Strategies for Increasing the Throughput of Genetic Screening: Lessons Learned from the COVID-19 Pandemic within a University Community
Abstract
1. Introduction
2. Materials and Methods
2.1. IPS COVID Lab Accreditation and Admission Proceedings
2.2. Sample Collection and Storage
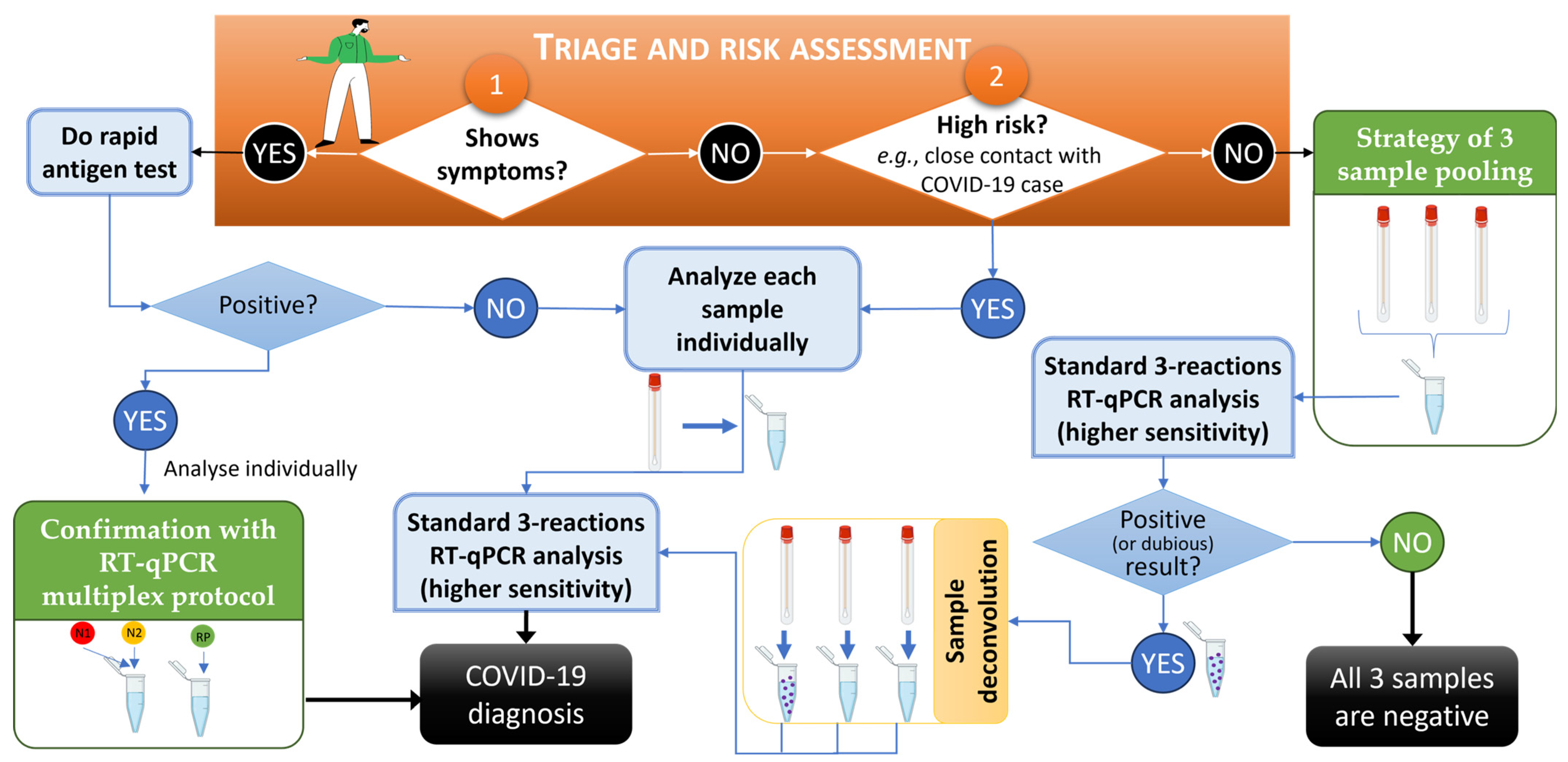
2.3. Sample Pooling and Procedure Validation
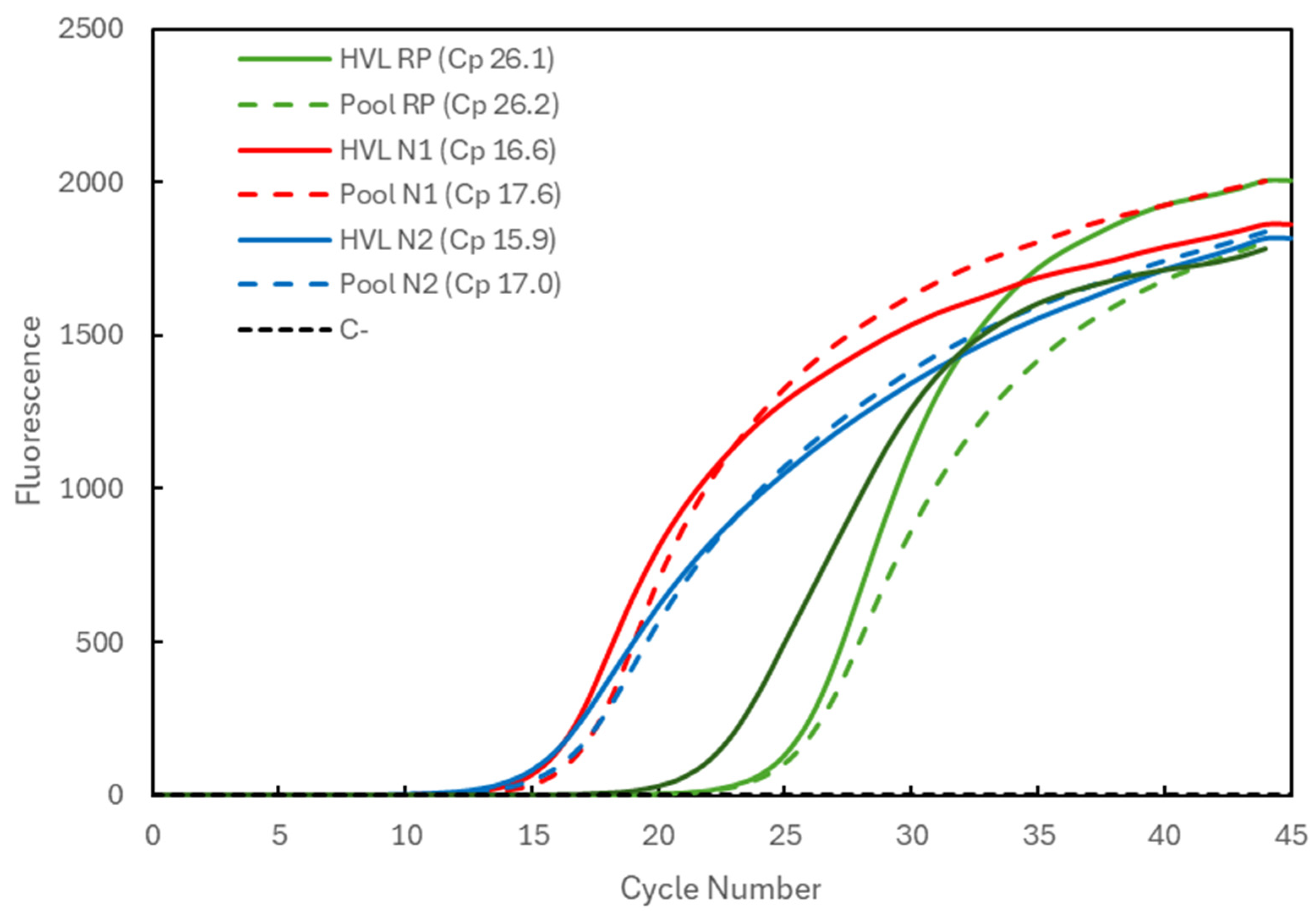
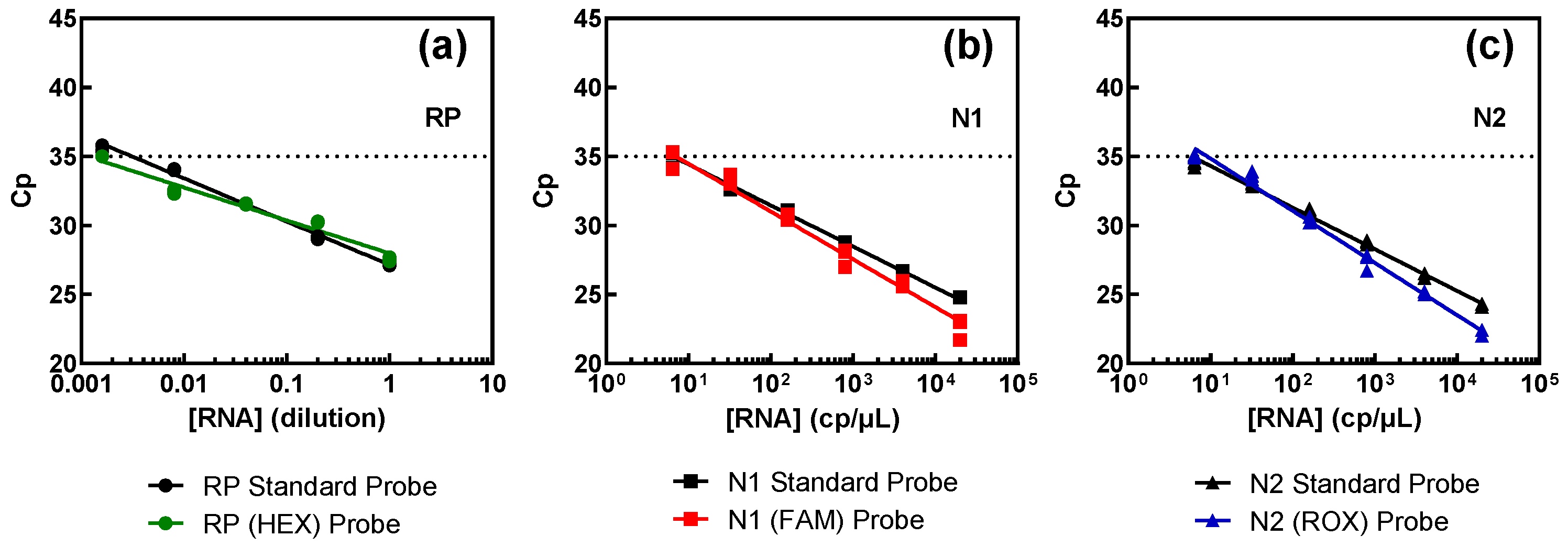
2.4. Multiplex RT-qPCR and Validation
3. Results and Discussion
3.1. Sample Pooling
3.2. Multiplex RT-qPCR
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Long, C.; Xu, H.; Shen, Q.; Zhang, X.; Fan, B.; Wang, C.; Zeng, B.; Li, Z.; Li, X.; Li, H. Diagnosis of the Coronavirus Disease (COVID-19): RRT-PCR or CT? Eur. J. Radiol. 2020, 126, 108961. [Google Scholar] [CrossRef]
- Direção Geral de Saúde (DGS) Portugal. COVID-19: Estratégia Nacional de Testes Para SARS-CoV-2; Direção Geral de Saúde: Lisboa, Portugal, 2022; pp. 1–14. [Google Scholar]
- Daniel, E.A.; Anbalagan, S.; Muthuramalingam, K.; Karunaianantham, R.; Karunakaran, L.P.; Nesakumar, M.; Selvachithiram, M.; Pattabiraman, S.; Natarajan, S.; Tripathy, S.P.; et al. Pooled Testing Strategies for SARS-CoV-2 Diagnosis: A Comprehensive Review. Diagn. Microbiol. Infect. Dis. 2021, 101, 115432. [Google Scholar] [CrossRef]
