Hydroponics with Microalgae and Cyanobacteria: Emerging Trends and Opportunities in Modern Agriculture
Abstract
1. Introduction
2. Microalgae as Biostimulants
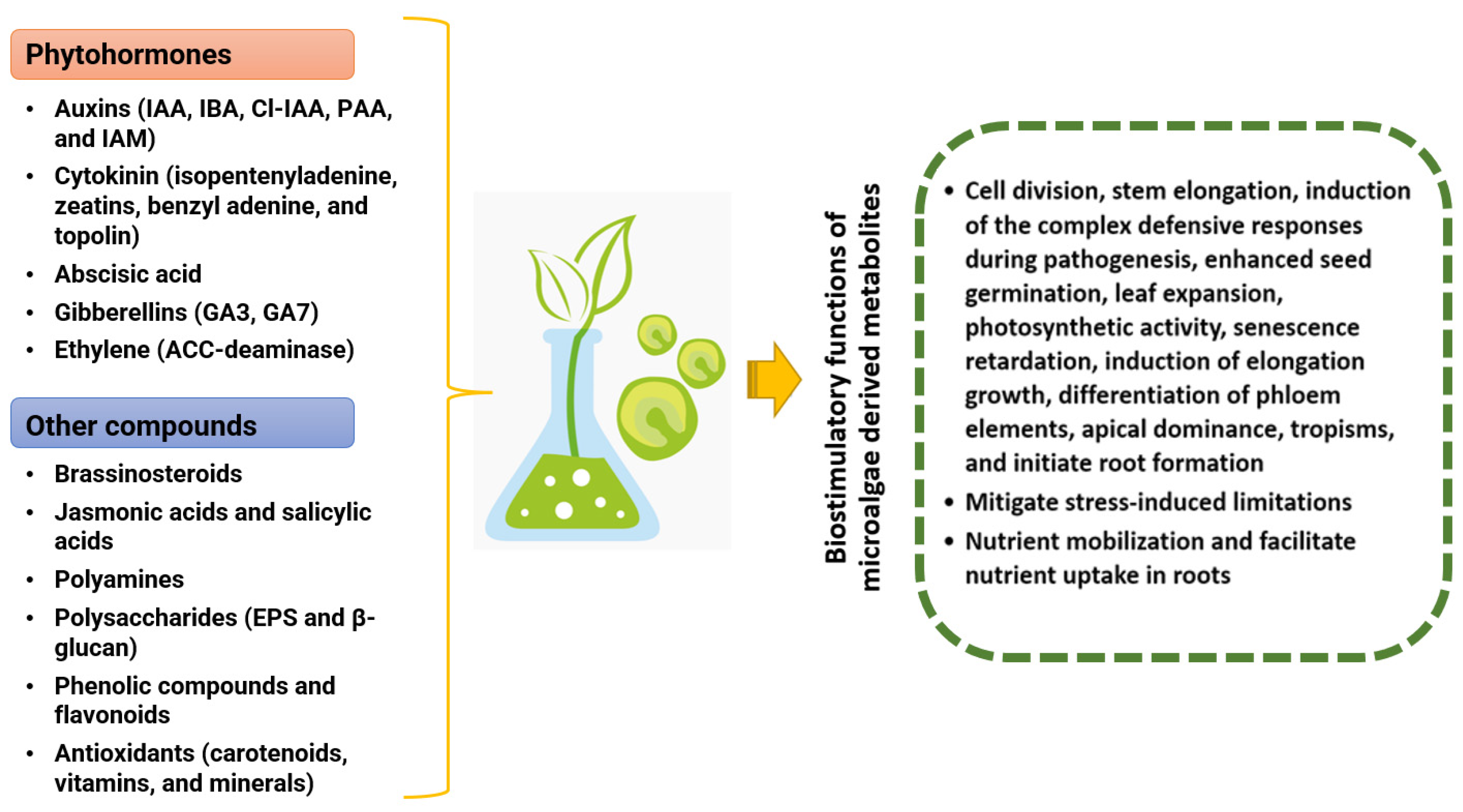
2.1. Phytohormones
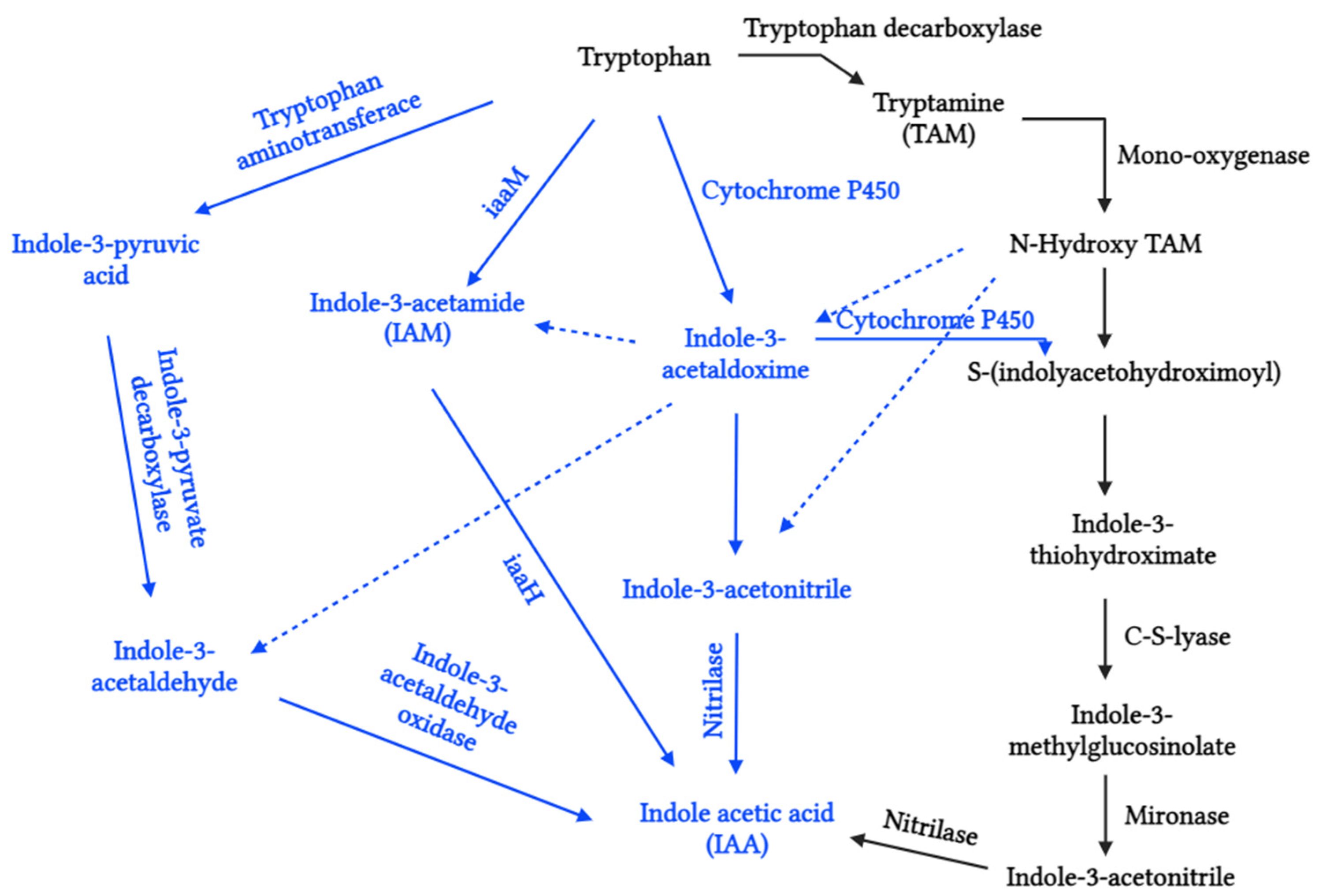
2.1.1. Auxins
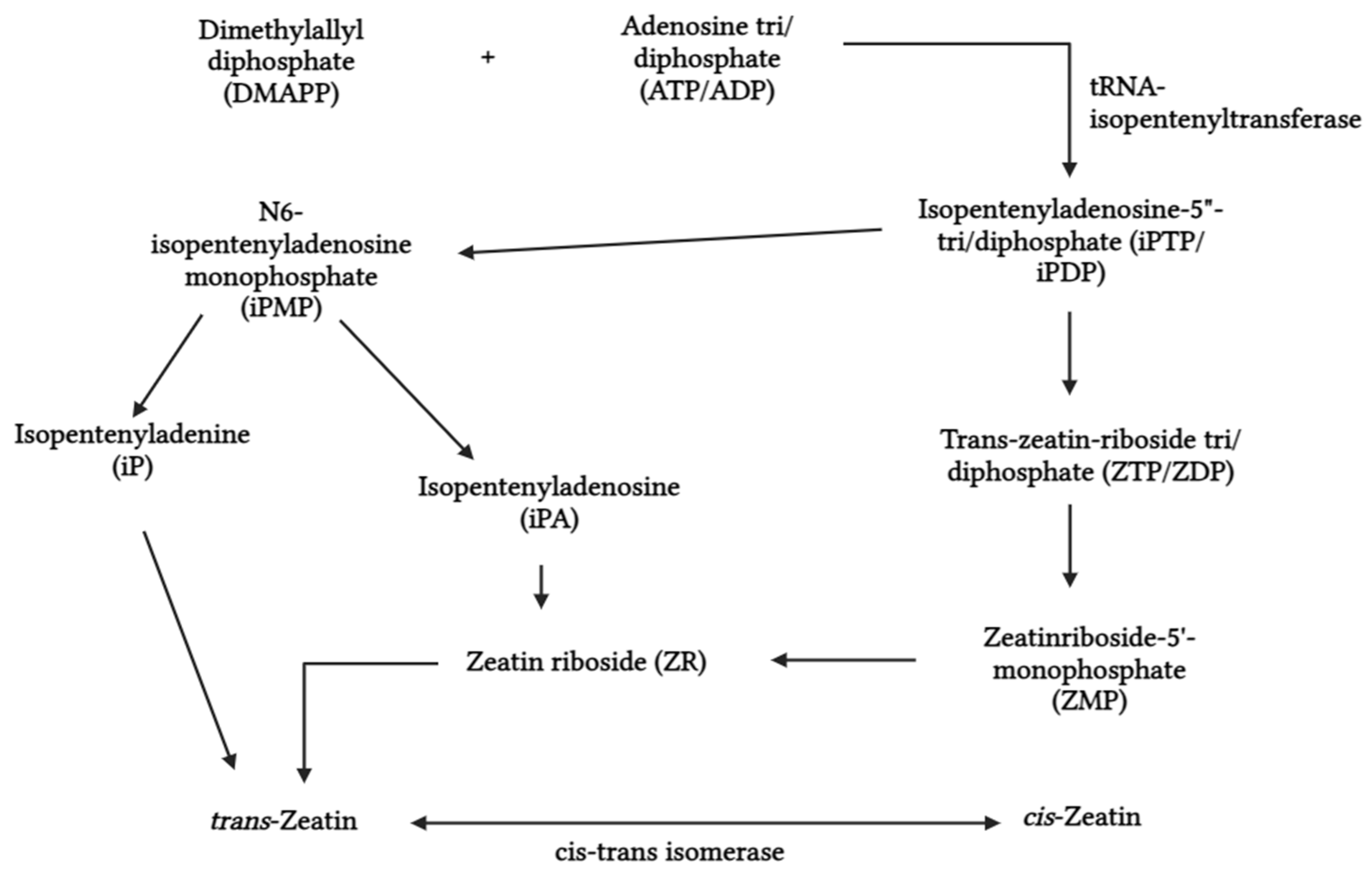
2.1.2. Cytokinins
2.1.3. Gibberellic Acid
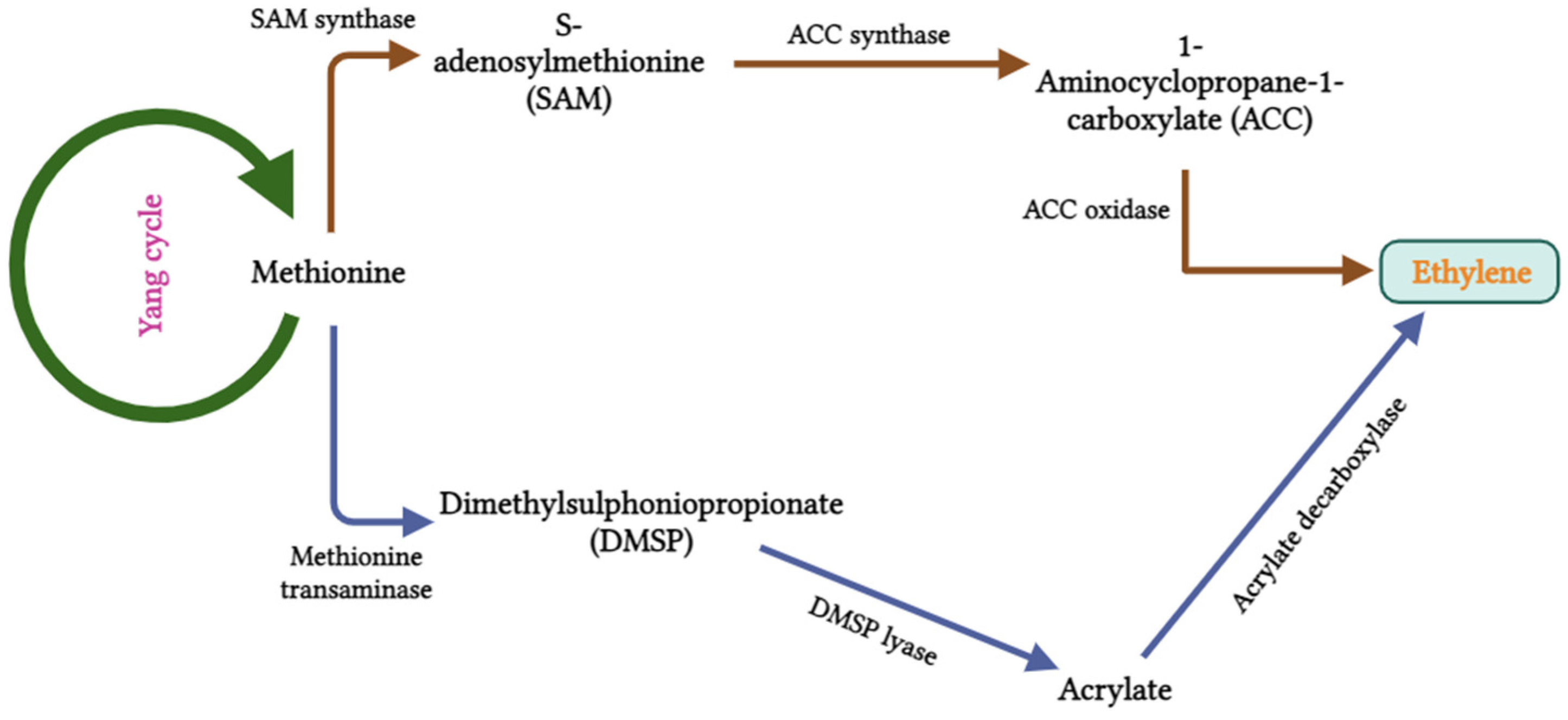
2.1.4. Ethylene
2.1.5. Abscisic Acid
2.1.6. Jasmonic and Salicylic Acids
2.2. Hormone-like Compounds as Biostimulants
2.2.1. Brassinosteroids
2.2.2. Polyamines
2.2.3. Polysaccharides
2.2.4. Phenolic Compounds
3. Abiotic Stress Tolerance
4. Modern Agriculture: Hi-Tech Indoor Farming
4.1. Hydroponics
Vertical System
4.2. Aeroponics
4.3. Aquaponics
5. Performance of Microalgae in Hydroponic Systems
5.1. Incorporating Microalgae and Hydroponics in Circular Bioeconomy and Sustainability
| Microalgae | Plants | N Removal | P Removal | Leaf Number | Fresh Weight | Dry Weight | Shoot Length | Root Length | Biomass Productivity | Biomass Yield | Other Results | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chlorella vulgaris | Swiss chard | TN: 92.41–97.48% | TP: 96.41–99.96% | 18.56% | 17.13% | - | 36.98% | - | - | [222] | ||
| C. vulgaris, Scenedesmus quadricauda | Tomato | - | - | - | 11.95 g | 0.90 g | 130% | 0.77–1.02 g L−1 | 0.019–022 g L−1 day−1 | [162] | ||
| Chlorella infusionum | Tomato | TN: 84% | TP: 44% | - | - | - | 22.95 g | - | 32–54.24 g dm−3 d−1 | [38] | ||
| Chlorella sp., Scenedesmus sp., Synechocystis sp., Spirulina sp. | Tomato | NO3: 41–84%, NH4: 88–99% | PO43⁻: 60–94% | 31–43% | 2.19–6.05 g | 0.16–0.50 g | 17.37–19.25 cm | 10.37–25.75 cm | 1.12–3.18 g | 0.066–0.149 g day−1 | K removal: 82–95%, | [39] |
| C. vulgaris | Lettuce | - | - | 17.75–20.25 plant−1 | 237.56–243.31 g plant−1 | 6.53–7.29 g plant−1 | - | - | - | - | - | [223] |
| C. vulgaris (UTEX 2714) | Arugula, Purple kohlrabi, Lettuce | TN: 94.6–97.6% | TP: 92.9% | - | - | - | 0.43–0.80 cm·d−1 | 0.43–1.85 cm·d−1 | 0.40–0.71 g·L−1 | 0.78–1.86 g·m−2·d−1 | Dissolved Oxygen: 7.89–8.23 g·mL−1, TDS removal: 56.7% |
5.2. Plant Growth Promotion
5.2.1. Productivity
5.2.2. Biomass
5.2.3. Plant Height
5.2.4. Leaf Count
5.2.5. Pigmentation
5.3. Nutrient Reduction
5.4. Dissolved Oxygen Content
6. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- UN Department of Economic and Social Affairs. Population Division. World Population Prospects: The 2017 Revision. Key Findings and Advance Tables. Working Paper No. ESA/P/WP/248. 2017. Available online: https://population.un.org/wpp/Publications/Files/WPP2017_KeyFindings.pdf (accessed on 23 December 2023).
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 29, 8. [Google Scholar] [CrossRef] [PubMed]
- Goh, Y.S.; Hum, Y.C.; Lee, Y.L.; Lai, K.W.; Yap, W.S.; Tee, Y.K. A meta-analysis: Food production and vegetable crop yields of hydroponics. Sci. Hortic. 2023, 321, 112339. [Google Scholar] [CrossRef]
- Rezaei, E.E.; Webber, H.; Asseng, S.; Boote, K.; Durand, J.L.; Ewert, F.; Martre, P.; MacCarthy, D.S. Climate change impacts on crop yields. Nat. Rev. Earth Environ. 2023, 4, 831–846. [Google Scholar] [CrossRef]
- Thomaier, S.; Specht, K.; Henckel, D.; Dierich, A.; Siebert, R.; Freisinger, U.B.; Sawicka, M. Farming in and on urban buildings: Present practice and specific novelties of zero-acreage farming (Zfarming). Renew. Agric. Food Syst. 2015, 30, 43–54. [Google Scholar] [CrossRef]
- Wang, L.; Ning, S.; Zheng, W.; Guo, J.; Li, Y.; Li, Y.; Chen, X.; Ben-Gal, A.; Wei, X. Performance analysis of two typical greenhouse lettuce production systems: Commercial hydroponic production and traditional soil cultivation. Front. Plant Sci. 2023, 14, 1165856. [Google Scholar] [CrossRef] [PubMed]
- Goodman, W.; Minner, J. Will the urban agricultural revolution be vertical and soilless? a case study of controlled environment agriculture in New York City. Land Use Policy 2019, 83, 160–173. [Google Scholar] [CrossRef]
- Savvas, D.; Gruda, N. Application of soilless culture technologies in the modern greenhouse industry—A review. Eur. J. Hortic. Sci. 2018, 83, 280–293. [Google Scholar] [CrossRef]
- Urrestarazu, M. State of the art and new trends of soilless culture in Spain and in emerging countries. Acta Hortic. 2013, 1013, 305–312. [Google Scholar] [CrossRef]
- Ferguson, S.D.; Saliga, R.; Omaye, S.T. Investigating the effects of hydroponic media on quality of greenhouse grown leafy greens. Int. J. Agric. Ext. 2014, 2, 227–234. [Google Scholar]
- Hydroponics Market Size, Share & Trends Analysis Report By Type (Aggregate Systems, Liquid Systems), By Crops (Tomatoes, Lettuce, Peppers, Cucumbers, Herbs), By Region, and Segment Forecasts, 2021–2028. 2021. Available online: https://www.researchandmarkets.com/reports/5457654/hydroponics-market-size-share-and-trends-analysis (accessed on 23 March 2024).
