Indoleamine 2,3-Dioxygenase Activity Is Increased in Myelodysplastic Syndrome Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Blood Sample Collection
2.2. Serum TRP Metabolite Measurements
2.3. Statistical Analyses
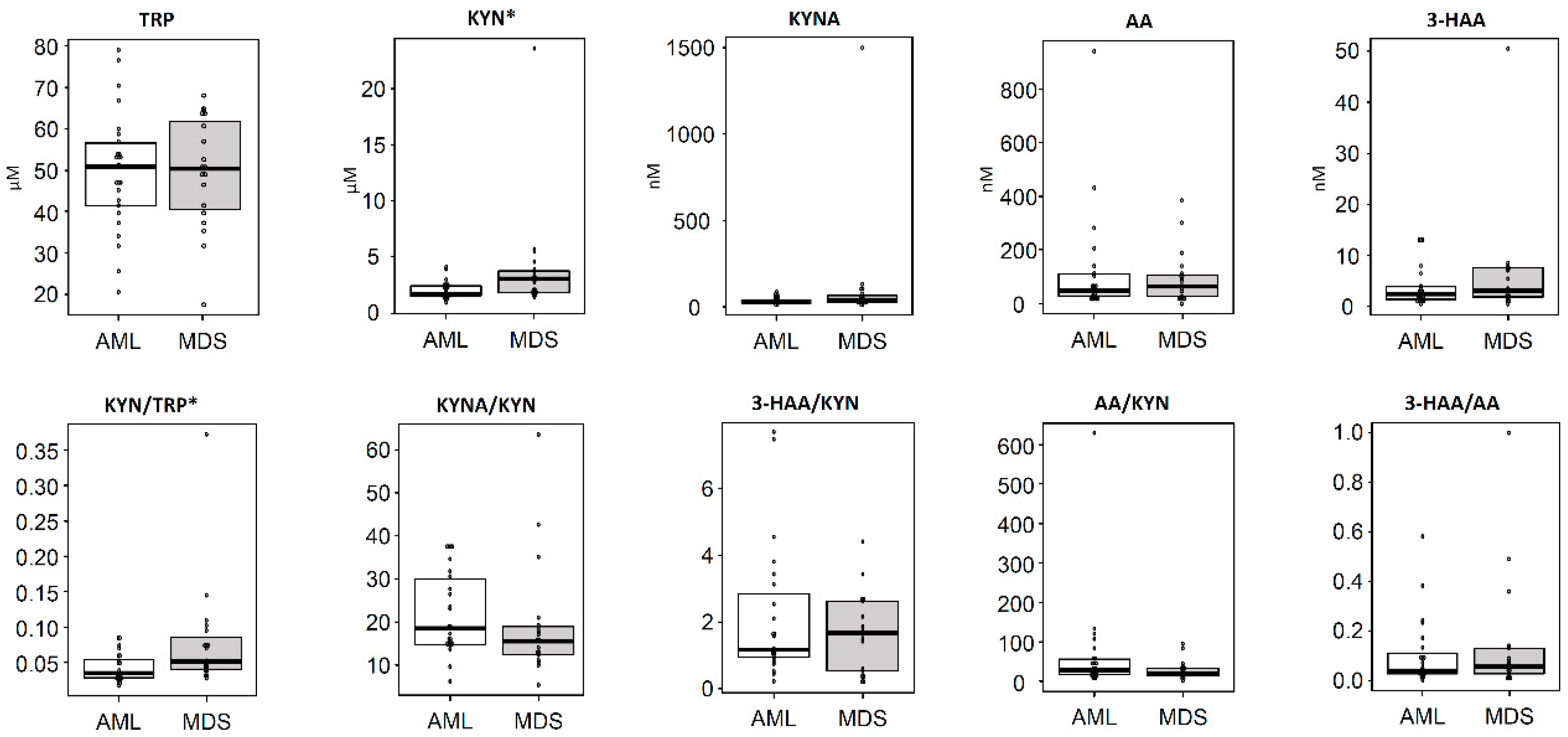
3. Results
3.1. Concentrations of Serum TRP Metabolites in Hematological Malignant Tumors
3.2. Differences in the Concentrations of TRP Metabolites According to Diagnostic Categories
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Uyttenhove, C.; Pilotte, L.; Théate, I.; Stroobant, V.; Colau, D.; Parmentier, N.; Boon, T.; Van Den Eynde, B.J. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat. Med. 2003, 9, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357, eaaf9794. [Google Scholar] [CrossRef] [PubMed]
- Munn, D.H.; Mellor, A.L. IDO in the Tumor Microenvironment: Inflammation, Counter-Regulation, and Tolerance. Trends Immunol. 2016, 37, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, G.C.; Mondal, A.; Dey, S.; Laury-Kleintop, L.D.; Muller, A.J. Inflammatory Reprogramming with IDO1 Inhibitors: Turning Immunologically Unresponsive ‘Cold’ Tumors ‘Hot’. Trends Cancer 2018, 4, 38–58. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, S.; Hara, T.; Tsurumi, H.; Hoshi, M.; Kanemura, N.; Goto, N.; Kasahara, S.; Shimizu, M.; Ito, H.; Saito, K.; et al. Indoleamine 2,3-dioxygenase in tumor tissue indicates prognosis in patients with diffuse large B-cell lymphoma treated with R-CHOP. Ann. Hematol. 2011, 90, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, S.; Hara, T.; Tsurumi, H.; Goto, N.; Saito, K.; Seishima, M.; Takami, T.; Moriwaki, H. Indoleamine 2,3-dioxygenase expression and serum kynurenine concentrations in patients with diffuse large B-cell lymphoma. Leuk. Lymphoma 2012, 53, 1143–1145. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.W.; Stoy, N.; Darlington, L.G. An expanding range of targets for kynurenine metabolites of tryptophan. Trends Pharm. Sci. 2013, 34, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Evangelisti, M.; De Rossi, P.; Rabasco, J.; Donfrancesco, R.; Lionetto, L.; Capi, M.; Sani, G.; Simmaco, M.; Nicoletti, F.; Villa, M.P. Changes in serum levels of kynurenine metabolites in paediatric patients affected by ADHD. Eur. Child Adolesc. Psychiatry 2017, 26, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, M.; Yamamoto, Y.; Kanayama, N.; Hasegawa, M.; Mouri, A.; Takemura, M.; Matsunami, H.; Miyauchi, T.; Tokura, T.; Kimura, H.; et al. Serum Metabolic Profiles of the Tryptophan-Kynurenine Pathway in the high risk subjects of major depressive disorder. Sci. Rep. 2020, 10, 1961. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Tan, G.; Wang, H.; Chen, P.; Hao, J.; Wang, Y. Identification of novel serum biomarker for the detection of acute myeloid leukemia based on liquid chromatography-mass spectrometry. J. Pharm. Biomed. Anal. 2019, 166, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Cheong, J.E.; Sun, L. Targeting the IDO1/TDO2-KYN-AhR Pathway for Cancer Immunotherapy—Challenges and Opportunities. Trends Pharmacol. Sci. 2018, 39, 307–325. [Google Scholar] [CrossRef] [PubMed]
