The Next Step for MRD in Myeloma? Treating MRD Relapse after First Line Treatment in the REMNANT Study
Abstract
1. Introduction
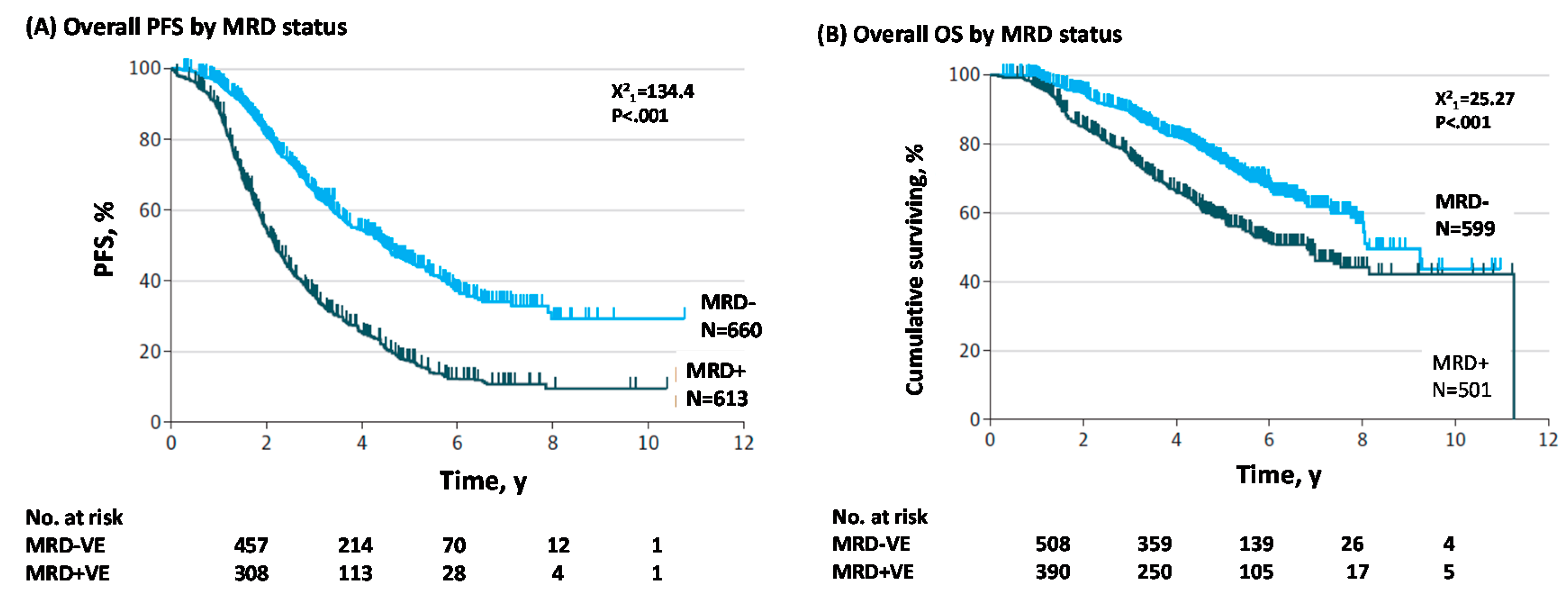
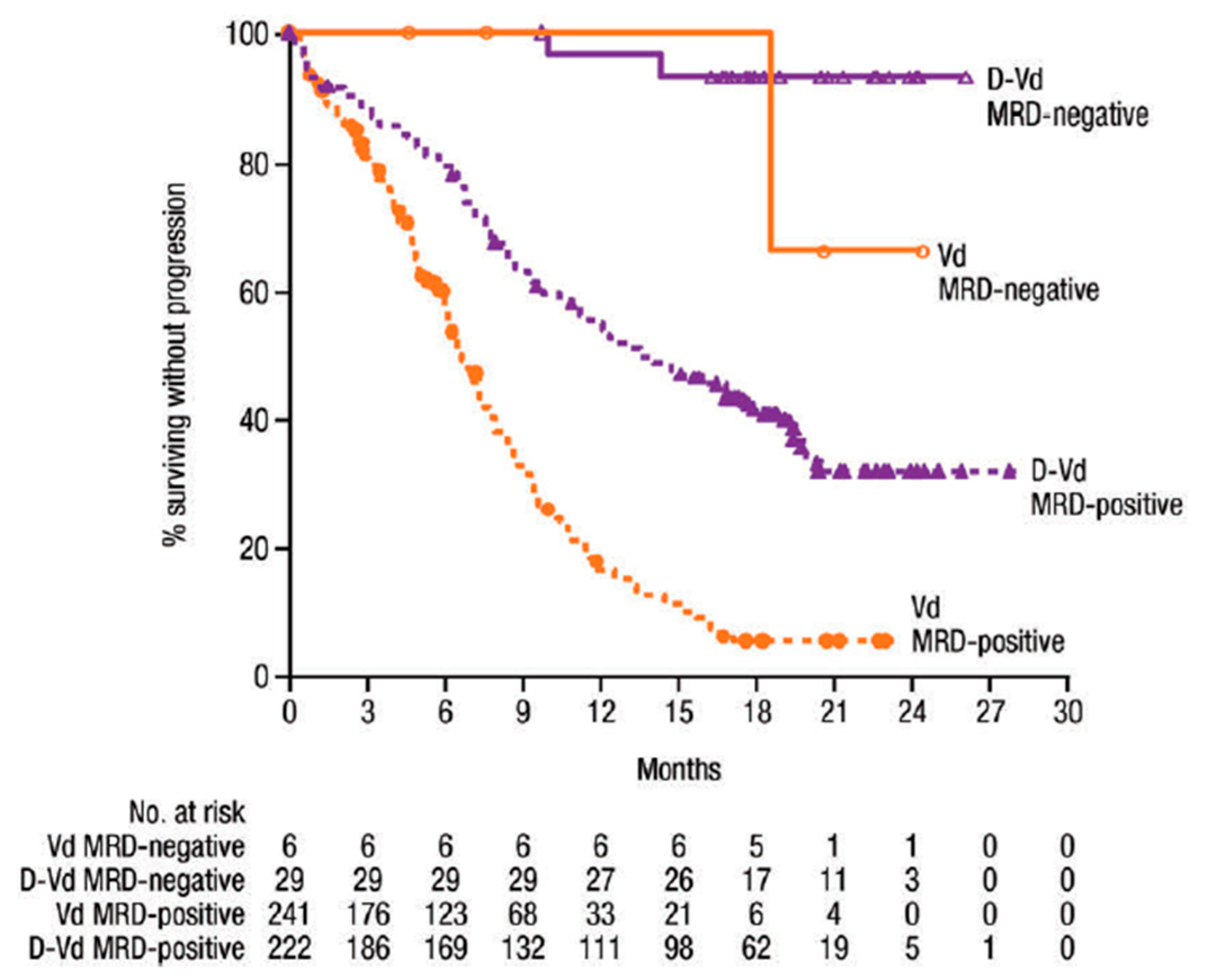
1.1. MRD Negativity Increase PFS and OS in NDMM and RRMM
1.2. MRD as a Surrogate Endpoint in Clinical Trials
2. Techniques for MRD Monitoring
2.1. How to Monitor MRD
2.2. PET-CT
2.3. Mass Spectrometry
3. MRD Trials
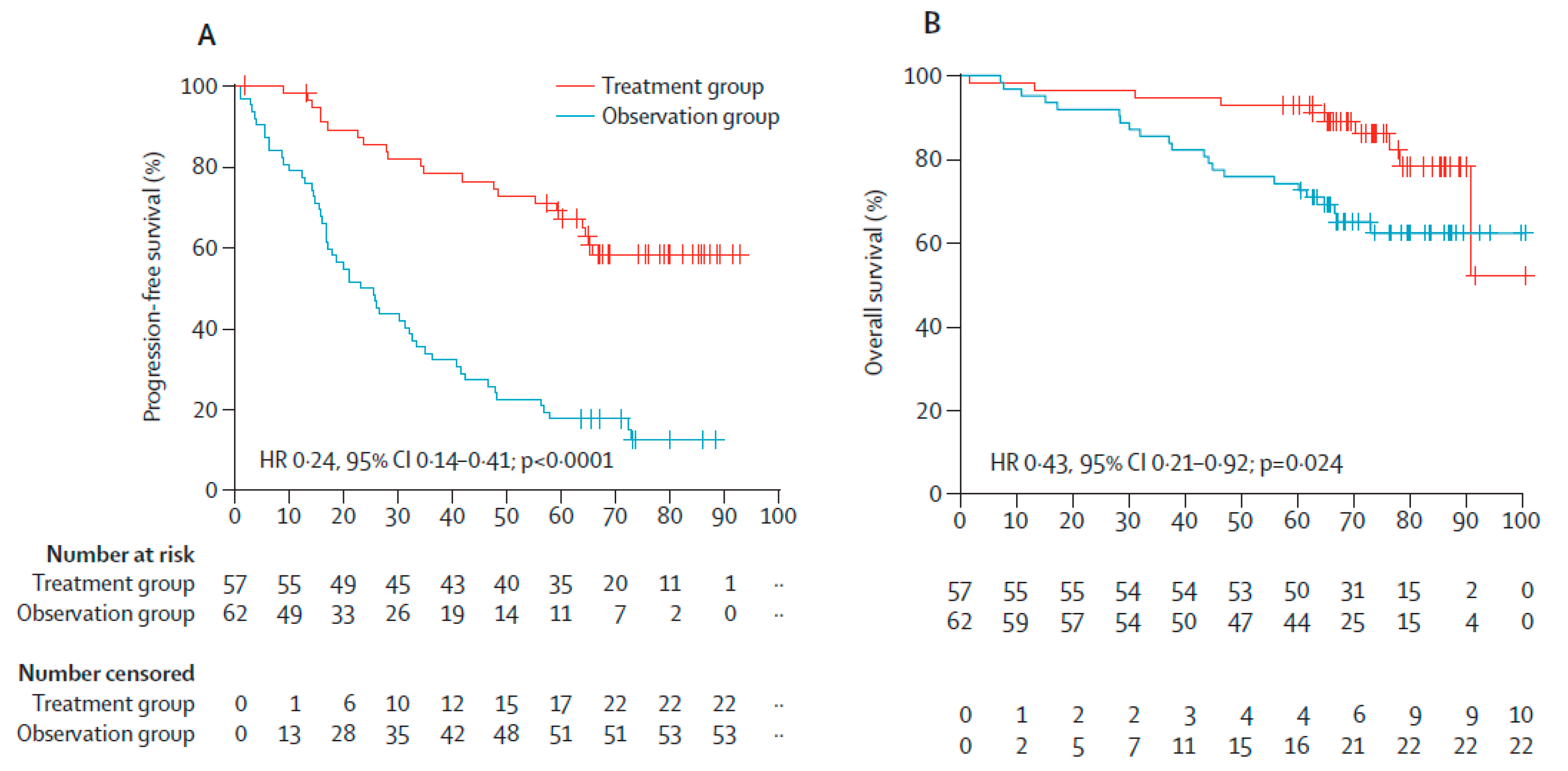
Delayed ASCT in NDMM Patients Who Are MRD- and MRD as Treatment Guidance in RRMM Patients
4. Early Treatment in Patients with SMM
Treatment of Patients with SMM
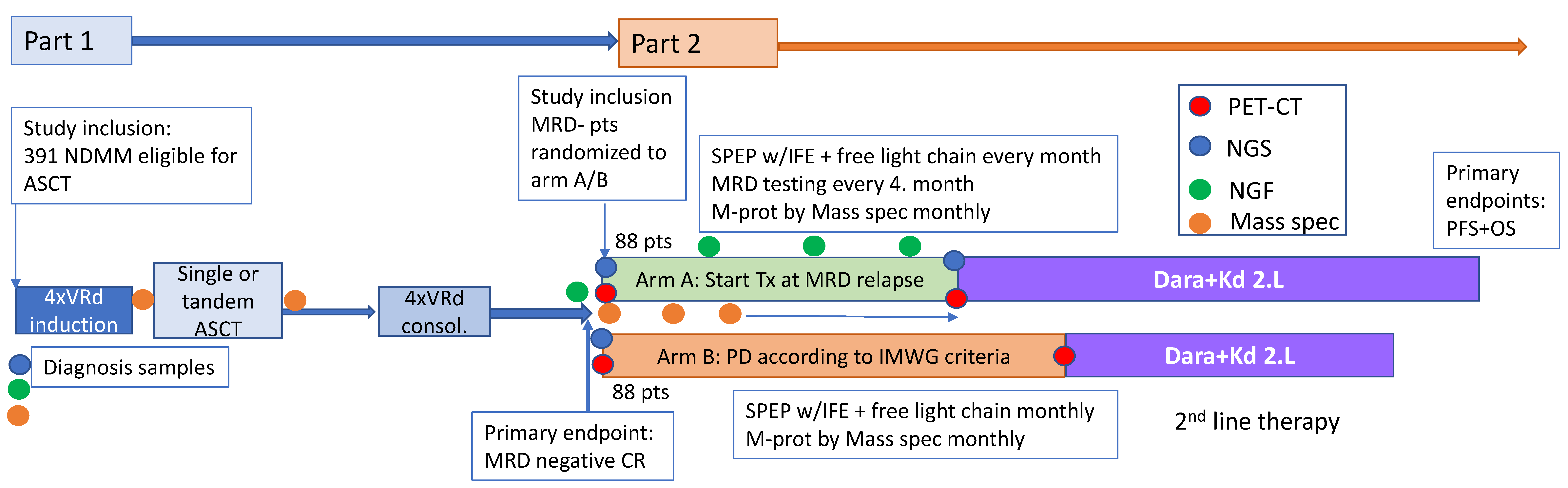
5. Relapse from MRD Negativity as Indication for Treatment: The REMNANT Study
5.1. First Line Treatment
5.2. Second Line Treatment
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Perrot, A.; Lauwers-Cances, V.; Corre, J.; Robillard, N.; Hulin, C.; Chretien, M.L.; Dejoie, T.; Maheo, S.; Stoppa, A.M.; Pegouri, B.; et al. Minimal residual disease negativity using deep sequencing is a major prognostic factor in multiple myeloma. Blood 2018, 132, 2456–2464. [Google Scholar] [CrossRef] [PubMed]
- Avet-Loiseau, H.; Ludwig, H.; Landgren, O.; Paiva, B.; Morris, C.; Yang, H.; Zhou, K.; Ro, S.; Mateoas, M.V. Minimal Residual Disease Status as a Surrogate Endpoint for Progression-free Survival in Newly Diagnosed Multiple Myeloma Studies: A Meta-analysis. Clin. Lymphoma Myeloma Leuk. 2020, 20, e30–e37. [Google Scholar] [CrossRef] [PubMed]
- Landgren, O.; Devlin, S.; Boulad, M.; Mailankody, S. Role of MRD status in relation to clinical outcomes in newly diagnosed multiple myeloma patients: A meta-analysis. Bone Marrow Transplant. 2016, 51, 1565–1568. [Google Scholar] [CrossRef] [PubMed]