- Tatas, K.; Al-Zoubi, A.; Christofides, N.; Zannettis, C.; Chrysostomou, M.; Panteli, S.; Antoniou, A. Reliable IoT-based monitoring and control of hydroponic systems. Technologies 2022, 10, 26. [Google Scholar] [CrossRef]
- Barbosa, G.L.; Gadelha, F.D.A.; Kublik, N.; Proctor, A.; Reichelm, L.; Weissinger, E.; Wohlleb, G.M.; Halden, R.U. Comparison of land, water, and energy requirements of lettuce grown using hydroponic vs. conventional agricultural methods. Int. J. Environ. Res. Public Health 2015, 12, 6879–6891. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.P. Pesticides, environment, and safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Rahman, K.M.A.; Zhang, D. Effects of fertilizer broadcasting on the excessive use of inorganic fertilizers and environmental sustainability. Sustainability 2018, 10, 759. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil. 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [PubMed]
- Ricci, M.; Tilbury, L.; Daridon, B.; Sukalac, K. General principles to justify plant biostimulant claims. Front Plant Sci. 2019, 10, 444124. [Google Scholar] [CrossRef] [PubMed]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Fact. 2018, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Tsaloglou, M.N. Microalgae: Current Research and Applications; Caister Academic Press: Poole, UK, 2016. [Google Scholar]
- Garcia-Gonzalez, J.; Sommerfeld, M. Biofertilizer and biostimulant properties of the microalga Acutodesmus dimorphus. J. Appl. Phycol. 2016, 28, 1051–1061. [Google Scholar] [CrossRef]
- El Arroussi, H.E.; Benhima, R.; Elbaouchi, A.; Sijilmassi, B.; Mernissi, N.E.; Aafsar, A.; Meftah-Kadmiri, I.; Bendaou, N.; Smouni, A. Dunaliella salina exopolysaccharides: A promising biostimulant for salt stress tolerance in tomato (Solanum lycopersicum). J. Appl. Phycol. 2018, 30, 2929–2941. [Google Scholar] [CrossRef]
- Rachidi, F.; Benhima, R.; Sbabou, L.; El Arroussi, H. Microalgae polysaccharides bio-stimulating effect on tomato plants: Growth and metabolic distribution. Biotechnol. Rep. 2020, 25, e00426. [Google Scholar] [CrossRef] [PubMed]
- Kapoore, R.V.; Wood, E.E.; Llewellyn, C.A. Algae biostimulants: A critical look at microalgal biostimulants for sustainable agricultural practices. Biotechnol. Adv. 2021, 49, 107754. [Google Scholar] [CrossRef] [PubMed]
- Parmar, P.; Kumar, R.; Neha, Y.; Srivatsan, V. Microalgae as next generation plant growth additives: Functions, applications, challenges and circular bioeconomy based solutions. Front. Plant Sci. 2023, 14, 1073546. [Google Scholar] [CrossRef] [PubMed]
- Chiaiese, P.; Corrado, G.; Colla, G.; Kyriacou, M.C.; Rouphael, Y. Renewable sources of plant biostimulation: Microalgae as a sustainable means to improve crop performance. Front. Plant Sci. 2018, 9, 1782. [Google Scholar] [CrossRef] [PubMed]
- Carillo, P.; Ciarmiello, L.F.; Woodrow, P.; Corrado, G.; Chiaiese, P.; Rouphael, Y. Enhancing sustainability by improving plant salt tolerance through macro-and micro-algal biostimulants. Biology 2020, 9, 253. [Google Scholar] [CrossRef] [PubMed]
- Colla, G.; Rouphael, Y. Microalgae: New source of plant biostimulants. Agronomy 2020, 10, 1240. [Google Scholar] [CrossRef]
- Braun, J.C.; Colla, L.M. Use of microalgae for the development of biofertilizers and biostimulants. Bioenergy Res. 2023, 16(1), 289–310. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Synergistic biostimulatory action: Designing the next generation of plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1655. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Korra, T.; Singh, U.; Singh, S.; Bisen, K. Microalgal based biostimulants as alleviator of biotic and abiotic stresses in crop plants. In New and Future Developments in Microbial Biotechnology and Bioengineering; Singh, H.B., Vaishnav, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 195–216. [Google Scholar]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Alvarez, A.L.; Weyers, S.L.; Goemann, H.M.; Peyton, B.M.; Gardner, R.D. Microalgae, soil and plants: A critical review of microalgae as renewable resources for agriculture. Algal Res. 2021, 54, 102200. [Google Scholar] [CrossRef]
- Kumar, M.; Prasanna, R.; Bidyarani, N.; Babu, S.; Mishra, B.K.; Kumar, A.; Adak, A.; Jauhari, S.; Yadav, K.; Singh, R.; et al. Evaluating the plant growth promoting ability of thermotolerant bacteria and cyanobacteria and their interactions with seed spice crops. Sci. Hortic. 2013, 164, 94–101. [Google Scholar] [CrossRef]
- Bharti, A.; Prasanna, R.; Kumar, G.; Kumar, A.; Nain, L. Co-cultivation of cyanobacteria for raising nursery of chrysanthemum using a hydroponic system. J. Appl. Phycol. 2019, 31, 3625–3635. [Google Scholar] [CrossRef]
- Kim, M.J.; Shim, C.K.; Kim, Y.K.; Ko, B.G.; Park, J.H.; Hwang, S.G.; Kim, B.H. Effect of biostimulator Chlorella fusca on improving growth and qualities of Chinese chives and spinach in organic farm. Plant Pathol. J. 2018, 34, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Zhou, Q. Co-cultivation of Chlorella spp. and tomato in a hydroponic system. Biomass Bioenergy 2017, 97, 132–138. [Google Scholar] [CrossRef]
- Supraja, K.V.; Behera, B.; Balasubramanian, P. Performance evaluation of hydroponic system for co-cultivation of microalgae and tomato plant. J. Clean. Prod. 2020, 272, 122823. [Google Scholar] [CrossRef]
- Agwa, O.K.; Ogugbue, C.J.; Williams, E.E. Field evidence of Chlorella vulgaris potentials as a biofertilizer for Hibiscus esculentus. Int. J. Agric. Res. 2017, 12, 181–189. [Google Scholar] [CrossRef]
- Schreiber, C.; Schiedung, H.; Harrison, L.; Briese, C.; Ackermann, B.; Kant, J.; Schrey, S.D.; Hofmann, D.; Singh, D.; Ebenhöh, O.; et al. Evaluating potential of green alga Chlorella vulgaris to accumulate phosphorus and to fertilize nutrient-poor soil substrates for crop plants. J. Appl. Phycol. 2018, 30, 2827–2836. [Google Scholar] [CrossRef]
- Grzesik, M.; Romanowska-duda, Z. Improvements in germination, growth, and metabolic activity of corn seedlings by grain conditioning and root application with cyanobacteria and microalgae. Polish J. Environ. Stud. 2014, 23, 1147–1153. [Google Scholar]
- Puglisi, I.; La Bella, E.; Rovetto, E.I.; Stevanato, P.; Fascella, G.; Baglieri, A. Morpho-biometric and biochemical responses in lettuce seedlings treated by different application methods of Chlorella vulgaris extract: Foliar spray or root drench? J. Appl. Phycol. 2022, 34, 889–901. [Google Scholar] [CrossRef]
- Dineshkumar, R.; Subramanian, J.; Gopalsamy, J.; Jayasingam, P.; Arumugam, A.; Kannadasan, S.; Sampathkumar, P. The impact of using microalgae as biofertilizer in maize (Zea mays L.). Waste Biomass Valorization 2019, 10, 1101–1110. [Google Scholar] [CrossRef]
- Rupawalla, Z.; Shaw, L.; Ross, I.L.; Schmidt, S.; Hankamer, B.; Wolf, J. Germination screen for microalgae-generated plant growth biostimulants. Algal Res. 2022, 66, 102784. [Google Scholar] [CrossRef]
- Coppens, J.; Grunert, O.; Van Den Hende, S.; Vanhoutte, I.; Boon, N.; Haesaert, G.; De Gelder, L. The use of microalgae as a high-value organic slow-release fertilizer results in tomatoes with increased carotenoid and sugar levels. J. Appl. Phycol. 2016, 28, 2367–2377. [Google Scholar] [CrossRef]
- Abedi Firoozjaei, M.H.; Hassani, S.B.; Nazifi, E.; Keypour, S. Study the effect of the terrestrial cyanobacterium nostoc commune aqueous extract on seed germination and seedling growth of rice. J. Phycol. Res. 2021, 5, 642–653. [Google Scholar]
- Godlewska, K.; Michalak, I.; Pacyga, P.; Baśladyńska, S.; Chojnacka, K. Potential applications of cyanobacteria: Spirulina platensis filtrates and homogenates in agriculture. World J. Microbiol. Biotechnol. 2019, 35, 80. [Google Scholar] [CrossRef] [PubMed]
- Thinh, N.Q. Influences of seed priming with Spirulina platensis extract on seed quality properties in black gram (Vigna mungo L.). Vietnam. J. Sci. Technol. Eng. 2021, 63, 36–41. [Google Scholar] [CrossRef]
- Alshehrei, F.; Al-Enazi, N.M.; Ameen, F. Vermicomposting amended with microalgal biomass and biochar produce phytopathogen-resistant seedbeds for vegetables. Biomass Conv. Bioref. 2021. [Google Scholar] [CrossRef]
- Ronga, D.; Biazzi, E.; Parati, K.; Carminati, D.; Carminati, E.; Tava, A. Microalgal biostimulants and biofertilisers in crop productions. Agronomy 2019, 9, 192. [Google Scholar] [CrossRef]
- Gonçalves, A.L. The use of microalgae and cyanobacteria in the improvement of agricultural practices: A review on their biofertilising, biostimulating and biopesticide roles. Appl. Sci. 2021, 11, 871. [Google Scholar] [CrossRef]
- Wijffels, R.H.; Kruse, O.; Hellingwerf, K.J. Potential of industrial biotechnology with cyanobacteria and eukaryotic microalgae. Curr. Opin. Biotechnol. 2013, 24, 405–413. [Google Scholar] [CrossRef]
- Gray, W.M. Hormonal Regulation of Plant Growth and Development. PLoS Biol. 2004, 2, e311. [Google Scholar] [CrossRef]
- Santner, A.; Calderon-Villalobos, L.I.A.; Estelle, M. Plant hormones are versatile chemical regulators of plant growth. Nat. Chem. Biol. 2009, 5, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Tarakhovskaya, E.R.; Maslov, Y.I.; Shishova, M.F. Phytohormones in algae. Russ. J. Plant Physiol. 2007, 54, 163–170. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, J. Phytohormones in microalgae: A new opportunity for microalgal biotechnology? Trends Plant Sci. 2015, 20, 273–282. [Google Scholar] [CrossRef] [PubMed]
- El Arroussi, H.; El Mernissi, N.; Benhima, R.; El Kadmiri, I.M.; Bendaou, N.; Smouni, A.; Wahby, I. Microalgae polysaccharides a promising plant growth biostimulant. J. Algal Biomass Util. 2016, 7, 55–63. [Google Scholar]
- Liu, J.; Qiu, W.; Song, Y. Stimulatory effect of auxins on the growth and lipid productivity of Chlorella pyrenoidosa and Scenedesmus quadricauda. Algal Res. 2016, 18, 273–280. [Google Scholar] [CrossRef]
- Cruz, C.G.; Vieira Costa, J.A. Identification of the phytohormones indole-3-acetic acid and trans-zeatin in microalgae. J. Chem. Technol. Biotechnol. 2023, 98, 1048–1056. [Google Scholar] [CrossRef]
- Bajguz, A.; Piotrowska-Niczyporuk, A. Synergistic effect of auxins and brassinosteroids on the growth and regulation of metabolite content in the green alga Chlorella vulgaris (Trebouxiophyceae). Plant Physiol. Biochem. 2013, 71, 290–297. [Google Scholar] [CrossRef]
- Singh, J.; Jain, D.; Agarwal, P.; Singh, R. Auxin and cytokinin synergism augmenting biomass and lipid production in microalgae Desmodesmus spp. JS07. Process Biochem. 2020, 95, 223–234. [Google Scholar] [CrossRef]
