Valorization of Second Cheese Whey Through Microalgae-Based Treatments: Advantages, Limits, and Opportunities
Abstract
1. Introduction
2. Characteristics and Classification of Dairy Wastewaters
3. Second Cheese Whey (SCW): Volumes, Challenges, and Opportunities
3.1. Volumes and Geographical Distribution of SCW
3.2. Environmental Impact of SCW
3.3. Industrial Applications of SCW
4. Microalgae for SCW Valorization: Concepts, Technologies, and Metabolisms
4.1. Definitions and Applications
4.2. Microalgal Cultivation Systems
4.3. Microalgal Photoautotrophy, Heterotrophy, and Mixotrophy
5. Microalgal Valorization of SCW: Case Studies and Regulatory Insights
5.1. Valorization of Dairy Wastewater (DWW) Through Microalgal Cultivation
5.2. Microalgal Cultivation on SCW: Current Studies
5.3. Microalgal Biomass from Treated SCW: Efficiency and Regulation
5.4. Economic Feasibility and LCA of SCW Valorization
6. Future Prospects
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | anaerobic digestion |
| AWW | agro-industrial wastewaters |
| BOD | Biochemical oxygen demand |
| CIP | cleaning-in-place |
| C-PC | C-phycocyanin |
| COD | chemical oxygen demand |
| CW | cheese whey |
| DHA | docosahexaenoic acid |
| DW | dry weight |
| DWW | dairy wastewater |
| EPA | eicosapentaenoic acid |
| EPS | extracellular polymeric substance |
| HRAP | high rate algal ponds |
| GHG | Greenhouse Gas |
| LCA | life cycle assessment |
| PBR | photobioreactors |
| PE | person equivalent |
| PHAs | polyhydroxyalkanoates |
| PUFAs | polyunsaturated fatty acids |
| SWP | second whey protein |
| SCW | second cheese whey |
| TSSs | total suspended solids |
| WPC | whey protein concentrate |
References
- Prazeres, A.R.; Carvalho, F.; Rivas, J. Cheese whey management: A review. J. Environ. Manag. 2012, 110, 48–68. [Google Scholar] [CrossRef] [PubMed]
- Smithers, G.W. Whey and whey proteins—From ‘gutter-to-gold’. Int. Dairy J. 2008, 18, 695–704. [Google Scholar] [CrossRef]
- Pires, A.F.; Marnotes, N.G.; Rubio, O.D.; Garcia, A.C.; Pereira, C.D. Dairy by-products: A review on the valorization of whey and second cheese whey. Foods 2021, 10, 1067. [Google Scholar] [CrossRef] [PubMed]
- Occhipinti, P.S.; Russo, N.; Foti, P.; Zingale, I.M.; Pino, A.; Romeo, F.V.; Randazzo, C.L.; Caggia, C. Current challenges of microalgae applications: Exploiting the potential of non-conventional microalgae species. J. Sci. Food Agric. 2024, 104, 823–3833. [Google Scholar] [CrossRef]
- Ryan, M.P.; Walsh, G. The biotechnological potential of whey. Rev. Environ. Sci. Biotechnol. 2016, 15, 479–498. [Google Scholar] [CrossRef]
- La Bella, E.; Occhipinti, P.S.; Puglisi, I.; Fragalà, F.; Saccone, R.; Russo, N.; Randazzo, C.L.; Caggia, C.; Baglieri, A. Comparative phycomeremediation performance of three microalgae species in two different magnitude of pollutants in wastewater from farm-house. Sustainability 2023, 15, 11644. [Google Scholar] [CrossRef]
- Vincenzi, A.; Maciel, M.J.; Burlani, E.L.; Oliveira, E.C.; Volpato, G.; Lehn, D.N.; Souza, C.D. Ethanol bio-production from ricotta cheese whey by several strains of the yeast Kluyveromyces. Am. J. Food Technol. 2014, 9, 281–291. [Google Scholar] [CrossRef]
- Uribe-Velázquez, T.; Díaz-Vázquez, D.; Barajas-Álvarez, P.; González-López, M.E.; Gradilla-Hernández, M.S.; Garcia-Amezquita, L.E.; Carrillo-Nieves, D.; García-Cayuela, T. From waste to value: Mitigating the environmental impact of whey in Jalisco, Mexico. J. Clean. Prod. 2025, 501, 145334. [Google Scholar] [CrossRef]
- Bintsis, T.; Papademas, P. Sustainable approaches in whey cheese production: A review. Dairy 2023, 4, 249–270. [Google Scholar] [CrossRef]
- Pereira, C.; Henriques, M.; Gomes, D.; Gomez-Zavaglia, A.; de Antoni, G. Novel functional whey-based drinks with great potential in the dairy industry. Food Technol. Biotechnol. 2015, 53, 307–314. [Google Scholar] [CrossRef]
- Kotoulas, A.; Agathou, D.; Triantaphyllidou, I.E.; Tatoulis, T.I.; Akratos, C.S.; Tekerlekopoulou, A.G.; Vayenas, D.V. Second cheese whey treatment using zeolite under continuous flow mode and its application on wheat growth. Water 2019, 11, 928. [Google Scholar] [CrossRef]
- Piskorz, A.; Pires, A.; Marnotes, N.G.; Gomes, D.; Henriques, M.; Pereira, C.D. Valorização do sorelho para a produção de molhos para saladas e de bebidas lácteas fermentadas—Parte 1. Tecnoalimentar 2019, 26, 26–29. [Google Scholar]
- Cassano, A.; Conidi, C.; Castro-Muñoz, R. Current and future applications of nanofiltration in food processing. In Separation of Functional Molecules in Food by Membrane Technology; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 305–348. [Google Scholar]
- Macedo, A.; Duarte, E.; Pinho, M. The role of concentration polarization in ultrafiltration of ovine cheese whey. J. Membr. Sci. 2011, 381, 34–40. [Google Scholar] [CrossRef]
- Macedo, A.; Duarte, E.; Fragoso, R. Assessment of the performance of three ultrafiltration membranes for fractionation of ovine second cheese whey. Int. Dairy J. 2015, 48, 31–37. [Google Scholar] [CrossRef]
- Pereira, C.D.; Diaz, O.; Cobos, A. Valorization of by-products from ovine cheese manufacture: Clarification by thermocalcic precipitation/microfiltration before ultrafiltration. Int. Dairy J. 2002, 12, 773–783. [Google Scholar] [CrossRef]
- Secchi, N.; Giunta, D.; Pretti, L.; García, M.R.; Roggio, T.; Mannazzu, I.; Catzeddu, P. Bioconversion of ovine scotta into lactic acid with pure and mixed cultures of lactic acid bacteria. J. Ind. Microbiol. Biotechnol. 2012, 39, 175–181. [Google Scholar] [CrossRef]
- Sommella, E.; Pepe, G.; Ventre, G.; Pagano, F.; Conte, G.M.; Ostacolo, C.; Campiglia, P. Detailed peptide profiling of “Scotta”: From a dairy waste to a source of potential health-promoting compounds. Dairy Sci. Technol. 2016, 96, 763–771. [Google Scholar] [CrossRef]
- Minhalma, M.; Magueijo, V.; Queiroz, D.P.; de Pinho, M.N. Optimization of “Serpa” cheese whey nanofiltration for effluent minimization and by-products recovery. J. Environ. Manag. 2007, 82, 200–206. [Google Scholar] [CrossRef]
- Zoppellari, F.; Bardi, L. Production of bioethanol from effluents of the dairy industry by Kluyveromyces marxianus. New Biotechnol. 2013, 30, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Rajeshwari, K.V.; Balakrishnan, M.; Kansal, A.; Lata, K.; Kishore, V.V.N. State-of-the-art of anaerobic digestion technology for industrial wastewater treatment. Renew. Sustain. Energy Rev. 2000, 4, 135–156. [Google Scholar] [CrossRef]
- Fancello, F.; Zara, G.; Hatami, F.; Scano, E.A.; Mannazzu, I. Unlocking the potential of second cheese whey: A comprehensive review on valorisation strategies. Rev. Environ. Sci. Biotechnol. 2024, 23, 411–441. [Google Scholar] [CrossRef]
- Martinez-Burgos, W.J.; Sydney, E.B.; Medeiros, A.B.P.; Magalhães, A.I.; de Carvalho, J.C.; Karp, S.G.; Soccol, C.R. Agro-industrial wastewater in a circular economy: Characteristics, impacts and applications for bioenergy and biochemicals. Bioresour. Technol. 2021, 341, 125795. [Google Scholar] [CrossRef]
- Tridge. Global Cheese Export Market Data. Available online: https://www.tridge.com (accessed on 12 July 2025).