- Munshi, N.C.; Avet-Loiseau, H.; Rawstron, A.C.; Owen, R.G.; Child, J.A.; Thakurta, A.; Sherrington, P.; Samur, M.K.; Geogieva, A.; Anderson, K.C.; et al. Association of Minimal Residual Disease With Superior Survival Outcomes in Patients With Multiple Myeloma: A Meta-analysis. JAMA Oncol. 2017, 3, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Prentice, R.L. Surrogate endpoints in clinical trials: Definition and operational criteria. Stat. Med. 1989, 8, 431–440. [Google Scholar] [CrossRef]
- Paiva, B.; Chandia, M.; Puig, N.; Vidriales, M.B.; Perez, J.J.; Lopez-Corral, L.; Ocio, E.M.; Garcia-Sanz, R.; Gutierrez, N.C.; Jimenez-Ubieto, A.; et al. The prognostic value of multiparameter flow cytometry minimal residual disease assessment in relapsed multiple myeloma. Haematologica 2015, 100, e53–e55. [Google Scholar] [CrossRef][Green Version]
- Spencer, A.; Lentzsch, S.; Weisel, K.; Avet-Loiseau, H.; Mark, T.M.; Spicka, I.; Masszi, T.; Lauri, B.; Levin, M.D.; Bosi, A.; et al. Daratumumab plus bortezomib and dexamethasone versus bortezomib and dexamethasone in relapsed or refractory multiple myeloma: Updated analysis of CASTOR. Haematologica 2018, 103, 2079–2087. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; San-Miguel, J.; Belch, A.; White, D.; Benboubker, L.; Cook, G.; Leiba, M.; Morotn, J.; Ho, P.J.; Kim, K.; et al. Daratumumab plus lenalidomide and dexamethasone versus lenalidomide and dexamethasone in relapsed or refractory multiple myeloma: Updated analysis of POLLUX. Haematologica 2018, 103, 2088–2096. [Google Scholar] [CrossRef]
- Landgren, O.; Iskander, K. Modern multiple myeloma therapy: Deep, sustained treatment response and good clinical outcomes. J. Intern. Med. 2017, 281, 365–382. [Google Scholar] [CrossRef]
- Landgren, O. Meeting report: Advances in minimal residual disease testing in multiple myeloma 2018. Adv. Cell Gene Ther. 2018, 2, 1–5. [Google Scholar] [CrossRef][Green Version]
