Development of PCR Methods for Detecting Wheat and Maize Allergens in Food
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Sample Preparation
2.2. DNA Extraction
2.3. Bioinformatic Analysis and Design of Oligonucleotide Primers
2.4. PCR Analysis
2.5. Statistical Analysis
3. Results
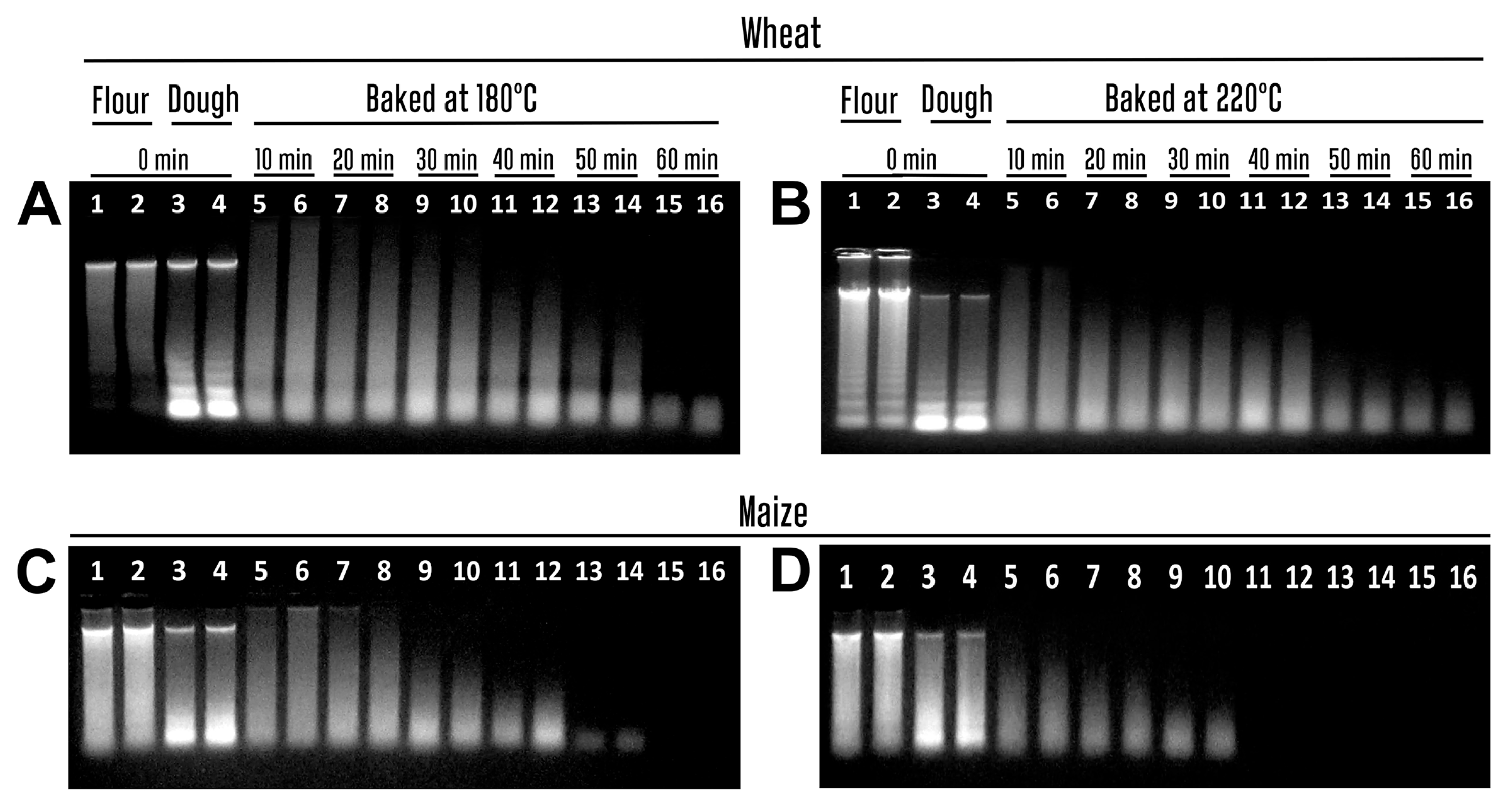
3.1. The Impact of Baking on the Wheat and Maize Genomic DNAs
3.2. Influence of Baking on the PCR Amplification of Wheat and Maize DNA
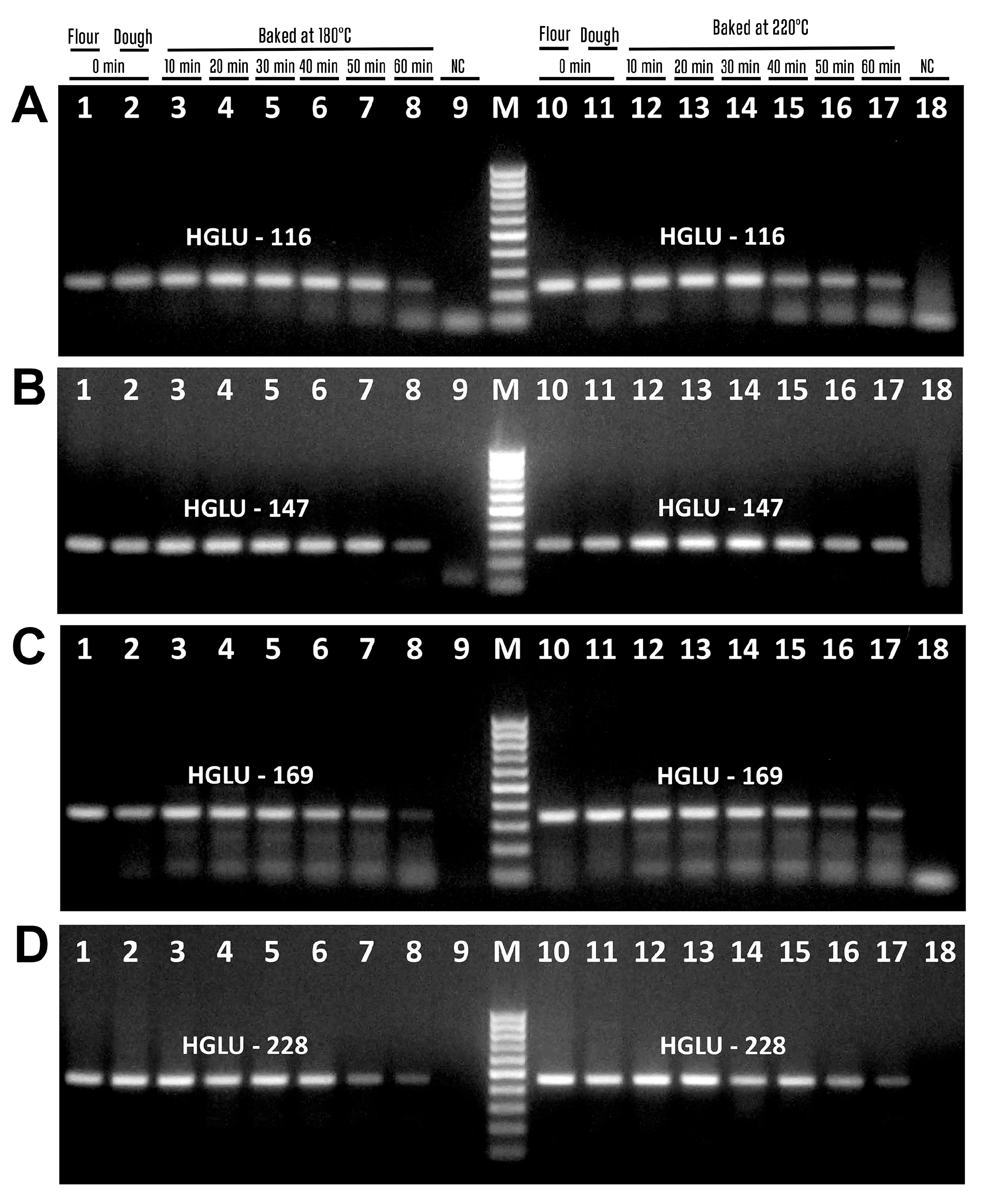
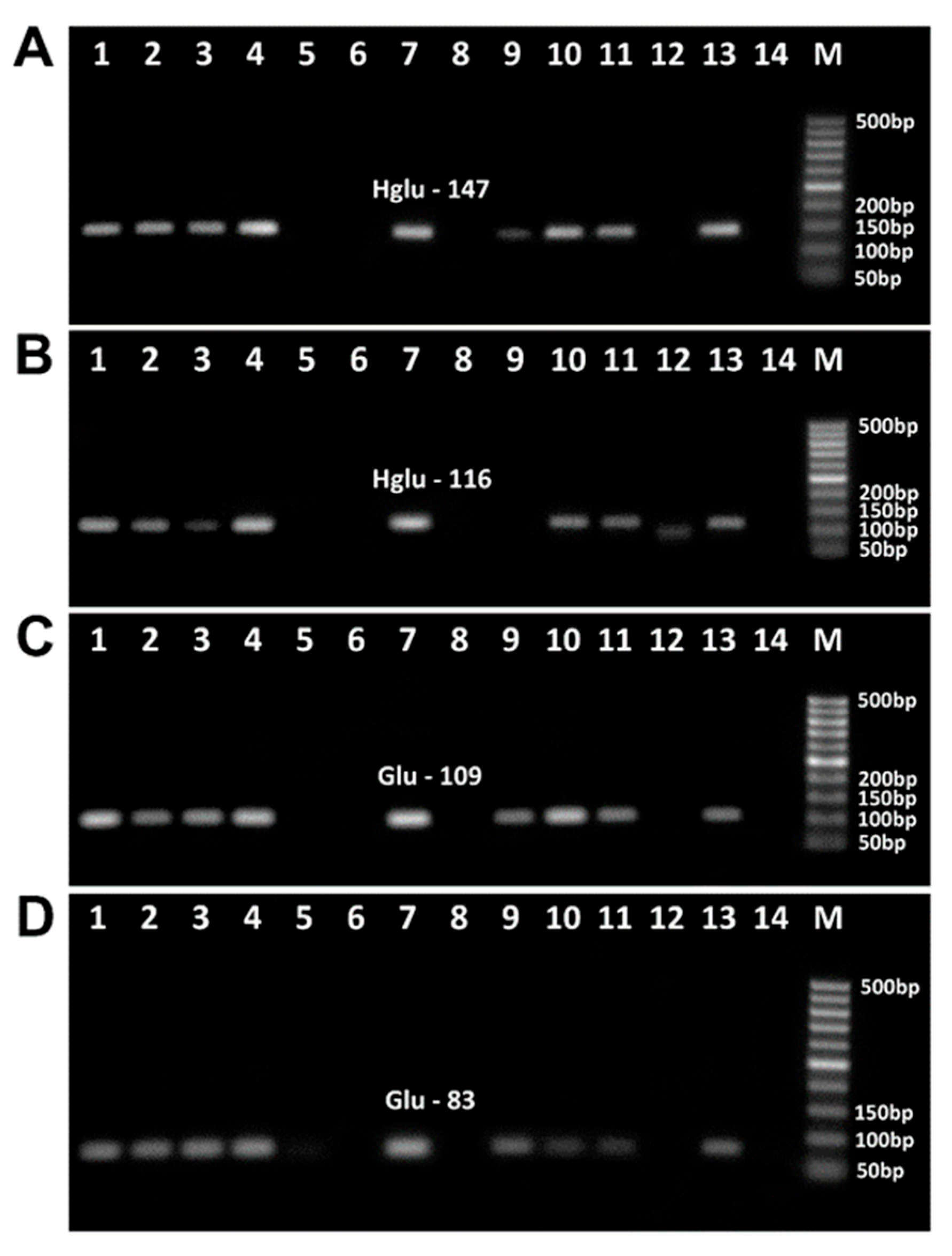
3.3. Impact of Baking on the PCR Detection of Wheat Allergens
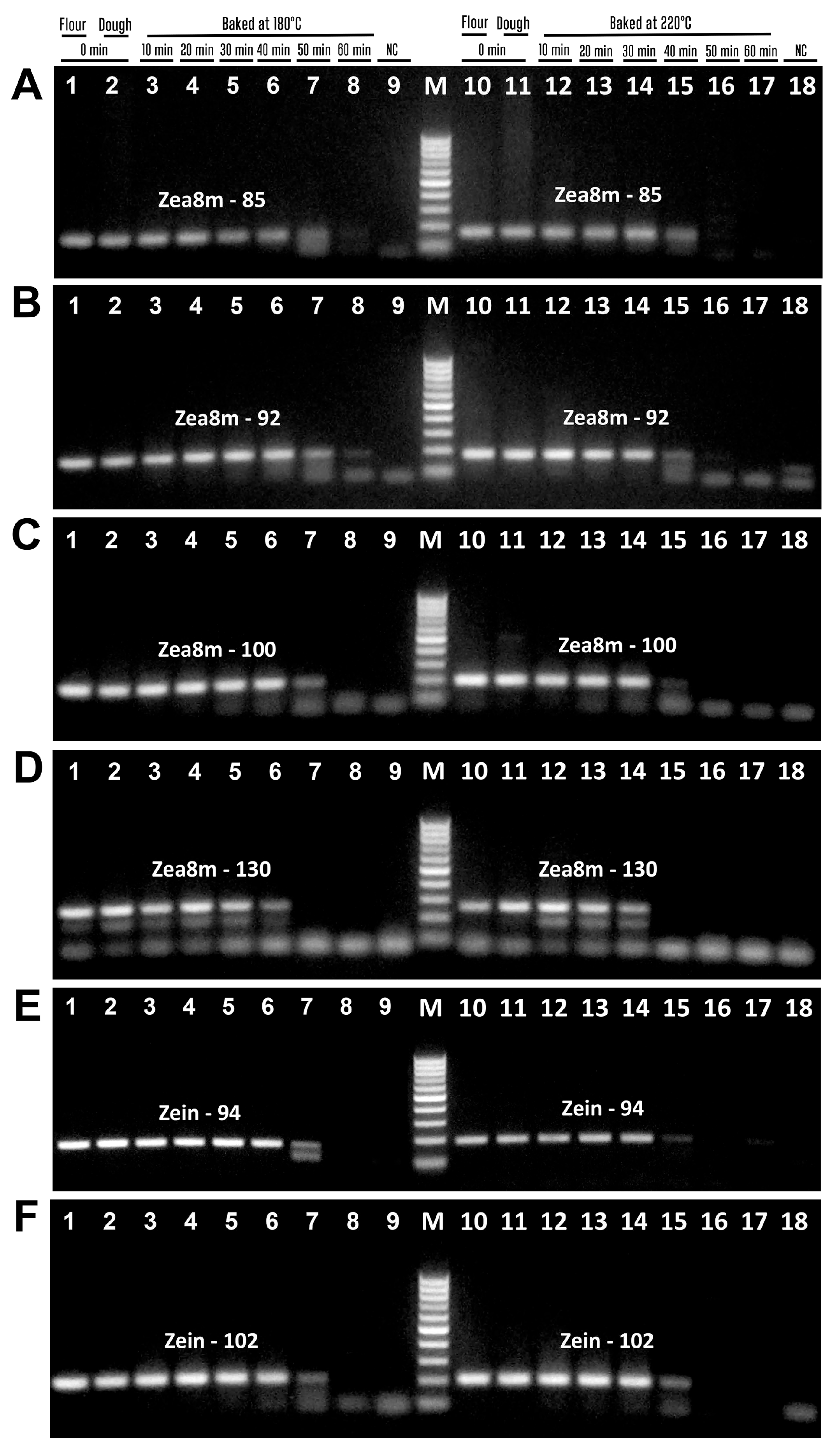
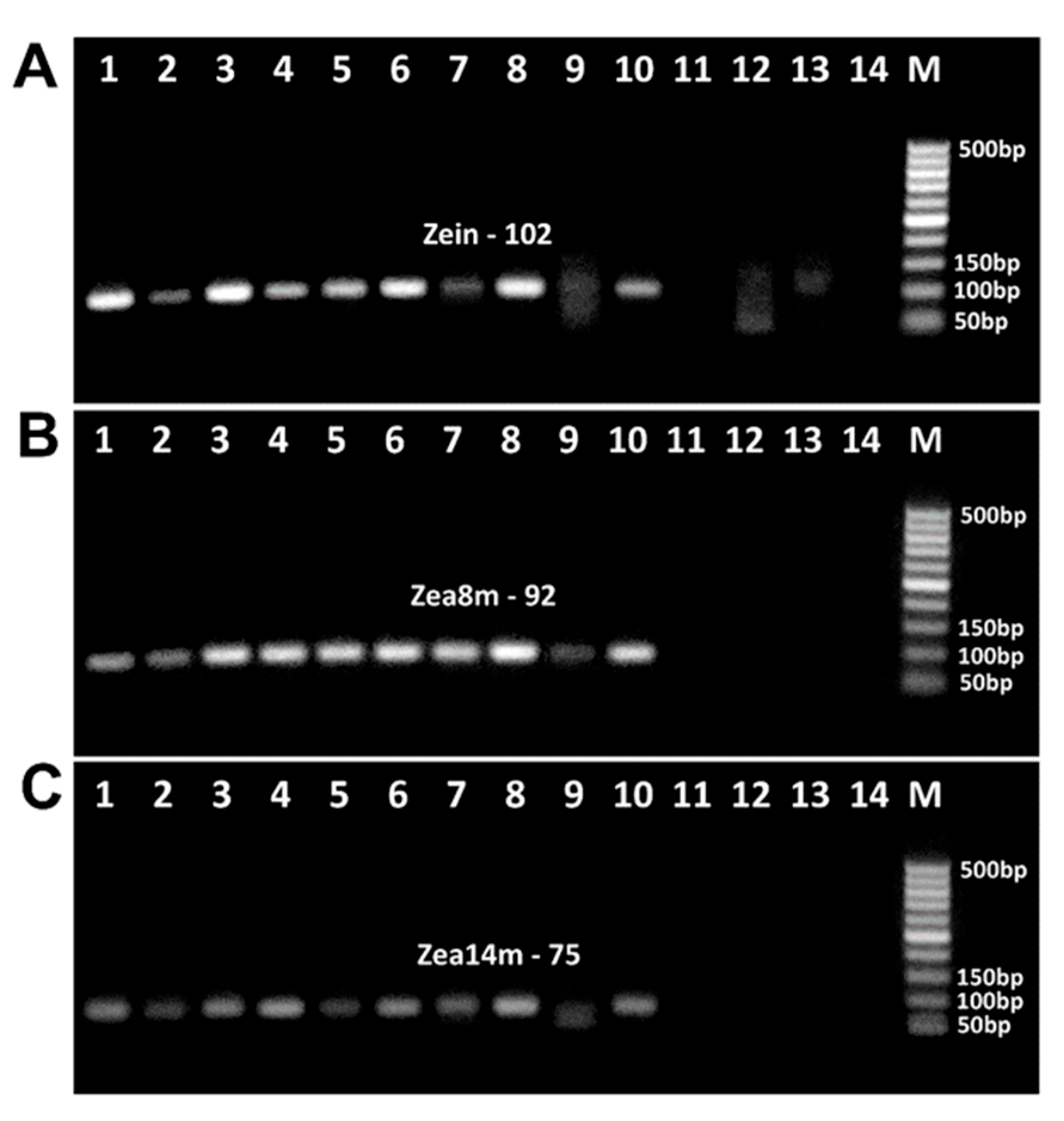
3.4. Impact of Baking on the PCR Detection of Maize Allergens
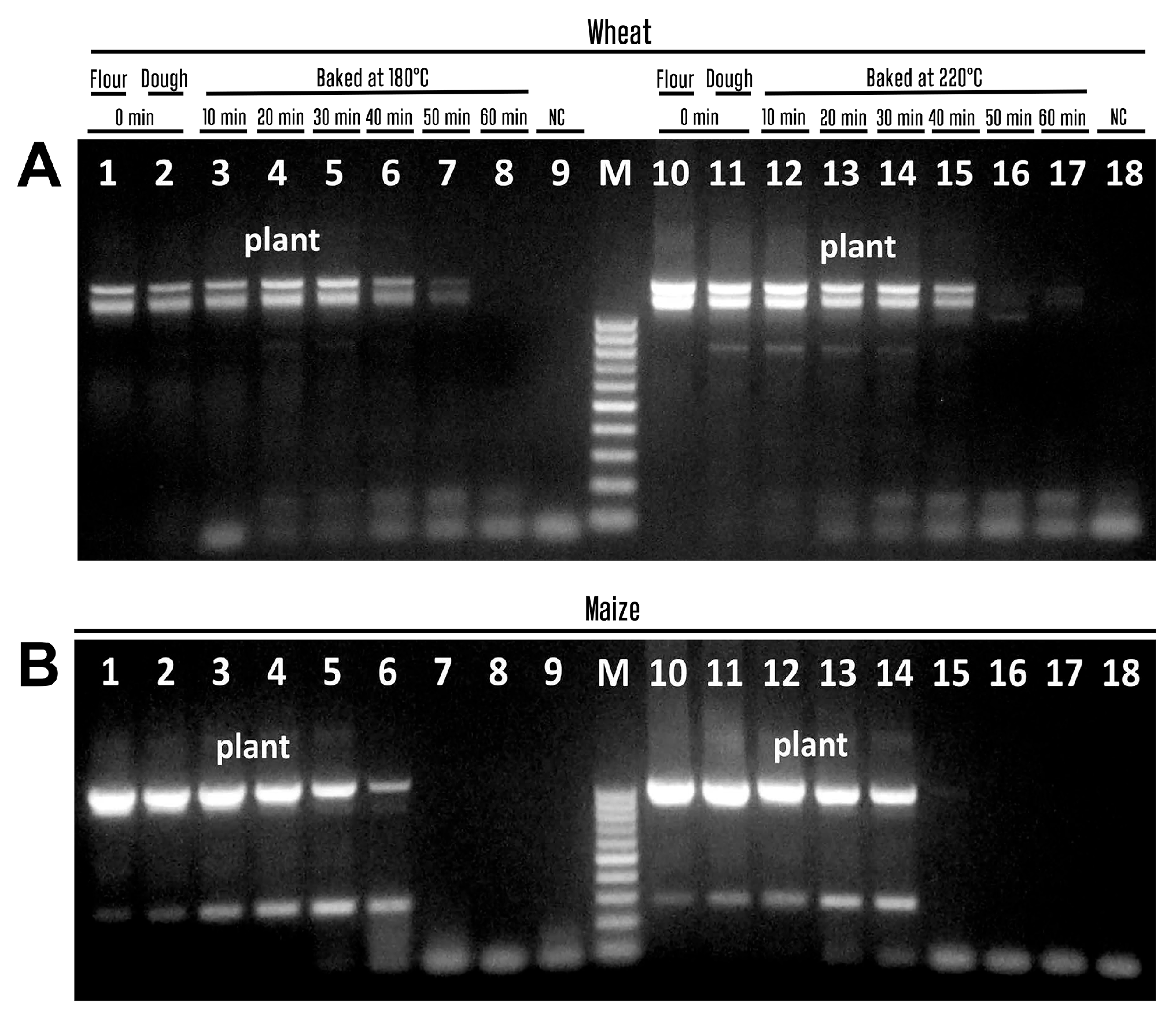
3.5. PCR Detection of Wheat and Maize Allergens in Food Products