- Mousavi, P.; Morowvat, M.H.; Montazeri-Najafabady, N.; Abolhassanzadeh, Z.; Mohagheghzadeh, A.; Hamidi, M.; Niazi, A.; Ghasemi, Y. Investigating the effects of phytohormones on growth and β-carotene production in a naturally isolates stain of Dunaliella salina. J. Appl. Pharm. Sci. 2016, 6, 164–171. [Google Scholar] [CrossRef]
- Chang, W.; Li, Y.; Qu, Y.; Liu, Y.; Zhang, G.; Zhao, Y.; Liu, S. Mixotrophic cultivation of microalgae to enhance the biomass and lipid production with synergistic effect of red light and phytohormone IAA. Renew. Energy 2022, 187, 819–828. [Google Scholar] [CrossRef]
- Udayan, A.; Arumugam, M. Selective enrichment of Eicosapentaenoic acid (20:5n-3) in N. oceanica CASA CC201 by natural auxin supplementation. Bioresour. Technol. 2017, 242, 329–333. [Google Scholar] [CrossRef]
- Trinh, C.T.; Tran, T.H.; Bui, T.V. Effects of plant growth regulators on the growth and lipid accumulation of Nannochloropsis oculata (droop) Hibberd. In AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2017; Volume 1878, p. 020017. [Google Scholar]
- Salama, E.S.; Jeon, B.H.; Chang, S.W.; Lee, S.H.; Roh, H.S.; Yang, I.S.; Kurade, M.B.; El-Dalatony, M.M.; Kim, D.H.; Kim, K.H.; et al. Interactive effect of indole-3-acetic acid and diethyl aminoethyl hexanoate on the growth and fatty acid content of some microalgae for biodiesel production. J. Clean. Prod. 2017, 168, 1017–1024. [Google Scholar] [CrossRef]
- Salama, E.S.; Kabra, A.N.; Ji, M.K.; Kim, J.R.; Min, B.; Jeon, B.H. Enhancement of microalgae growth and fatty acid content under the influence of phytohormones. Bioresour. Technol. 2014, 172, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Kozlova, T.A.; Hardy, B.P.; Krishna, P.; Levin, D.B. Effect of phytohormones on growth and accumulation of pigments and fatty acids in the microalgae Scenedesmus quadricauda. Algal Res. 2017, 27, 325–334. [Google Scholar] [CrossRef]
- Yu, Z.; Pei, H.; Jiang, L.; Hou, Q.; Nie, C.; Zhang, L. Phytohormone addition coupled with nitrogen depletion almost tripled the lipid productivities in two algae. Bioresour. Technol. 2018, 247, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Renuka, N.; Guldhe, A.; Singh, P.; Ansari, F.A.; Rawat, I.; Bux, F. Evaluating the potential of cytokinins for biomass and lipid enhancement in microalga Acutodesmus obliquus under nitrogen stress. Energy Convers. Manag. 2017, 140, 14–23. [Google Scholar] [CrossRef]
- Parsaeimehr, A.; Mancera-Andrade, E.I.; Robledo-Padilla, F.; Iqbal, H.M.; Parra-Saldivar, R. A chemical approach to manipulate the algal growth, lipid content and high-value alpha-linolenic acid for biodiesel production. Algal Res. 2017, 26, 312–322. [Google Scholar] [CrossRef]
- Piotrowska-Niczyporuk, A.; Bajguz, A.; Zambrzycka, E.; Godlewska-Żyłkiewicz, B. Phytohormones as regulators of heavy metal biosorption and toxicity in green alga Chlorella vulgaris (Chlorophyceae). Plant Physiol. Biochem. 2012, 52, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Ramphal, K.; Lewis, A.; Trzaskalski, N.A.; Kisiala, A.; Morrison, E.N.; Narine, S.S.; Emery, R.J.N. Phytohormonal impacts on fatty acid profiles in Chlorella vulgaris Beijerinck: Endogenous identification and exogenous application of cytokinins and abscisic acid. J. Appl. Phycol. 2023, 35, 2205–2218. [Google Scholar] [CrossRef]
- Tiwari, S.; Patel, A.; Prasad, S.M. Kinetin alleviates chromium toxicity on growth and PS II photochemistry in Nostoc muscorum by regulating antioxidant system. Ecotoxicol. Environ. Saf. 2018, 161, 296–304. [Google Scholar] [CrossRef]
- González-Garcinuño, Á.; Sánchez-Álvarez, J.M.; Galán, M.A.; Martin del Valle, E.M. Understanding and optimizing the addition of phytohormones in the culture of microalgae for lipid production. Biotechnol. Prog. 2016, 32, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Tao, H.; Wen, X.; Geng, Y.; Li, Y. Enhanced growth and lipid production of Chlorella pyrenoidosa by plant growth regulator GA3. Fresenius Environ. Bull. 2015, 24, 3414–3419. [Google Scholar]
- Madani, N.S.H.; Shamsaie Mehrgan, M.; Hosseini Shekarabi, S.P.; Pourang, N. Regulatory effect of gibberellic acid (GA3) on the biomass productivity and some metabolites of a marine microalga, Isochrysis galbana. J. Appl. Phycol. 2021, 33, 255–262. [Google Scholar] [CrossRef]
- Arora, S.; Mishra, G. Effect of gibberellin, methyl jasmonate and myoinositol on biomass and eicosapentaenoic acid productivities in the eustigmatophyte Monodopsis subterranea CCALA 830. J. Appl. Phycol. 2021, 33, 287–299. [Google Scholar] [CrossRef]
- Kim, S.H.; Lim, S.R.; Hong, S.J.; Cho, B.K.; Lee, H.; Lee, C.G.; Choi, H.K. Effect of ethephon as an ethylene-releasing compound on the metabolic profile of Chlorella vulgaris. J. Agric. Food Chem. 2016, 64, 4807–4816. [Google Scholar] [CrossRef]
- Vo, T.T.; Lee, C.; Han, S.I.; Kim, J.Y.; Kim, S.; Choi, Y.E. Effect of the ethylene precursor, 1-aminocyclopropane-1-carboxylic acid on different growth stages of Haematococcus pluvialis. Bioresour. Technol. 2016, 220, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, X.; Zhao, Y.; Gao, H.; Li, L.; Zhang, Y.; Yu, X. Myo-inositol facilitates astaxanthin and lipid coproduction in Haematococcus pluvialis by regulating oxidative stress and ethylene signalling. Bioresour. Technol. 2022, 366, 128222. [Google Scholar] [CrossRef] [PubMed]
- Qiao, T.; Gu, D.; Zhu, L.; Zhao, Y.; Zhong, D.; Yu, X. Coupling of myo-inositol with salinity regulates ethylene-induced microalgal lipid hyperproduction in molasses wastewater. Sci. Total Environ. 2022, 818, 151765. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qiu, W.; Song, Y.; Peng, H.; Zhao, Y. The growth and lipid productivity of Chlorella pyrenoidosa enhanced by plant hormones under ammonium stress. Environ. Prog. Sustain. Energy 2017, 36, 1187–1193. [Google Scholar] [CrossRef]
- Norlina, R.; Norashikin, M.N.; Loh, S.H.; Aziz, A.A.; Cha, T.S. Exogenous abscisic acid supplementation at early stationary growth phase triggers changes in the regulation of fatty acid biosynthesis in Chlorella vulgaris UMT-M1. Appl. Biochem. Biotechnol. 2020, 191, 1653–1669. [Google Scholar] [CrossRef]
- Kozlova, T.A.; Kartashov, A.V.; Zadneprovskaya, E.; Krapivina, A.; Zaytsev, P.; Chivkunova, O.B.; Solovchenko, A.E. Effect of abscisic acid on growth, fatty acid profile, and pigment composition of the Chlorophyte Chlorella (Chromochloris) zofingiensis and its co-culture microbiome. Life 2023, 13, 452. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Li, Q.; Chen, C.; Zhang, Y.; Liu, Y.; Xu, L.; Zhou, Y.; Li, C.; Zhou, D.; Rittmann, B.E. Benzoic and salicylic acid are the signaling molecules of Chlorella cells for improving cell growth. Chemosphere 2021, 265, 129084. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Z.; Peng, Y.; Zhang, Y.; Li, Q.; Sun, D. Salicylic acid enhances cell growth, fatty acid and astaxanthin production in heterotrophic Chromochloris zofingiensis without reactive oxygen species elevation. Biotechnol. Biofuels 2024, 17, 1. [Google Scholar] [CrossRef]
- Raman, V.; Ravi, S. Effect of salicylic acid and methyl jasmonate on antioxidant systems of Haematococcus pluvialis. Acta Physiol. Plant. 2011, 33, 1043–1049. [Google Scholar] [CrossRef]
- Mc Gee, D.; Archer, L.; Parkes, R.; Fleming, G.T.; Santos, H.M.; Touzet, N. The role of methyl jasmonate in enhancing biomass yields and bioactive metabolites in Stauroneis spp. (Bacillariophyceae) revealed by proteome and biochemical profiling. J. Proteom. 2021, 249, 104381. [Google Scholar] [CrossRef]
- Górka, B.; Lipok, J.; Wieczorek, P.P. Biologically active organic compounds, especially plant promoters, in algae extracts and their potential application in plant cultivation. In Marine Algae Extracts: Processes, Products, and Applications; Kim, S.K., Chojnacka, K., Eds.; Wiley: New York, NY, USA, 2015; pp. 659–680. [Google Scholar]
- Rathod, S.G.; Bhushan, S.; Mantri, V.A. Phytohormones and pheromones in the phycology literature: Benchmarking of data-set and developing critical tools of biotechnological implications for commercial aquaculture industry. Phycology 2024, 4, 1–36. [Google Scholar] [CrossRef]
- Sergeeva, E.; Liaimer, A.; Bergman, B. Evidence for production of the phytohormone indole-3-acetic acid by cyanobacteria. Planta 2002, 215, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Taiz, L.; Zeiger, E. Plant Physiology, 2nd ed.; Sinauer Associates: Sunderland, UK, 1998. [Google Scholar]
- Wang, Y.; Mopper, S.; Hasenstein, K.H. Effects of salinity on endogenous ABA, IAA, JA, and SA in Iris hexagona. J. Chem. Ecol. 2001, 27, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Werner, T.; Motika, V.; Strnad, M.; Schmulling, T. Regulation of plants growth by cytokinin. Proc. Natl. Acad. Sci. USA 2001, 98, 10487–10492. [Google Scholar] [CrossRef]
- Pan, S.; Jeevanandam, J.; Danquah, M.K. Benefits of algal extracts in sustainable agriculture. In Grand Challenges in Algae Biotechnology; Springer: Cham, Switzerland, 2019; pp. 501–534. [Google Scholar]
- Tan, C.Y.; Dodd, I.C.; Chen, J.E.; Phang, S.M.; Chin, C.F.; Yow, Y.Y.; Ratnayeke, S. Regulation of algal and cyanobacterial auxin production, physiology, and application in agriculture: An overview. J. Appl. Phycol. 2021, 33, 2995–3023. [Google Scholar] [CrossRef]
- Stirk, W.A.; Ördög, V.; Novák, O.; Rolčík, J.; Strnad, M.; Bálint, P.; van Staden, J. Auxin and cytokinin relationships in 24 microalgal strains1. J. Phycol. 2013, 49, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Plaza, B.M.; Gómez-Serrano, C.; Acién-Fernández, F.G.; Jimenez-Becker, S. Effect of microalgae hydrolysate foliar application (Arthrospira platensis and Scenedesmus spp.) on petunia x hybrida growth. J. Appl. Phycol. 2018, 30, 2359–2365. [Google Scholar] [CrossRef]
- Mazhar, S.; Cohen, J.D.; Hasnain, S. Auxin producing non-heterocystous Cyanobacteria and their impact on the growth and endogenous auxin homeostasis of wheat. J. Basic Microbiol. 2013, 53, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Elakbawy, W.M.; Shanab, S.M.M.; Shalaby, E.A. Enhancement of plant growth regulators production from microalgae cultivated in treated sewage wastewater (TSW). BMC Plant Biol. 2022, 22, 377. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, N.; Prasanna, R.; Sood, A.; Jaiswal, P.; Nayak, S.; Kaushik, B. Physiological characterization and electron microscopic investigation of cyanobacteria associated with wheat rhizosphere. Folia Microbiol. 2009, 54, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Ordog, V.; Stirk, W.A.; Van Staden, J.; Novak, O.; Strnad, M. Endogenous cytokinins in three genera of microalgae from the Chlorophyta. J. Phycol. 2004, 40, 88–95. [Google Scholar] [CrossRef]