- Estikomah, S.A.; Susilowati, A.; Masykuri, M. Cheese Whey Wastewater: Characterization and Value. KnE Soc. Sci. 2023, 8, 465–474. [Google Scholar] [CrossRef]
- De Almeida, M.P.G.; Mockaitis, G.; Weissbrodt, D.G. Got whey? Sustainability endpoints for the dairy industry through resource biorecovery. Fermentation 2023, 9, 897. [Google Scholar] [CrossRef]
- Casallas-Ojeda, M.; Cabeza, I.; Sanchez, N.; Caicedo-Concha, D.M.; Astals, S. Cheese whey and dairy manure anaerobic co-digestion at psychrophilic conditions: Technical and environmental evaluation. Environ. Res. 2024, 251, 118525. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, N.; Forleo, M.B.; Salimei, E. Environmental impacts of a dairy cheese chain including whey feeding: An Italian case study. J. Clean. Prod. 2017, 140, 881–889. [Google Scholar] [CrossRef]
- Hogg, D. Costs for Municipal Waste Management in the EU; Final Report to Directorate General Environment; European Commission, Eunomia Research & Consulting Ltd.: Bristol, UK, 2002. [Google Scholar]
- Bacenetti, J.; Bava, L.; Schievano, A.; Zucali, M. Whey protein concentrate (WPC) production: Environmental impact assessment. J. Food Eng. 2018, 224, 139–147. [Google Scholar] [CrossRef]
- Kim, D.; Thoma, G.; Nutter, D.; Milani, F.; Ulrich, R.; Norris, G. Life cycle assessment of cheese and whey production in the USA. Int. J. Life Cycle Assess. 2013, 18, 1019–1035. [Google Scholar] [CrossRef]
- Żyłka, R.; Karolinczak, B.; Dąbrowski, W. Structure and indicators of electric energy consumption in dairy wastewater treatment plant. Sci. Total Environ. 2021, 782, 146599. [Google Scholar] [CrossRef]
- Petersen, S.O.; Blanchard, M.; Chadwick, D.; Del Prado, A.; Edouard, N.; Mosquera, J.; Sommer, S.G. Manure management for greenhouse gas mitigation. Animal 2013, 7, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Alnajdi, S.; Beni, A.N.; Alsaati, A.A.; Luhar, M.; Childress, A.E.; Warsinger, D.M. Practical minimum energy use of seawater reverse osmosis. Joule 2024, 8, 3088–3105. [Google Scholar] [CrossRef]
- García-Casas, V.E.; Seiquer, I.; Pardo, Z.; Haro, A.; Recio, I.; Olías, R. Antioxidant potential of the sweet whey-based beverage colada after the digestive process and relationships with the lipid and protein fractions. Antioxidants 2022, 11, 1827. [Google Scholar] [CrossRef] [PubMed]
- Pescuma, M.; Hébert, E.M.; Mozzi, F.; De Valdez, G.F. Functional fermented whey-based beverage using lactic acid bacteria. Int. J. Food Microbiol. 2010, 141, 73–81. [Google Scholar] [CrossRef]
- Joshi, J.; Gururani, P.; Vishnoi, S.; Srivastava, A. Whey based beverages: A review. Octa J. Biosci. 2020, 8, 30–37. [Google Scholar]
- El-Aidie, S.A.; Khalifa, G.S. Innovative applications of whey protein for sustainable dairy industry: Environmental and technological perspectives—A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13319. [Google Scholar] [CrossRef]
- Optimum Nutrition. Gold Standard 100% Whey—Product Information. Available online: https://www.optimumnutrition.com (accessed on 16 July 2025).
- MyProtein. Impact Whey Protein—Official Product Information. 2025. Available online: https://www.myprotein.com (accessed on 16 July 2025).
- Foods Ingredients. Arla Foods Ingredients Targets Acid Whey Elimination with High-Yield Solution. Available online: https://www.arlafoodsingredients.com/about/press-releases/arla-foods-ingredients-targets-acid-whey-elimination-with-high-yield-solution (accessed on 16 July 2025).
- Glanbia Nutritionals. Lactose Production for Infant Formula and Food Applications. Available online: https://www.glanbianutritionals.com/en (accessed on 16 July 2025).
- Addai, F.P.; Lin, F.; Wang, T.; Kosiba, A.A.; Sheng, P.; Yu, F.; Gu, J.; Zhou, Y.; Shi, H. Technical integrative approaches to cheese whey valorization towards sustainable environment. Food Funct. 2020, 11, 8407–8423. [Google Scholar] [CrossRef]
- Tesfaw, A. The current trends of bioethanol production from cheese whey using yeasts: Biological and economical perspectives. Front. Energy Res. 2023, 11, 1183035. [Google Scholar] [CrossRef]
- Dragone, G.; Mussatto, S.I.; Almeida e Silva, J.B.; Teixeira, J.A. Optimal fermentation conditions for maximizing the ethanol production by Kluyveromyces fragilis from cheese whey powder. Biomass Bioenergy 2011, 35, 1977–1982. [Google Scholar] [CrossRef]
- Asunis, F.; De Gioannis, G.; Francini, G.; Lombardi, L.; Muntoni, A.; Polettini, A.; Spiga, D. Environmental life cycle assessment of polyhydroxyalkanoates production from cheese whey. J. Waste Manag. 2021, 132, 31–43. [Google Scholar] [CrossRef]
