from 18% to 33% [6,30,31,32]. The crucial role of innate and adaptive immunity in controlling and clearing SARS-CoV-2 cases and determining disease severity was acknowledged early. It was observed that hematological patients at a higher risk of mortality had a lower rate of seroconversion of anti-SARS-CoV-2 reactive antibodies (SARS-CoV-2-RA) after contracting COVID-19, with seroconversion rates ranging from 50% to 80%. This contrasted with solid tumor patients and healthy individuals, who had a seroconversion rate exceeding 90% [7,8,9]. The seroconversion rate was even lower in those receiving chemoimmunotherapy, anti-CD-20 therapy, chimeric antigen receptor (CAR)-T cell therapy, or HSCT [10,11]. Another key observation was the antibody level waning after COVID-19, which could have hindered antibody-mediated protection against SARS-CoV-2 re-infection [8], despite instances of antibody responses lasting more than 6 months after COVID-19 [13]. Although a considerable proportion of HSCT recipients can mount an immune response after infection, the absolute lymphocyte count (ALC) at the time of infection was negatively correlated with antibody production [12].
3.2. Cellular Response
3.2.1. Lymphocytopenia
Lymphopenia during COVID-19 was a common phenomenon in the general population. This indicates that SARS-CoV-2 infection impairs lymphocyte proliferation, increases apoptosis, and causes lymphocyte migration into tissues [33]. Lymphopenia has been linked to a more severe disease course and higher mortality rates in multiple studies [34,35], and it was also observed in HSCT recipients [30]. Given the immunocompromised state of HSCT recipients and the significant impact of viral infections on post-transplantation immune reconstitution, it is crucial to understand how the adaptive immune response works in COVID-19 patients who have undergone HSCT [36,37,38,39,40]. SARS-CoV-2 infection does not specifically target an immune lymphocyte subset; rather, it leads to a marked reduction across lymphocyte populations [41,42,43,44]. In healthy populations, cellular immunity during COVID-19 starts from the NK cells through cytokine production (mainly IL-6) and the direct lysis of infected cells, followed by CD8+ T cell destruction of infected cells and CD4+ T cells stimulating B cells to produce antibodies [45]. However, subtle differences are present in baseline lymphocytopenia HSCT recipients. Phenotypic evaluation of 20 patients without HSCT who recovered from COVID-19 revealed a slight increase in the CD3 T cell percentage, with a reduction in CD19 B cells compared with the controls with no COVID-19 cases [46]. Lymphocytopenia is a prevalent condition among HSCT recipients, particularly during the early transplant phase. However, the 25 HSCT recipients testing positive for SARS-CoV-2 exhibited a further reduction in the absolute lymphocyte count (ALC) compared with their pre-COVID-19 baseline within a week of diagnosis [47]. This lymphocyte reduction involved all subsets, particularly a trend in the CD4/CD8 ratio toward a relative increase in CD4+ T cells (predominantly effector memory cells), while the CD8+ T cells showed a T-cell effector memory (CCR7–CD45RA+) phenotype. This data may reflect that the more severe lymphopenia observed in HSCT patients after primoinfection could be driven by higher senescence of the terminally differentiated CD8+ T cells in the context of lower baseline lymphopoiesis and maturation after transplantation.
3.2.2. Virus-Specific T Cell Response
Despite lymphopenia, long-lasting SARS-CoV-2-specific T cell responses were observed early on in immunocompromised patients after primary infection, albeit at a lower rate than in the healthy controls [13]. The magnitude of long-lived SARS-CoV-2-specific T-cell responses correlated significantly with the number of CD4+ T cells and natural killer (NK) cells [13]. The importance of developing a specific T-cell response, particularly in the setting of impaired humoral immunity, was demonstrated in two cohorts of adults with hematologic malignancy, where higher CD8+ T cell counts were associated with improved overall survival, even when some patients did not develop SARS-CoV-2-RA. These facts suggest that specific CD8+ T cells likely compensate for a deficient humoral response and may influence clinical recovery after COVID-19 [27]. Although a specific T cell response could be generated in most patients, it would not develop in all HSCT recipients. In the HSCT population, prolonged SARS-CoV-2 shedding has been well documented [6,48]. The primary factors contributing to prolonged viral shedding are closely associated with compromised B or T cell function, resulting from chemotherapeutic agents used before transplantation or the conditioning regimen, in addition to immunosuppressive medications prescribed to prevent or treat graft-vs-host disease (GvHD). Among immunochemotherapy agents, CD-20 monoclonal antibodies (i.e., rituximab) have been associated with prolonged (more than 3 weeks) asymptomatic shedding [49]. While prolonged fragment RNA shedding is often from non-viable viruses in healthy patients, HSCT recipients could shed viable SARS-CoV-2 for several months after initial infection [50] and even develop clinical relapse with the same or evolved strain types, as reported in other immunocompromised patients [51,52]. This issue is of the utmost importance as it raises concerns related to the risk of community and health care facility transmissibility, delaying both regular clinical monitoring and the initiation of other immunosuppressive treatments.
In conclusion, most unvaccinated cell therapy recipients demonstrated both humoral and cellular responses after SARS-CoV-2 primoinfection, but these responses were less robust than in the healthy population, due to immunosuppression-related impaired baseline immune function. This lower level of immunity translated into more severe and recurrent disease, as well as longer viral shedding, highlighting the importance of prioritizing research on prophylactic and therapeutic strategies in this vulnerable population in future pandemics (see Figure 1).
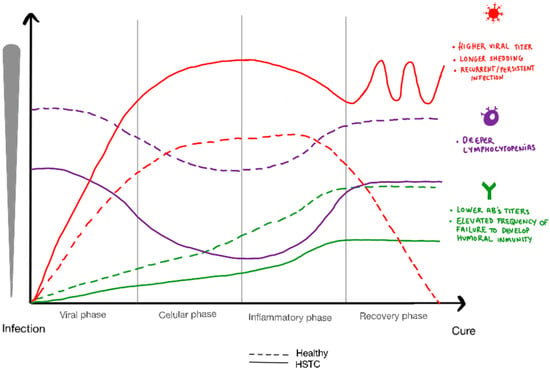
Figure 1.
Graphical representation of viral load, absolute lymphocyte count and antibody response in hematopoietic transplant recipients compared to healthy population.
4. Serological Response to Full (Two-Dose) mRNA SARS-CoV-2 Vaccination
The availability of different SARS-CoV-2 vaccines by late 2020 highly impacted the course of the pandemic in terms of reduced severity and mortality. New mRNA-based vaccines demonstrated higher immunogenicity than adenoviral vector-based vaccines in the general population [53], providing the rationale for offering these compounds to immunosuppressed patients worldwide. In fact, more than 80% of hematological malignancy patients received mRNA vaccines in most series [14]. Therefore, this review focuses only on available data on mRNA compounds.