- To, J.P.; Kieber, J.J. Cytokinin signaling: Two-cEomponents and more. Trends Plant Sci. 2008, 13, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.D.; Karas, B.J.; Sato, S.; Tabata, S.; Amyot, L.; Szczyglowski, K. A cytokinin perception mutant colonized by Rhizobium in the absence of nodule organogenesis. Science 2007, 315, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Mazepa, E.; Malburg, B.V.; Mógor, G.; de Oliveira, A.C.; Amatussi, J.O.; Corrêa, D.O.; Lemos, J.S.; Ducatti, D.R.B.; Duarte, M.E.R.; Mógor, Á.F.; et al. Plant growth biostimulant activity of the green microalga Desmodesmus subspicatus. Algal Res. 2021, 59, 102434. [Google Scholar] [CrossRef]
- Lu, Y.; Tarkowská, D.; Turečková, V.; Luo, T.; Xin, Y.; Li, J.; Wang, Q.; Jiao, N.; Strnad, M.; Xu, J. Antagonistic roles of abscisic acid and cytokinin during response to nitrogen depletion in oleaginous microalga Nannochloropsis oceanica expand the evolutionary breadth of phytohormone function. Plant J. 2014, 80, 52–68. [Google Scholar] [CrossRef]
- Yamaguchi, S. Gibberellin metabolism and its regulation. Annu. Rev. Plant Biol. 2008, 59, 225–251. [Google Scholar] [CrossRef] [PubMed]
- Tuna, A.L.; Kaya, C.; Dikilitas, M.; Higgs, D. The combined effects of gibberellic acid and salinity on some antioxidant enzyme activities, plant growth parameters and nutritional status in maize plants. Environ. Exp. Bot. 2008, 62, 1–9. [Google Scholar] [CrossRef]
- Stirk, W.A.; Bálint, P.; Tarkowská, D.; Novák, O.; Strnad, M.; Ördög, V.; van Staden, J. Hormone profiles in microalgae: Gibberellins and brassinosteroids. Plant Physiol. Biochem. 2013, 70, 348–353. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, A.H.; Osman, M.E.H.E.-S.; Gheda, S.F. Influence of the aqueous extracts of Ulva lactuca and Chlorella kessleri on growth and yield of Vicia faba. Arch. Hydrobiol. Suppl. Algol. Stud. 2005, 116, 213–229. [Google Scholar] [CrossRef]
- Rodríguez, A.; Stella, A.; Storni, M.; Zulpa, G.; Zaccaro, M. Effects of cyanobacterial extracellular products and gibberellic acid on salinity tolerance in Oryza sativa L. Saline Syst. 2006, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zeng, H.; Bartocci, P.; Fantozzi, F.; Yan, Y. Phytohormones and effects on growth and metabolites of microalgae: A review. Fermentation 2018, 4, 25. [Google Scholar] [CrossRef]
- Sponsel, V.M.; Hedden, P. Gibberellin biosynthesis and inactivation. In Plant Hormones; Davies, P.J., Ed.; Springer: Dordrecht, The Netherlands, 2010; pp. 63–94. [Google Scholar]
- Bleecker, A.B.; Kende, H. Ethylene: A gaseous signal molecule in plants. Annu. Rev. Cell Dev. Biol. 2000, 16, 1–18. [Google Scholar] [CrossRef]
- Hao, D.; Sun, X.; Ma, B.; Zhang, J.; Guo, H. 6—Ethylene. In Hormone Metabolism and Signaling in Plants; Li, J., Li, C., Smith, S.M., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 203–241. [Google Scholar]
- Tsavkelova, E.A.; Klimova, S.Y.; Cherdyntseva, T.A.; Netrusov, A.I. Hormones and hormone-like substances of microorganisms: A review. Appl. Biochem. Microbiol. 2006, 42, 229–235. [Google Scholar] [CrossRef]
- Zavřel, T.; Knoop, H.; Steuer, R.; Jones, P.R.; Červený, J.; Trtílek, M. A quantitative evaluation of ethylene production in the recombinant cyanobacterium Synechocystis spp. PCC 6803 harboring the ethylene-forming enzyme by membrane inlet mass spectrometry. Bioresour. Technol. 2016, 202, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, F.; Carbonell, V.; Cossu, M.; Correddu, D.; Jones, P.R. Ethylene synthesis and regulated expression of recombinant protein in Synechocystis spp. PCC 6803. PLoS ONE 2012, 7, e50470. [Google Scholar] [CrossRef]
- Ungerer, J.; Tao, L.; Davis, M.; Ghirardi, M.; Maness, P.-C.; Yu, J. Sustained photosynthetic conversion of CO2 to ethylene in recombinant cyanobacterium Synechocystis 6803. Energy Environ. Sci. 2012, 5, 8998–9006. [Google Scholar] [CrossRef]
- Xiong, W.; Morgan, J.; Ungerer, J.; Wang, B.; Maness, P.; Yu, J. The plasticity of cyanobacterial metabolism supports direct CO2 conversion to ethylene. Nat. Plants 2015, 1, 15053. [Google Scholar] [CrossRef]
- Nambara, E. Abscisic Acid. In Encyclopedia of Applied Plant Sciences, 2nd ed.; Thomas, B., Murray, B.G., Murphy, D.J., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 361–366. [Google Scholar]
- Agehara, S.; Leskovar, D.I. Growth suppression by exogenous abscisic acid and uniconazole for prolonged marketability of tomato transplants in commercial conditions. HortScience 2017, 52, 606–611. [Google Scholar] [CrossRef]
- Yamazaki, H.; Nishijima, T.; Koshioka, M. Effects of (+)-s-abscisic acid on the quality of stored cucumber and tomato seedlings. HortScience 1995, 30, 80–82. [Google Scholar] [CrossRef]
- Khasin, M.; Cahoon, R.E.; Alvarez, S.; Beckeris, R.; Eyun, S.; Jia, Q.; Riethoven, J.J.; Nickerson, K.W.; Riekhof, W.R. Synthesis, secretion, and perception of abscisic acid regulates stress responses in Chlorella sorokiniana. bioRxiv 2017. [Google Scholar] [CrossRef]
- McAdam, S.A.; Brodribb, T.J.; Ross, J.J. Shoot-derived abscisic acid promotes root growth. Plant Cell Environ. 2016, 39, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Tamaoki, D.; Seo, S.; Yamada, S.; Kano, A.; Miyamoto, A.; Shishido, H.; Miyoshi, S.; Taniguchi, S.; Akimitsu, K.; Gomi, K. Jasmonic acid and salicylic acid activate a common defense system in rice. Plant Signal. Behav. 2013, 8, e24260. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Tsuda, K. Salicylic acid and jasmonic acid crosstalk in plant immunity. Essays Biochem. 2022, 66, 647–656. [Google Scholar] [PubMed]
- Ghorbel, M.; Brini, F. Role of brassinosteroids in regulating physiological and molecular aspects of plants under abiotic stress. In The Role of Growth Regulators and Phytohormones in Overcoming Environmental Stress; Sharma, A., Pandey, S., Bhardwaj, R., Zheng, B., Tripathi, D.K., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 197–233. [Google Scholar]
- Zhu, B.C.; Su, J.; Cham, M.C.; Verma, D.P.S.; Fan, Y.L.; Wu, R. Overexpression of pyrroline-5-carboxylate synthetase gene and analysis of tolerance to water stress and salt stress in transgenic rice. Plant Sci. 1998, 139, 41–48. [Google Scholar] [CrossRef]
- Singh, I.; Shono, M. Physiological and molecular effects of 24-epibrassinolide, a brassinosteroid, on thermotolerance of tomato. Plant Growth Regul. 2005, 47, 111–119. [Google Scholar] [CrossRef]
- El-Bassiony, A.M.; Ghoname, A.A.; El-Awadi, M.E.; Fawzy, Z.F.; Gruda, N. Ameliorative effects of brassinosteroids on growth and productivity of snap beans grown under high temperature. Gesunde Pflanz. 2012, 64, 175–182. [Google Scholar] [CrossRef]
- Bajguz, A. Isolation and characterization of brassinosteroids from algal cultures of Chlorella vulgaris Beijerinck (Trebouxiophyceae). J. Plant Physiol. 2009, 166, 1946–1949. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xing, S.; Sun, X. Effects of polyamines on hormones contents and the relationship with the flower bud differentiation in chrysanthemum. Plant Physiol. J. 2014, 50, 1195–1202. [Google Scholar]
- Xu, L. The effect of polyamine on flower bud differentiation and bud germination of chrysanthemum. Shandong Agric. Univ. 2015, 2, 31–36. [Google Scholar]
- Mustafavi, S.H.; Naghdi Badi, H.; Sekara, A.; Mehrafarin, A.; Janda, T.; Ghorbanpour, M.; Rafiee, H. Polyamines and their possible mechanisms involved in plant physiological processes and elicitation of secondary metabolites. Acta Physiol. Plant 2018, 40, 102. [Google Scholar] [CrossRef]
- Wang, W.; Paschalidis, K.; Feng, J.C.; Song, J.; Liu, J.H. Polyamine catabolism in plants: A universal process with diverse functions. Front. Plant Sci. 2019, 10, 561. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Lin, H.J. Polyamines in microalgae: Something borrowed, something new. Mar. Drugs 2019, 17, 1. [Google Scholar] [CrossRef] [PubMed]
- Mógor, Á.F.; Ördög, V.; Lima, G.P.P.; Molnár, Z.; Mógor, G. Biostimulant properties of cyanobacterial hydrolysate related to polyamines. J. Appl. Phycol. 2018, 30, 453–460. [Google Scholar] [CrossRef]
- Udayan, A.; Arumugam, M.; Pandey, A. Nutraceuticals from algae and cyanobacteria. In Algal Green Chemistry; Rastogi, R.P., Madamwar, D., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 65–89. [Google Scholar]
- Synytsya, A.; Sushytskyi, L.; Saloň, I.; Babayeva, T.; Čopíková, J. Intracellular and extracellular carbohydrates in microalgae. In Handbook of Food and Feed from Microalgae; Jacob-Lopes, E., Queiroz, M.I., Maroneze, M.M., Zepka, L.Q., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 87–102. [Google Scholar]
- Chanda, M.J.; Merghoub, N.; El Arroussi, H. Microalgae polysaccharides: The new sustainable bioactive products for the development of plant bio-stimulants? World J. Microbiol. Biotechnol. 2019, 35, 177. [Google Scholar] [CrossRef]
- Adamuchio-Oliveira, L.G.; Mazaro, S.M.; Mógor, G.; Sant’Anna-Santos, B.F.; Mógor, Á.F. Chitosan associated with chelated copper applied on tomatoes: Enzymatic and anatomical changes related to plant defense responses. Sci. Hortic. 2020, 271, 109431. [Google Scholar] [CrossRef]
- Matos, Â.P.; da Silva, T.; Sant’Anna, E.S. The feasibility of using inland desalination concentrate (DC) as an alternative substrate for Spirulina platensis mass cultivation. Waste Biomass Valorization 2021, 12, 3193–3203. [Google Scholar] [CrossRef]
- Zainan, N.H.; Sapardi, M.A.M.; Ho, B.C.H.; Siajam, S.I.; Kamal, S.M.M.; Danquah, M.K.; Harun, R. Kinetic and thermodynamic characterization of amino acids generation via subcritical water reaction of microalgae Nannochloropsis spp. biomass. Biomass Convers. Biorefinery 2022, 12, 2001–2014. [Google Scholar] [CrossRef]
- Bulut, O.; Akın, D.; Sönmez, Ç.; Öktem, A.; Yücel, M.; Öktem, H.A. Phenolic compounds, carotenoids, and antioxidant capacities of a thermo-tolerant Scenedesmus spp. (Chlorophyta) extracted with different solvents. J. Appl. Phycol. 2019, 31, 1675–1683. [Google Scholar] [CrossRef]