- Gul, I.; Zhai, S.; Zhong, X.; Chen, Q.; Yuan, X.; Du, Z.; Chen, Z.; Raheem, M.A.; Deng, L.; Leeansyah, E.; et al. Angiotensin-Converting Enzyme 2-Based Biosensing Modalities and Devices for Coronavirus Detection. Biosensors 2022, 12, 984. [Google Scholar] [CrossRef]
- Pavia, C.S.; Plummer, M.M. The Evolution of Rapid Antigen Detection Systems and Their Application for COVID-19 and Other Serious Respiratory Infectious Diseases. J. Microbiol. Immunol. Infect. 2021, 54, 776–786. [Google Scholar] [CrossRef]
- Dorfman, R. The Detection of Defective Members of Large Populations. Ann. Math. Stat. 1943, 14, 436–440. [Google Scholar] [CrossRef]
- Hanel, R.; Thurner, S. Boosting Test-Efficiency by Pooled Testing for SARS-CoV-2-Formula for Optimal Pool Size. PLoS ONE 2020, 15, e0240652. [Google Scholar] [CrossRef]
- Abdalhamid, B.; Bilder, C.R.; McCutchen, E.L.; Hinrichs, S.H.; Koepsell, S.A.; Iwen, P.C. Assessment of Specimen Pooling to Conserve SARS CoV-2 Testing Resources. Am. J. Clin. Pathol. 2020, 153, 715–718. [Google Scholar] [CrossRef]
- Baggio, F.; Hetzel, U.; Prähauser, B.; Dervas, E.; Michalopoulou, E.; Thiele, T.; Kipar, A.; Hepojoki, J. A Multiplex RT-PCR Method for the Detection of Reptarenavirus Infection. Viruses 2023, 15, 2313. [Google Scholar] [CrossRef]
- Chung, H.Y.; Jian, M.J.; Chang, C.K.; Lin, J.C.; Yeh, K.M.; Chen, C.W.; Chiu, S.K.; Wang, Y.H.; Liao, S.J.; Li, S.Y.; et al. Novel Dual Multiplex Real-Time RT-PCR Assays for the Rapid Detection of SARS-CoV-2, Influenza A/B, and Respiratory Syncytial Virus Using the BD MAX Open System. Emerg. Microbes Infect. 2021, 10, 161–166. [Google Scholar] [CrossRef]