- Muto, T.; Walker, C.S.; Choi, K.; Hueneman, K.; Smith, M.A.; Gul, Z.; Garcia-Manero, G.; Ma, A.; Zheng, Y.; Starczynowski, D.T. Adaptive response to inflammation contributes to sustained myelopoiesis and confers a competitive advantage in myelodysplastic syndrome HSCs. Nat. Immunol. 2020, 21, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Brunner, A.M.; Weng, S.; Cronin, A.; Fathi, A.T.; Habib, A.R.; Stone, R.; Graubert, T.; Steensma, D.P.; Abel, G.A. Impact of lenalidomide use among non-transfusion dependent patients with myelodysplastic syndromes. Am. J. Hematol. 2018, 93, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Komrokji, R.S.; Wei, S.; Mailloux, A.W.; Zhang, L.; Padron, E.; Sallman, D.; Lancet, J.E.; Tinsley, S.; Nardelli, L.A.; Pinilla-Ibarz, J.; et al. A Phase II Study to Determine the Safety and Efficacy of the Oral Inhibitor of Indoleamine 2,3-Dioxygenase (IDO) Enzyme INCB024360 in Patients with Myelodysplastic Syndromes. Clin. Lymphoma Myeloma Leuk. 2019, 19, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Constantinidou, A.; Alifieris, C.; Trafalis, D.T. Targeting Programmed Cell Death -1 (PD-1) and Ligand (PD-L1): A new era in cancer active immunotherapy. Pharmacol. Ther. 2019, 194, 84–106. [Google Scholar] [CrossRef] [PubMed]
- Im, A.; Pavletic, S.Z. Immunotherapy in hematologic malignancies: Past, present, and future. J. Hematol. Oncol. 2017, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Chow, V.A.; Shadman, M.; Gopal, A.K. Translating anti-CD19 CAR T-cell therapy into clinical practice for relapsed/refractory diffuse large B-cell lymphoma. Blood 2018, 132, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Brochez, L.; Chevolet, I.; Kruse, V. The rationale of indoleamine 2,3-dioxygenase inhibition for cancer therapy. Eur. J. Cancer 2017, 76, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, S.; Narala, N.; Huye, L.; Yagyu, S.; Savoldo, B.; Dotti, G.; Heslop, H.E.; Brenner, M.K.; Rooney, C.M.; Ramos, C.A. Tumor indoleamine 2,3-dioxygenase (IDO) inhibits CD19-CAR T cells and is downregulated by lymphodepleting drugs. Blood 2015, 125, 3905–3916. [Google Scholar] [CrossRef] [PubMed]
- Botticelli, A.; Cerbelli, B.; Lionetto, L.; Zizzari, I.; Salati, M.; Pisano, A.; Federica, M.; Simmaco, M.; Nuti, M.; Marchetti, P. Can IDO activity predict primary resistance to anti-PD-1 treatment in NSCLC. J. Transl. Med. 2018, 16, 219. [Google Scholar] [CrossRef] [PubMed]
