Extramedullary Hematopoiesis in Myelodysplastic Syndromes: A Systematic Literature Review
Abstract
1. Introduction
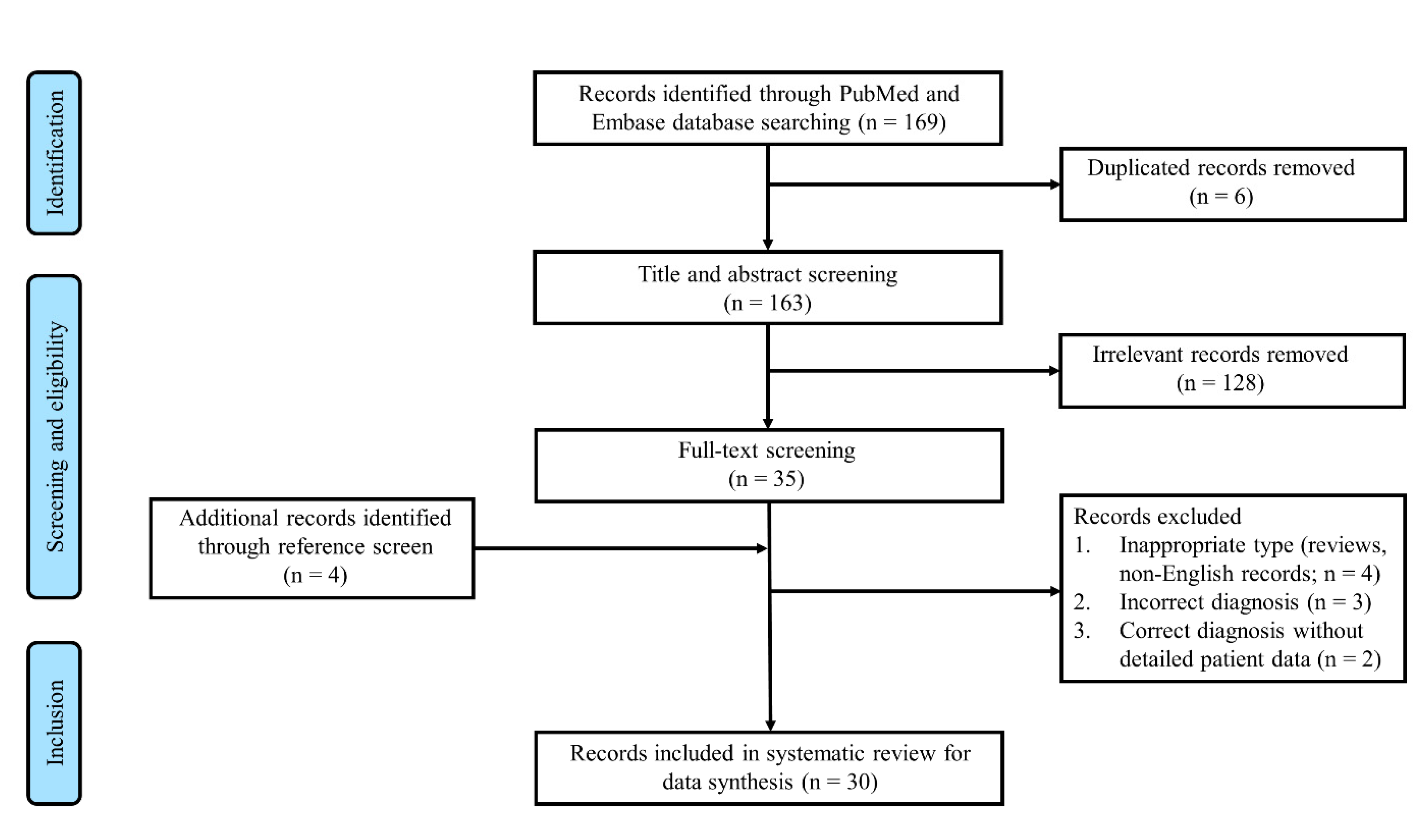
2. Methods
3. Results and Discussion
Funding
Conflicts of Interest
Abbreviations
| MDS | Myelodysplastic syndromes |
| EMH | Extramedullary hematopoiesis |
References
- O’Malley, D.P. Benign extramedullary myeloid proliferations. Mod. Pathol. 2007, 20, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.A.; Li, C.-Y.; Mesa, R.A.; Tefferi, A. Nonhepatosplenic extramedullary hematopoiesis: Associated diseases, pathology, clinical course, and treatment. Mayo Clin. Proc. 2003, 78, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Barraco, D.; Lasho, T.L.; Gangat, N.; Finke, C.; Elala, Y.C.; Pardanani, A.; Tefferi, A. Leukocytosis and presence of CALR mutation is associated with non-hepatosplenic extramedullary hematopoiesis in primary myelofibrosis. Blood Cancer J. 2016, 6, e436. [Google Scholar] [CrossRef] [PubMed]
- Heffez, D.S.; Sawaya, R.; Udvarhelyi, G.B.; Mann, R. Spinal epidural extramedullary hematopoiesis with cord compression in a patient with refractory sideroblastic anemia. Case report. J. Neurosurg. 1982, 57, 399–406. [Google Scholar] [CrossRef]
- Bennett, J.M.; Catovsky, D.; Daniel, M.T.; Flandrin, G.; Galton, D.A.; Gralnick, H.R.; Sultan, C. Proposals for the classification of the myelodysplastic syndromes. Br. J. Haematol. 1982, 51, 189–199. [Google Scholar] [CrossRef]
- Fan, N.; Lavu, S.; Hanson, C.A.; Tefferi, A. Extramedullary hematopoiesis in the absence of myeloproliferative neoplasm: Mayo Clinic case series of 309 patients. Blood Cancer J. 2018, 8, 119. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef]
- Walker, A.N.; Feldman, P.S.; Walker, G.K. Fine needle aspiration of thoracic extramedullary hematopoiesis. Acta Cytol. 1983, 27, 170–172. [Google Scholar]
- Dibbern, D.A.; Loevner, L.A.; Lieberman, A.P.; Salhany, K.E.; Freese, A.; Marcotte, P.J. MR of thoracic cord compression caused by epidural extramedullary hematopoiesis in myelodysplastic syndrome. AJNR Am. J. Neuroradiol. 1997, 18, 363–366. [Google Scholar]