- Sampaio, A.; Silva, V.; Poeta, P.; Aonofriesei, F. Vibrio spp.: Life strategies, ecology, and risks in a changing environment. Diversity 2022, 14, 97. [Google Scholar] [CrossRef]
- Zhou, X.; Hua, X.; Huang, L.; Xu, Y. Bio-utilization of cheese manufacturing wastes (cheese whey powder) for bioethanol and specific product (galactonic acid) production via a two-step bioprocess. Bioresour. Technol. 2019, 272, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Rawat, I.; Kumar, R.R.; Mutanda, T.; Bux, F. Dual role of microalgae: Phycoremediation of domestic wastewater and biomass production for sustainable biofuels production. Appl. Energy 2011, 88, 3411–3424. [Google Scholar] [CrossRef]
- Choudhary, S.; Tripathi, S.; Poluri, K.M. Microalgal-based bioenergy: Strategies, prospects, and sustainability. Energy Fuels 2022, 36, 14584–14612. [Google Scholar] [CrossRef]
- Bleakley, S.; Hayes, M. Algal proteins: Extraction, application, and challenges concerning production. Foods 2017, 6, 33. [Google Scholar] [CrossRef]
- Boukid, F.; Castellari, M. Algae as nutritional and functional food sources. Foods 2022, 12, 122. [Google Scholar] [CrossRef]
- Jaiswal, K.K.; Dutta, S.; Banerjee, I.; Pohrmen, C.B.; Kumar, V. Photosynthetic microalgae–based carbon sequestration and generation of biomass in biorefinery approach for renewable biofuels for a cleaner environment. Biomass Convers. Bior. 2023, 13, 7403–7421. [Google Scholar] [CrossRef]
- Gupta, S.; Marchetti, J.M. Co-cultivation of high-value microalgae species with filamentous microalgae for dairy wastewater treatment. npj Clean Water 2024, 7, 119. [Google Scholar] [CrossRef]
- Occhipinti, P.S.; Del Signore, F.; Canziani, S.; Caggia, C.; Mezzanotte, V.; Ferrer-Ledo, N. Mixotrophic and heterotrophic growth of Galdieria sulphuraria using buttermilk as a carbon source. J. Appl. Phycol. 2023, 35, 2631–2643. [Google Scholar] [CrossRef]
- Usman, M.; Amin, M.; Kamal, I.; Shahid, A.; Xu, J.; Alam, M.A.; Boopathy, R. Algae-mediated resource recovery from urban wastewater. Curr. Pollut. Rep. 2023, 9, 243–258. [Google Scholar] [CrossRef]
- Amorim, M.L.; Soares, J.; Vieira, B.B.; de Oliveira Leite, M.; Rocha, D.N.; Aleixo, P.E.; Falconí, J.H.H.; Xavier Júnior, M.d.L.; Albino, L.F.T.; Martins, M.A. Pilot-scale biorefining of Scenedesmus obliquus for the production of lipids and proteins. Sep. Purif. Technol. 2021, 270, 118775. [Google Scholar] [CrossRef]
- Jagadevan, S.; Banerjee, A.; Banerjee, C.; Guria, C.; Tiwari, R.; Baweja, M.; Shukla, P. Recent developments in synthetic biology and metabolic engineering in microalgae towards biofuel production. Biotechnol. Biofuels 2018, 11, 185. [Google Scholar] [CrossRef] [PubMed]
- Borowitzka, M.A. High-value products from microalgae—Their development and commercialisation. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Hasnain, M.; Abideen, Z.; Hashmi, S.; Naz, S.; Munir, N. Assessing the potential of nutrient deficiency for enhancement of biodiesel production in algal resources. Biofuels 2023, 14, 1–34. [Google Scholar] [CrossRef]
- Kichouh-Aiadi, S.; López-Rosales, L.; Gallardo-Rodríguez, J.J.; Cerón-García, M.C.; Sánchez-Mirón, A.; García-Camacho, F. Effects of hormones on the growth and metabolite production of Amphidinium carterae under carbon sufficient and carbon limited conditions. Algal Res. 2025, 85, 103810. [Google Scholar] [CrossRef]
- Kichouh-Aiadi, S.; Gallardo-Rodríguez, J.J.; Cerón-García, M.C.; López-Rosales, L.; Sánchez-Mirón, A.; García-Camacho, F. Selecting phytohormone treatments through Grey Relational and Principal Component Analysis for microalgal bioactives production. Algal Res. 2025, 90, 104152. [Google Scholar] [CrossRef]
- Udayan, A.; Sirohi, R.; Sreekumar, N.; Sang, B.I.; Sim, S.J. Mass cultivation and harvesting of microalgal biomass: Current trends and future perspectives. Bioresour. Technol. 2022, 344, 126406. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.S.; Lee, S.Y.; Chew, K.W.; Lam, M.K.; Lim, J.W.; Ho, S.H.; Show, P.L. A review on microalgae cultivation and harvesting, and their biomass extraction processing using ionic liquids. Bioengineered 2020, 11, 116–129. [Google Scholar] [CrossRef]
- Stark, M.; O’Gara, I. An introduction to photosynthetic microalgae. Disruptive Sci. Technol. 2012, 1, 65–67. [Google Scholar] [CrossRef]
- Ugwu, U.; Aoyagi, H. Microalgal culture systems: An insight into their designs, operation and applications. Biotechnology 2012, 11, 127–132. [Google Scholar] [CrossRef]