Numerous immunoassays allowing quantitative assessment of SARS-CoV-2-S binding antibodies have been marketed, with potential differences in analytical design, performance characteristics, the immunoglobulin class measured, and the type of SARS-CoV-2 antigen to which the target antibodies are directed (i.e., receptor-binding domain (RBD), with the S protein in its trimeric conformation, or the S1 or S2 subunits). Although immunoassays may return discordant qualitative or quantitative results (the latter occurring despite calibration to the first WHO SARS-CoV-2 antibody international standard), many tests tend to correlate in terms of antibody levels measured. Taking this limitation into consideration, several early studies (employing different immunoassays) confirmed the initial hypothesis of lower SARS-CoV-2-RA seroconversion rates after full vaccination in hematological malignancy patients (including HSCT recipients) compared with the healthy controls [14] or even solid tumor patients [15]. Note that the seroconversion rate (>70%) in HSCT recipients [16,17,54,55,56] largely exceeds prior experiences with common antigen-based vaccines (i.e., the conjugate pneumococcal vaccine, influenza vaccine, or hepatitis B virus vaccines), which showed at most 50% immunogenicity [57,58]. This fact highlights an improved immunogenicity with mRNA-based compounds in this population, which should pave the way for future research on mRNA-based vaccines against other relevant pathogens that increase morbidity and mortality in these immunocompromised patients.
Identifying conditions associated with impaired immunogenicity is crucial to improve antibody response by customizing the current vaccine schedules in this population. In allo-HSCT recipients, older age, lymphopenia < 1 × 109/L, active GvHD, corticosteroids use, ongoing immunosuppressant drugs, or vaccination within the first year of a transplant were associated with lower seroconversion rates [16,17]. In addition, recipients allografted from alternative donors (unrelated donor or human leukocyte antigen (HLA) haploidentical donors) showed lower antibody levels after full vaccination, emphasizing the role of HLA matching in antigen recognition and efficient antibody production [16,19]. In the ASCT setting, non-Hodgkin’s lymphoma (NHL) and active corticosteroid therapy were associated with lower seroconversion rates [16]. It is likely that recipients harboring these conditions may require early booster or different vaccination schedules with higher or additional doses.
An important factor in the serological response to vaccination in the allo-HSCT setting includes a potential clinical benefit through adoptive transfer of specific SARS-CoV-2 immune memory function from a vaccinated stem cell donor to a recipient [59]. However, the limited data preclude the possibility of recommending an extra vaccine dose administration to volunteer donors before HSC donation. Nonetheless, the time from transplant to vaccination is likely more important than the donor’s vaccination status. Immune recovery after transplantation has been demonstrated to correlate with the humoral immune response to vaccination [18]. Although ASTCT and EBMT guidelines suggest delaying COVID-19 vaccination until at least three months after allogeneic or autologous HSCT [60,61], the optimal timing and schedule of COVID-19 vaccination after these types of transplantation remain to be redefined and should be tailored according to the conditions influencing vaccine responses.
In summary, mRNA compounds have been proven to be highly immunogenic, even in the early phase of HSCT. The factors influencing humoral immunogenicity should be identified to further recommend boosters or monoclonal anti-SARS-CoV-2 antibodies with neutralizing activity against the circulant strain to prevent severe COVID-19.
5. Antibody Waning after Full Vaccination and the Booster Effect
Although both natural infections and mRNA-based vaccinations produce a satisfactory humoral immunogenicity, current thinking is that antibody titers will invariably wane over time, which in turn could be associated with a significant loss of protection and increased probability of future infections [62]. This has been analyzed in the HSCT setting, and as in the general population [63,64], antibody waning was detected as early as three months after full vaccination [22,65], which formed the basis for booster vaccine guidance in these patients. Antibody waning was common in all cell therapy procedures, although subtle differences should be taken into account according to post-transplant treatment requirements, donor type, HLA matching, and immunosuppression factors [22]. In this regard, antibody waning is more pronounced in allo-HSCT recipients under immunosuppressive drugs and with low lymphocyte counts in the peripheral blood at the time of vaccination [23]. Identifying the factors associated with faster antibody decay is of the utmost importance to tailor booster dose administration. An additional issue concerns SARS-CoV-2′s evolution through different variants of concern (VOCs) able to evade the neutralization activity of vaccine-induced SARS-CoV-2-RA, even when the higher antibody levels after a booster vaccine still remain [66]. As with influenza virus vaccines, mRNA vaccines should be updated yearly, in line with current circulating VOCs.
Diverse studies have analyzed the effect of a booster vaccine dose in cell therapy procedures. A booster dose was able to increase the antibody levels in all cell therapy scenarios, reaching antibody levels even higher than those observed at 3–6 weeks after full vaccination [22]. Boosters were able to achieve comparable antibody titers in allo-HSCT recipients with different donor types [22]. In addition, a significant proportion (>50%) of poor responders after a two-dose mRNA vaccination achieved adequate antibody levels after the booster [17,19,22,59,67]. However, this rate was clearly inferior in the poorly responding CAR T cell therapy recipients experiencing long-lasting B cell aplasia (from 0% to 24%) [22,68]. A heterologous vaccination regimen combining mRNA and an adenoviral-vectored vaccine led to a serological response in 31% of hemato-oncological patients who failed to respond after a previous double dose of an mRNA vaccine [69]. The effect and durability of the SARS-CoV-2-RA titers through a natural booster delivered by a breakthrough SARS-CoV-2 infection remain to be determined in HSCT. A graphical representation of serological response in pre-vaccination COVID-19 and non-infected and booster effect in antibody titers in HSCT recipients and healthy population is provided in Figure 2.
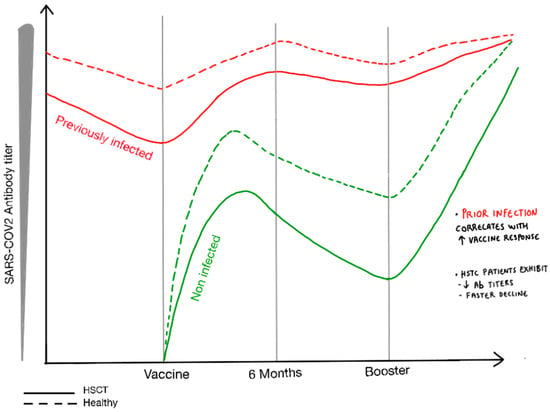
Figure 2.
Graphical representation of the serological response following complete vaccination and booster administration in HSCT recipients and the general population, accounting for pre-vaccination COVID-19 cases and non-infected individuals.
Antibody decline was observed in the first three months after full vaccination. Poorly responding HSCT recipients could benefit from a booster dose, yet the booster benefit is debatable in poorly responding CAR T therapy recipients and may require a different approach.
6. SARS-CoV-2-Specific T Cell Response after Full Vaccination and a Booster
Regarding specific T cell response rates, HSCT recipients showed a specific T cell response in 19–82.3% of cases [26,54,70,71]. The conditions associated with a lower specific T cell response included active GvHD, the need for immunosuppressant drugs, and low CD3+ and CD4+ counts [26]. In a cohort of 46 allo-HSCT patients, a strong response of specific anti-SARS-CoV-2-CD4+ T cells was observed after a two-dose mRNA vaccine in 89% of the serological responders and 40% of non-responders, associated with a predominant IFN or TNF + cytokine profile assessed by intracellular staining of stimulated T cells and in 85% through the detection of IFN-secreting cells upon stimulation with SARS-CoV-2 spike peptides [24]. A study evaluating T cell responses in 17 allo-HSCT patients found a CD4+ T cell response in 29.4% of patients after one dose and 70.6% after two doses, whereas a CD8+ T cell response was seen in 17.6% after one dose and 52.3% after two doses [71]. These rates increased up to 80% in 20 allo-HSCT recipients after one booster dose. Although the proportion of patients with specific T cells did not vary significantly between the second and third doses, an increase in antigen-specific, cell-mediated immunity was observed after the third dose, notably in IL-2 monofunctional and polyfunctional CD4+ T cells [19]. With two boosters (four vaccine doses), specific T cell frequencies became comparable between HSCT recipients and one boosted healthy control cohort, suggesting a potential benefit of a second booster [25]. Specific T cells against SARS-CoV-2 persisted more than six months after the booster and were similar to those of the healthy controls [72,73]. Specific CD4+ T cells predominated late after vaccination, whereas specific CD8+ T cells were more evident earlier after the booster. This is consistent with the activation of cytotoxic effector cells, with a shorter persistence and recall for the CD4+ memory T cells in subjects further away from the last challenge. A positive CD4+ T cell response was observed in 55–80% of allo-HSCT recipients, while the frequencies of CD4+ polyfunctional T cells increased significantly after the third dose [19].