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erenstein, O.; Jaleta, M.; Mottaleb, K.A.; Sonder, K.; Donovan, J.; Braun, H.-J. Global Trends in Wheat Production, Consumption and Trade. In Wheat Improvement; Springer: Cham, Switzerland, 2022; pp. 47–66. [Google Scholar] [CrossRef]
- Gupta, O.P.; Kumar, S.; Pandey, A.; Khan, M.K.; Singh, S.K.; Singh, G.P. Wheat Science: Nutritional and Anti-Nutritional Properties, Processing, Storage, Bioactivity, and Product Development, 1st ed.; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar] [CrossRef]
- Grote, U.; Fasse, A.; Nguyen, T.T.; Erenstein, O. Food Security and the Dynamics of Wheat and Maize Value Chains in Africa and Asia. Front. Sustain. Food Syst. 2021, 4, 617009. [Google Scholar] [CrossRef]
- Erenstein, O.; Jaleta, M.; Sonder, K.; Mottaleb, K.; Prasanna, B.M. Global Maize Production, Consumption and Trade: Trends and R&D Implications. Food Secur. 2022, 14, 1295–1319. [Google Scholar] [CrossRef]
- Cappelli, A.; Cini, E. Challenges and Opportunities in Wheat Flour, Pasta, Bread, and Bakery Product Production Chains: A Systematic Review of Innovations and Improvement Strategies to Increase Sustainability, Productivity, and Product Quality. Sustainability 2021, 13, 2608. [Google Scholar] [CrossRef]
- Sanodiya, P.; Bhusal, K.P.; Mishra, N.K. Maize Nutritional Quality and Value Addition: A Brief Overview. Maize J. 2022, 11, 75–82. [Google Scholar]
- Wong, G.W.-K. Food Allergies around the World. Front. Nutr. 2024, 11, 1373110. [Google Scholar] [CrossRef]
- Florsheim, E.B.; Sullivan, Z.A.; Khoury-Hanold, W.; Medzhitov, R. Food Allergy as a Biological Food Quality Control System. Cell 2021, 184, 1440–1454. [Google Scholar] [CrossRef]
- Seth, D.; Poowutikul, P.; Pansare, M.; Kamat, D. Food Allergy: A Review. Pediatr. Ann. 2020, 49, e50–e58. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO); World Health Organization (WHO); Codex Alimentarius Commission. General Standard for the Labelling of Prepackaged Foods (CXS 1-1985). 1985. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/fr/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B1-1985%252FCXS_001e.pdf (accessed on 15 February 2024).
- European Union. Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the Provision of Food Information to Consumers; European Parliament and of The Council: Strasbourg, France, 2011; Available online: https://eur-lex.europa.eu/eli/reg/2011/1169/oj (accessed on 10 March 2024).