- Rogers, J.N.; Rosenberg, J.N.; Guzman, B.J.; Oh, V.H.; Mimbela, L.E.; Ghassemi, A.; Betenbaugh, M.J.; Oyler, G.A.; Donohue, M.D. A critical analysis of paddlewheel-driven raceway ponds for algal biofuel production at commercial scales. Algal Res. 2014, 4, 76–88. [Google Scholar] [CrossRef]
- White, R.; Ryan, R. Long-term cultivation of algae in open-raceway ponds: Lessons from the field. Ind. Biotechnol. 2015, 11, 213–220. [Google Scholar] [CrossRef]
- Posten, C. Design principles of photo-bioreactors for cultivation of microalgae. Eng. Life Sci. 2009, 9, 165–177. [Google Scholar] [CrossRef]
- Mishra, A.K.; Kaushik, M.S.; Tiwari, D. Nitrogenase and hydrogenase: Enzymes for nitrogen fixation and hydrogen production in cyanobacteria. In Cyanobacteria: From Basic Science to Applications; Mishra, A.K., Tiwari, D.N., Rai, A.N., Eds.; Academic Press; Elsevier: Cambridge, MA, USA, 2019; pp. 173–191. [Google Scholar]
- Torzillo, G.; Chini Zittelli, G. Tubular photobioreactors. In Algal Biorefineries; Prokop, A., Bajpai, R., Zappi, M., Eds.; Springer: Cham, Switzerland, 2015; pp. 187–212. [Google Scholar]
- Mohan, S.V.; Rohit, M.; Subhash, G.V.; Chandra, R.; Devi, M.P.; Butti, S.K.; Rajesh, K. Algal oils as biodiesel. In Biofuels from Algae, 2nd ed.; Pandey, A., Chang, J.S., Soccol, C.R., Lee, D.J., Chisti, Y., Eds.; Elsevier: Cambridge, MA, USA, 2019; pp. 287–323. [Google Scholar]
- Yan, C.; Zhang, Q.; Xue, S.; Sun, Z.; Wu, X.; Wang, Z.; Lu, Y.; Cong, W. A novel low-cost thin-film flat plate photobioreactor for microalgae cultivation. Biotechnol. Bioprocess Eng. 2016, 21, 103–109. [Google Scholar] [CrossRef]
- Vo, H.N.P.; Ngo, H.H.; Guo, W.; Nguyen, T.M.H.; Liu, Y.; Liu, Y.; Nguyen, D.D.; Chang, S.W. A critical review on designs and applications of microalgae-based photobioreactors for pollutants treatment. Sci. Total Environ. 2019, 651, 1549–1568. [Google Scholar] [CrossRef] [PubMed]
- Do, S.; Du, Z.Y. Exploring the impact of environmental conditions and bioreactors on microalgae growth and applications. Energies 2024, 17, 5218. [Google Scholar] [CrossRef]
- Hamid Nour, A.; Mokaizh, A.A.B.; Alazaiza, M.Y.; Bashir, M.J.; Mustafa, S.E.; Baarimah, A.O. Innovative strategies for microalgae-based bioproduct extraction in biorefineries: Current trends and future solutions integrating wastewater treatment. Sustainability 2024, 16, 10565. [Google Scholar] [CrossRef]
- Mehariya, S.; Goswami, R.K.; Verma, P.; Lavecchia, R.; Zuorro, A. Integrated approach for wastewater treatment and biofuel production in microalgae biorefineries. Energies 2021, 14, 2282. [Google Scholar] [CrossRef]
- Xu, H.; Miao, X.; Wu, Q. High quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters. J. Biotechnol. 2006, 126, 499–507. [Google Scholar] [CrossRef]
- Liang, Z.C.; Liang, M.H.; Jiang, J.G. Transgenic microalgae as bioreactors. Crit. Rev. Food Sci. Nutr. 2020, 60, 3195–3213. [Google Scholar] [CrossRef]
- Das, S.; Nath, K.; Chowdhury, R. Comparative studies on biomass productivity and lipid content of a novel blue-green algae during autotrophic and heterotrophic growth. Environ. Sci. Pollut. Res. 2021, 28, 12107–12118. [Google Scholar] [CrossRef]
- Abreu, A.P.; Fernandes, B.; Vicente, A.A.; Teixeira, J.; Dragone, G. Mixotrophic cultivation of Chlorella vulgaris using industrial dairy waste as organic carbon source. Bioresour. Technol. 2012, 118, 61–66. [Google Scholar] [CrossRef]
- Li, T.; Xu, J.; Gao, B.; Xiang, W.; Li, A.; Zhang, C. Morphology, growth, biochemical composition and photosynthetic performance of Chlorella vulgaris (Trebouxiophyceae) under low and high nitrogen supplies. Algal Res. 2016, 16, 481–491. [Google Scholar] [CrossRef]
- Castillo, T.; Ramos, D.; García-Beltrán, T.; Brito-Bazan, M.; Galindo, E. Mixotrophic cultivation of microalgae: An alternative to produce high-value metabolites. Biochem. Eng. J. 2021, 176, 108183. [Google Scholar] [CrossRef]
- Chen, C.Y.; Yeh, K.L.; Aisyah, R.; Lee, D.J.; Chang, J.S. Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: A critical review. Bioresour. Technol. 2011, 102, 71–81. [Google Scholar] [CrossRef]
- Wu, G.; Chong, J.W.R.; Khoo, K.S.; Tang, D.Y.Y.; Show, P.L. Upcycling food waste for microalgae cultivation toward lipid production in a closed-loop and system-integrated circular bioeconomy. Biotechnol. Biofuels Bioprod. 2025, 18, 74. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.K.; Joun, J.; Sim, S.J. A sustainable mixotrophic microalgae cultivation from dairy wastes for carbon credit, bioremediation and lucrative biofuels. Bioresour. Technol. 2020, 313, 123681. [Google Scholar] [CrossRef]