- Flores-Montero, J.; Sanoja-Flores, L.; Paiva, B.; Puig, N.; Garcia-Sanchez, O.; Bottcher, S.; van der Velden, V.H.J.; Perez-Moran, J.J.; Vidriales, M.B.; Garcia-Sanz, R.; et al. Next Generation Flow for highly sensitive and standardized detection of minimal residual disease in multiple myeloma. Leukemia 2017, 31, 2094–2103. [Google Scholar] [CrossRef] [PubMed]
- Biancon, G.; Gimondi, S.; Vendramin, A.; Carniti, C.; Corradini, P. Noninvasive Molecular Monitoring in Multiple Myeloma Patients Using Cell-Free Tumor DNA: A Pilot Study. J. Mol. Diagn. 2018, 20, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Thoren, K.L. Mass spectrometry methods for detecting monoclonal immunoglobulins in multiple myeloma minimal residual disease. Semin. Hematol. 2018, 55, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Cavo, M.; Terpos, E.; Nanni, C.; Moreau, P.; Lentzsch, S.; Zweegman, S.; Hillengass, J.; Engelhardt, M.; Usmani, S.Z.; Vesole, D.H.; et al. Role of (18)F-FDG PET/CT in the diagnosis and management of multiple myeloma and other plasma cell disorders: A consensus statement by the International Myeloma Working Group. Lancet Oncol. 2017, 18, e206–e217. [Google Scholar] [CrossRef]
- Moreau, P.; Attal, M.; Caillot, D.; Macro, M.; Karlin, L.; Garderet, L.; Facon, T.; Benboubker, L.; Escoffre-Barbe, M.; Stoppa, A.M.; et al. Prospective Evaluation of Magnetic Resonance Imaging and [(18)F]Fluorodeoxyglucose Positron Emission Tomography-Computed Tomography at Diagnosis and Before Maintenance Therapy in Symptomatic Patients With Multiple Myeloma Included in the IFM/DFCI 2009 Trial: Results of the IMAJEM Study. J. Clin. Oncol. 2017, 35, 2911–2918. [Google Scholar]
- Hillengass, J.; Usmani, S.; Rajkumar, S.V.; Durie, B.G.M.; Mateos, M.V.; Lonial, S.; Joao, C.; Anderson, K.C.; Garcia-Sanz, R.; Riva, E.; et al. International myeloma working group consensus recommendations on imaging in monoclonal plasma cell disorders. Lancet Oncol. 2019, 20, e302–e312. [Google Scholar] [CrossRef]