- Hancock, J.C.; Prchal, J.T.; Bennett, J.M.; Listinsky, C.M. Trilineage extramedullary myeloid cell tumor in myelodysplastic syndrome. Arch. Pathol. Lab. Med. 1997, 121, 520–523. [Google Scholar]
- Kraus, M.D.; Bartlett, N.L.; Fleming, M.D.; Dorfman, D.M. Splenic pathology in myelodysplasia: A report of 13 cases with clinical correlation. Am. J. Surg. Pathol. 1998, 22, 1255–1266. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.; Maldjian, P.; Simmons, M.Z. Extramedullary hematopoiesis presenting as a focal splenic mass: A case report. Abdom Imaging 2004, 29, 710–712. [Google Scholar] [CrossRef] [PubMed]
- Vaunois, B.; Breyton, M.; Seigneurin, D.; Boutonnat, J. Intra-serous haematopoiesis. In Vivo 2005, 19, 407–415. [Google Scholar]
- Dagdas, S.; Ozet, G.; Alanoglu, G.; Ayli, M.; Gokmen Akoz, A.; Erekul, S. Unusual extramedullary hematopoiesis in a patient receiving granulocyte colony-stimulating factor. Acta Haematol. 2006, 116, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Haniffa, M.A.; Wilkins, B.S.; Blasdale, C.; Simpson, N.B. Cutaneous extramedullary hemopoiesis in chronic myeloproliferative and myelodysplastic disorders. J. Am. Acad. Dermatol. 2006, 55, S28–S31. [Google Scholar] [CrossRef]
- Mateen, F.J.; Harding, S.R.; Saxena, A. Extensive myocardial infiltration by hemopoietic precursors in a patient with myelodysplastic syndrome. BMC Blood Disord 2006, 6, 4. [Google Scholar] [CrossRef]
- Di Ieva, A.; Aimar, E.; Tancioni, F.; Levi, D.; Debernardi, A.; Pisano, P.; Rahal, D.; Nozza, A.; Magagnoli, M.; Gaetani, P. Focal extra-axial hemorrahagic mass with subdural hemorrhage secondare to extramedullary hematopoiesis in idiopathic myelodysplastic sindrome. J. Neurosurg. Sci. 2007, 51, 29–32. [Google Scholar]
- An Astonishing Discovery in A Patient with Abdominal Fullness and Anemia. J. Hosp. Med. 2012, 7 (Suppl. 2). Available online: https://shmabstracts.org/abstract/an-astonishing-discovery-in-a-patient-with-abdominal-fullness-and-anemia/ (accessed on 15 May 2022).