- Sarıtaş, S.; Kalkan, A.E.; Yılmaz, K.; Gurdal, S.; Göksan, T.; Witkowska, A.M.; Karav, S. Biological and nutritional applications of microalgae. Nutrients 2024, 17, 93. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Yadav, P.; Kumar, I.; Solanki, M.K.; Roychowdhury, R.; Kumar, A.; Gupta, R.K. Advancement of abiotic stresses for microalgal lipid production and its bioprospecting into sustainable biofuels. Sustainability 2023, 15, 13678. [Google Scholar] [CrossRef]
- Yu, Q.; Chen, X.; Ai, S.; Wang, X.; He, J.; Gao, Z.; Huang, F. Comprehensive transcriptomic and metabolomic insights into simultaneous CO2 sequestration and nitrate removal by the Chlorella vulgaris and Pseudomonas sp. consortium. Environ. Res. 2024, 259, 119540. [Google Scholar] [CrossRef]
- Acién, F.G.; Fernández, J.M.; Magán, J.J.; Molina, E. Production cost of a real microalgae production plant and strategies to reduce it. Biotechnol. Adv. 2012, 30, 1344–1353. [Google Scholar] [CrossRef]
- Silva, S.C.; Dias, M.M.; Barreiro, M.F. What is next in microalgae research. In Microalgae-Based Systems: Process Integration and Process Intensification Approaches; Jacob-Lopes, E., Rodrigues Dias, R., Queiroz Zepka, L., Eds.; De Gruyter: Berlin, Germany; Boston, MA, USA, 2023; Chapter 81. [Google Scholar]
- Ding, J.; Zhao, F.; Cao, Y.; Xing, L.; Liu, W.; Mei, S.; Li, S. Cultivation of microalgae in dairy farm wastewater without sterilization. Int. J. Phytoremediat. 2015, 17, 222–227. [Google Scholar] [CrossRef]
- Zimermann, J.D.A.F.; Sydney, E.B.; Cerri, M.L.; de Carvalho, I.K.; Schafranski, K.; Sydney, A.C.N.; Demiate, I.M. Growth kinetics, phenolic compounds profile and pigments analysis of Galdieria sulphuraria cultivated in whey permeate in shake-flasks and stirred-tank bioreactor. J. Water Process. Eng. 2020, 38, 101598. [Google Scholar] [CrossRef]
- Daneshvar, E.; Zarrinmehr, M.J.; Koutra, E.; Kornaros, M.; Farhadian, O.; Bhatnagar, A. Sequential cultivation of microalgae in raw and recycled dairy wastewater: Microalgal growth, wastewater treatment and biochemical composition. Bioresour. Technol. 2019, 273, 556–564. [Google Scholar] [CrossRef]
- Zulekha, R.; Mubashar, M.; Sultan, M.M.; Wang, Z.; Li, J.; Zhang, X. An assessment of the autotrophic/heterotrophic synergism in microalgae under mixotrophic mode and its contribution in high-rate phosphate recovery from wastewater. Bioresour. Technol. 2024, 413, 131450. [Google Scholar] [CrossRef] [PubMed]
- Kusmayadi, A.; Lu, P.H.; Huang, C.Y.; Leong, Y.K.; Yen, H.W.; Chang, J.S. Integrating anaerobic digestion and microalgae cultivation for dairy wastewater treatment and potential biochemicals production from the harvested microalgal biomass. Chemosphere 2022, 291, 133057. [Google Scholar] [CrossRef]
- Brar, A.; Kumar, M.; Pareek, N. Comparative appraisal of biomass production, remediation, and bioenergy generation potential of microalgae in dairy wastewater. Front. Microbiol. 2019, 10, 678. [Google Scholar] [CrossRef]
- Choi, H.J. Dairy wastewater treatment using microalgae for potential biodiesel application. Environ. Eng. Res. 2016, 21, 393–400. [Google Scholar] [CrossRef]
- Costa, J.A.V.; Cruz, C.G.; da Rosa, A.P.C. Insights into the technology utilized to cultivate microalgae in dairy effluents. Biocatal. Agric. Biotechnol. 2021, 35, 102106. [Google Scholar] [CrossRef]
- Pang, N.; Gu, X.; Chen, S.; Kirchhoff, H.; Lei, H.; Roje, S. Exploiting mixotrophy for improving productivities of biomass and co-products of microalgae. Renew. Sustain. Energy Rev. 2019, 112, 450–460. [Google Scholar] [CrossRef]
- Sloth, J.K.; Jensen, H.C.; Pleissner, D.; Eriksen, N.T. Growth and phycocyanin synthesis in the heterotrophic microalga Galdieria sulphuraria on substrates made of food waste from restaurants and bakeries. Bioresour. Technol. 2017, 238, 296–305. [Google Scholar] [CrossRef]
- Rahman, D.Y.; Sarian, F.D.; van der Maarel, M.J.E.C. Biomass and phycocyanin content of heterotrophic Galdieria sulphuraria 074G under maltodextrin and granular starches–feeding conditions. J. Appl. Phycol. 2020, 32, 51–57. [Google Scholar] [CrossRef]
- Casá, N.E.; Lois-Milevicich, J.; Alvarez, P.; Mateucci, R.; de Escalada Pla, M. Chlorella vulgaris cultivation using ricotta cheese whey as substrate for biomass production. J. Appl. Phycol. 2022, 34, 745–756. [Google Scholar] [CrossRef]
- Ribeiro, J.E.S.; Martini, M.; Altomonte, I.; Salari, F.; Nardoni, S.; Source, C.; Andreucci, A. Production of Chlorella protothecoides biomass, chlorophyll and carotenoids using the dairy industry by-product scotta as a substrate. Biocatal. Agric. Biotechnol. 2017, 11, 207–213. [Google Scholar] [CrossRef]