- Andriopoulos, V.; Gkioni, M.D.; Koutra, E.; Mastropetros, S.G.; Lamari, F.N.; Hatziantoniou, S.; Kornaros, M. Total phenolic content, biomass composition, and antioxidant activity of selected marine microalgal species with potential as aquaculture feed. Antioxidants 2022, 11, 1320. [Google Scholar] [CrossRef] [PubMed]
- Haoujar, I.; Cacciola, F.; Abrini, J.; Mangraviti, D.; Giuffrida, D.; El Majdoub, Y.O.; Kounnoun, A.; Miceli, N.; Taviano, M.F.; Mondello, L.; et al. The contribution of carotenoids, phenolic compounds, and flavonoids to the antioxidative properties of marine microalgae isolated from Mediterranean Morocco. Molecules 2019, 24, 4037. [Google Scholar] [CrossRef] [PubMed]
- Skinner, C.; Gattinger, A.; Krauss, M.; Krause, H.M.; Mayer, J.; van der Heijden, M.G.A.; Mäder, P. The impact of long-term organic farming on soil-derived greenhouse gas emissions. Sci. Rep. 2019, 9, 1702. [Google Scholar] [CrossRef] [PubMed]
- Payen, F.T.; Evans, D.L.; Falagán, N.; Hardman, C.A.; Kourmpetli, S.; Liu, L.; Marshall, R.; Mead, B.R.; Davies, J.A.C. How much food can we grow in urban areas? Food production and crop yields of urban agriculture: A meta-analysis. Earth’s Future 2022, 10, e2022EF002748. [Google Scholar] [CrossRef] [PubMed]
- Treftz, C.; Omaye, S.T. Hydroponics: Potential for augmenting sustainable food production in non-arable regions. Nutr. Food Sci. 2016, 46, 672–684. [Google Scholar] [CrossRef]
- Al-Kodmany, K. The vertical farm: A review of developments and implications for the vertical city. Buildings 2018, 8, 24. [Google Scholar] [CrossRef]
- Martin, M.; Molin, E. Environmental assessment of an urban vertical hydroponic farming system in Sweden. Sustainability 2018, 11, 4124. [Google Scholar] [CrossRef]
- Rehman, S.; Chattha, M.U.; Khan, I.; Mahmood, A.; Hassan, M.U.; Al-Huqail, A.A.; Salem, M.Z.M.; Ali, H.M.; Hano, C.; El-Esawi, M.A. Exogenously applied trehalose augments cadmium stress tolerance and yield of mung bean (Vigna radiata L.) grown in soil and hydroponic systems through reducing Cd uptake and enhancing photosynthetic efficiency and antioxidant defense systems. Plants 2022, 11, 822. [Google Scholar] [CrossRef] [PubMed]
- Aires, A. Hydroponic Production Systems: Impact on Nutritional Status and Bioactive Compounds of Fresh Vegetables; InTech: London, UK, 2018. [Google Scholar] [CrossRef]
- Al-Kodmany, K. Sustainable tall buildings: Cases from the global south. Int. J. Archit. Res. 2016, 10, 52–66. [Google Scholar] [CrossRef]
- Harris, D. Hydroponics: A Practical Guide for the Soilless Grower, 2nd ed.; New Holland Publishing: London, UK, 1992. [Google Scholar]
- Despommier, D. Farming up the city: The rise of urban vertical farms. Trends Biotechnol. 2013, 31, 388–389. [Google Scholar] [CrossRef] [PubMed]
- Cometti, N.N.; Bremenkamp, D.M.; Galon, K.; Hell, L.R.; Zanotelli, M.F. Cooling and concentration of nutrient solution in hydroponic lettuce crop. Hortic. Bras. 2013, 31, 287–292. [Google Scholar] [CrossRef]
- Cardoso, F.B.; Martinez, H.E.; Silva, D.J.; Milagres, C.D.; Barbosa, J.G. Yield and quality of tomato grown in a hydroponic system, with different planting densities and number of bunches per plant. Pesqui. Agropecu. Trop. 2018, 48, 340–349. [Google Scholar] [CrossRef]
- Barone, V.; Puglisi, I.; Fragalà, F.; Lo Piero, A.R.; Giuffrida, F.; Baglieri, A. Novel bioprocess for the cultivation of microalgae in hydroponic growing system of tomato plants. J. Appl. Phycol. 2019, 31, 465–470. [Google Scholar] [CrossRef]
- Ran, M.; Lu, Y.; Wu, J.; Li, J. Mitigating antimony toxicity in rice (Oryza sativa L.) through exogenous selenium supplementation: A comparative study of seed priming, hydroponics, and foliar spray methods. J. Plant Growth Regul. 2023, 43, 816–828. [Google Scholar] [CrossRef]
- Trevisan, S.; Manoli, A.; Quaggiotti, S. A novel biostimulant, belonging to protein hydrolysates, mitigates abiotic stress effects on maize seedlings grown in hydroponics. Agronomy 2019, 9, 28. [Google Scholar] [CrossRef]
- Nguyen, D.T.P.; Lu, N.; Kagawa, N.; Kitayama, M.; Takagaki, M. Short-term root-zone temperature treatment enhanced the accumulation of secondary metabolites of hydroponic coriander (Coriandrum sativum L.) grown in a plant factory. Agronomy 2020, 10, 413. [Google Scholar] [CrossRef]
- Ramezani, S.; Yousefshahi, B.; Ramezan, D.; Zargar, M.; Pakina, E.; Bayat, M. Selenium, iodine, and supplementary blue light enriched fenugreek (Trigonella foenum-gracum L.) in terms of biochemical quality, mineral uptake, and trace elements accumulation in a hydroponic system. Agriculture 2023, 13, 2009. [Google Scholar] [CrossRef]
- Rattan, S.; Partap, M.; Kumar, S.; Warghat, A.R. Nutrient feeding approach enhances the vegetative growth biomass, volatile oil composition, and myristicin content in hydroponically cultivated Petroselinum crispum (Mill.) Nyman. J. Appl. Res. Med. 2022, 26, 100359. [Google Scholar] [CrossRef]
- Loera-Muro, A.; Troyo-Diéguez, E.; Murillo-Amador, B.; Barraza, A.; Caamal-Chan, G.; Lucero-Vega, G.; Nieto-Garibay, A. Effects of vermicompost leachate versus inorganic fertilizer on morphology and microbial traits in the early development growth stage in mint (Mentha spicata L.) and rosemary (Rosmarinus officinalis L.) plants under closed hydroponic system. Horticulturae 2021, 7, 100. [Google Scholar] [CrossRef]
- Khater, E.; Bahnasawy, A.; Abass, W.; Morsy, O.; Shaban, Y.; Egela, M. Production of basil (Ocimum basilicum L.) under different soilless cultures. Sci. Rep. 2021, 11, 12754. [Google Scholar]
- Juárez-Rosete, C.R.; Aguilar-Castillo, J.A.; Aburto-González, C.A.; Alejo-Santiago, G. Biomass production, nutritional requirement of nitrogen, phosphorus and potassium, and concentration of the nutrient solution in oregano. Rev. Chapingo Ser. Hortic. 2019, 25, 17–28. [Google Scholar] [CrossRef]
- Sharma, D.; Partap, M.; Warghat, A.R.; Bhargava, B. Hydroponic cultivation enhances the morpho-physiological traits and quality flower production in three cultivars (marigold scarlet red, marigold orange, and marigold yellow) of French marigold (Tagetes patula L.). Sci. Hortic. 2024, 327, 112803. [Google Scholar]
- Alvarado-Camarillo, D.; Valdez-Aguilar, L.A.; Castillo-González, A.M.; Trejo-Téllez, L.I.; Martínez-Amador, S.Y. Biomass, nitrogen and potassium dynamics in hydroponic rose production. Acta Agric. Scand. B Soil Plant Sci. 2018, 68, 719–726. [Google Scholar] [CrossRef]
- Francato Zancheta, A.C.; De Abreu, C.; Bachiega Zambrosi, F.C.; de Magalhães Erismann, N.; Andrade Lagôa, A.M.M. Cadmium accumulation by jack-bean and sorghum in hydroponic culture. Int. J. Phytoremediat. 2015, 17, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, W.; Liu, Y.; Li, S.; Yao, W.; Sun, X.; Li, S.; Ma, L.; Sun, J.; Yang, Q.; et al. De novo hydroponics system efficiency for the cuttings of alfalfa (Medicago sativa L.). Physiol. Mol. Biol. Plants 2021, 27, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Menn, W.G.; McBee, G.G. A study of certain nutritional requirements for tifgreen bermudagrass (Cynodon dactylon × C. Transvaalensis L.) utilizing a hydroponic system. Agron. J. 1970, 62, 192–194. [Google Scholar] [CrossRef]
- Choi, S.-H.; Kim, D.-Y.; Lee, S.Y.; Chang, M.-S. Growth and quality of strawberry (Fragaria ananassa Dutch. cvs. ‘Kuemsil’) affected by nutrient solution supplying control system using drainage rate in hydroponic systems. Horticulturae 2022, 8, 1059. [Google Scholar] [CrossRef]
- Dasgan, H.Y.; Yilmaz, D.; Zikaria, K.; Ikiz, B.; Gruda, N.S. Enhancing the yield, quality and antioxidant content of lettuce through innovative and eco-friendly biofertilizer practices in hydroponics. Horticulturae 2023, 9, 1274. [Google Scholar] [CrossRef]
- Levine, C.P.; Mattson, N.S. Potassium-deficient nutrient solution affects the yield, morphology, and tissue mineral elements for hydroponic baby leaf spinach (Spinacia oleracea L.). Horticulturae 2021, 7, 213. [Google Scholar] [CrossRef]
- Isnainun, E.; Tini, E.; Suwarto, S. Growth and results of three varieties celery (Apium graveolens L.) with addition of alternative nutrition in the hydroponic floating system. Agroland Agric. Sci. J. 2021, 8, 91–98. [Google Scholar] [CrossRef]
- Lombardi, T.; Bertacchi, A.; Pistelli, L.; Pardossi, A.; Pecchia, S.; Toffanin, A.; Sanmartin, C. Biological and agronomic traits of the main halophytes widespread in the mediterranean region as potential new vegetable crops. Horticulturae 2022, 8, 195. [Google Scholar] [CrossRef]
- Olfati, J.A.; Saadatian, M.; Moqbeli, E. Optimisation of nitrogen and potassium for Aloe vera (L.) Burm.f. in a soilless culture system. S. Afr. J. Plant Soil 2015, 32, 249–252. [Google Scholar] [CrossRef]
- Ignatius, A.; Arunbabu, V.; Neethu, J.; Ramasamy, E.V. Rhizofiltration of lead using an aromatic medicinal plant Plectranthus amboinicus cultured in a hydroponic nutrient film technique (NFT) system. Environ. Sci. Pollut. Res. 2014, 21, 13007–13016. [Google Scholar] [CrossRef] [PubMed]
- Partap, M.; Sharma, D.; Deekshith, H.N.; Thakur, M.; Verma, V.; Bhargava, B. Microgreen: A tiny plant with superfood potential. J. Funct. Foods 2023, 107, 105697. [Google Scholar] [CrossRef]
- Nicola, S.; Egea-Gilabert, C.; Niñirola, D.; Conesa, E.; Pignata, G.; Fontana, E.; Fernández, J.A. Nitrogen and aeration levels of the nutrient solution in soilless cultivation systems as important growing conditions affecting inherent quality of baby leaf vegetables: A review. Acta Hortic. 2015, 1099, 167–177. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Rizvi, H.; Rinklebe, J.; Tsang, D.C.W.; Meers, E.; Ok, Y.S.; Ishaque, W. Phytomanagement of heavy metals in contaminated soils using sunflower: A review. Crit. Rev. Environ. Sci. Technol. 2016, 46, 1498–1528. [Google Scholar] [CrossRef]
- Cheng, X.; Chen, C.; Hu, Y.; Wang, J. Response of Amaranthus tricolor to cesium stress in hydroponic system: Growth, photosynthesis and cesium accumulation. Chemosphere 2022, 307, 135754. [Google Scholar] [CrossRef]
- Kawatra, N.; Jha, G.; Dubey, A. Study of the phytochemical profile of hydroponically cultivated buckwheat (Fagopyrum esculentum Moench) at different phenological stages. Biochem. Syst. Ecol. 2023, 107, 104612. [Google Scholar] [CrossRef]
- Sabatino, L.; Ntatsi, G.; Iapichino, G.; D’Anna, F.; De Pasquale, C. Effect of selenium enrichment and type of application on yield, functional quality and mineral composition of curly endive grown in a hydroponic system. Agronomy 2019, 9, 207. [Google Scholar] [CrossRef]