- Fiocchi, A.; Risso, D.; DunnGalvin, A.; González Díaz, S.N.; Monaci, L.; Fierro, V.; Ansotegui, I.J. Food Labeling Issues for Severe Food Allergic Patients. World Allergy Organ. J. 2021, 14, 100598. [Google Scholar] [CrossRef]
- Holzhauser, T.; Johnson, P.; Hindley, J.P.; O’Connor, G.; Chan, C.-H.; Costa, J.; Fæste, C.K.; Hirst, B.J.; Lambertini, F.; Miani, M.; et al. Are Current Analytical Methods Suitable to Verify VITAL® 2.0/3.0 Allergen Reference Doses for EU Allergens in Foods? Food Chem. Toxicol. 2020, 145, 111709. [Google Scholar] [CrossRef]
- de la Cruz, S.; López-Calleja, I.; Martín, R.; González, I.; Alcocer, M.; García, T. Recent Advances in the Detection of Allergens in Foods. In Methods in Molecular Biology; Springer: New York, NY, USA, 2017; pp. 263–295. [Google Scholar] [CrossRef]
- Shin, J.H.; Reddy, Y.V.M.; Park, T.J.; Park, J.P. Recent Advances in Analytical Strategies and Microsystems for Food Allergen Detection. Food Chem. 2022, 371, 131120. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Upadhyay, A.; Rafiq, S.M.; Nayik, G.A. Recent Advances in Detection of Food Allergens. J. Postharvest Technol. 2020, 08, 22–36. [Google Scholar]
- Senyuva, H.Z.; Jones, I.B.; Sykes, M.; Baumgartner, S. A Critical Review of the Specifications and Performance of Antibody and DNA-Based Methods for Detection and Quantification of Allergens in Foods. Food Addit. Contam. Part A 2019, 36, 507–547. [Google Scholar] [CrossRef]
- Okolie, C.L.; Aryee, A.N.A.; Udenigwe, C.C. Detection and Deactivation of Allergens in Food. In Proteins in Food Processing; Elsevier: Amsterdam, The Netherlands, 2018; pp. 367–387. [Google Scholar] [CrossRef]
- Sharma, G.M.; Khuda, S.E.; Parker, C.H.; Eischeid, A.C.; Pereira, M. Detection of Allergen Markers in Food: Analytical Methods. In Food Safety: Innovative Analytical Tools for Safety Assessment; Food and Drug Administration: Rockville, MD, USA, 2017; Volume 6, pp. 65–121. [Google Scholar] [CrossRef]
- Sajali, N.; Wong, S.C.; Hanapi, U.K.; Abu Bakar @ Jamaluddin, S.; Tasrip, N.A.; Mohd Desa, M.N. The Challenges of DNA Extraction in Different Assorted Food Matrices: A Review. J. Food Sci. 2018, 83, 2409–2414. [Google Scholar] [CrossRef]
- Bergerová, E.; Godálová, Z.; Siekel, P. Combined Effects of Temperature, Pressure and Low pH on the Amplification of DNA of Plant Derived Foods. Czech J. Food Sci. 2011, 29, 337–345. [Google Scholar] [CrossRef]
- Hrnčírová, Z.; Bergerová, E.; Siekel, P. Effects of Technological Treatment on DNA Degradation in Selected Food Matrices of Plant Origin. J. Food Nutr. Res. 2008, 47, 23–28. [Google Scholar]
- Pi, X.; Zhu, L.; Liu, J.; Zhang, B. Effect of Thermal Processing on Food Allergenicity: Mechanisms, Application, Influence Factor, and Future Perspective. J. Agric. Food Chem. 2024, 72, 20225–20240. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.; Villa, C.; Grazina, L.; Mafra, I. Single-Tube Nested Real-Time PCR versus Normalised Real-Time PCR for the Quantification of Allergenic Cashew Nut in Foods: Impact of Thermal Processing and Matrix. Food Chem. 2022, 397, 133778. [Google Scholar] [CrossRef]
- Liu, W.; Wu, Y.; Wang, J.; Wang, Z.; Gao, J.; Yuan, J.; Chen, H. A Meta-Analysis of the Prevalence of Wheat Allergy Worldwide. Nutrients 2023, 15, 1564. [Google Scholar] [CrossRef]
- Neyer, A.; Dölle-Bierke, S.; Höfer, V.; Grünhagen, J.; Beyer, K.; Worm, M. Prevalence and Clinical Symptoms of Wheat Allergy in Adults and Adolescents in Central Europe. Clin. Exp. Allergy 2025, 55, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Cianferoni, A. Wheat Allergy: Diagnosis and Management. JAA 2016, 13, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Asri, N.; Rostami-Nejad, M.; Anderson, R.P.; Rostami, K. The Gluten Gene: Unlocking the Understanding of Gluten Sensitivity and Intolerance. TACG 2021, 14, 37–50. [Google Scholar] [CrossRef]
- Li, Y.; Fu, J.; Shen, Q.; Yang, D. High-Molecular-Weight Glutenin Subunits: Genetics, Structures, and Relation to End Use Qualities. Int. J. Mol. Sci. 2020, 22, 184. [Google Scholar] [CrossRef]
- Løvik, A.; Skodje, G.; Bratlie, J.; Brottveit, M.; Lundin, K.E.A. Diet Adherence and Gluten Exposure in Coeliac Disease and Self-Reported Non-Coeliac Gluten Sensitivity. Clin. Nutr. 2017, 36, 275–280. [Google Scholar] [CrossRef]
- Osorio, C.E.; Mejías, J.H.; Rustgi, S. Gluten Detection Methods and Their Critical Role in Assuring Safe Diets for Celiac Patients. Nutrients 2019, 11, 2920. [Google Scholar] [CrossRef]
- Lu, Y.; Ji, H.; Chen, Y.; Li, Z.; Timira, V. A Systematic Review on the Recent Advances of Wheat Allergen Detection by Mass Spectrometry: Future Prospects. Crit. Rev. Food Sci. Nutr. 2023, 63, 12324–12340. [Google Scholar] [CrossRef]
- Verma, A.; Gatti, S.; Galeazzi, T.; Monachesi, C.; Padella, L.; Baldo, G.; Annibali, R.; Lionetti, E.; Catassi, C. Gluten Contamination in Naturally or Labeled Gluten-Free Products Marketed in Italy. Nutrients 2017, 9, 115. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, K.-M.; Kang, C.-S.; Yoon, M.; Jang, K.-C.; Choi, C. Development of PCR-Based Markers for Identification of Wheat HMW Glutenin Glu-1Bx and Glu-1By Alleles. BMC Plant Biol. 2024, 24, 395. [Google Scholar] [CrossRef] [PubMed]