- Zamagni, E.; Patriarca, F.; Nanni, C.; Zannetti, B.; Englaro, E.; Pezzi, A.; Tacchetti, P.; Buttignol, S.; Perrone, G.; Brioli, A.; et al. Prognostic relevance of 18-F FDG PET/CT in newly diagnosed multiple myeloma patients treated with up-front autologous transplantation. Blood 2011, 118, 5989–5995. [Google Scholar] [CrossRef]
- North, S.; Barnidge, D.; Brusseau, S.; Patel, R.; Haselton, M.; Du Chateau, B.; Wallis, G.; Harding, S.; Sakrikar, D.; Ashby, J. QIP-MS: A specific, sensitive, accurate, and quantitative alternative to electrophoresis that can identify endogenous m-proteins and distinguish them from therapeutic monoclonal antibodies in patients being treated for multiple myeloma. Clin. Chim. Acta 2019, 493, S379–S433. [Google Scholar] [CrossRef]
- Eveillard, M.; Hultcrantz, M.; Lesokhin, A.; Mailankody, S.; Smith, E.; Hassoun, H.; Shah, U.A.; Lu, S.; Korde, N.; Landgren, O.; et al. MALDI-TOF Mass Spectrometry in Serum for the Follow-up of Newly Diagnosed Multiple Myeloma Patients Treated with Daratumumab-Based Combination Therapy. Blood 2019, 134 (Suppl. 1), 4377. [Google Scholar] [CrossRef]
- Sidana, S.; Tandon, N.; Dispenzieri, A.; Gertz, M.A.; Buadi, F.K.; Lacy, M.Q.; Dingli, D.; Fonder, A.L.; Hayman, S.R.; Hobbs, M.A. Relapse after complete response in newly diagnosed multiple myeloma: Implications of duration of response and patterns of relapse. Leukemia 2019, 33, 730–738. [Google Scholar] [CrossRef]
- Ferrero, S.; Ladetto, M.; Drandi, D.; Cavallo, F.; Genuardi, E.; Urbano, M.; Caltagirone, S.; Grasso, M.; Rossini, F.; Guglielmalli, T.; et al. Long-term results of the GIMEMA VEL-03-096 trial in MM patients receiving VTD consolidation after ASCT: MRD kinetics’ impact on survival. Leukemia 2015, 29, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Oliva, S. Minimal residual disease after transplantation or lenalidomide based consolidation in myeloma patients: A prospective analysis. OncoTarget 2016, 1, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mateos, M.V.; Hernandez, M.T.; Giraldo, P.; de la Rubia, J.; de Arriba, F.; Corral, L.L.; Rosignol, L.; Paiva, B.; Palomera, L.; Bargay, J.; et al. Lenalidomide plus dexamethasone versus observation in patients with high-risk smouldering multiple myeloma (QuiRedex): Long-term follow-up of a randomised, controlled, phase 3 trial. Lancet Oncol. 2016, 17, 1127–1136. [Google Scholar] [CrossRef]
- Korde, N.; Roschewski, M.; Zingone, A.; Kwok, M.; Manasanch, E.E.; Bhutani, M.; Tageja, N.; Kazandjian, D.; Mailankody, S.; Wu, P.; et al. Treatment With Carfilzomib-Lenalidomide-Dexamethasone With Lenalidomide Extension in Patients With Smoldering or Newly Diagnosed Multiple Myeloma. JAMA Oncol. 2015, 1, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Lonial, S.; Jacobus, S.; Fonseca, R.; Weiss, M.; Kumar, S.; Orlowski, R.Z.; Kaufman, J.L.; Yacoub, A.M.; Buadi, F.K.; O’Brien, T.; et al. Randomized Trial of Lenalidomide Versus Observation in Smoldering Multiple Myeloma. J. Clin. Oncol. 2020, 38, 1126–1137. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.L.; Shen, K.N.; Wang, J.N.; Huo, L.Q.; Li, J.; Cao, X.X. Early or deferred treatment of smoldering multiple myeloma: A meta-analysis on randomized controlled studies. Cancer Manag. Res. 2019, 11, 5599–5611. [Google Scholar] [CrossRef]
- Witzig, T.E.; Laumann, K.M.; Lacy, M.Q.; Hayman, S.R.; Dispenzieri, A.; Kumar, S.; Reeder, C.B.; Roy, V.; Lust, J.A.; Gertz, M.A.; et al. A phase III randomized trial of thalidomide plus zoledronic acid versus zoledronic acid alone in patients with asymptomatic multiple myeloma. Leukemia 2013, 27, 220–225. [Google Scholar] [CrossRef]