Specific T cell responses after SARS-CoV-2 vaccination were frequently observed in cell therapy recipients, even in those who did not seroconvert after full vaccination. Preliminary data suggest that a CD8+ T cell response was observed early after vaccination, whereas a CD4+ T cell response was more evident later on. The relationship between the levels of T cell subset responses (CD4+ and CD8+), viral clearance kinetics, and asymptomatic or symptomatic SARS-CoV-2 infection remain to be analyzed. Likewise, the HLA hierarchy determining which SARS-CoV-2 epitopes efficiently trigger specific CD8+ and CD4+ T cell responses is still unclear [74]. Furthermore, potential SARS-CoV-2 T cell cross-reactivity (likely related to a prior seasonal coronavirus) might confound analysis of the simple relationship between vaccine response and protection. Another critical aspect is the applicability and reliability of novel methods providing rapid estimation of the quantity, diversity, and function of SARS-CoV-2-specific T cells [75,76].
7. Clinical Efficacy and Immune Determination of Breakthrough SARS-CoV-2 Infections
Encouraging preliminary data suggest that SARS-CoV-2 vaccination has played a major role in reducing the severity of breakthrough COVID-19 in these immunocompromised patients, with mortality dropping to 12% in retrospective case-registry series during the alpha VOC [77] or to less than 5% before the Omicron period [20,78] and notably to <2% during Omicron [29]. Similarly, the rate of asymptomatic SARS-CoV-2 infection has increased from 8% during the ancestral SARS-CoV-2 strain waves [30] to nearly 50% after mass vaccination [20,29]. Although the first generation of SARS-CoV-2 vaccines failed to prevent SARS-CoV-2 community transmission, protection against severe disease still remained high [28]. In fact, the estimated one-year cumulative incidence of breakthrough SARS-CoV-2 infections after full vaccination was 18% in a Spanish prospective cohort including more than 1500 patients with different hematological disorders prospectively followed for development of breakthrough infections [29]. This series included more than 500 HSCT recipients, whose one-year cumulative incidence of breakthrough infections was 15%, with no reported deaths among these 72 breakthrough cases [22,29]. These epidemiological data suggest that despite vaccination and boosters, HSCT recipients are likely contract COVID-19 several times over the following years. In fact, administrating booster doses did not result in a reduction in breakthrough infections [29]. The risk factors for a higher breakthrough infection incidence included the use of corticosteroids or immunosuppressive drugs without corticosteroids and myeloablative intensity conditioning [22]. It is of note that the antibody titers were positively correlated with the risk of a breakthrough infection and its severity, a finding not limited to the early (3–6 weeks after full vaccination) period [20] but also present when the antibodies were determined at later timepoints [29]. In this large series, a cutoff of 250 binding antibody units (BAUs)/mL of SARS-CoV-2-RA was predictive of severe COVID-19 and mortality. No patients died when the antibody levels were above this threshold [20,29], supporting current worldwide health authority policies focused on boosters in immunocompromised patients.
The mortality rate in hematological malignancy patients has declined due to mass vaccination, viral evolution with less virulent VOCs, and advances in detection, supportive care, and treatment. Nonetheless, immunosuppressed patients have a higher incidence of breakthrough infections after vaccination. Although not routine, serological monitoring should guide booster doses and prophylactic strategies. The effect of a specific T cell response on the risk of breakthrough SARS-CoV-2 infections in this population remains to be determined.
8. Hybrid Immunity (Vaccines and Natural Infections)
In healthy patients, hybrid immunity (conferred from both natural infections and vaccination) induces serum-binding and neutralizing antibody responses that are markedly more potent, durable, and resilient to the spike mutations observed in different SARS-CoV-2 variants than those of subjects who received only two vaccine doses [28,79,80,81]. Indirect data suggest that this could also be the case in hematological malignancy patients. In fact, hematological patients (including HSCT recipients) fully vaccinated after COVID-19 showed higher seroconversion rates in multivariate analysis [16] and higher antibody titers compared with SARS-CoV-2-naïve fully vaccinated patients [20], irrespective of current or past treatments, disease type and status, or the ALC at the time of vaccination, as well as better serological response after having COVID-19 [21]. Additionally, those with pre-vaccination COVID-19 showed a trend to a lower incidence of breakthrough cases [29].
As in the general population, the duration of hybrid humoral immunity indicates longer stability in the antibody titers (at least for six months after complete vaccination), suggesting that this group may not require an additional vaccine dose or at least not as early as those vaccinated without a prior SARS-CoV-2 case [22]. There are no available data on SARS-CoV-2-RA waning when a SARS-CoV-2 case occurs after vaccination, although similar behavior could be expected.
9. Adoptive Transfer of Specific T Cells against SARS-CoV-2
Adaptive and innate immune responses are essential for SARS-CoV-2 control and clearance. The viral phase of COVID-19 is higher and longer in immunocompromised patients than in the general population [82], emphasizing the central role of cellular immunity. Interestingly, the counts of all T cell subsets recovered dramatically in most patients who cleared the virus, in contrast to those with persistent SARS-CoV-2 shedding [83,84].
Consistent with the increasing use of cell therapy, several research groups have focused their efforts on restoring virus-specific T cell immunity through the transfer of adoptive immune cell therapy [85,86]. Clinical-grade SARS-CoV-2-specific T cells may be generated by stimulation with SARS-CoV-e peptides (MACS GMP PepTivator SARS-CoV-2) and fast selection using CliniMACS Prodigy and the CliniMACS Cytokine Capture System (IFN-gamma) [87,88]. Specific T cells can also be expanded in vitro from recovered healthy donors [89]. In order to reduce the alloreactivity, central memory, and effector memory, T cell subsets containing SARS-CoV-2-specific T cells may be the preferred option. More sophisticated approaches have been explored, such as CRISPR-Cas9 gene editing of cytotoxic T lymphocytes (CTLs) to obtain tacrolimus-resistant SARS-CoV-2-specific T cells and glucocorticoid resistance in SARS-CoV-2 CTLs [90,91]. Recent data have shown that the large-scale clinical cell isolation, production, and biobank of CD45RA- T cell-containing SARS-CoV-2 IFN-g+ T cells using a CliniMACS Plus device was feasible, cheap, and safe, with a balanced immune response, accelerated lymphocyte cell recovery, and decreased proinflammatory parameters [85,86,92].