| Total | n = 157 |
|---|---|
| Age | 67 (15–93) |
| males | 94 (60%) |
| Tryptophan (µM) | 45.84 (8.94 3–91.06) |
| Kynurenine (µM) | 2.00 (0.44–23.57) |
| Kynurenic acid (nM) | 32.98 (3.65–1498.91) |
| Anthranilic acid (nM) | 74.89 (0.37–943.19) |
| 3-Hydroxyanthranilic acid (nM) | 3.19 (0.33–50.48) |
| Kynurenine/Tryptophan ratio | 0.047 (0.017–0.473) |
| Kynurenic acid/Kynurenine ratio | 16.34 (1.59–374.52) |
| 3-Hydroxyanthranilic acid/Kynurenine ratio | 1.64 (0.10–14.45) |
| Anthranilic acid/Kynurenine ratio | 35.2 (0.19–687.05) |
| 3-Hydroxyanthranilic acid/Anthranilic acid ratio | 0.041 (0.001–2.277) |
| Gender | Age | p | ||||
|---|---|---|---|---|---|---|
| males (n = 94) | females (n = 63) | p | <60 (n = 42) | ≧60 (n = 115) | ||
| TRP (µM) | 47.46 (15.63–88.62) | 41.43 (8.94–91.06) | <0.001 | 47.49 (20.61–91.06) | 45.83 (8.94–88.62) | 0.679 |
| KYN (µM) | 2.10 (0.71–23.57) | 1.82 (0.44–10.34) | 0.083 | 1.71 (0.44–4.84) | 2.11 (0.60–23.57) | 0.025 |
| KYNA (nM) | 34.37 (3.65–1498.91) | 32.25 (13.36–278.09) | 0.368 | 29.58 (11.05–272.58) | 35.84 (3.65–1498.91) | 0.034 |
| AA (nM) | 78.04 (5.90–943.19) | 70.12 (0.37–583.99) | 0.933 | 64.15 (0.37–391.07) | 76.92 (12.16–943.19) | 0.237 |
| 3-HAA (nM) | 3.29 (0.27–50.48) | 2.78 (0.33–20.95) | 0.226 | 2.58 (0.33–13.43) | 3.49 (0.27–50.48) | 0.003 |
| KYN/TRP | 0.04 (0.02–0.47) | 0.05 (0.02–0.35) | 0.727 | 0.04 (0.02–0.16) | 0.05 (0.02–0.47) | 0.011 |
| KYNA/KYN | 15.77 (1.59–374.52) | 16.61 (6.59–110.33) | 0.404 | 16.12 (5.42–304.55) | 16.48 (1.59–374.52) | 0.736 |
| 3-HAA/KYN | 1.72 (0.19–14.45) | 1.53 (0.10–8.19) | 0.658 | 1.50 (0.10–9.44) | 1.72 (0.10–14.45) | 0.078 |
| AA/KYN | 32.13 (4.14–628.80) | 41.84 (0.19–687.05) | 0.220 | 43.81 (0.19–269.59) | 33.02 (4.60–687.05) | 0.687 |
| 3-HAA/AA | 0.049 (0.001–2.277) | 0.034 (0.002–1.000) | 0.300 | 0.028 (0.002–2.277) | 0.049 (0.001–1.059) | 0.187 |
| Diagnosis | AML (n = 25) | MDS (n = 19) | MM (n = 24) | DLBCL (n = 65) | FL (n = 24) | p |
|---|---|---|---|---|---|---|
| Age | 63 (17–83) | 72 (15–89) | 67 (45–87) | 70 (27 93) | 63 (49–83) | 0.064 * |
| Males | 16 (64%) | 14 (74%) | 11 (46%) | 41 (63%) | 11 (46%) | 0.30 ** |
| Tryptophan (µM) | 50.9 (20.61–79.07) | 50.34 (17.51–67.81) | 37.95 (8.94–63.26) | 42.96 (15.63–88.62) | 46.06 (22.02–91.06) | 0.012 * |
| Kynurenine (µM) | 1.68 (0.84–4.09) | 3.03 (1.36–23.57) | 1.44 (0.85–11.53) | 2.12 (0.60–8.24) | 2.26 (0.44–2.91) | <0.01* |
| Kynurenic acid (nM) | 35.3 (14.38–86.52) | 39.06 (15.21–1498.91) | 26.22 (4.00–278.09) | 32.87 (12.72–344.56) | 32.29 (3.65–53.83) | 0.17 * |
| Anthranilic acid (nM) | 46.78 (14.85–943.19) | 63.23 (0.37–385.80) | 59.97 (18.99–583.99) | 95.72 (5.90–639.49) | 47.53 (11.90–391.07) | 0.52 * |