- Giovanardi, M.; Baldisserotto, C.; Daglia, M.; Ferroni, L.; Sabia, A.; Pancaldi, S. Morpho-physiological aspects of Scenedesmus acutus PVUW12 cultivated with a dairy industry waste and after starvation. Plant Biosyst. 2016, 50, 767–775. [Google Scholar] [CrossRef]
- Russo, G.L.; Langellotti, A.L.; Oliviero, M.; Baselice, M.; Sacchi, R.; Masi, P. Valorization of second cheese whey through cultivation of extremophile microalga Galdieria sulphuraria. AIMS Environ. Sci. 2021, 8, 435–448. [Google Scholar] [CrossRef]
- Abiusi, F.; Trompetter, E.; Pollio, A.; Wijffels, R.H.; Janssen, M. Acid tolerant and acidophilic microalgae: An underexplored world of biotechnological opportunities. Front. Microbiol. 2022, 13, 820907. [Google Scholar] [CrossRef]
- Luo, H.; Almatrafi, E.; Wang, W.; Yang, Y.; Huang, D.; Xiong, W.; Zhang, C. Insight into the effect of pyrolysis temperature on photoreactivity of biochar-derived dissolved organic matter: Impacts of aromaticity and carbonyl groups. Sci. Total Environ. 2023, 871, 162048. [Google Scholar] [CrossRef]
- Goswami, R.K.; Agrawal, K.; Shah, M.P.; Verma, P. Bioremediation of heavy metals from wastewater: A current perspective on microalgae-based future. Lett. Appl. Microbiol. 2022, 75, 701–717. [Google Scholar] [CrossRef]
- Magalhães, I.B.; Ferreira, J.; de Siqueira Castro, J.; de Assis, L.R.; Calijuri, M.L. Agro-industrial wastewater-grown microalgae: A techno-environmental assessment of open and closed systems. Sci Total Environ. 2022, 834, 155282. [Google Scholar] [CrossRef]
- Das, C.; Naseera, K.; Ram, A.; Meena, R.M.; Ramaiah, N. Bioremediation of tannery wastewater by a salt-tolerant strain of Chlorella vulgaris. J. Appl. Phycol. 2017, 29, 235e243. [Google Scholar] [CrossRef]
- Leng, L.; Li, W.; Chen, J.; Leng, S.; Chen, J.; Wei, L.; Huang, H. Co-culture of fungi-microalgae consortium for wastewater treatment: A review. Bioresour. Technol. 2021, 330, 125008. [Google Scholar] [CrossRef]
- Abonyi, M.N.; Obi, C.C.; Nwabanne, J.T.; Aniagor, C.O. Emerging and ecofriendly biological methods for agricultural wastewater treatment. Environ. Syst. Res. 2024, 13, 45. [Google Scholar] [CrossRef]
- Tang, Y.; Alam, M.S.; Konhauser, K.O.; Alessi, D.S.; Xu, S.; Tian, W.; Liu, Y. Influence of pyrolysis temperature on production of digested sludge biochar and its application for ammonium removal from municipal wastewater. J. Clean. Prod. 2019, 209, 927e936. [Google Scholar] [CrossRef]
- Lutzu, G.A.; Ciurli, A.; Chiellini, C.; Di Caprio, F.; Concas, A.; Dunford, N.T. Latest developments in wastewater treatment and biopolymer production by microalgae. J. Environ. Chem. Eng. 2021, 9, 104926. [Google Scholar] [CrossRef]
- Occhipinti, P.S.; Russo, N.; Foti, P.; Pino, A.; Randazzo, C.L.; Pollio, A.; Caggia, C. An indigenous microalgal pool from a constructed wetland as an alternative strategy for Escherichia coli removal in urban wastewater. J. Sci. Food Agric. 2025, 105, 1288–1297. [Google Scholar] [CrossRef] [PubMed]
- Council of the European Communities. Council Directive 91/271/EEC of 21 May 1991 Concerning Urban Waste-Water Treatment. 1991, Volume 135, pp. 40–52. Available online: https://eur-lex.europa.eu/eli/dir/1991/271/oj/eng (accessed on 1 August 2025).
- European Parliament; Council of the European Union. Regulation (EU) 2020/741 of the European Parliament and of the Council of 25 May 2020 on Minimum Requirements for Water Reuse. 2023, Volume 177, pp. 32–55. Available online: https://eur-lex.europa.eu/eli/reg/2020/741/oj/eng (accessed on 1 August 2025).
- Pandey, A.; Srivastava, S.; Kumar, S. Development and cost-benefit analysis of a novel process for biofuel production from microalgae using pre-treated high-strength fresh cheese whey wastewater. Environ. Sci. Pollut. Res. 2020, 27, 23963–23980. [Google Scholar] [CrossRef]
- Riaño, B.; Blanco, S.; Becares, E.; García-González, M.C. Bioremediation and biomass harvesting of anaerobic digested cheese whey in microalgal-based systems for lipid production. Ecol. Eng. 2016, 97, 40–45. [Google Scholar] [CrossRef]
- European Union. Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying Down Rules on the Making Available on the Market of EU Fertilising Products; Publications Office of the European Union: Luxembourg, 2019. [Google Scholar]
- Kolesovs, S.; Semjonovs, P. Microalgal conversion of whey and lactose-containing substrates: Current state and challenges. Biodegradation 2023, 34, 405–416. [Google Scholar] [CrossRef]
- European Union. Regulation (EC) No. 767/2009 of the European Parliament and of the Council of 13 July 2009 on the Placing on the Market and Use of Feed; Publications Office of the European Union: Luxembourg, 2009. [Google Scholar]