10. Future Directions
One of the most critical lessons learned from this pandemic is the urgent need to prioritize prophylactic and therapeutic research at early stages of the pandemic to protect these vulnerable populations. Moving forward, there is an urgent need to examine the epidemiology and consequences of contracting SARS-CoV-2 in HSCT recipients in the Omicron era. This requires exploring methods to evaluate specific T cell immunity and its role in protecting HSCT recipients. In addition, efforts should focus on enhancing vaccine-induced humoral responses, developing monoclonal antibody prophylaxis for poor responders, and advancing T cell therapy research. Addressing these critical areas of research could prove instrumental in mitigating the impact of future pandemics and safeguarding the health of at-risk populations.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We want to thanks Ana Facal from the hematology service of Hospital Universitario and polytechnic La Fe of Valencia Spain for her invaluable assistance in creating the graphical representations.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cordonnier, C.; Einarsdottir, S.; Cesaro, S.; Di Blasi, R.; Mikulska, M.; Rieger, C.; de Lavallade, H.; Gallo, G.; Lehrnbecher, T.; Engelhard, D.; et al. European Conference on Infections in Leukaemia group. Vaccination of haemopoietic stem cell transplant recipients: Guidelines of the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect. Dis. 2019, 19, e200–e212. [Google Scholar] [CrossRef] [PubMed]
- Styczyński, J.; Tridello, G.; Koster, L.; Iacobelli, S.; van Biezen, A.; van der Werf, S.; Mikulska, M.; Gil, L.; Cordonnier, C.; Ljungman, P.; et al. Infectious Diseases Working Party EBMT. Death after hematopoietic stem cell transplantation: Changes over calendar year time, infections and associated factors. Bone Marrow Transplant. 2020, 55, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Strasfeld, L. Respiratory Virus Infections of the Stem Cell Transplant Recipient and the Hematologic Malignancy Patient. Infect. Dis. Clin. N. Am. 2019, 33, 523–544. [Google Scholar] [CrossRef] [PubMed]
- Pérez, A.; Montoro, J.; Hernani, R.; Lorenzo, I.; Hernández-Boluda, J.C.; Giménez, E.; Gómez, M.D.; Balaguer-Roselló, A.; Gonzalez-Barberá, E.; Guerreiro, M.; et al. Assessment of immunodeficiency scoring index performance in enterovirus/rhinovirus respiratory infection after allogeneic hematopoietic stem cell transplantation. Transpl. Infect. Dis. 2020, 22, e13301. [Google Scholar] [CrossRef]
- Langerbeins, P.; Hallek, M. COVID-19 in patients with hematologic malignancy. Blood 2022, 140, 236–252. [Google Scholar] [CrossRef]
- Ljungman, P.; de la Camara, R.; Mikulska, M.; Tridello, G.; Aguado, B.; Zahrani, M.A.; Apperley, J.; Berceanu, A.; Bofarull, R.M.; Calbacho, M.; et al. COVID-19 and stem cell transplantation; results from an EBMT and GETH multicenter prospective survey. Leukemia 2021, 35, 2885–2894. [Google Scholar] [CrossRef]
- Fujii, T.; Hagihara, M.; Mitamura, K.; Nakashima, S.; Ohara, S.; Uchida, T.; Inoue, M.; Okuda, M.; Yasuhara, A.; Murakami, J.; et al. Anti-SARS-CoV-2 IgG in COVID-19 Patients with Hematological Diseases: A Single-center, Retrospective Study in Japan. Intern. Med. 2022, 61, 1681–1686. [Google Scholar] [CrossRef]
- Candoni, A.; Pizzano, U.; Fabris, M.; Curcio, F.; Fanin, R. Seroconversion and kinetic of anti SARS-CoV-2 antibodies in 25 patients with hematological malignancies who recovered from SARS-CoV-2 infection. Hematol. Oncol. 2021, 39, 428–431. [Google Scholar] [CrossRef]
- El Fakih, R.; Haroon, A.; Alfraih, F.; Al-Khabori, M.K.; Alzahrani, M.; Alhuraiji, A.; Hamadah, A.; AlJohani, N.I.; Alahmari, B.; Essa, M.F.; et al. Clinical course and outcomes of COVID-19 in hematopoietic cell transplant patients, a regional report from the middle East. Bone Marrow Transplant. 2021, 56, 2144–2151. [Google Scholar] [CrossRef]
- Thakkar, A.; Pradhan, K.; Jindal, S.; Cui, Z.; Rockwell, B.; Shah, A.P.; Hamadah, A.; AlJohani, N.I.; Alahmari, B.; Essa, M.F.; et al. Patterns of seroconversion for SARS-CoV2-IgG in patients with malignant disease and association with anticancer therapy. Nat. Cancer 2021, 2, 392–399. [Google Scholar] [CrossRef]
- Passamonti, F.; Romano, A.; Salvini, M.; Merli, F.; Porta, M.G.D.; Bruna, R.; Coviello, E.; Romano, I.; Cairoli, R.; Lemoli, R.; et al. COVID-19 elicits an impaired antibody response against SARS-CoV-2 in patients with haematological malignancies. Br. J. Haematol. 2021, 195, 371–377. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.S.; Kim, T.Y.; Lee, D.G.; Kim, D.W. Lymphopenia as a biological predictor of outcomes in COVID-19 patients: A nationwide cohort study. Cancers 2021, 13, 471. [Google Scholar] [CrossRef]
- Sjöwall, J.; Hjorth, M.; Gustafsson, A.; Göransson, R.; Larsson, M.; Waller, H.; Nordgren, J.; Nilsdotter-Augustinsson, Å.; Nyström, S. SARS-CoV-2 Specific Antibody Response and T Cell-Immunity in Immunocompromised Patients up to Six Months Post COVID: A Pilot Study. J. Clin. Med. 2022, 11, 3535. [Google Scholar] [CrossRef]
- Maneikis, K.; Šablauskas, K.; Ringelevičiūtė, U.; Vaitekėnaitė, V.; Čekauskienė, R.; Kryžauskaitė, L.; Naumovas, D.; Banys, V.; Pečeliūnas, V.; Beinortas, T.; et al. Immunogenicity of the BNT162b2 COVID-19 mRNA vaccine and early clinical outcomes in patients with haematological malignancies in Lithuania: A national prospective cohort study. Lancet Haematol. 2021, 8, e583–e592. [Google Scholar] [CrossRef]
- Ehmsen, S.; Asmussen, A.; Jeppesen, S.S.; Nilsson, A.C.; Osterlev, S.; Vestergaard, H.; Justesen, U.S.; Johansen, I.S.; Frederiksen, H.; Ditzel, H.J. Antibody and T cell immune responses following mRNA COVID-19 vaccination in patients with cancer. Cancer Cell 2021, 39, 1034–1036. [Google Scholar] [CrossRef]