- Kawakami, T.; Kimura, S.; Kato, M.; Mizoguchi, M.; Soma, Y. Transforming growth factor-beta overexpression in cutaneous extramedullary hematopoiesis of a patient with myelodysplastic syndrome associated with myelofibrosis. J. Am. Acad. Dermatol. 2008, 58, 703–706. [Google Scholar] [CrossRef]
- Monti, L.; Romano, D.G.; Gozzetti, A.; Di Pietro, G.; Miracco, C.; Cerase, A. Myelodysplasia presenting as thoracic spinal epidural extramedullary hematopoiesis: A rare treatable cause of spinal cord myelopathy. Skeletal Radiol. 2012, 41, 611–614. [Google Scholar] [CrossRef]
- Buccisano, F.; Maurillo, L.; Neri, B.; Masala, S.; Mauriello, A.; Del Principe, M.I.; Ditto, C.; Sarlo, C.; Cefalo, M.; Di Caprio, L.; et al. Thoracic cord compression caused by epidural extramedullary hematopoiesis during erythroid-stimulating agent therapy in two patients with myelodysplastic syndromes. J. Clin. Oncol. 2013, 31, e189–e191. [Google Scholar] [CrossRef]
- Martinez-Losada, C.; Alhambra-Exposito, M.R.; Sanchez-Sanchez, R.; Casaño, J.; Tenorio-Jimenez, C.; Sanchez-Garcia, J. Dysplastic extramedullary haematopoeisis with ringed sideroblasts mimicking adrenal adenoma. Histopathology 2013, 63, 738–739. [Google Scholar] [CrossRef] [PubMed]
- Pinato, D.J.; Tan, W.; Gately, A. An unexpected cause of pulmonary cannonball lesion. J. Thorac. Oncol. 2014, 9, 259. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; Shapey, J.; Pohl, U.; Vindlacheruvu, R. Intracranial extramedullary haematopoiesis: A case report. Br. J. Neurosurg. 2015, 29, 734–736. [Google Scholar] [CrossRef]
- Tan, L.A.; Deutsch, H. Severe spinal cord compression due to extramedullary hematopoiesis. Br. J. Neurosurg. 2015, 29, 737–738. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Singhal, A.L.; Sachdeva, A. Extramedullary Pulmonary Hematopoiesis Presenting as Spontaneous Hemothorax in a Patient with Myelodysplastic Syndrome. In D39. Helter Skelter: Unusual Causes of Hemoptysis and Hemorrhage; American Thoracic Society: Denver, CO, USA, 2015; p. A5852. [Google Scholar]
- Kazemimood, R.; Meinke, K.; Hamedani, F.S.; Eliaszadeh, S.; Levy, B. An Unusual Presentation of Extramedullary Hematopoiesis as Multiple Nodular Lesions in Liver and Spleen of a 75-Year-Old Man: Case Report and Literature Review. Am. J. Clin. Pathol. 2015, 144, A005. [Google Scholar] [CrossRef][Green Version]
- Sawada, H.; Higuchi, T.; Koyamada, R.; Okada, S. Myelodysplastic Syndrome Developing Presacral Extramedullary Hematopoiesis with Atypical MRI Findings. Intern. Med. 2017, 56, 1213–1217. [Google Scholar] [CrossRef][Green Version]
- Takano, H.; Takahashi, K.; Taki, K. Myelodysplastic hematopoiesis mimicking the bone marrow in a mediastinal myelolipoma. Clin. Case Rep. 2017, 5, 385–388. [Google Scholar] [CrossRef]
- Belay, A.A.; Bellizzi, A.M.; Stolpen, A.H. The role of T2*-weighted gradient echo in the diagnosis of tumefactive intrahepatic extramedullary hematopoiesis in myelodysplastic syndrome and diffuse hepatic iron overload: A case report and review of the literature. J. Med. Case Repl 2018, 12, 9. [Google Scholar] [CrossRef]
- Kapatia, G.; Kaur, A.; Rastogi, P.; Sreedharanunni, S.; Gupta, P.; Rohilla, M.; Gupta, N.; Srinivasan, R.; Rajwanshi, A.; Dey, P. Extramedullary hematopoiesis: Clinical and cytological features. Diagn. Cytopathol. 2020, 48, 191–196. [Google Scholar] [CrossRef]