- Richardson, P.G.; Weller, E.; Lonial, S.; Jakubowiak, A.J.; Jagannath, S.; Raje, N.S.; Avigan, D.E.; Xie, W.; Ghobrial, I.M.; Schlossman, R.L.; et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood 2010, 116, 679–686. [Google Scholar] [CrossRef]
- Durie, B.G.M.; Hoering, A.; Abidi, M.H.; Rajkumar, S.V.; Epstein, J.; Kahanic, S.P.; Thakuri, M.; Reu, F.; Reynolds, C.M.; Sexton, R.; et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): A randomised, open-label, phase 3 trial. Lancet 2017, 389, 519–527. [Google Scholar] [CrossRef]
- Attal, M.; Lauwers-Cances, V.; Hulin, C.; Leleu, X.; Caillot, D.; Escoffre, M.; Arnulf, B.; Macro, M.; Behadj, K.; Garderet, L.; et al. Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma. N. Engl. J. Med. 2017, 376, 1311–1320. [Google Scholar] [CrossRef]
- Rosinol, L.; Oriol, A.; Rios, R.; Sureda, A.; Blanchard, M.J.; Hernandez, M.T.; Martinez-Martinez, R.; Moraleda, J.M.; Jarque, I.; Bargay, J.; et al. Bortezomib, lenalidomide, and dexamethasone as induction therapy prior to autologous transplant in multiple myeloma. Blood 2019, 134, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Roussel, M.; Lauwers-Cances, V.; Robillard, N.; Hulin, C.; Leleu, X.; Benboubker, L.; Marit, G.; Moreau, P.; Pegourie, B.; Caillot, D.; et al. Front-line transplantation program with lenalidomide, bortezomib, and dexamethasone combination as induction and consolidation followed by lenalidomide maintenance in patients with multiple myeloma: A phase II study by the Intergroupe Francophone du Myelome. J. Clin. Oncol. 2014, 32, 2712–2717. [Google Scholar] [PubMed]
- Blocka, J.; Hielscher, T.; Goldschmidt, H.; Hillengass, J. Response improvement rather than response status after 1st ASCT is a significant prognostic factor for survival benefit from tandem compared to single ASCT in multiple myeloma patients. Biol. Blood Marrow Transplant. 2020, 26, 1280–1287. [Google Scholar] [CrossRef] [PubMed]