- Piñana, J.L.; López-Corral, L.; Martino, R.; Montoro, J.; Vazquez, L.; Pérez, A.; Martin-Martin, G.; Facal-Malvar, A.; Ferrer, E.; Pascual, M.J.; et al. SARS-CoV-2-reactive antibody detection after SARS-CoV-2 vaccination in hematopoietic stem cell transplant recipients: Prospective survey from the Spanish Hematopoietic Stem Cell Transplantation and Cell Therapy Group. Am. J. Hematol. 2022, 97, 30–42. [Google Scholar] [CrossRef]
- Redjoul, R.; Le Bouter, A.; Beckerich, F.; Fourati, S.; Maury, S. Antibody response after second BNT162b2 dose in allogeneic HSCT recipients. Lancet 2021, 398, 298–299. [Google Scholar] [CrossRef]
- Tamari, R.; Politikos, I.; Knorr, D.A.; Vardhana, S.A.; Young, J.C.; Marcello, L.T.; Doddi, S.; Devlin, S.M.; Ramanathan, L.V.; Pessin, M.S.; et al. Predictors of humoral response to SARS-CoV-2 vaccination after hematopoietic cell transplantation and CAR T-cell therapy. Blood Cancer Discov. 2021, 2, 577–585. [Google Scholar] [CrossRef]
- Kimura, M.; Ferreira, V.H.; Kothari, S.; Pasic, I.; Mattsson, J.I.; Kulasingam, V.; Humar, A.; Mah, A.; Delisle, J.S.; Ierullo, M.; et al. Safety and Immunogenicity after a Three-Dose SARS-CoV-2 Vaccine Schedule in Allogeneic Stem Cell Transplant Recipients. Transplant. Cell Ther. 2022, 28, 706.e1–706.e10. [Google Scholar] [CrossRef]
- Piñana, J.L.; López-Corral, L.; Martino, R.; Vazquez, L.; Pérez, A.; Martin-Martin, G.; Gago, B.; Sanz-Linares, G.; Sanchez-Salinas, A.; Villalon, L.; et al. SARS-CoV-2 vaccine response and rate of breakthrough infection in patients with hematological disorders. J. Hematol. Oncol. 2022, 15, 54. [Google Scholar] [CrossRef]
- Piñana, J.L.; Garcia-Sanz, R.; Martino, R.; Garcia-Roa, M.; Martin-Martin, G.A.; Risco-Gálvez, I.; Tormo, M.; Martinez-Barranco, P.; Marcos-Corrales, S.; Calabuig, M.; et al. Booster effect after SARS-CoV-2 vaccination in immunocompromised hematology patients with prior COVID-19. Blood Adv. 2022, 6, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Piñana, J.L.; Martino, R.; Vazquez, L.; López-Corral, L.; Pérez, A.; Chorão, P.; Avendaño-Pita, A.; Pascual, M.J.; Sánchez-Salinas, A.; Sanz-Linares, G.; et al. SARS-CoV-2-reactive antibody waning, booster effect and breakthrough SARS-CoV-2 infection in hematopoietic stem cell transplant and cell therapy recipients at one year after vaccination. Bone Marrow Transplant. 2023, 58, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, M.; Redjoul, R.; Le Bouter, A.; Beckerich, F.; Robin, C.; Parinet, V.; Pautas, C.; Menouche, D.; Bouledroua, S.; Roy, L.; et al. Determinants of SARS-CoV-2 waning immunity in allogeneic hematopoietic stem cell transplant recipients. J. Hematol. Oncol. 2022, 15, 27. [Google Scholar] [CrossRef] [PubMed]
- Clémenceau, B.; Guillaume, T.; Coste-Burel, M.; Peterlin, P.; Garnier, A.; Le Bourgeois, A.; Jullien, M.; Ollier, J.; Grain, A.; Béné, M.C.; et al. SARS-CoV-2 T-cell responses in allogeneic hematopoietic stem cell recipients following two doses of BNT162b2 mRNA vaccine. Vaccines 2022, 10, 448. [Google Scholar] [CrossRef] [PubMed]
- Clémenceau, B.; Le Bourgeois, A.; Guillaume, T.; Coste-Burel, M.; Peterlin, P.; Garnier, A.; Jullien, M.; Ollier, J.; Grain, A.; Béné, M.C.; et al. Strong SARS-CoV-2 T-Cell Responses after One or Two COVID-19 Vaccine Boosters in Allogeneic Hematopoietic Stem Cell Recipients. Cells 2022, 11, 3010. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, M.; Roldán, E.; Fernández-Naval, C.; Villacampa, G.; Martinez-Gallo, M.; Medina-Gil, D.; Peralta-Garzón, S.; Pujadas, G.; Hernández, C.; Pagès, C.; et al. Cellular and humoral immunogenicity of the mRNA-1273 SARS-CoV-2 vaccine in patients with hematologic malignancies. Blood Adv. 2022, 6, 774–784. [Google Scholar] [CrossRef]
- Bange, E.M.; Han, N.A.; Wileyto, P.; Kim, J.Y.; Gouma, S.; Robinson, J.; Greenplate, A.R.; Hwee, M.A.; Porterfield, F.; Owoyemi, O.; et al. CD8 T cells compensate for impaired humoral immunity in COVID-19 patients with hematologic cancer. Nat Med. 2021, 27, 1280–1289. [Google Scholar] [CrossRef]
- Ssentongo, P.; Ssentongo, A.E.; Voleti, N.; Groff, D.; Sun, A.; Ba, D.M.; Nunez, J.; Parent, L.J.; Chinchilli, V.M.; Paules, C.I. SARS-CoV-2 vaccine effectiveness against infection, symptomatic and severe COVID-19: A systematic review and meta-analysis. BMC Infect. Dis. 2022, 22, 439. [Google Scholar] [CrossRef]
- Piñana, J.L.; Vazquez, L.; Calabuig, M.; López-Corral, L.; Martin-Martin, G.; Villalon, L.; Sanz-Linares, G.; Conesa-Garcia, V.; Sanchez-Salinas, A.; Gago, B.; et al. One-year breakthrough SARS-CoV-2 infection and correlates of protection in fully vaccinated hematological patients. Blood Cancer J. 2023, 13, 8. [Google Scholar] [CrossRef]
- Piñana, J.L.; Martino, R.; García-García, I.; Parody, R.; Morales, M.D.; Benzo, G.; Gómez-Catalan, I.; Coll, R.; De La Fuente, I.; Luna, A.; et al. Infectious Complications Subcommittee of the Spanish Hematopoietic Stem Cell Transplantation and Cell Therapy Group (GETH). Risk factors and outcome of COVID-19 in patients with hematological malignancies. Exp. Hematol. Oncol. 2020, 9, 21. [Google Scholar] [CrossRef]
- García-Suárez, J.; de la Cruz, J.; Cedillo, Á.; Llamas, P.; Duarte, R.; Jiménez-Yuste, V.; Hernández-Rivas, J.Á.; Gil-Manso, R.; Kwon, M.; Sánchez-Godoy, P.; et al. Impact of hematologic malignancy and type of cancer therapy on COVID-19 severity and mortality: Lessons from a large population-based registry study. J. Hematol. Oncol. 2020, 13, 133. [Google Scholar] [CrossRef]