- Koliopoulos, P.; Kayange, N.M.; Daniel, T.; Huth, F.; Gröndahl, B.; Medina-Montaño, G.C.; Pretsch, L.; Klüber, J.; Schmidt, C.; Züchner, A.; et al. Multiplex-RT-PCR-ELISA Panel for Detecting Mosquito-Borne Pathogens: Plasmodium Sp. Preserved and Eluted from Dried Blood Spots on Sample Cards. Malar. J. 2021, 20, 66. [Google Scholar] [CrossRef]
- Gene LinkTM Fluorescent Dyes Applications- Gene Link TM. Available online: https://www.genelink.com/oligo_modifications_reference/OMR_mod_category_applications.asp?mod_sp_cat_id=18 (accessed on 10 May 2024).
- Tombuloglu, H.; Sabit, H.; Al-Khallaf, H.; Kabanja, J.H.; Alsaeed, M.; Al-Saleh, N.; Al-Suhaimi, E. Multiplex Real-Time RT-PCR Method for the Diagnosis of SARS-CoV-2 by Targeting Viral N, RdRP and Human RP Genes. Sci. Rep. 2022, 12, 2853. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Laboratory Testing for Coronavirus Disease (COVID-19) in Suspected Human Cases: Interim Guidance, 19 March 2020; World Health Organization: Geneva, Switzerland, 2020; 7p. [Google Scholar]
- Mahmoud, S.A.; Ibrahim, E.; Thakre, B.; Teddy, J.G.; Raheja, P.; Ganesan, S.; Zaher, W.A. Evaluation of Pooling of Samples for Testing SARS-CoV-2 for Mass Screening of COVID-19. BMC Infect. Dis. 2021, 21, 360. [Google Scholar] [CrossRef]