| 3-Hydroxyanthranilic acid (nM) | 2.38 (0.41–13.07) | 3.04 (0.37–50.48) | 2.18 (0.27–10.59) | 3.44 (0.33–23.34) | 4.36 (0.61–24.99) | 0.021 * |
| KYN/TRP | 0.03 (0.02–0.09) | 0.05 (0.03–0.37) | 0.04 (0.02–0.35) | 0.05 (0.02–0.47) | 0.04 (0.02–0.08) | 0.052 * |
| KYNA/KYN | 18.62 (5.97–37.49) | 15.57 (5.42–63.58) | 16.40 (3.63–304.55) | 16.24 (5.00–374.52) | 16.35 (1.59–110.33) | 0.43 * |
| 3-HAA/KYN | 1.17 (0.26–7.67) | 1.68 (0.19–4.39) | 1.32 (0.25–5.15) | 1.72 (0.10–14.45) | 1.92 (0.26–11.04) | 0.39 * |
| AA/KYN | 27.91 (9.20–628.80) | 20.66 (0.19–94.30) | 35.09 (7.17–687.05) | 46.51 (4.14–255.54) | 29.33 (5.01–1500.85) | 0.013 * |
| 3-HAA/AA | 0.042 (0.001–0.577) | 0.057 (0.007–1.000) | 0.037 (0.004–0.495) | 0.036 (0.002–2.277) | 0.082 (0.007–1.059) | 0.54 * |
| Diagnosis | AML (n = 25) | MDS (n = 19) | p |
|---|---|---|---|
| Age | 59 (17–83) | 67 (15–89) | 0.144 |
| Males | 16 (64%) | 14 (74%) | 0.53 |
| Total bilirubin (mg/dL) | 0.7 (0.1–1.3) | 0.9 (0.3–2.8) | 0.33 |
| Aspartate transaminase (IU/L) | 23 (11–55) | 23 (6–49) | 0.983 |
| Alanine aminotransferase (IU/L) | 22 (6–49) | 20 (5–66) | 0.684 |
| Lactate dehydrogenase (U/L) | 300 (131–797) | 281 (122–517) | 0.715 |
| Creatinine (mg/dL) | 0.81 (1.57–0.40) | 1.15 (0.44–5.50) | 0.155 |
| Blood urea nitrogen (mg/dL) | 16.5 (6.6–37.2) | 15.9 (5.2–25.4) | 0.769 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamaguchi, K.; Ninomiya, S.; Matsumoto, T.; Nakamura, N.; Nakamura, H.; Kitagawa, J.; Kanemura, N.; Hara, T.; Fujigaki, S.; Yamamoto, Y.; et al. Indoleamine 2,3-Dioxygenase Activity Is Increased in Myelodysplastic Syndrome Patients. Hemato 2020, 1, 77-85. https://doi.org/10.3390/hemato1020011
Yamaguchi K, Ninomiya S, Matsumoto T, Nakamura N, Nakamura H, Kitagawa J, Kanemura N, Hara T, Fujigaki S, Yamamoto Y, et al. Indoleamine 2,3-Dioxygenase Activity Is Increased in Myelodysplastic Syndrome Patients. Hemato. 2020; 1(2):77-85. https://doi.org/10.3390/hemato1020011
Chicago/Turabian StyleYamaguchi, Kimihiro, Soranobu Ninomiya, Takuro Matsumoto, Nobuhiko Nakamura, Hiroshi Nakamura, Junichi Kitagawa, Nobuhiro Kanemura, Takeshi Hara, Suwako Fujigaki, Yasuko Yamamoto, and et al. 2020. "Indoleamine 2,3-Dioxygenase Activity Is Increased in Myelodysplastic Syndrome Patients" Hemato 1, no. 2: 77-85. https://doi.org/10.3390/hemato1020011
APA StyleYamaguchi, K., Ninomiya, S., Matsumoto, T., Nakamura, N., Nakamura, H., Kitagawa, J., Kanemura, N., Hara, T., Fujigaki, S., Yamamoto, Y., Saito, K., Tsurumi, H., & Shimizu, M. (2020). Indoleamine 2,3-Dioxygenase Activity Is Increased in Myelodysplastic Syndrome Patients. Hemato, 1(2), 77-85. https://doi.org/10.3390/hemato1020011