- Sharma, A.; Bhatt, N.S.; St Martin, A.; Abid, M.B.; Bloomquist, J.; Chemaly, R.F.; Dandoy, C.; Gauthier, J.; Gowda, L.; Perales, M.A.; et al. Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: An observational cohort study. Lancet Haematol. 2021, 8, e185–e193. [Google Scholar] [CrossRef]
- Notarbartolo, S.; Ranzani, V.; Bandera, A.; Gruarin, P.; Bevilacqua, V.; Putignano, A.R.; Gobbini, A.; Galeota, E.; Manara, C.; Bombaci, M.; et al. Integrated longitudinal immunophenotypic, transcriptional and repertoire analyses delineate immune responses in COVID-19 patients. Sci. Immunol. 2021, 6, eabg5021. [Google Scholar] [CrossRef]
- Chen, L.; Pang, P.; Qi, H.; Yan, K.; Ren, Y.; Ma, M.; Cao, R.; Li, H.; Hu, C.; Li, Y.; et al. Evaluation of Spike Protein Epitopes by Assessing the Dynamics of Humoral Immune Responses in Moderate COVID-19. Front. Immunol. 2022, 13, 770982. [Google Scholar] [CrossRef]
- Kalicińska, E.; Szymczak, D.; Zińczuk, A.; Adamik, B.; Smiechowicz, J.; Skalec, T.; Nowicka-Suszko, D.; Biernat, M.; Bogucka-Fedorczuk, A.; Rybka, J.; et al. Immunosuppression as a Hallmark of Critical COVID-19: Prospective Study. Cells 2021, 10, 1293. [Google Scholar] [CrossRef]
- van Heijst, J.W.J.; Ceberio, I.; Lipuma, L.B.; Samilo, D.W.; Wasilewski, G.D.; Gonzales, A.M.; Nieves, J.L.; van den Brink, M.R.; Perales, M.A.; Pamer, E.G. Quantitative assessment of T cell repertoire recovery after hematopoietic stem cell transplantation. Nat. Med. 2013, 19, 372–377. [Google Scholar] [CrossRef]
- Muraro, P.A.; Robins, H.; Malhotra, S.; Howell, M.; Phippard, D.; Desmarais, C.; de Paula Alves Sousa, A.; Griffith, L.M.; Lim, N.; Nash, R.A.; et al. T cell repertoire following autologous stem cell transplantation for multiple sclerosis. J. Clin. Investig. 2014, 124, 1168–1172. [Google Scholar] [CrossRef]
- de Koning, C.; Admiraal, R.; Nierkens, S.; Boelens, J.J. Human herpesvirus 6 viremia affects T-cell reconstitution after allogeneic hematopoietic stem cell transplantation. Blood Adv. 2018, 2, 428–432. [Google Scholar] [CrossRef]
- Kanakry, C.G.; Coffey, D.G.; Towlerton, A.M.; Vulic, A.; Storer, B.E.; Chou, J.; Yeung, C.C.; Gocke, C.D.; Robins, H.S.; O’Donnell, P.V.; et al. Origin and evolution of the T cell repertoire after posttransplantation cyclophosphamide. JCI Insight 2016, 1, e86252. [Google Scholar] [CrossRef]
- Suessmuth, Y.; Mukherjee, R.; Watkins, B.; Koura, D.T.; Finstermeier, K.; Desmarais, C.; Stempora, L.; Horan, J.T.; Langston, A.; Qayed, M.; et al. CMV reactivation drives posttransplant T-cell reconstitution and results in defects in the underlying TCRβ repertoire. Blood 2015, 125, 3835–3850. [Google Scholar] [CrossRef]
- Mazzoni, A.; Mukherjee, R.; Watkins, B.; Koura, D.T.; Finstermeier, K.; Desmarais, C.; Mencarini, J.; Caporale, R.; Peruzzi, B.; Antonelli, A.; et al. Impaired immune cell cytotoxicity in severe COVID-19 is IL-6 dependent. J. Clin. Investig. 2020, 130, 4694–4703. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Gao, Y.; Wang, G.; Song, G.; Liu, S.; Sun, D.; Xu, Y.; Tian, Z. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol. Immunol. 2020, 17, 533–535. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Kamińska, D.; Dęborska-Materkowska, D.; Kościelska-Kasprzak, K.; Mazanowska, O.; Remiorz, A.; Poznański, P.; Durlik, M.; Krajewska, M. Immunity after COVID-19 Recovery and Vaccination: Similarities and Differences. Vaccines 2022, 10, 1068. [Google Scholar] [CrossRef]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell 2020, 181, 1489–1501.e15. [Google Scholar] [CrossRef]
- Shah, G.L.; DeWolf, S.; Lee, Y.J.; Tamari, R.; Dahi, P.B.; Lavery, J.A.; Ruiz, J.; Devlin, S.M.; Cho, C.; Peled, J.U.; et al. Favorable outcomes of COVID-19 in recipients of hematopoietic cell transplantation. J. Clin. Investig. 2020, 130, 6656–6667. [Google Scholar] [CrossRef]
- Fernandez-Ruiz, M.; Aguado, J.M. Severe acute respiratory syndrome coronavirus 2 infection in the stem cell transplant recipient—Clinical spectrum and outcome. Curr. Opin. Infect. Dis. 2021, 34, 654–662. [Google Scholar] [CrossRef]
- Avanzato, V.A.; Matson, M.J.; Seifert, S.N.; Pryce, R.; Williamson, B.N.; Anzick, S.L.; Barbian, K.; Judson, S.D.; Fischer, E.R.; Martens, C.; et al. Case study: Prolonged infectious SARS-CoV-2 shedding from an asymptomatic immunocompromised individual with cancer. Cell 2020, 183, 1901–1912.e9. [Google Scholar] [CrossRef]
- Aydillo, T.; Gonzalez-Reiche, A.S.; Aslam, S.; van de Guchte, A.; Khan, Z.; Obla, A.; Dutta, J.; van Bakel, H.; Aberg, J.; García-Sastre, A.; et al. Shedding of viable SARS-CoV-2 after immunosuppressive therapy for cancer. N. Engl. J. Med. 2020, 383, 2586–2588. [Google Scholar] [CrossRef]
- Thornton, C.S.; Huntley, K.; Berenger, B.M.; Bristow, M.; Evans, D.H.; Fonseca, K.; Franko, A.; Gillrie, M.R.; Lin, Y.-C.; Povitz, M.; et al. Prolonged SARS-CoV-2 infection following rituximab treatment: Clinical course and response to therapeutic interventions correlated with quantitative viral cultures and cycle threshold values. Antimicrob. Resist. Infect. Control 2022, 11, 28. [Google Scholar] [CrossRef]
- Morel, A.; Imbeaud, S.; Scemla, A.; Pere, H.; Fourgeaud, J.; Amrouche, L.; Robillard, N.; Planas, D.; Puech, J.; Simon, S.; et al. Severe relapse of SARS-CoV-2 infection in a kidney transplant recipient with negative nasopharyngeal SARS-CoV-2 RT-PCR after rituximab. Am. J. Transplant. 2022, 22, 2099–2103. [Google Scholar] [CrossRef]
- Coronavirus: Information & Resources. Available online: https://www.nationaljewish.org/patients-visitors/patient-info/important-updates/coronavirus-information-and-resources/covid-19-vaccines/vaccine-articles/side-by-side-comparison-covid-19-vaccine (accessed on 1 January 2023).