- Mastrianni, D.; Falivena, R.; Brooks, T.; McDermott, B.; Tan, J.; Vandell, R.; Holland, M. Pooled Testing for SARS-CoV-2 in Hospitalized Patients. J. Hosp. Med. 2020, 15, 538–539. [Google Scholar] [CrossRef]
- Ball, J.; McNally, A. Pooled Testing for SARS-CoV-2 Could Provide the Solution to UK’s Testing Strategy. BMJ 2020, 371, m4312. [Google Scholar] [CrossRef]
- Mishra, B.; Behera, B.; Mohanty, M.; Ravindra, A.; Ranjan, J. Challenges and Issues of SARS-CoV-2 Pool Testing. Lancet Infect. Dis. 2020, 20, 1233. [Google Scholar] [CrossRef]
- Afzal, A. Molecular Diagnostic Technologies for COVID-19: Limitations and Challenges. J. Adv. Res. 2020, 26, 149–159. [Google Scholar] [CrossRef]
- Arevalo-Rodriguez, I.; Buitrago-Garcia, D.; Simancas-Racines, D.; Zambrano-Achig, P.; Del Campo, R.; Ciapponi, A.; Sued, O.; Martinez-García, L.; Rutjes, A.W.; Low, N.; et al. False-Negative Results of Initial RT-PCR Assays for COVID-19: A Systematic Review. PLoS ONE 2020, 15, e0242958. [Google Scholar] [CrossRef]
- Lohse, S.; Pfuhl, T.; Berkó-Göttel, B.; Rissland, J.; Geißler, T.; Gärtner, B.; Becker, S.L.; Schneitler, S.; Smola, S. Pooling of Samples for Testing for SARS-CoV-2 in Asymptomatic People. Lancet Infect. Dis. 2020, 20, 1231–1232. [Google Scholar] [CrossRef]
- Agoti, C.N.; Mutunga, M.; Lambisia, A.W.; Kimani, D.; Cheruiyot, R.; Kiyuka, P.; Lewa, C.; Gicheru, E.; Tendwa, M.; Said Mohammed, K.; et al. Pooled Testing Conserves SARS-CoV-2 Laboratory Resources and Improves Test Turn-around Time: Experience on the Kenyan Coast. Wellcome Open Res. 2021, 5, 186. [Google Scholar] [CrossRef]