- Výrostková, J.; Regecová, I.; Zigo, F.; Marcinčák, S.; Kožárová, I.; Kováčová, M.; Bertová, D. Detection of Gluten in Gluten-Free Foods of Plant Origin. Foods 2022, 11, 2011. [Google Scholar] [CrossRef] [PubMed]
- García-García, A.; Madrid, R.; Sohrabi, H.; De La Cruz, S.; García, T.; Martín, R.; González, I. A Sensitive and Specific Real-Time PCR Targeting DNA from Wheat, Barley and Rye to Track Gluten Contamination in Marketed Foods. LWT 2019, 114, 108378. [Google Scholar] [CrossRef]
- Martín-Fernández, B.; Costa, J.; Oliveira, M.B.P.P.; López-Ruiz, B.; Mafra, I. Screening New Gene Markers for Gluten Detection in Foods. Food Control 2015, 56, 57–63. [Google Scholar] [CrossRef]
- Scharf, A.; Kasel, U.; Wichmann, G.; Besler, M. Performance of ELISA and PCR Methods for the Determination of Allergens in Food: An Evaluation of Six Years of Proficiency Testing for Soy (Glycine max L.) and Wheat Gluten (Triticum aestivum L.). J. Agric. Food Chem. 2013, 61, 10261–10272. [Google Scholar] [CrossRef]
- Frank, K.; Miró, K.; Nagy, T.; Marincs, F. Development of a PCR-Based DNA Marker for Glu-1By Alleles in the Old Hungarian Bánkúti Wheat. Mol. Breed. 2017, 37, 120. [Google Scholar] [CrossRef]
- Park, H.-S.; Nahm, D.-H. Identification of IgE-Binding Components in Occupational Asthma Caused by Corn Dust. Ann. Allergy Asthma Immunol. 1997, 79, 75–79. [Google Scholar] [CrossRef]
- Guillen, D.; Barranco, P.; Palacín, A.; Quirce, S. Occupational Rhinoconjunctivitis Due to Maize in a Snack Processor: A Cross-Reactivity Study Between Lipid Transfer Proteins From Different Cereals and Peach. Allergy Asthma Immunol. Res. 2014, 6, 470. [Google Scholar] [CrossRef][Green Version]
- Aruanno, A.; Urbani, S.; Frati, F.; Nucera, E. LTP Allergy/Sensitization in a Pediatric Population. Allergol. Immunopathol. 2020, 48, 763–770. [Google Scholar] [CrossRef]
- Jimenez-Lopez, J.C.; Kotchoni, S.O.; Gachomom, E.W.; Castro-López, A.J.; Rodríguez-García, M.I.; Alché, J.D. Molecular Features of Maize Allergens and Their Implications in Human Health. In Maize: Cultivation, Uses and Health Benefits; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2012. [Google Scholar]
- Pastorello, E. Lipid-Transfer Protein Is the Major Maize Allergen Maintaining IgE-Binding Activity after Cooking at 100 °C, as Demonstrated in Anaphylactic Patients and Patients with Positive Double-Blind, Placebo-Controlled Food Challenge Results. J. Allergy Clin. Immunol. 2003, 112, 775–783. [Google Scholar] [CrossRef]
- Leoni, C.; Volpicella, M.; Dileo, M.C.G.; Gattulli, B.A.R.; Ceci, L.R. Chitinases as Food Allergens. Molecules 2019, 24, 2087. [Google Scholar] [CrossRef] [PubMed]
- Volpicella, M.; Leoni, C.; Fanizza, I.; Distaso, M.; Leoni, G.; Farioli, L.; Naumann, T.; Pastorello, E.; Ceci, L.R. Characterization of Maize chitinase-A, a Tough Allergenic Molecule. Allergy 2017, 72, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, S.E.; McClain, S.; Thelen, J.J. Development of an Isoform-Specific Tandem Mass Spectrometry Assay for Absolute Quantitation of Maize Lipid Transfer Proteins. J. Agric. Food Chem. 2015, 63, 821–828. [Google Scholar] [CrossRef]
- Kuppannan, K.; Albers, D.R.; Schafer, B.W.; Dielman, D.; Young, S.A. Quantification and Characterization of Maize Lipid Transfer Protein, A Food Allergen, by Liquid Chromatography with Ultraviolet and Mass Spectrometric Detection. Anal. Chem. 2011, 83, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, C.; Planchon, S.; Renaut, J.; Oliveira, M.M.; Batista, R. Characterization of Maize Allergens—MON810 vs. Its Non-Transgenic Counterpart. J. Proteom. 2012, 75, 2027–2037. [Google Scholar] [CrossRef]
- Bitskinashvili, K.; Kutateladze, T.; Vishnepolsky, B.; Ninidze, T.; Karseladze, M.; Datukishvili, N. Analysis of Genetically Modified Maize Allergens by PCR. Bull. Georgian Natl. Acad. Sci. 2022, 16, 62–68. [Google Scholar]
- Datukishvili, N.; Gabriadze, I.; Kutateladze, T.; Karseladze, M.; Vishnepolsky, B. Comparative Evaluation of DNA Extraction Methods for Food Crops. Int. J. Food Sci. Technol. 2010, 45, 1316–1320. [Google Scholar] [CrossRef]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A Tool to Design Target-Specific Primers for Polymerase Chain Reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef]
- Kalendar, R.; Lee, D.; Schulman, A.H. Java Web Tools for PCR, in Silico PCR, and Oligonucleotide Assembly and Analysis. Genomics 2011, 98, 137–144. [Google Scholar] [CrossRef]
- Duan, Y.; Pi, Y.; Li, C.; Jiang, K. An Optimized Procedure for Detection of Genetically Modified DNA in Refined Vegetable Oils. Food Sci. Biotechnol. 2021, 30, 129–135. [Google Scholar] [CrossRef]
- Taberlet, P.; Gielly, L.; Pautou, G.; Bouvet, J. Universal Primers for Amplification of Three Non-Coding Regions of Chloroplast DNA. Plant Mol. Biol. 1991, 17, 1105–1109. [Google Scholar] [CrossRef]
- Bitskinashvili, K.; Gabriadze, I.; Kutateladze, T.; Vishnepolsky, B.; Mikeladze, D.; Datukishvili, N. Effects of Thermal-Acid Treatment on Degradation and Amplification of Wheat and Maize DNA. J. Food Nutr. Res. 2018, 57, 242–251. [Google Scholar]
- Gabriadze, I.; Kutateladze, T.; Vishnepolsky, B.; Karseladze, M.; Datukishvili, N. Application of PCR-Based Methods for Rapid Detection of Corn Ingredients in Processed Foods. IJNFS 2014, 3, 199. [Google Scholar] [CrossRef]