- Ram, R.; Hagin, D.; Kikozashvilli, N.; Freund, T.; Amit, O.; Bar-On, Y.; Beyar-Katz, O.; Shefer, G.; Moshiashvili, M.M.; Karni, C.; et al. Safety and Immunogenicity of the BNT162b2 mRNA COVID-19 Vaccine in Patients after Allogeneic HCT or CD19-based CART therapy-A Single-Center Prospective Cohort Study. Transplant. Cell Ther. 2021, 27, 788–794. [Google Scholar] [CrossRef]
- Dhakal, B.; Abedin, S.M.; Fenske, T.S.; Chhabra, S.; Ledeboer, N.; Hari, P.; Hamadani, M. Response to SARS-CoV-2 vaccination in patients after hematopoietic cell transplantation and CAR-T cell therapy. Blood 2021, 138, 1278–1281. [Google Scholar] [CrossRef]
- Chevallier, P.; Coste-Burel, M.; Le Bourgeois, A.; Peterlin, P.; Garnier, A.; Béné, M.C.; Imbert, B.M.; Drumel, T.; Le Gouill, S.; Moreau, P.; et al. Safety and immunogenicity of a first dose of SARS-CoV-2 mRNA vaccine in allogeneic hematopoietic stem-cells recipients. EJHaem 2021, 2, 520–524. [Google Scholar] [CrossRef]
- Cherif, H.; Hoglund, M.; Pauksens, K. Adjuvanted influenza A (H1N1) 2009 vaccine in patients with hematological diseases: Good safety and immunogenicity even in chemotherapy-treated patients. Eur. J. Haematol. 2013, 90, 413–419. [Google Scholar] [CrossRef]
- Hahn, M.; Schnitzler, P.; Schweiger, B.; Kunz, C.; Ho, A.D.; Goldschmidt, H.; Schmitt, M. Efficacy of single versus boost vaccination against influenza virus in patients with multiple myeloma. Haematologica 2015, 100, e285–e288. [Google Scholar] [CrossRef]
- Leclerc, M.; Fourati, S.; Menouche, D.; Challine, D.; Maury, S. Allogeneic haematopoietic stem cell transplantation from SARS-CoV-2 positive donors. Lancet Haematol. 2021, 8, e167–e169. [Google Scholar] [CrossRef]
- ASH-ASTCT COVID-19 Vaccination for HCT and CAR T Cell Recipients Frequently Asked Questions. Version 5.0. Available online: https://www.hematology.org/covid-19/ash-astct-covid-19-vaccination-for-hct-and-car-t-cell-recipients (accessed on 22 March 2022).
- COVID-19 and BMT—EBMT: COVID-19 Vaccines. Version 7, 3 October 2021. Available online: https://www.ebmt.org/sites/default/files/2021-10/COVID%20vaccines%20version%207.22%20-%202021-10-03.pdf (accessed on 1 January 2023).
- Townsend, J.P.; Hassler, H.B.; Sah, P.; Galvani, A.P.; Dornburg, A. The durability of natural infection and vaccine-induced immunity against future infection by SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2022, 119, e2204336119. [Google Scholar] [CrossRef]
- Widge, A.T.; Rouphael, N.G.; Jackson, L.A.; Anderson, E.J.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. mRNA-1273 Study Group. Durability of Responses after SARS-CoV-2 mRNA-1273 Vaccination. N. Engl. J. Med. 2021, 384, 80–82. [Google Scholar] [CrossRef]
- Doria-Rose, N.; Suthar, M.S.; Makowski, M.; O’Connell, S.; McDermott, A.B.; Flach, B.; Ledgerwood, J.E.; Mascola, J.R.; Graham, B.S.; Lin, B.C.; et al. mRNA-1273 Study Group. Antibody Persistence through 6 Months after the Second Dose of mRNA-1273 Vaccine for COVID-19. N. Engl. J. Med. 2021, 384, 2259–2261. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Cicin-Sain, C.; Pasin, C.; Epp, S.; Audigé, A.; Müller, N.J.; Nilsson, J.; Bankova, A.; Wolfensberger, N.; Vilinovszki, O.; et al. Antibody Response to SARS-CoV-2 Vaccination in Patients following Allogeneic Hematopoietic Cell Transplantation. Transplant. Cell Ther. 2022, 28, 214.e1–214.e11. [Google Scholar] [CrossRef] [PubMed]
- Nemet, I.; Kliker, L.; Lustig, Y.; Zuckerman, N.; Erster, O.; Cohen, C.; Kreiss, Y.; Alroy-Preis, S.; Regev-Yochay, G.; Mendelson, E.; et al. Third BNT162b2 Vaccination Neutralization of SARS-CoV-2 Omicron Infection. N. Engl. J. Med. 2022, 386, 492–494. [Google Scholar] [CrossRef] [PubMed]
- Ahmed-Belkacem, A.; Redjoul, R.; Brillet, R.; Ahnou, N.; Leclerc, M.; López-Molina, D.S.; Soulier, A.; Gourgeon, A.; Rodriguez, C.; Maury, S.; et al. Third Early “Booster” Dose Strategy in France of bnt162b2 SARS-CoV-2 Vaccine in Allogeneic Hematopoietic Stem Cell Transplant Recipients Enhances Neutralizing Antibody Responses. Viruses 2022, 14, 1928. [Google Scholar] [CrossRef] [PubMed]
- Uyemura, B.S.; Abid, M.A.; Suelzer, E.; Abid, M.B. Efficacy of SARS-CoV-2 primary and booster vaccine doses in CAR-T recipients—Targeting the target antigen. Bone Marrow Transplant. 2022, 57, 1727–1731. [Google Scholar] [CrossRef]
- Reimann, P.; Ulmer, H.; Mutschlechner, B.; Benda, M.; Severgnini, L.; Volgger, A.; Lang, T.; Atzl, M.; Huynh, M.; Gasser, K.; et al. Efficacy and Safety of Heterologous Booster Vaccination with Ad26.COV2.S after BNT162b2 MRNA COVID-19 Vaccine in Haemato-Oncological Patients with No Antibody Response. Br. J. Haematol. 2022, 196, 577–584. [Google Scholar] [CrossRef]
- Lindemann, M.; Klisanin, V.; Thümmler, L.; Fisenkci, N.; Tsachakis-Mück, N.; Ditschkowski, M.; Schwarzkopf, S.; Klump, H.; Reinhardt, H.C.; Horn, P.A.; et al. Humoral and cellular vaccination responses against SARS-CoV-2 in hematopoietic stem cell transplant recipients. Vaccines 2021, 9, 1075. [Google Scholar] [CrossRef]
- Harrington, P.; Doores, K.J.; Saha, C.; Saunders, J.; Child, F.; Dillon, R.; Saglam, S.; Raj, K.; McLornan, D.; Avenoso, D.; et al. Repeated vaccination against SARS-CoV-2 elicits robust polyfunctional T cell response in allogeneic stem cell transplantation recipients. Cancer Cell 2021, 39, 1448–1449. [Google Scholar] [CrossRef]
- Zuo, J.; Dowell, A.C.; Pearce, H.; Verma, K.; Long, H.M.; Begum, J.; Aiano, F.; Amin-Chowdhury, Z.; Hoschler, K.; Brooks, T.; et al. Robust SARS-CoV-2-specific T cell immunity is maintained at 6 months following primary infection. Nat. Immunol. 2021, 22, 620–626. [Google Scholar] [CrossRef]
- Bilich, T.; Nelde, A.; Heitmann, J.S.; Maringer, Y.; Roerden, M.; Bauer, J.; Rieth, J.; Wacker, M.; Peter, A.; Hörber, S.; et al. T cell and antibody kinetics delineate SARS-CoV-2 peptides mediating long-term immune responses in COVID-19 convalescent individuals. Sci. Transl. Med. 2021, 13, eabf7517. [Google Scholar] [CrossRef]