- Pikovski, A.; Bentele, K. Pooling of Coronavirus Tests under Unknown Prevalence. Epidemiol. Infect. 2020, 148, e183. [Google Scholar] [CrossRef]
- Yelin, I.; Aharony, N.; Tamar, E.S.; Argoetti, A.; Messer, E.; Berenbaum, D.; Shafran, E.; Kuzli, A.; Gandali, N.; Shkedi, O.; et al. Evaluation of COVID-19 RT-QPCR Test in Multi Sample Pools. Clin. Infect. Dis. 2020, 71, 2073–2078. [Google Scholar] [CrossRef] [PubMed]
- National Center for Immunization and Respiratory Diseases (U.S.); Division of Viral Diseases. Evaluating and Testing Persons for Coronavirus Disease 2019 (COVID-19); U. S. Centers for Disease Control and Prevention: Atlanta, GA, USA, 2020. Available online: https://stacks.cdc.gov/view/cdc/87523 (accessed on 24 November 2021).
- D’Arienzo, M.; Coniglio, A. Assessment of the SARS-CoV-2 Basic Reproduction Number, R0, Based on the Early Phase of COVID-19 Outbreak in Italy. Biosaf. Health 2020, 2, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Grohmann, L.; Barbante, A.; Eriksson, R.; Gatto, F.; Georgieva, T.; Huber, I.; Hulin, J.; Köppel, R.; Marchesi, U.; Marmin, L.; et al. Guidance Document on Multiplex Real-Time PCR Methods; European Comission–Joint Research Center: Brussels, Belgium, 2021; Available online: https://data.europa.eu/doi/10.2760/243914 (accessed on 5 January 2022).