- Costa, J.; Silva, I.; Villa, C.; Mafra, I. A Novel Single-Tube Nested Real-Time PCR Method to Quantify Pistachio Nut as an Allergenic Food: Influence of Food Matrix. J. Food Compos. Anal. 2023, 115, 105042. [Google Scholar] [CrossRef]
- Pietsch, K.; Waiblinger, H.U.; Brodmann, P.; Wurz, A. Screeningverfahren Zur Identizierung Gentechnisch Veraenderter Pflanzlicher Lebensmittel. Dtsch. Lebensm.-Rundsch. 1997, 93, 35–38. [Google Scholar]
- Halabi, K.; Shafir, A.; Mayrose, I. PloiDB: The Plant Ploidy Database. New Phytol. 2023, 240, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.A.; Feldman, M. Evolution and Origin of Bread Wheat. Plant Cell 2022, 34, 2549–2567. [Google Scholar] [CrossRef] [PubMed]
- Gaut, B.S.; Le Thierry d’Ennequin, M.; Peek, A.S.; Sawkins, M.C. Maize as a Model for the Evolution of Plant Nuclear Genomes. Proc. Natl. Acad. Sci. USA 2000, 97, 7008–7015. [Google Scholar] [CrossRef]
- Pourmohammadi, K.; Abedi, E.; Hashemi, S.M.B. Gliadin and Glutenin Genomes and Their Effects on the Technological Aspect of Wheat-Based Products. Curr. Res. Food Sci. 2023, 7, 100622. [Google Scholar] [CrossRef] [PubMed]
- Swanson-Wagner, R.A.; Eichten, S.R.; Kumari, S.; Tiffin, P.; Stein, J.C.; Ware, D.; Springer, N.M. Pervasive Gene Content Variation and Copy Number Variation in Maize and Its Undomesticated Progenitor. Genome Res. 2010, 20, 1689–1699. [Google Scholar] [CrossRef]
- Cazares-Álvarez, J.E.; Báez-Astorga, P.A.; Arroyo-Becerra, A.; Maldonado-Mendoza, I.E. Genome-Wide Identification of a Maize Chitinase Gene Family and the Induction of Its Expression by Fusarium verticillioides (Sacc.) Nirenberg (1976) Infection. Genes 2024, 15, 1087. [Google Scholar] [CrossRef]
- Fang, C.; Wu, S.; Li, Z.; Pan, S.; Wu, Y.; An, X.; Long, Y.; Wei, X.; Wan, X. A Systematic Investigation of Lipid Transfer Proteins Involved in Male Fertility and Other Biological Processes in Maize. Int. J. Mol. Sci. 2023, 24, 1660. [Google Scholar] [CrossRef]




| Primer | Sequence 5′→3′ | Target Gene/ GenBank ID | Amplicon Size (bp) | Reference |
|---|---|---|---|---|
| Hglu-116f | AGGCTAAACAAACCTTACCGT | Wheat HMW-GS | 116 | This study |
| Hglu-116r | TTGTGATGCTCGGTGTTGTG | X61009.1 | ||
| Hglu-147f | TAGGCTAAACAAACCTTACCGTG | Wheat HMW-GS | 147 | This study |
| Hglu-147r | CGGTGGACTGTCGGTGAAT | X61009.1 | ||
| Hglu-169f | GGCATGCCGACAGGTCGTA | Wheat HMW-GS | 169 | This study |
| Hglu-169r | ACTTTGTTGGAGTTGCTGTGGT | X61009.1 | ||
| Hglu-228f | CAATGGCTGCAATCAGGGT | Wheat HMW-GS | 228 | This study |
| Hglu-228r | AACATGGTATGGGCTGTCGT | X61009.1 | ||
| Glu-83f | AATCCCGCTATGAGGCAATC | Wheat LMW-GS | 83 | This study |
| Glu-83f | AGATTGGATGGAACCCTGAAC | U86029.1 | ||
| Glu-93f | TTGCAGCCACACCAGATAG | Wheat LMW-GS | 93 | This study |
| Glu-93r | GTACAACGGCACATTAACACTG | U86029.1 | ||
| Glu-109f | GGTGGTTCCTGGGCTACTATAA | Wheat LMW-GS | 109 | This study |
| Glu-109r | GGAAGGTCTTCATGGTGGATTG | U86029.1 | ||
| Glu-259f | CAAGGTATTCCTCCAGCAGTGCAGC | Wheat LMW-GS | 259 | [56] |
| Glu-259r | GGGTTGGGAAACACATTGGCCCA | U86029.1 | ||
| zea14m-75f | GCCTCAACGCCGGTAAC | Maize Zea m 14 | 75 | This study |
| zea14m-75f | TGGAGGTGCTGATGGTGTA | DQ147199.1 | ||
| zea14m87f | GCCTCAACGCCGGTAAC | Maize Zea m 14 | 87 | This study |
| zea14m87r | TGGAGCAGTCGGTGGAG | DQ147199.1 | ||
| zea14m134f | CGCCCTGCATCTCCTAC | Maize Zea m 14 | 134 | This study |
| zea14m134r | AGCGTTCTTGAGGCAGTT | DQ147199.1 | ||
| zea8m85f | TCAACGGCATCAAGAACCA | Maize Zea m 8 | 85 | This study |
| zea8m85r | TTGTTGACGGCGCTCAG | GQ856537.1 | ||
| zea8m92f | AACGTGGCTAACGTGGTC | Maize Zea m 8 | 92 | This study |
| zea8m92r | CTCCGGGTGTAGAAGTTCTTG | GQ856537.1 | ||
| zea8m-100f | GGTGCGAACGTGGCTAAC | Maize Zea m 8 | 100 | This study |
| zea8m100r | CGCTCCGGGTGTAGAAGT | GQ856537.1 | ||
| zea8m-130f | GAACGTGGCTAACGTGGTCA | Maize Zea m 8 | 130 | [50] |
| zea8m-130r | GAAGCCCGGGTACTTGTTGA | GQ856537.1 | ||
| zein-94f | GACGATTCCACCCATGTTCTTA | Maize zein | 94 | This study |
| zein-94r | CGATGGCATGTCAACTCATTATTC | U25674.1 | ||
| zein102f | ACACCACCGACCATGGCAGC | Maize zein | 102 | [57] |
| zein102r | TGGTGGCAAGTGCGCTGGAA | U25674.1 | ||
| 18S-167f | GCAAGACCGAAACTCAAAGGA | 18S rRNA | 167 | [54] |
| 18S-167r | ACGACAGCCATGCAGCACC | X16077 | ||
| plant1 | CGAAATCGGTAGACGCTACG | Chloroplast genome | 500–600 | [55] |
| plant2 | GGGGATAGAGGGACTTGAAC | 000932.1 |
| Target Gene | Cycles | Initial Denaturation | Denaturation | Annealing | Elongation | Final Extension |
|---|---|---|---|---|---|---|
| Chloroplast genome | 35 | 95 °C for 4 min | 95 °C for 30 s | 62 °C for 30 s | 72 °C for 2 min | 72 °C for 5 min |
| 18S rRNA | 35 | 95 °C for 4 min | 95 °C for 40 s | 56 °C for 45 s | 72 °C for 45 s | 72 °C for 5 min |
| Maize Zein | 40 | 95 °C for 3 min | 95 °C for 30 s | 65 °C for 30 s | 72 °C for 35 s | 72 °C for 3 min |
| Maize Zea m 8 Maize Zea m 14 | 40 | 95 °C for 3 min | 95 °C for 30 s | 60 °C for 30 s | 72 °C for 35 s | 72 °C for 5 min |
| Wheat LMW glutenin | 35 | 94 °C 2 min | 94 °C 30 s | 60 °C 30 s | 72 °C 1 min | 72 °C 3 min |
| Wheat HMW glutenin | 35 | 94 °C 3 min | 95 °C 40 s | 56 °C 45 s | 72 °C 45 s | 72 °C 5 min |
| Samples | DNA Concentration (ng/μL) DNA Yield (ng/mg Food) | A260/A280 | A260/A230 | |