- Wellington, D.; Yin, Z.; Kessler, B.M.; Dong, T. Immunodominance complexity: Lessons yet to be learned from dominant T cell responses to SARS-CoV-2. Curr. Opin. Virol. 2021, 50, 183–191. [Google Scholar] [CrossRef]
- Schwarz, M.; Torre, D.; Lozano-Ojalvo, D.; Tan, A.T.; Tabaglio, T.; Mzoughi, S.; Sanchez-Tarjuelo, R.; Le Bert, N.; Lim, J.M.E.; Hatem, S.; et al. Rapid, scalable assessment of SARS-CoV-2 cellular immunity by whole-blood PCR. Nat. Biotechnol. 2022, 40, 1680–1689. [Google Scholar] [CrossRef]
- Dalai, S.C.; Dines, J.N.; Snyder, T.M.; Gittelman, R.M.; Eerkes, T.; Vaney, P.; Howard, S.; Akers, K.; Skewis, L.; Monteforte, A.; et al. Clinical validation of a novel T-cell receptor sequencing assay for identification of recent or prior SARS-CoV-2 infection. Clin. Infect. Dis. 2022, 75, 2079–2087. [Google Scholar] [CrossRef]
- Pagano, L.; Salmanton-García, J.; Marchesi, F.; López-García, A.; Lamure, S.; Itri, F.; Gomes-Silva, M.; Dragonetti, G.; Falces-Romero, I.; van Doesum, J.; et al. COVID-19 in vaccinated adult patients with hematological malignancies: Preliminary results from EPICOVIDEHA. Blood 2022, 139, 1588–1592. [Google Scholar] [CrossRef]
- Shaw, B.; Shortt, J.; Low, M.; Rogers, B.; Kaplan, Z.; Fedele, P.; Gregory, G.; Vilcassim, S.; Gilbertson, M.; Grigoriadis, G.; et al. Low mortality in vaccinated immunocompromised haematology patients infected with SARS-CoV-2. Intern. Med. J. 2022, 52, 2172–2175. [Google Scholar] [CrossRef] [PubMed]
- Walls, A.C.; Sprouse, K.R.; Bowen, J.E.; Joshi, A.; Franko, N.; Navarro, M.J.; Stewart, C.; Cameroni, E.; McCallum, M.; Goecker, E.A.; et al. SARS-CoV-2 breakthrough infections elicit potent, broad, and durable neutralizing antibody responses. Cell 2022, 185, 872–880.e3. [Google Scholar] [CrossRef]
- Altarawneh, H.N.; Chemaitelly, H.; Ayoub, H.H.; Tang, P.; Hasan, M.R.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Coyle, P.; Al-Kanaani, Z.; et al. Effects of Previous Infection and Vaccination on Symptomatic Omicron Infections. N. Engl. J. Med. 2022, 387, 21–34. [Google Scholar] [CrossRef]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.S.; Ash, N.; Alroy-Preis, S.; Huppert, A.; Milo, R. Protection and Waning of Natural and Hybrid Immunity to SARS-CoV-2. N. Engl. J. Med. 2022, 386, 2201–2212. [Google Scholar] [CrossRef]
- Arcani, R.; Colle, J.; Cauchois, R.; Koubi, M.; Jarrot, P.A.; Jean, R.; Boyer, A.; Lachamp, J.; Tichadou, A.; Couderc, A.L.; et al. Clinical characteristics and outcomes of patients with haematologic malignancies and COVID-19 suggest that pro-longed SARS-CoV-2 carriage is an important issue. Ann. Hematol. 2021, 100, 2799–2803. [Google Scholar] [CrossRef]
- Jiang, M.; Guo, Y.; Luo, Q.; Huang, Z.; Zhao, R.; Liu, S.; Li, J.; Wan, L. T-cell subset counts in peripheral blood can be used as discriminatory biomarkers for diagnosis and severity prediction of coronavirus disease 2019. J. Infect. Dis. 2020, 222, 198–202. [Google Scholar] [CrossRef]
- Huang, W.; Berube, J.; McNamara, M.; Saksena, S.; Hartman, M.; Arshad, T.; Bornheimer, S.J.; O’Gorman, M. Lymphocyte subset counts in COVID-19 patients: A meta-analysis. Cytom. A 2020, 97, 772–776. [Google Scholar] [CrossRef] [PubMed]
- García-García, I.; Guerra-García, P.; Ferreras, C.; Borobia, A.M.; Carcas, A.J.; Queiruga-Parada, J.; Vicario, J.L.; Mirones, I.; Solano, C.; Eguizabal, C.; et al. A phase I/II dose-escalation multi-center study to evaluate the safety of infusion of natural killer cells or memory T cells as adoptive therapy in coronavirus pneumonia and/or lymphopenia: RELEASE study protocol. Trials 2021, 22, 674. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Martínez, A.; Mora-Rillo, M.; Ferreras, C.; Guerra-García, P.; Pascual-Miguel, B.; Mestre-Durán, C.; Borobia, A.M.; Carcas, A.J.; Queiruga-Parada, J.; García, I.; et al. Phase I dose-escalation single centre clinical trial to evaluate the safety of infusion of memory T cells as adoptive therapy in COVID-19 (RELEASE). EClinicalMedicine 2021, 39, 101086. [Google Scholar] [CrossRef] [PubMed]
- Leung, W.; Soh, T.G.; Linn, Y.C.; Low, J.G.; Loh, J.; Chan, M.; Chng, W.J.; Koh, L.P.; Poon, M.L.; Ng, K.P.; et al. Rapid production of clinical-grade SARS-CoV-2 specific T cells. Adv. Cell Gene Ther. 2020, 3, e101. [Google Scholar] [CrossRef] [PubMed]
- Bonifacius, A.; Tischer-Zimmermann, S.; Santamorena, M.M.; Mausberg, P.; Schenk, J.; Koch, S.; Barnstorf-Brandes, J.; Gödecke, N.; Martens, J.; Goudeva, L.; et al. Rapid Manufacturing of Highly Cytotoxic Clinical-Grade SARS-CoV-2-specific T Cell Products Covering SARS-CoV-2 and Its Variants for Adoptive T Cell Therapy. Front. Bioeng. Biotechnol. 2022, 10, 867042. [Google Scholar] [CrossRef]
- Guerreiro, M.; Aguilar-Gallardo, C.; Montoro, J.; Francés-Gómez, C.; Latorre, V.; Luna, I.; Planelles, D.; Carrasco, M.P.; Gómez, M.D.; González-Barberá, E.M.; et al. Adoptive transfer of ex vivo expanded SARS-CoV-2-specific cytotoxic lymphocytes: A viable strategy for COVID-19 immunosuppressed patients? Transpl. Infect. Dis. 2021, 23, e13602. [Google Scholar] [CrossRef]
- Basar, R.; Uprety, N.; Ensley, E.; Daher, M.; Klein, K.; Martinez, F.; Aung, F.; Shanley, M.; Hu, B.; Gokdemir, E.; et al. Generation of glucocorticoid-resistant SARS-CoV-2 T cells for adoptive cell therapy. Cell Rep. 2021, 36, 109432. [Google Scholar] [CrossRef]
- Peter, L.; Wendering, D.J.; Schlickeiser, S.; Hoffmann, H.; Noster, R.; Wagner, D.L.; Zarrinrad, G.; Münch, S.; Picht, S.; Schulenberg, S.; et al. Tacrolimus-resistant SARS-CoV-2-specific T cell products to prevent and treat severe COVID-19 in immunosuppressed patients. Mol. Ther. Methods Clin. Dev. 2022, 25, 52–73. [Google Scholar] [CrossRef]
- Ferreras, C.; Pascual-Miguel, B.; Mestre-Durán, C.; Navarro-Zapata, A.; Clares-Villa, L.; Martín-Cortázar, C.; De Paz, R.; Marcos, A.; Vicario, J.L.; Balas, A.; et al. SARS-CoV-2-Specific Memory T Lymphocytes from COVID-19 Convalescent Donors: Identification, Biobanking, and Large-Scale Production for Adoptive Cell Therapy. Front. Cell Dev. Biol. 2021, 9, 620730. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).