- Konishi, T.; Doki, N.; Takaki, Y.; Igarashi, A.; Ohashi, K. Presacral extramedullary hematopoiesis under treatment with an erythropoietin-stimulating agent for myelodysplasia. Int. J. Hematol. 2019, 109, 1–2. [Google Scholar] [CrossRef]
- Satoh, T.; Kayano, H. Myelodysplastic syndrome with ring sideroblasts presenting as postmediastinal extramedullary hematopoiesis. Blood 2020, 136, 1213. [Google Scholar] [CrossRef] [PubMed]
- Kamran, S.; Al-Obaidi, A.; Al-Khazraji, Y.; Alderson, J.; Reddy, P.S. Obstructive Jaundice Secondary to Extramedullary Hematopoiesis. Cureus 2021, 13, e17927. [Google Scholar] [CrossRef] [PubMed]
- Asou, C.; Maeda, T.; Ishikawa, M.; Okamura, D.; Kohri, M.; Takahashi, N.; Tsukasaki, K.; Sakaguchi, H.; Satoh, T.; Kayano, H.; et al. Paravertebral extramedullary hematopoiesis in a case of myelodysplastic syndrome with ring sideroblasts and an SF3B1 mutation. Int. J. Hematol. 2022, 115, 898–901. [Google Scholar] [CrossRef] [PubMed]
- Ternes, L.; Giangiacomo, F.; Nassif, I. An Unusual Case of Myelodysplastic Syndrome with Intrahepatic Extramedullary Hematopoiesis Leading to Liver Failure. Cureus 2022, 14, e22733. [Google Scholar] [CrossRef]
- Ramdohr, F.; Monecke, A.; Jentzsch, M.; Zehrfeld, T.; Borte, G.; Schwind, S.; Franke, G.N.; Metzeler, K.H.; Platzbecker, U.; Vucinic, V. Extramedullary Clonal Hematopoiesis with Indeterminate Potential. Clin. Lymphoma Myeloma Leuk. 2021, 21, e696–e698. [Google Scholar] [CrossRef]
- Malcovati, L.; Stevenson, K.; Papaemmanuil, E.; Neuberg, D.; Bejar, R.; Boultwood, J.; Bowen, D.T.; Campbell, P.J.; Ebert, B.L.; Fenaux, P.; et al. SF3B1-mutant MDS as a distinct disease subtype: A proposal from the International Working Group for the Prognosis of MDS. Blood 2020, 136, 157–170. [Google Scholar] [CrossRef]
| Variables 1 | All (n = 41) |
|---|---|
| Baseline characteristics | |
| Age (years) | 70 (64–76) |
| Male (%) | 27 (71.1) (n = 38) |
| Hemoglobin (g/dL) | 8.2 (7.4–9.4) (n = 20) |
| MDS subtype (%) | |
| Ring sideroblasts | 11 (30.6) |
| Excess blasts | 5 (12.2) |
| EMH onset (%) | |
| Symptomatic onset | 27 (65.9) |
| After MDS diagnosis | 27 (73.0) (n = 37) |
| Temporal relationship with prior growth factor use | 4 (9.8) |
| EMH location (%) | |
| Spleen and/or liver | 15 (36.6) |
| Paravertebral region | 10 (24.4) |
| Other sites 2 | 16 (39.0) |
| EMH treatment (%) | (n = 38) |
| Observation | 20 (52.6) |
| Surgery | 14 (36.8) |
| Radiation | 5 (13.2) |
| MDS treatment (%) | (n = 27) |
| Transfusion | 17 (63.0) |
| Recombinant erythropoietin | 5 (18.5) |
| Granulocyte-colony stimulating factor | 2 (7.4) |
| Lenalidomide or thalidomide | 2 (7.4) |
| Azacitidine | 1 (3.7) |
| Outcomes | (n = 36) |
| Death at last follow-up | 19 (52.8) |
| Secondary acute myeloid leukemia | 4 (11.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Shi, Y. Extramedullary Hematopoiesis in Myelodysplastic Syndromes: A Systematic Literature Review. Hemato 2022, 3, 569-573. https://doi.org/10.3390/hemato3030039
Wang C, Shi Y. Extramedullary Hematopoiesis in Myelodysplastic Syndromes: A Systematic Literature Review. Hemato. 2022; 3(3):569-573. https://doi.org/10.3390/hemato3030039
Chicago/Turabian StyleWang, Chen, and Yiyun Shi. 2022. "Extramedullary Hematopoiesis in Myelodysplastic Syndromes: A Systematic Literature Review" Hemato 3, no. 3: 569-573. https://doi.org/10.3390/hemato3030039
APA StyleWang, C., & Shi, Y. (2022). Extramedullary Hematopoiesis in Myelodysplastic Syndromes: A Systematic Literature Review. Hemato, 3(3), 569-573. https://doi.org/10.3390/hemato3030039