|---|---|---|---|---|
| Wheat flour | 154.12 ± 15.63 | 1.88 ± 0.00 | 2.189 ± 0.01 | |
| Dough | 136.30 ± 3.00 | 1.88 ± 0.01 | 2.27 ± 0.01 | |
| Baked at 180 °C | 10 min | 115.95 ± 5.54 | 1.89 ± 0.01 | 2.09 ± 0.08 |
| 20 min | 97.34 ± 0.64 | 1.88 ± 0.01 | 2.05 ± 0.07 | |
| 30 min | 102.83 ± 7.84 | 1.87 ± 0.02 | 2.18 ± 0.00 | |
| 40 min | 91.53 ± 17.93 | 1.87 ± 0.01 | 2.08 ± 0.01 | |
| 50 min | 68.21 ± 8.36 | 1.85 ± 0.01 | 2.05 ± 0.00 | |
| 60 min | 33.195 ± 6.71 | 1.84 ± 0.03 | 1.90 ± 0.25 | |
| Dough | 135.69 ± 17.37 | 1.88 ± 0.01 | 2.26 ± 0.02 | |
| Baked at 220 °C | 10 min | 125.35 ± 4.98 | 1.89 ± 0.00 | 2.20 ± 0.04 |
| 20 min | 120.80 ± 10.34 | 1.89 ± 0.01 | 2.19 ± 0.01 | |
| 30 min | 121.36 ± 2.46 | 1.91 ± 0.01 | 2.15 ± 0.03 | |
| 40 min | 95.43 ± 4.52 | 1.89 ± 0.00 | 2.09 ± 0.06 | |
| 50 min | 45.58 ± 1.30 | 1.85 ± 0.00 | 1.93 ± 0.01 | |
| 60 min | 35.34 ± 3.95 | 1.87 ± 0.01 | 1.91 ± 0.03 |
| Samples | DNA Concentration (ng/μL) DNA Yield (ng/mg Food) | A260/A280 | A260/A230 | |
|---|---|---|---|---|
| Maize flour | 81.59 ± 1.90 | 1.82 ± 0.04 | 2.46 ± 0.25 | |
| Dough | 59.61 ± 5.58 | 1.80 ± 0.02 | 2.57 ± 0.16 | |
| Baked at 180 °C | 10 min | 45.40 ± 3.21 | 1.88 ± 0.13 | 2.38 ± 0.42 |
| 20 min | 50.46 ± 0.64 | 1.88 ± 0.02 | 2.51 ± 0.27 | |
| 30 min | 42.93 ± 8.11 | 1.84 ± 0.03 | 2.94 ± 0.22 | |
| 40 min | 30.00 ± 11.18 | 1.89 ± 0.09 | 2.56 ± 0.19 | |
| 50 min | 8.89 ± 0.10 | 1.85 ± 0.05 | 3.95 ± 2.90 | |
| 60 min | 1.65 ± 1.80 | 1.33 ± 0.97 | 1.43 ± 2.19 | |
| Dough | 66.06 ± 5.01 | 1.80 ± 0.03 | 2.94 ± 0.23 | |
| Baked at 220 °C | 10 min | 44.13 ± 3.58 | 1.88 ± 0.02 | 2.77 ± 0.63 |
| 20 min | 33.69 ± 0.24 | 1.81 ± 0.07 | 3.36 ± 0.06 | |
| 30 min | 32.45 ± 3.65 | 1.82 ± 0.03 | 2.81 ± 0.19 | |
| 40 min | 18.00 ± 5.35 | 1.20 ± 0.16 | 0.61 ± 0.02 | |
| 50 min | 11.29 ± 1.63 | 1.29 ± 0.09 | 0.65 ± 0.01 | |
| 60 min | 2.36 ± 1.57 | 1.14 ± 0.27 | 0.46 ± 0.05 |
| Target Gene | Amplicon Name | Amplicon Size (bp) | Baking Temp (°C) | Baking Time (min) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | 40 | 50 | 60 | ||||
| Wheat HMW-GS | HGLU-116 | 116 | 180 °C | + | + | + | + | + | + | + |
| 220 °C | + | + | + | + | + | + | + | |||
| Hglu-147 | 147 | 180 °C | + | + | + | + | + | + | + | |
| 200 °C | + | + | + | + | + | + | + | |||
| Hglu-169 | 169 | 180 °C | + | + | + | + | + | + | + | |
| 200 °C | + | + | + | + | + | + | + | |||
| Hglu-228 | 228 | 180 °C | + | + | + | + | + | + | + | |
| 200 °C | + | + | + | + | + | + | + | |||
| Wheat LMW-GS | Glu-83 | 83 | 180 °C | + | + | + | + | + | + | + |
| 200 °C | + | + | + | + | + | + | + | |||
| Glu-93 | 93 | 180 °C | + | + | + | + | + | + | + | |
| 220 °C | + | + | + | + | + | + | + | |||
| Glu-109f | 109 | 180 °C | + | + | + | + | + | + | + | |
| 220 °C | + | + | + | + | + | + | + | |||
| Glu-259f | 259 | 180 °C | + | + | + | + | + | + | + | |
| 220 °C | + | + | + | + | + | + | - | |||
| Maize Zea m 14 | zea14m-75 | 75 | 180 °C | + | + | + | + | + | + | - |
| 220 °C | + | + | + | + | + | - | - | |||
| zea14m87 | 87 | 180 °C | + | + | + | + | + | - | - | |
| 220 °C | + | + | + | + | - | - | - | |||
| zea14m134 | 134 | 180 °C | + | + | + | + | - | - | - | |
| 220 °C | + | + | + | - | - | - | - | |||
| Maize Zea m 8 | zea8m85 | 85 | 180 °C | + | + | + | + | + | + | - |
| 220 °C | + | + | + | + | + | - | - | |||
| zea8m92f | 92 | 180 °C | + | + | + | + | + | + | + | |
| 220 °C | + | + | + | + | + | - | - | |||
| zea8m-100f | 100 | 180 °C | + | + | + | + | + | + | - | |
| 220 °C | + | + | + | + | + | - | - | |||
| zea8m-130 | 130 | 180 °C | + | + | + | + | + | - | - | |
| 220 °C | + | + | + | + | - | - | - | |||
| Maize zein | zein-94f | 94 | 180 °C | + | + | + | + | + | + | - |
| 220 °C | + | + | + | + | + | - | - | |||
| zein102f | 102 | 180 °C | + | + | + | + | + | + | - | |
| 220 °C | + | + | + | + | + | - | - | |||
| 18S rRNA | Maize and wheat 18S-167f | 167 | 180 °C | + | + | + | + | + | + | + |
| 220 °C | + | + | + | + | + | + | + | |||
| Chloroplast genome | Maize plant | 500 | 180 °C | + | + | + | + | + | - | - |
| 220 °C | + | + | + | + | - | - | - | |||
| Chloroplast genome | Wheat plant | 600–700 | 180 °C | + | + | + | + | + | + | - |
| 220 °C | + | + | + | + | + | - | - | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ninidze, T.; Koberidze, T.; Bitskinashvili, K.; Kutateladze, T.; Vishnepolsky, B.; Datukishvili, N. Development of PCR Methods for Detecting Wheat and Maize Allergens in Food. BioTech 2025, 14, 78. https://doi.org/10.3390/biotech14040078
Ninidze T, Koberidze T, Bitskinashvili K, Kutateladze T, Vishnepolsky B, Datukishvili N. Development of PCR Methods for Detecting Wheat and Maize Allergens in Food. BioTech. 2025; 14(4):78. https://doi.org/10.3390/biotech14040078
Chicago/Turabian StyleNinidze, Tata, Tamar Koberidze, Kakha Bitskinashvili, Tamara Kutateladze, Boris Vishnepolsky, and Nelly Datukishvili. 2025. "Development of PCR Methods for Detecting Wheat and Maize Allergens in Food" BioTech 14, no. 4: 78. https://doi.org/10.3390/biotech14040078
APA StyleNinidze, T., Koberidze, T., Bitskinashvili, K., Kutateladze, T., Vishnepolsky, B., & Datukishvili, N. (2025). Development of PCR Methods for Detecting Wheat and Maize Allergens in Food. BioTech, 14(4), 78. https://doi.org/10.3390/biotech14040078