- European Union. Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on Undesirable Substances in Animal Feed; Publications Office of the European Union: Luxembourg, 2002. [Google Scholar]
- Sánchez-Zurano, A.; Villaró-Cos, S.; Ciardi, M.; Acién-Fernández, F.G.; Fernández-Sevilla, J.M.; Lafarga, T. Assessment of the mixotrophic production of Chlorella vulgaris using milk whey as a nutrient source. J. Appl. Phycol. 2024, 36, 87–100. [Google Scholar] [CrossRef]
- European Union. Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on Novel Foods; Publications Office of the European Union: Luxembourg, 2015. [Google Scholar]
- European Union. Regulation (EC) No. 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products; Publications Office of the European Union: Luxembourg, 2009. [Google Scholar]
- Li, G.; Hu, R.; Wang, N.; Yang, T.; Xu, F.; Li, J.; Lyu, T. Cultivation of microalgae in adjusted wastewater to enhance biofuel production and reduce environmental impact: Pyrolysis performances and life cycle assessment. J. Clean. Prod. 2022, 355, 131768. [Google Scholar] [CrossRef]
- Mu, D.; Min, M.; Krohn, B.; Mullins, K.A.; Ruan, R.; Hill, J. Life cycle environmental impacts of wastewater-based algal biofuels. Environ. Sci. Technol. 2014, 48, 11696–11704. [Google Scholar] [CrossRef] [PubMed]
- Crippa, I.; Dolci, G.; Grosso, M.; Rigamonti, L. Life Cycle Assessment of Microalgal Biomass Valorization from a Wastewater Treatment Process. Waste Biomass Valorization 2025, 16, 525–541. [Google Scholar] [CrossRef]
- Quinn, J.C.; Davis, R. The potentials and challenges of algae based biofuels: A review of the techno-economic, life cycle, and resource assessment modeling. Bioresour. Technol. 2015, 184, 444–452. [Google Scholar] [CrossRef] [PubMed]
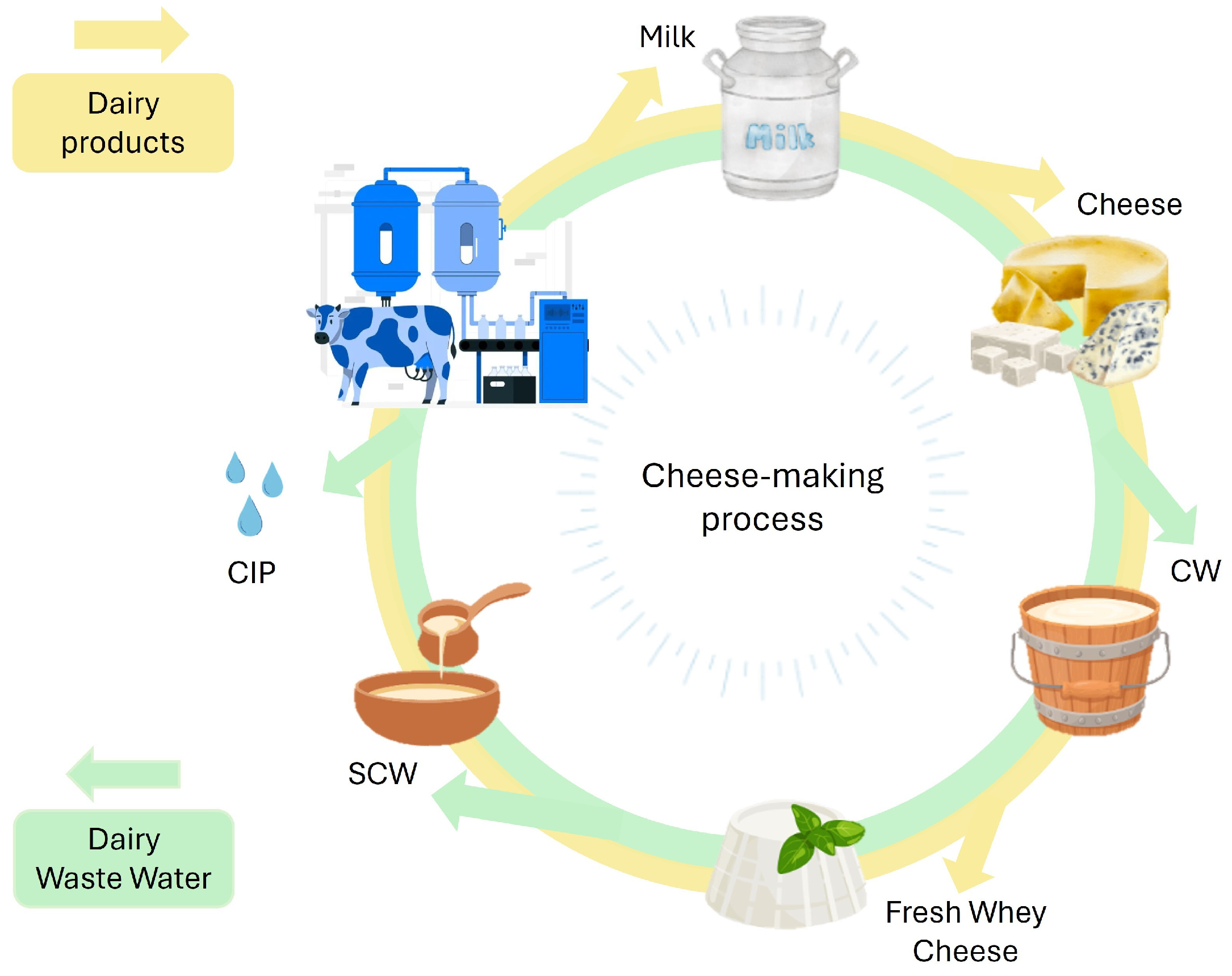
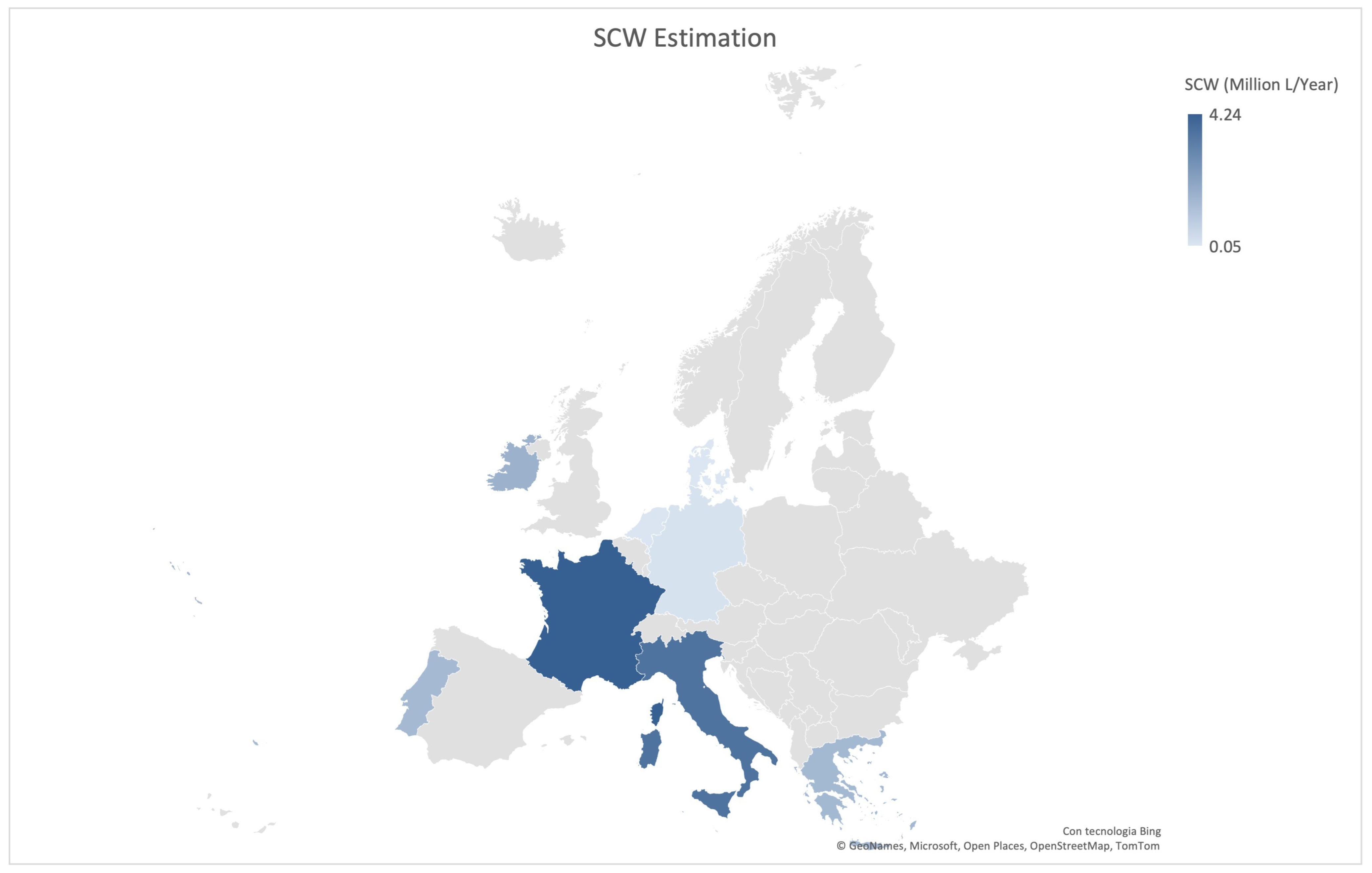
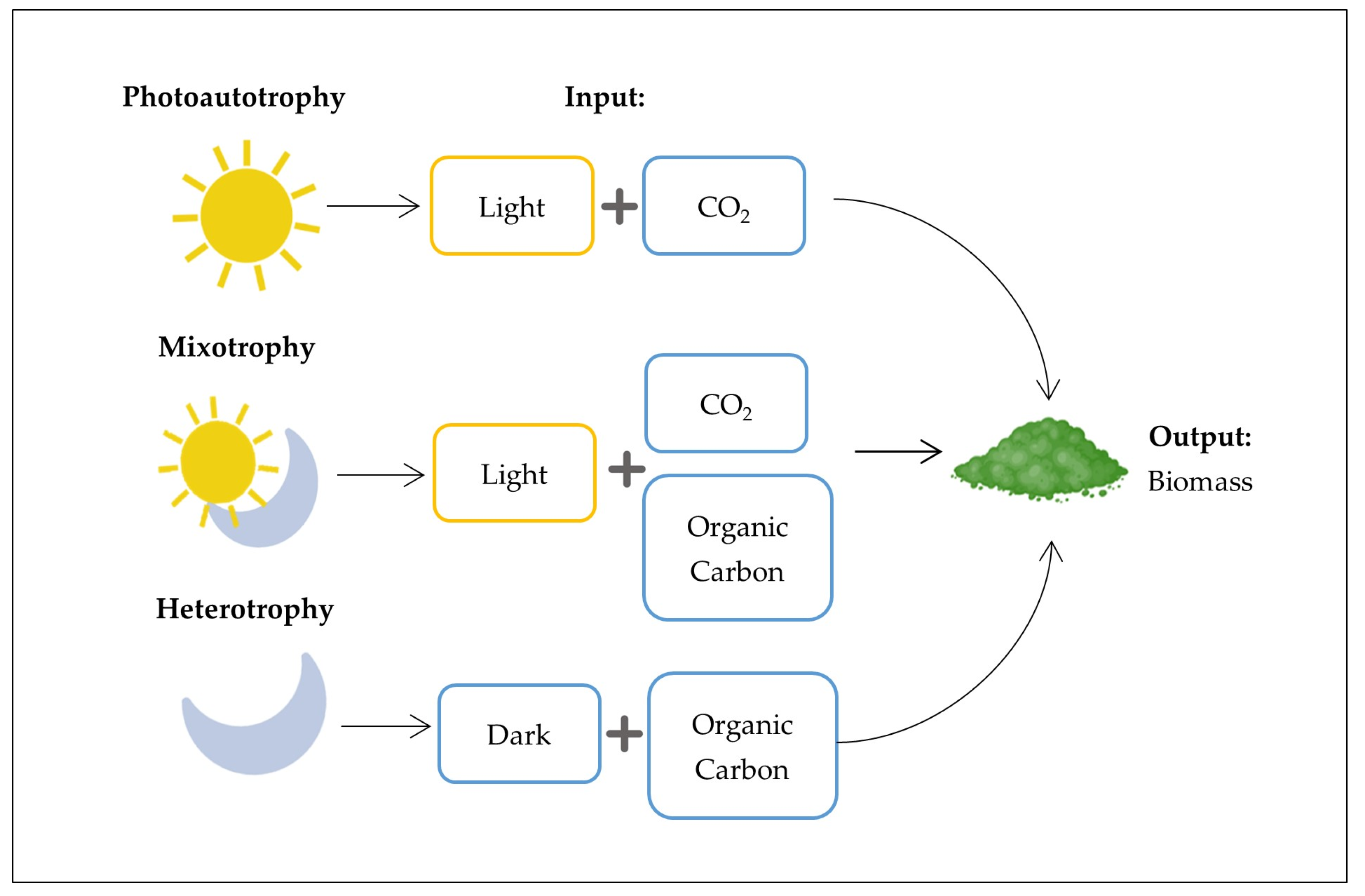
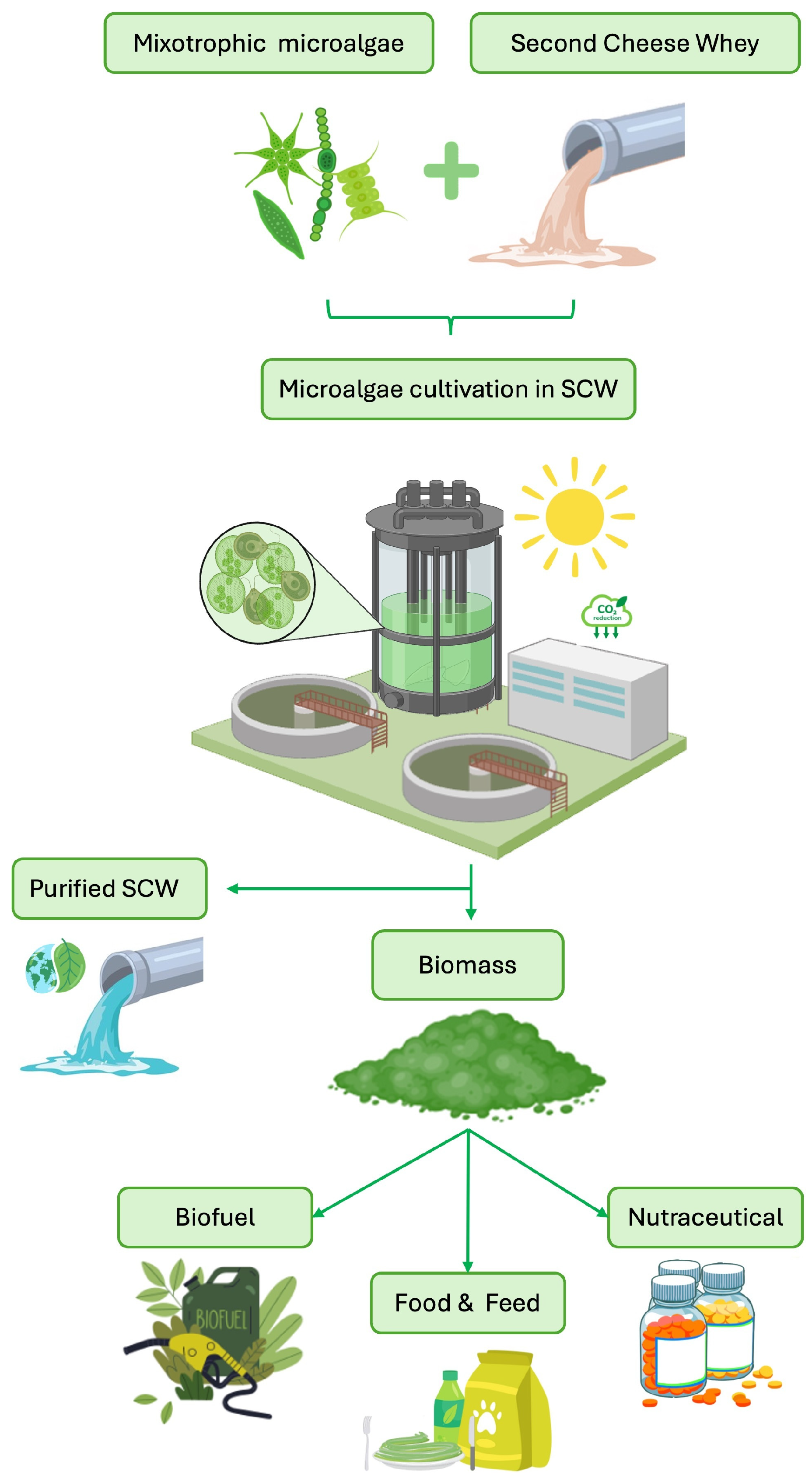
| Type of SCW | pH | COD (g/L) | BOD (g/L) | Lactose (%) | Proteins (%) | Salts (%) | Dry Matter (%) | Notes | References |
|---|---|---|---|---|---|---|---|---|---|
| From cow’s milk | ~6.0 | ~60 | ~45 | 4.8–5.0 | 0.1–0.2 | 1.0–1.1 | ~6.0 | Average data from small dairies | [14] |
| From sheep’s milk | 6.2–6.5 | n.d. | n.d. | 4.5–5.0 | ~0.5 | ~0.5 | ~6.7 | Richer composition compared to cow SCW | [18] |
| From mixed milk | 6.0–6.5 | 50–70 | n.d. | 4.7–5.2 | 0.2–0.3 | 0.9–1.1 | ~6.5 | Variability linked to the type of milk | [17] |
| From cow’s milk | ~6.1 | n.d. | n.d. | ~5.0 | ~0.2 | ~1.1 | ~6.0 | Brazilian study | [16] |
| Valorization Strategy | End-Product Applications | Opportunities/Strengths | Challenges/Limits | Industrial Perspective | References |
|---|---|---|---|---|---|
| Anaerobic digestion | Biogas, renewable energy | Established technology; renewable energy incentives; reduces GHG emissions | High capital cost; requires skilled operation | Already applied in the agro-industry | [27,47] |
| Bioethanol & galactonic acid production | Biofuels, chemical intermediates | Renewable alternative fuels; valorize lactose | Process optimization needed; market competitiveness | Pilot-scale; potential in biorefineries | [48] |
| Lactic acid fermentation | Bioplastics, food additives | Bio-based alternative chemicals | Process optimization; market competition | Early-stage research | [3] |
| Microalgae cultivation | Algal biomass (nutraceuticals, biofuels) | Combines treatment & biomass production; sunlight-driven | Scalability; contamination risks; harvesting | Research stage: promising for low-impact valorization | [26,46] |
| Microbial fermentation substrate | Enzymes, bioactive compounds | Supports microbial growth; biotechnological interest | Process standardization; economic viability | Applied in niche biotech sectors | [3] |
| PHA (bioplastic) production | Biodegradable plastics | Circular bio-based product; reduces fossil plastic dependency | Low yields; high production & extraction costs | Pilot-scale; emerging market | [46] |
| Prebiotic oligosaccharide synthesis | Functional food ingredients | Nutraceutical interest: high-value products | Complex purification; limited demand | Niche applications; limited scale | [43] |
| Probiotic bacteria substrate | Starter cultures, probiotics | Sustainable bio-production; waste valorization | Composition variability; small-scale use | Applied in dairy industries; niche applications | [3] |
| Refined lactose extraction | Food and pharma ingredient | High lactose recovery; ingredient market | Costly separation technologies | Industrial in large plants; costly for SMEs | [43] |