- Cavo, M.; Petrucci, M.T.; Di Raimondo, F.; Zamagni, E.; Gamberi, B.; Crippa, C.; Marzocchi, G.; Grasso, M.; Ballanti., S.; Vincelli, D.I. Upfront Single Versus Double Autologous Stem Cell Transplantation for Newly Diagnosed Multiple Myeloma: An Intergroup, Multicenter, Phase III Study of the European Myeloma Network (EMN02/HO95 MM Trial); American Society of Hematology: Washington, WA, USA, 2016. [Google Scholar]
- McCarthy, P.L.; Holstein, S.A.; Petrucci, M.T.; Richardson, P.G.; Hulin, C.; Tosi, P.; Bringhen, S.; Musto, P.; Anderson, K.C.; Caillot, D.; et al. Lenalidomide Maintenance After Autologous Stem-Cell Transplantation in Newly Diagnosed Multiple Myeloma: A Meta-Analysis. J. Clin. Oncol. 2017, 35, 3279–3289. [Google Scholar] [CrossRef]
- Kumar, S.K.; Lee, J.H.; Lahuerta, J.J.; Morgan, G.; Richardson, P.G.; Crowley, J.; Haessler, J.; Feather, J.; Hoering, A.; Moreau, P.; et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: A multicenter international myeloma working group study. Leukemia 2012, 26, 149–157. [Google Scholar] [CrossRef]
- Chari, A.; Martinez-Lopez, J.; Mateos, M.-V.; Bladé, J.; Benboubker, L.; Oriol, A.; Arnulf, B.; Rodriguez-Otero, P.; Pineiro, L.; Jakubowiak, A.; et al. Daratumumab plus carfilzomib and dexamethasone in patients with relapsed or refractory multiple myeloma. Blood 2019, 134, 421–431. [Google Scholar] [CrossRef]
- Usmani, S.Z.; Quach, H.; Mateos, M.-V.; Landgren, O.; Leleu, X.; Siegel, D.S.; Weisel, K.; Yang, H.; Klippel, Z.K.; Zahlten-Kumeli, A.; et al. Carfilzomib, Dexamethasone, and Daratumumab versus Carfilzomib and Dexamethasone for the Treatment of Patients with Relapsed or Refractory Multiple Myeloma (RRMM): Primary Analysis Results from the Randomized, Open-Label, Phase 3 Study Candor (NCT03158688). Blood 2019, 134. [Google Scholar] [CrossRef]