- Ceccanti, C.; Landi, M.; Incrocci, L.; Pardossi, A.; Guidi, L. Suitability of hydroponically-grown Rumex acetosa L. as fresh-cut produce. Horticulturae 2020, 6, 4. [Google Scholar] [CrossRef]
- Liu, H.; Shi, Z.; Li, J.; Zhao, P.; Qin, S.; Nie, Z. The impact of phosphorus supply on selenium uptake during hydroponics experiment of winter wheat (Triticum aestivum) in China. Front. Plant Sci. 2018, 9, 373. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shang, M.; Xu, J.; Zhao, P.; Li, T.; Yu, X. Enhanced astaxanthin production from a novel strain of Haematococcus pluvialis using fulvic acid. Process Biochem. 2015, 50, 2072–2077. [Google Scholar] [CrossRef]
- Castrejón Valdez, M.; De La Cruz Quispe, J.; Mendoza Común, V.E.; Sumarriva-Bustinza, L.A.; De La Cruz-Rojas, L.A.; More López, J.M.; Espinoza-Quispe, C.E.; Rojas-Felipe, E.; Caira Mamani, C.M.; Yaulilahua-Huacho, R. Effect of rhizobium and gibberellin on the production of hydroponic green forage of red clover (Trifolium pratense L.) variety quiñequeli. Braz. J. Biol. 2023, 83, e274345. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Dunn, B.L.; Payton, M.; Brandenberger, L. Selection of fertilizer and cultivar of sweet pepper and eggplant for hydroponic production. Agronomy 2019, 9, 433. [Google Scholar] [CrossRef]
- Karavidas, I.; Ntatsi, G.; Vougeleka, V.; Karkanis, A.; Ntanasi, T.; Saitanis, C.; Agathokleous, E.; Ropokis, A.; Sabatino, L.; Tran, F.; et al. Agronomic practices to increase the yield and quality of common bean (Phaseolus vulgaris L.): A systematic review. Agronomy 2022, 12, 271. [Google Scholar] [CrossRef]
- Puccinelli, M.; Carmassi, G.; Botrini, L.; Bindi, A.; Rossi, L.; Fierro-Sañudo, J.F.; Pardossi, A.; Incrocci, L. Growth and mineral relations of Beta vulgaris var. cicla and Beta vulgaris ssp. maritima cultivated hydroponically with diluted seawater and low nitrogen level in the nutrient solution. Horticulturae 2022, 8, 638. [Google Scholar] [CrossRef]
- Samba, N.; Nunomura, O.; Lu, N.; Johkan, M.; Nakano, A.; Tsukagoshi, S. Cucumber (Cucumis sativus L.) growth and productivity under solar radiation-based quantitative nutrient management in hydroponic system. Agronomy 2024, 14, 296. [Google Scholar] [CrossRef]
- Gao, S.; Liu, X.; Liu, Y.; Cao, B.; Chen, Z.; Xu, K. Comparison of the effects of LED light quality combination on growth and nutrient accumulation in green onion (Allium fistulosum L.). Protoplasma 2021, 258, 753–763. [Google Scholar] [CrossRef]
- Touliatos, D.; Dodd, I.C.; McAinsh, M. Vertical farming increases lettuce yield per unit area compared to conventional horizontal hydroponics. Food Energy Secur. 2016, 5, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Despommier, D. Encyclopedia of Food and Agricultural Ethics (Vertical Farms in Horticulture); Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Muller, A.; Ferré, M.; Engel, S.; Gattinger, A.; Holzkämper, A.; Huber, R.; Müller, M.; Six, J. Can soil-less crop production be a sustainable option for soil conservation and future agriculture? Land Use Policy 2017, 69, 102–105. [Google Scholar] [CrossRef]
- Lakhiar, I.A.; Gao, J.; Syed, T.N.; Chandio, F.A.; Buttar, N.A. Modern plant cultivation technologies in agriculture under controlled environment: A review on aeroponics. J. Plant Interact. 2018, 13, 338–352. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Tang, B.; Gu, M. Growth responses and root characteristics of lettuce grown in aeroponics, hydroponics, and substrate culture. Horticulturae 2018, 4, 35. [Google Scholar] [CrossRef]
- Espinal, C.A.; Matulić, D. Recirculating Aquaculture Technologies. In Aquaponics Food Production Systems; Goddek, S., Joyce, A., Kotzen, B., Burnell, G.M., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Maucieri, C.; Barbera, A.C.; Vymazal, J.; Borin, M. A review on the main affecting factors of greenhouse gases emission in constructed wetlands. Agric. Forest Meteorol. 2017, 236, 175–193. [Google Scholar] [CrossRef]
- Munguia-Fragozo, P.; Alatorre-Jacome, O.; Rico-Garcia, E.; Torres-Pacheco, I.; Cruz-Hernandez, A.; Ocampo-Velazquez, R.V. Perspective for aquaponic systems: “omic” technologies for microbial community analysis. BioMed Res. Int. 2015, 1, 480386. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Hu, Z.; Zhang, J.; Xie, H.; Liang, S.; Wang, J.; Yan, R. Attempts to improve nitrogen utilization efficiency of aquaponics through nitrifies addition and filler gradation. Environ. Sci. Pollut. Res. 2016, 23, 6671–6679. [Google Scholar] [CrossRef] [PubMed]
- Andriani, Y.; Dhahiyat, Y.; Zahidah, I.Z. The effect of stocking density ratio of fish on water plant productivity in aquaponics culture. NUS Biosci. 2017, 9, 31–35. [Google Scholar] [CrossRef]
- Yahia, E.M.; García-Solís, P.; Celis, M.E.M. Contribution of fruits and vegetables to human nutrition and health. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Yahia, E.M., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 19–45. [Google Scholar]
- Rouphael, Y.; Kyriacou, M.C.; Petropoulos, S.A.; De Pascale, S.; Colla, G. Improving vegetable quality in controlled environments. Sci. Hortic. 2018, 234, 275–289. [Google Scholar] [CrossRef]
- Bisbis, M.B.; Gruda, N.; Blanke, M. Potential impacts of climate change on vegetable production and product quality: A review. J. Clean. Prod. 2017, 170, 1602–1620. [Google Scholar] [CrossRef]
- Huo, S.; Liu, J.; Addy, M.; Chen, P.; Necas, D.; Cheng, P.; Li, K.; Chai, H.; Liu, Y.; Ruan, R. The influence of microalgae on vegetable production and nutrient removal in greenhouse hydroponics. J. Clean. Prod. 2020, 243, 118563. [Google Scholar] [CrossRef]
- Sathasivam, R.; Radhakrishnan, R.; Hashem, A.; Abd Allah, E.F. Microalgae metabolites: A rich source for food and medicine. Saudi J. Biol. Sci. 2019, 26, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Lind, O.P.; Hultberg, M.; Bergstrand, K.J.; Larsson-Jönsson, H.; Caspersen, S.; Asp, H. Biogas digestate in vegetable hydroponic production: pH dynamics and pH management by controlled nitrification. Waste Biomass Valorization 2021, 12, 123–133. [Google Scholar] [CrossRef]
- Stegmann, P.; Londo, M.; Junginger, M. The circular bioeconomy: Its elements and role in European bioeconomy clusters. Resour. Conserv. Recycl. 2020, X, 100029. [Google Scholar] [CrossRef]
- Ajeng, A.A.; Rosli, N.S.M.; Abdullah, R.; Yaacob, J.S.; Qi, N.C.; Loke, S.P. Resource recovery from hydroponic wastewaters using microalgae-based biorefineries: A circular bioeconomy perspective. J. Biotechnol. 2022, 360, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Renuka, N.; Guldhe, A.; Prasanna, R.; Singh, P.; Bux, F. Microalgae as multi-functional options in modern agriculture: Current trends, prospects and challenges. Biotechnol. Adv. 2018, 36, 1255–1273. [Google Scholar] [CrossRef] [PubMed]
- Behera, B.; Balasubramanian, P. Natural plant extracts as an economical and ecofriendly alternative for harvesting microalgae. Bioresour. Technol. 2019, 283, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Camargo, E.C.; Casali, C.A.; Lombardi, A.T.; Lima, M.I.S. Coupling microalgal cultures with hydroponics: Prospection for clean biotechnology processes. J. Algal Biomass Util. 2015, 6, 88–94. [Google Scholar]
- Salazar, J.; Santana-Sánchez, A.; Näkkilä, J.; Sirin, S.; Allahverdiyeva, Y. Complete N and P removal from hydroponic greenhouse wastewater by Tetradesmus obliquus: A strategy for algal bioremediation and cultivation in Nordic countries. Algal Res. 2023, 70, 102988. [Google Scholar] [CrossRef]
- Abdel-Raouf, N.; Al-Homaidan, A.; Ibraheem, I. Microalgae and wastewater treatment. Saudi J. Biol. Sci. 2012, 19, 257–275. [Google Scholar] [CrossRef] [PubMed]
- Žunić, V.; Jafari, T.H.; Grabić, J.; Đurić, S.; Stamenov, D. Hydroponic systems: Exploring the balance between co-cultivation of Chlorella vulgaris and Swiss chard (Beta vulgaris L. subsp. cicla). J. Appl. Phycol. 2022, 34, 903–913. [Google Scholar] [CrossRef]
- Ergun, O.; Dasgan, H.Y.; Isık, O. Effects of microalgae Chlorella vulgaris on hydroponically grown lettuce. Acta Hortic. 2020, 1273, 169–176. [Google Scholar] [CrossRef]
- Cortés-Jiménez, D.; Gómez-Guzmán, A.; Iturriaga, G.; Suárez, R.; Montero Alpírez, G.; Escalante, F.M.E. Microorganisms associated to tomato seedlings growing in saline culture act as osmoprotectant. Braz. J. Microbiol. 2014, 45, 613–620. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Escalante, F.M.; Cortés-Jiménez, D.; Tapia-Reyes, G.; Suárez, R. Immobilized microalgae and bacteria improve salt tolerance of tomato seedlings grown hydroponically. J. Appl. Phycol. 2015, 27, 1923–1933. [Google Scholar] [CrossRef]
- Lefever, K.; Laubscher, C.P.; Ndakidemi, P.A.; Nchu, F. Effects of pH and phosphorus concentrations on the chlorophyll responses of Salvia chamelaeagnea (Lamiaceae) grown in hydroponics. In Chlorophyll; InTech: London, UK, 2017. [Google Scholar] [CrossRef]
- Sapkota, S.; Sapkota, S.; Liu, Z. Effects of nutrient composition and lettuce cultivar on crop production in hydroponic culture. Horticulturae 2019, 5, 72. [Google Scholar] [CrossRef]
- Mattner, S.W.; Milinkovic, M.; Arioli, T. Increased growth response of strawberry roots to a commercial extract from Durvillaea potatorum and Ascophyllum nodosum. J. Appl. Phycol. 2018, 30, 2943–2951. [Google Scholar] [CrossRef]
- Santoro, D.F.; Puglisi, I.; Sicilia, A.; Baglieri, A.; La Bella, E.; Lo Piero, A.R. Transcriptomic profile of lettuce seedlings (Lactuca sativa) response to microalgae extracts used as biostimulant agents. AoB Plants 2023, 15, plad043. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.A.; Chatzieustratiou, E.; Constantopoulou, E.; Kapotis, G. Yield and quality of lettuce and rocket grown in floating culture system. Not. Bot. Horti Agrobot. Cluj-Napoca 2016, 44, 603–612. [Google Scholar] [CrossRef]
- Fraile-Robayo, R.D.; Álvarez-Herrera, J.G.; Reyes, M.; Johana, A.; Álvarez-Herrera, O.F.; Fraile-Robayo, A.L. Evaluation of the growth and quality of lettuce (Lactuca sativa L.) in a closed recirculating hydroponic system. Agron. Colomb. 2017, 35, 216–222. [Google Scholar] [CrossRef]
- Nurzyńska-Wierdak, R. The effect of nitrogen fertilization on yield and chemical composition of garden rocket (Eruca sativa Mill.) in autumn cultivation. Acta Sci. Pol. Hortorum Cultus 2006, 5, 53–63. [Google Scholar]
- Sublett, W.; Barickman, T.; Sams, C. The effect of environment and nutrients on hydroponic lettuce yield, quality, and phytonutrients. Horticulturae 2018, 4, 48. [Google Scholar] [CrossRef]