- Gul, I.; Liu, C.; Yuan, X.; Du, Z.; Zhai, S.; Lei, Z.; Chen, Q.; Raheem, M.A.; He, Q.; Hu, Q.; et al. Current and Perspective Sensing Methods for Monkeypox Virus. Bioengineering 2022, 9, 571. [Google Scholar] [CrossRef]
- Chen, Q.; Gul, I.; Liu, C.; Lei, Z.; Li, X.; Raheem, M.A.; He, Q.; Haihui, Z.; Leeansyah, E.; Zhang, C.Y.; et al. CRISPR–Cas12-based Field-deployable System for Rapid Detection of Synthetic DNA Sequence of the Monkeypox Virus Genome. J. Med. Virol. 2023, 95, e28385. [Google Scholar] [CrossRef]


| Fluorophore | Excitation Max (nm) | Emission Max (nm) | Extinction Coefficient * | Color ** | Quencher |
|---|---|---|---|---|---|
| FAM | 495 | 520 | 75,850 | Yellow-Green | BHQ-1 |
| HEX | 535 | 556 | 98,000 | Yellow | TAMRA |
| ROX | 575 | 602 | 82,000 | Orange-Red | BHQ-2 |
| Gene Target | Fluorophore 5′ | Sequence (5′ → 3′) | Quencher 3′ |
|---|---|---|---|
| RP | HEX | TTC TGA CCT GAA GGC TCT GCG CG | TAMRA |
| N1 | FAM | ACC CCG CAT TAC GTT TGG TGG ACC | BHQ-1 |
| N2 | ROX | ACA ATT TGC CCC CAG CGC TTC AG | BHQ-2 |
| RP 1 | N1 | N2 | ||||
|---|---|---|---|---|---|---|
| Probe | Standard | Multiplex | Standard | Simplex | Standard | Simplex |
| Efficiency | 109 ± 2% | 126 ± 14% | 117 ± 3% | 95 ± 4% | 114 ± 3% | 84 ± 2% |
| Detection limit (Cp < 35) | DF 2 625 | DF 2 625 | ≈6.4 cp/µL 3 DF 2 15,625 | ≈6.4 cp/µL 3 DF 2 15,625 | ≈6.4 cp/µL 3 DF 2 15,625 | ≈6.4 cp/µL 3 DF 2 15,625 |
| [RNA] (cp/µL) | Dilutions Performed in RNA Matrix * | Dilutions Performed in Water | ||||||
|---|---|---|---|---|---|---|---|---|
| Triplex (RP + N1 + N2) | Duplex (N1 + N2) | Duplex (RP + N1) | Duplex (RP + N2) | Triplex (RP + N1 + N2) | Duplex (N1 + N2) | Duplex (RP + N1) | Duplex (RP + N2) | |
| 20,000 |  |  |  |  |  |  |  |  |
| 4000 |  |  |  |  |  |  |  |  |
| 800 |  |  |  |  |  |  |  |  |
| 160 |  |  |  |  |  |  |  |  |
| 32 |  |  |  |  |  |  |  |  |
| 6.4 |  |  |  |  |  |  |  |  |
 —100% of the target is present;
—100% of the target is present;  —75% of the target is present;
—75% of the target is present; 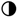 —50% of the target is present;
—50% of the target is present;  —25% of the target is present; and
—25% of the target is present; and  0% is present (no target detected) from a total of 4–6 replicates.
0% is present (no target detected) from a total of 4–6 replicates.Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miguel, F.; Baleizão, A.R.; Gomes, A.G.; Caria, H.; Serralha, F.N.; Justino, M.C. Strategies for Increasing the Throughput of Genetic Screening: Lessons Learned from the COVID-19 Pandemic within a University Community. BioTech 2024, 13, 26. https://doi.org/10.3390/biotech13030026
Miguel F, Baleizão AR, Gomes AG, Caria H, Serralha FN, Justino MC. Strategies for Increasing the Throughput of Genetic Screening: Lessons Learned from the COVID-19 Pandemic within a University Community. BioTech. 2024; 13(3):26. https://doi.org/10.3390/biotech13030026
Chicago/Turabian StyleMiguel, Fernanda, A. Raquel Baleizão, A. Gabriela Gomes, Helena Caria, Fátima N. Serralha, and Marta C. Justino. 2024. "Strategies for Increasing the Throughput of Genetic Screening: Lessons Learned from the COVID-19 Pandemic within a University Community" BioTech 13, no. 3: 26. https://doi.org/10.3390/biotech13030026
APA StyleMiguel, F., Baleizão, A. R., Gomes, A. G., Caria, H., Serralha, F. N., & Justino, M. C. (2024). Strategies for Increasing the Throughput of Genetic Screening: Lessons Learned from the COVID-19 Pandemic within a University Community. BioTech, 13(3), 26. https://doi.org/10.3390/biotech13030026