| Succinic acid production | Biopolymers, green chemicals | Circular chemical production; green chemistry | Low yields; strain development | Research stage: promising for green industries | [3] |
| Metabolism | Species | Light & Carbon Source | Productivity (g L−1 d−1) | Recovery/Waste Utilization Rate (%) | Limitations/Costs | Applications | References |
|---|---|---|---|---|---|---|---|
| Photoautotrophic | Chlorella sp., Dunaliella salina | Light (natural or artificial) + CO2 (inorganic) | ~0.1–0.2 | N and P removal 80–95%; COD/BOD reduction 60–85% | Low input costs (light, CO2), but low productivity makes biomass more expensive; downstream processing accounts for 50–70% of total costs | Biofuels, CO2 mitigation, wastewater treatment | [56,76,89] |
| Heterotrophic | Chlorella protothecoides, Crypthecodinium cohnii | No light + Glucose, acetate, glycerol (organic carbon) | ~0.3–1.5 | N removal 85–93%; COD removal 65–80% | High costs due to sterile conditions and organic substrates; using agri-food wastewater as carbon source reduces carbon cost by up to 70% compared to glucose | Nutraceuticals, lipids, industrial pigments | [78,80] |
| Mixotrophic | C. vulgaris, Tetraselmis sp., Nannochloropsis salina | Light (natural or artificial) + CO2 + Glucose (organic carbon) | ~0.4–1.8 | COD 84–90%; N 80–95%; P 90–98% | Intermediate costs; economic advantage from coupling wastewater treatment with biomass production; carbon source costs reduced by 60–70% when using agri-food wastewater | Integrated biorefineries, SCW valorization, high-value compounds | [23,81,82] |
| Microalgae | Trophic Mode | SCW Treatment | Biomass Yield (g/L) | Key Metabolites | Strengths | Weaknesses | References |
|---|---|---|---|---|---|---|---|
| C. vulgaris | Mixotrophic | Dilution, pH adjustment, nutrient supplementation | 1.6 | Proteins | Good growth protein accumulation | Requires pH and nutrient adjustment | [103] |
| C. protothecoides | Mixotrophic | Autoclaving, filtration, dilution, nutrient supplementation | 3.6 | Chlorophyll, β-carotene, lutein | High pigment yield, effective nutrient use | Requires sterilization, sensitive to contamination | [104] |
| S. acutus | Mixotrophic | Dilution, nutrient supplementation | 0.9 | Lipids, pigments | High pigment production, improved lipids under starvation | Requires dilution and nutrient supplementation, lower yield | [105] |
| G. sulphuraria | Heterotrophic | None | 1.8 | Biomass content | Biomass rich in organic compounds, no pretreatment needed, high tolerance to acidity | Requires low pH, limited application range | [106] |
| Valorization Pathway | Relevant Regulation | Main Requirements | Feasibility | References |
|---|---|---|---|---|
| Bioenergy (biodiesel, biogas, bioethanol) | No specific EU regulation | None for non-food use; quality control for emissions and residues | High, widely feasible | [119,120] |
| Agricultural use (fertilizer, biostimulant) | Regulation (EU) 2019/1009 | Limits on heavy metals, pathogens, and persistent organic pollutants | Moderate, requires post-processing to meet criteria | [122] |
| Feed | Regulation (EC) No 767/2009 and Directive 2002/32/EC | Traceability, contaminant thresholds (e.g., heavy metals, mycotoxins), hygienic processing | Moderate to low, subject to strict monitoring | [125] |
| Human applications (nutraceuticals, cosmetics, food) | Regulation (EU) 2015/2283; Regulation (EC) No 1223/2009 | Use of approved sources; safety validation; pharmaceutical-grade purification | Low, not allowed without advanced purification and validation | [122,125] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sciuto, G.; Russo, N.; Randazzo, C.L.; Caggia, C. Valorization of Second Cheese Whey Through Microalgae-Based Treatments: Advantages, Limits, and Opportunities. BioTech 2025, 14, 79. https://doi.org/10.3390/biotech14040079
Sciuto G, Russo N, Randazzo CL, Caggia C. Valorization of Second Cheese Whey Through Microalgae-Based Treatments: Advantages, Limits, and Opportunities. BioTech. 2025; 14(4):79. https://doi.org/10.3390/biotech14040079
Chicago/Turabian StyleSciuto, Gloria, Nunziatina Russo, Cinzia L. Randazzo, and Cinzia Caggia. 2025. "Valorization of Second Cheese Whey Through Microalgae-Based Treatments: Advantages, Limits, and Opportunities" BioTech 14, no. 4: 79. https://doi.org/10.3390/biotech14040079
APA StyleSciuto, G., Russo, N., Randazzo, C. L., & Caggia, C. (2025). Valorization of Second Cheese Whey Through Microalgae-Based Treatments: Advantages, Limits, and Opportunities. BioTech, 14(4), 79. https://doi.org/10.3390/biotech14040079