- Ciriello, M.; Formisano, L.; Soteriou, G.A.; Kyratzis, A.; De Pascale, S.; Kyriacou, M.C.; Rouphael, Y. Differential response to NaCl osmotic stress in sequentially harvested hydroponic red and green basil and the role of calcium. Front. Plant Sci. 2022, 13, 799213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, M.; Fan, Y.; Zhang, L.; Wang, H. Using microalgae to reduce the use of conventional fertilizers in hydroponics and soil-based cultivation. Sci. Total Environ. 2024, 912, 169424. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Liu, L.; Wang, Q.; Duanmu, D.; Qiu, B. Editorial: Algal photosynthesis. Front. Microbiol. 2023, 13, 1112301. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Edmundson, S.; Huesemann, M. Oxygen stress mitigation for microalgal biomass productivity improvement in outdoor raceway ponds. Algal Res. 2022, 68, 102901. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, L.; Dixon, M. An upper limit for elevated root zone dissolved oxygen concentration for tomato. Sci. Hortic. 2007, 113, 162–165. [Google Scholar] [CrossRef]


| Microalgae | Plants | Outcomes | Reference | ||||
|---|---|---|---|---|---|---|---|
| Germination | Shoot/Root Length | Plant Biomass | Nutrient Content | Other Results | |||
| Live cell suspensions or fresh biomass | |||||||
| Anabaena laxa, Calothrix elenkinii | Coriandrum sativum, Cuminum cyminum, Foeniculum vulgare | + | + | + | Increased peroxidase activity in shoots and roots and antifungal activities against Macrophomina phaseolina and Fusarium moniliforme | [35] | |
| Anabaena torulosa, Trichormus doliolum, A. laxa | Chrysanthemum morifolium | + | + | Enhanced leaf pigments, IAA production, and PEP carboxylase activity | [36] | ||
| Chlorella fusca | Spinacia oleracea | + | + | Increased plant yield, leaf width, thickness and number, and resistance to gray mold disease | [37] | ||
| Chlorella infusionum | Solanum lycopersicum | + | + | + | [38] | ||
| Chlorella sp., Scenedesmus sp., Synechocystis sp., Spirulina sp. | S. lycopersicum | + | + | + | + | Enhanced chlorophyll pigments and dissolved oxygen | [39] |
| Chlorella vulgaris | Hibiscus esculentus | + | + | Increased number of flower buds | [40] | ||
| C. vulgaris | Triticum aestivum L. | + | + | Increased plant growth, leaf area, and root hair production | [41] | ||
| Microcystis aeruginosa, Anabaena sp., Chlorella sp. | Zea mays | + | + | Inhibited the growth of pathogenic bacteria and fungi | [42] | ||
| Dry biomass, cell extracts, or hydrolysates | |||||||
| Tetradesmus dimorphus | S. lycopersicum | + | + | + | + | Increased number of flowers and branches | [22] |
| C. vulgaris | Lactuca sativa L. | + | + | + | + | Increased leaf chlorophyll, carotenoid, and protein content | [43] |
| C. vulgaris, Limnospira platensis | Z. mays L. | + | Enhanced early seedling growth and improved yield characteristics | [44] | |||
| Chlorococcum sp., Micractinium sp., Scenedesmus sp., Chlorella sp. | S. oleracea L. | + | + | + | Synthesis of cytokinins (trans-zeatin, DHZR, tZMP, iP, iPA, and iPAMP), gibberellins (GA1, GA3, GA4, GA20, and GA29), auxin (IAA), and abscisic acid (ABA) | [45] | |
| Nannochloropsis oculata | S. lycopersicum cv. Maxifort | + | + | + | Improved the fruit quality through an increase in sugar and carotenoid contents | [46] | |
| Nostoc commune | Oryza sativa cv. Shiroodi L. | + | + | + | [47] | ||
| L. platensis | Raphanus sativus | + | + | + | Enhanced leaf pigments | [48] | |
| L. platensis | Vigna mungo L. | + | + | + | + | [49] | |
| Ulothrix sp., Pinnularia sp., and Oscillatoria sp. | S. lycopersicum, Capsicum annuum, Solanum melongena | + | + | + | Improved disease resistance | [50] | |
| Species | Metabolites | Targets Promoted | Reference |
|---|---|---|---|
| Auxin | |||
| Auxenochlorella pyrenoidosa, Scenedesmus quadricauda | Indole-3-acetic acid (IAA), indole-3-butyric acid (IBA) | Lipid content and production | [59] |
| C. fusca, C. vulgaris, Scenedesmus obliquus, Synechococcus nidulans, Spirulina sp. LEB 18 | IAA | Carbohydrate, protein | [60] |
| C. vulgaris | IAA, IBA, phenylacetic acid (PAA) | Cell divisions, proteins, chlorophylls, monosaccharides | [61] |
| Desmodesmus sp. | IAA, IBA, IPA | Biomass, lipids, fatty acids | [62] |
| Dunaliella salina | IAA | Growth, β-carotene | [63] |
| C. vulgaris | IAA | Biomass, lipid content and productivity | [64] |
| Nannochloropsis oceanica | IAA | Growth, lipid | [65] |
| N. oculata | IAA | Cell division, chlorophyll-a | [66] |
| S. obliquusi, Pilidiocystis multispora, C. vulgaris | IAA | Growth, PUFAs | [67] |
| S. obliquus | IAA | Growth, fatty acid, protein, carbohydrate content | [68] |
| S. quadricauda | Auxins | Cell divisions, growth, biomass, chlorophyll, carotenoids, fatty acids | [69] |
| Scenedesmus sp., Chlorella sorokiniana | IBA, NAA | Lipid | [70] |
| Cytokinin | |||
| Tetradesmus obliquus | Kinetin, zeatin | Biomass, lipid, carbohydrate | [71] |
| C. fusca, C. vulgaris, S. obliquus, S. nidulans, Spirulina sp. LEB 18 | Trans-zeatin | Carbohydrate, protein | [60] |
| Auxenochlorella protothecoides | Cytokinin | Biomass, lipid | [72] |
| C. vulgaris | Zeatin | Cell divisions, carotenoids | [73] |
| C. vulgaris | Benzyladenine, trans-zeatin, 2-methylthio-trans-zeatin | α-Linolenic, linoleic, palmitic, oleic, and stearic acids | [74] |
| Desmodesmus sp. | 6-benzylaminopurine, Thidiazuron | Biomass, lipids, fatty acids | [62] |
| D. salina | Kinetin | Growth, β-carotene | [63] |
| Nostoc muscorum | Kinetin | Biomass, carotenoids | [75] |
| Gibberellic acid | |||
| Chlorella ellipsoidea | Gibberellic acid (GA) | Growth, lipid | [76] |
| A. pyrenoidosa | GA3 | Growth, lipid | [77] |
| C. vulgaris | GA | Cell divisions, carotenoid | [73] |
| Isochrysis galbana | GA3 | Biomass, chlorophyll a, protein, lipid, PUFAs | [78] |
| Monodopsis subterranea | GA | Biomass, total fatty acid, eicosapentaenoic acid | [79] |
| N. oculata | GA | Cell diameter, lipid | [66] |
| Ethylene | |||
| C. vulgaris | Ethephon | SFAs, a-tocopherol, c-aminobutyric acid, asparagine, proline | [80] |
| Haematococcus lacustris | 1-Aminocyclopropane-1-carboxylic acid (ACC) | Astaxanthin | [81] |
| H. lacustris | Ethylene | Astaxanthin, lipid | [82] |
| Monoraphidium sp. | Ethylene | Lipid | [83] |
| Abscisic acid | |||
| A. pyrenoidosa | Abscisic acid (ABA) | Lipid | [84] |
| C. vulgaris | ABA | Biomass, total fatty acid | [85] |
| C. vulgaris | ABA | Fatty acids | [74] |
| Chromochloris zofingiensis | ABA | Growth, fatty acid, pigmentation | [86] |
| D. salina | ABA | Growth, β-carotene | [63] |
| Salicylic acid | |||
| Chlorella sp. | Salicylic acid (SA) | Cell growth | [87] |
| C. zofingiensis | SA | Cell growth, total fatty acids, astaxanthin | [88] |
| H. lacustris | SA | Biomass, astaxanthin | [89] |
| Jasmonic acid | |||
| C. vulgaris | Jasmonic acid (JA) | Cell divisions, carotenoid | [73] |
| H. lacustris | Methyl jasmonate (MJ) | β-Carotene, lutein | [89] |
| M. subterranea | MJ | Biomass, total fatty acid, eicosapentaenoic acid | [79] |
| Stauroneis sp. | MJ | Lipids and pigments | [90] |
| Microalgae | Plants | Stress | Tolerance |
|---|---|---|---|
| Dunaliella salina, Phaeodactylum tricornutum | Bell pepper | Salinity | Reduced production of superoxide radicals, decreased lipid peroxidation, and increased antioxidant enzyme activity. |
| D. salina | Wheat | Salinity | Improved seed germination and coleoptile height. Enhanced the accumulation of proline and ROS antioxidant enzymes like catalase (CAT), peroxidase (POD), and superoxide dismutase (SOD). |
| Nannochloris sp. | Tomato | Water stress | Enhanced root length, leaf number, and leaf area. |
| Chlorella vulgaris | Guar | Drought | Increased shoot length, fresh and dry weights of shoot and root. Stimulated the accumulation of relative water content, total phenolic content, and ROS scavengers, such as SOD, CAT, ascorbate peroxidase (APX), and glutathione reductase (GR). |
| C. vulgaris | Onion | Drought | Increased growth parameters, nutrients, and accumulation of carbohydrates. |
| C. vulgaris | Guar | Salinity | Increased photosynthetic pigments and induced antioxidant enzymes, such as SOD, CAT, GR, and APX, and decreased MDA, NA+, and Ca− ions. |
| Crops | Crop Names | References |
|---|---|---|
| Cereals | O. sativa, Z. mays | [163,164] |
| Condiments/herbs | Coriandrum sativum, Trigonella foenum-graecum, Petroselinum crispum, Mentha piperita, Rosmarinus officinalis, Ocimum basilicum, Origanum vulgare | [165,166,167,168,169,170] |
| Flower/ornamental crops | Tagetes sp., Rosa sp., Dianthus sp., Chrysanthemum sp. | [36,171,172] |
| Fodder crops | Sorghum bicolor, Medicago sativa, Cynodon dactylon, Axonopus sp. | [173,174,175] |
| Fruits | Fragaria ananassa | [176] |
| Leafy vegetables | L. sativa, S. oleracea, Apium graveolens, Atriplex sp. | [177,178,179,180] |
| Medicinal crops | Aloe perfoliata, Coleus sp. | [181,182] |
| Microgreens | R. sativus, Brassica oleracea, Lepidium sativum, Eruca sativa, Daucus carota, Helianthus annuus, Amaranthus sp., Fagopyrum esculentum, Ocimum basilicum, Rumex acetosa, T. aestivum, Medicago sativa, Brassica sp., Trifolium sp. | [3,152,169,183,184,185,186,187,188,189,190,191,192] |
| Vegetables | S. lycopersicum, Capsicum sp., S. melongena L., Phaseolus vulgaris, Beta vulgaris, Cucumis sp., Allium fistulosum L. | [39,193,194,195,196,197] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renganathan, P.; Puente, E.O.R.; Sukhanova, N.V.; Gaysina, L.A. Hydroponics with Microalgae and Cyanobacteria: Emerging Trends and Opportunities in Modern Agriculture. BioTech 2024, 13, 27. https://doi.org/10.3390/biotech13030027
Renganathan P, Puente EOR, Sukhanova NV, Gaysina LA. Hydroponics with Microalgae and Cyanobacteria: Emerging Trends and Opportunities in Modern Agriculture. BioTech. 2024; 13(3):27. https://doi.org/10.3390/biotech13030027
Chicago/Turabian StyleRenganathan, Prabhaharan, Edgar Omar Rueda Puente, Natalia V. Sukhanova, and Lira A. Gaysina. 2024. "Hydroponics with Microalgae and Cyanobacteria: Emerging Trends and Opportunities in Modern Agriculture" BioTech 13, no. 3: 27. https://doi.org/10.3390/biotech13030027
APA StyleRenganathan, P., Puente, E. O. R., Sukhanova, N. V., & Gaysina, L. A. (2024). Hydroponics with Microalgae and Cyanobacteria: Emerging Trends and Opportunities in Modern Agriculture. BioTech, 13(3), 27. https://doi.org/10.3390/biotech13030027