- Moreau, P.; Mateos, M.-V.; Berenson, J.R.; Weisel, K.; Lazzaro, A.; Song, K.; Dimopoulos, M.A.; huang, M.; Zahlten-Kumeli, A.; Stewart, A.K. Once weekly versus twice weekly carfilzomib dosing in patients with relapsed and refractory multiple myeloma (ARROW): Interim analysis results of a randomised, phase 3 study. Lancet Oncol. 2018, 19, 953–964. [Google Scholar] [CrossRef]
| Euroflow | NGS | MALDI ToF Mass Spectrometry [13] | |
|---|---|---|---|
| Sensitivity | 10−5 | 10−6 | Not defined—Method under development |
| Diagnosis sample needed | No | Yes | Preferred |
| Testing duration | Rapid | 5–10 days | 2 h |
| Standardization | Easy | Mostly service based | Traceable to DA470K |
| Bioinformatics experience needed | No | Yes | No |
| Sample type | Bone marrow | Bone marrow | Serum/Plasma/Urine |
| Cost | Acceptable | High | Acceptable |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasmussen, A.-M.; Askeland, F.B.; Schjesvold, F. The Next Step for MRD in Myeloma? Treating MRD Relapse after First Line Treatment in the REMNANT Study. Hemato 2020, 1, 36-48. https://doi.org/10.3390/hemato1020008
Rasmussen A-M, Askeland FB, Schjesvold F. The Next Step for MRD in Myeloma? Treating MRD Relapse after First Line Treatment in the REMNANT Study. Hemato. 2020; 1(2):36-48. https://doi.org/10.3390/hemato1020008
Chicago/Turabian StyleRasmussen, Anne-Marie, Frida Bugge Askeland, and Fredrik Schjesvold. 2020. "The Next Step for MRD in Myeloma? Treating MRD Relapse after First Line Treatment in the REMNANT Study" Hemato 1, no. 2: 36-48. https://doi.org/10.3390/hemato1020008
APA StyleRasmussen, A.-M., Askeland, F. B., & Schjesvold, F. (2020). The Next Step for MRD in Myeloma? Treating MRD Relapse after First Line Treatment in the REMNANT Study. Hemato, 1(2), 36-48. https://doi.org/10.3390/hemato1020008






