An Efficient and Rapid Protocol for Somatic Shoot Organogenesis from Juvenile Hypocotyl-Derived Callus of Castor Bean cv. Zanzibar Green
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
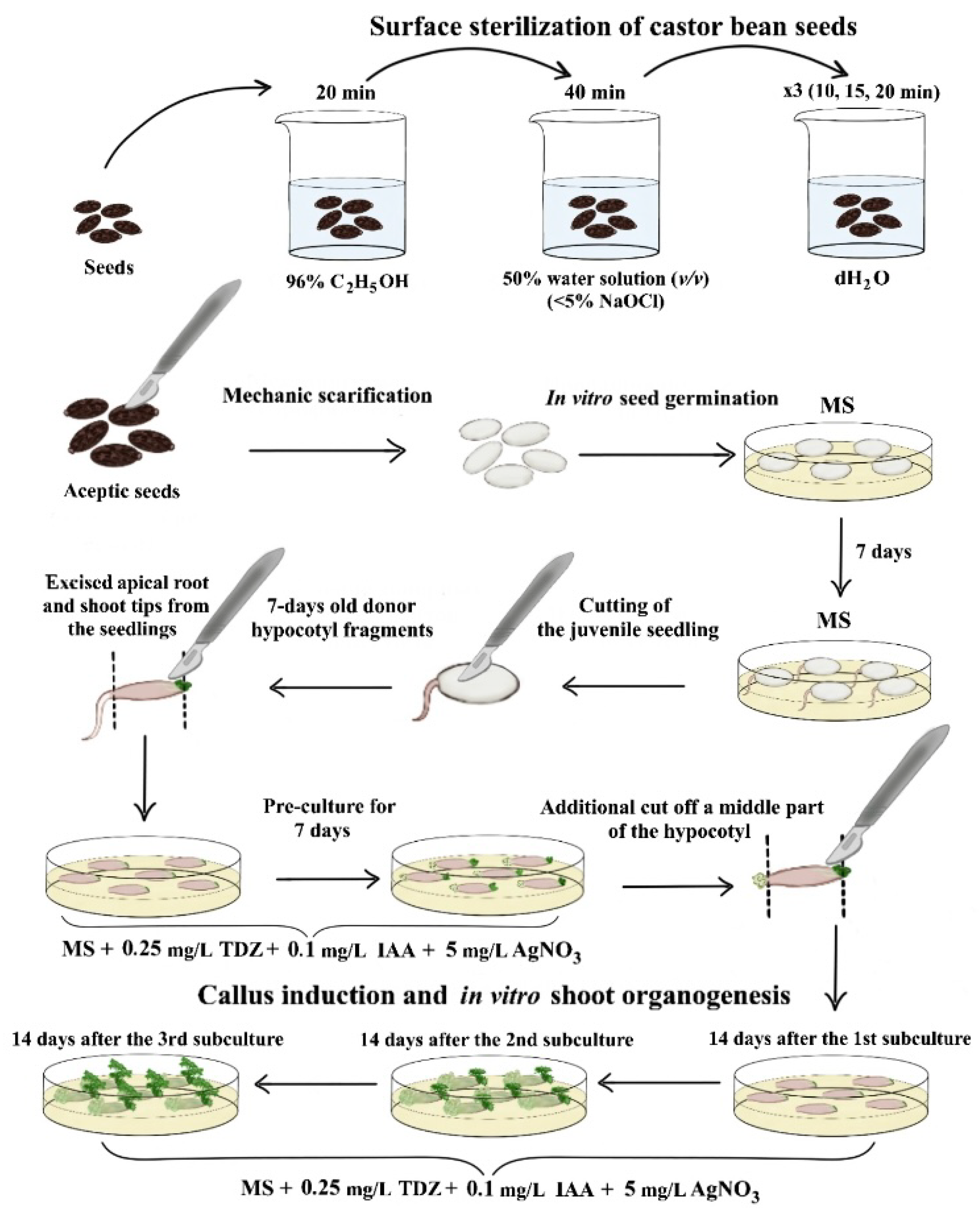
2.2. Obtaining Aseptic Donor Seedlings. Culture Conditions
2.3. In Vitro Callus Induction and Somatic Shoot Organogenesis
2.4. In Vitro Root Induction of Regenerants and Plantlets Adaptation to Soil Conditions
2.5. Estimation of the Efficiency of an In Vitro Morphogenetic Responses
2.6. Statistical Processing of Experimental Data
3. Results
3.1. Influence of Culture Medium Composition on Explant Viability
3.2. Effects of Explant Source and Culture Medium Composition on In Vitro Callus Induction
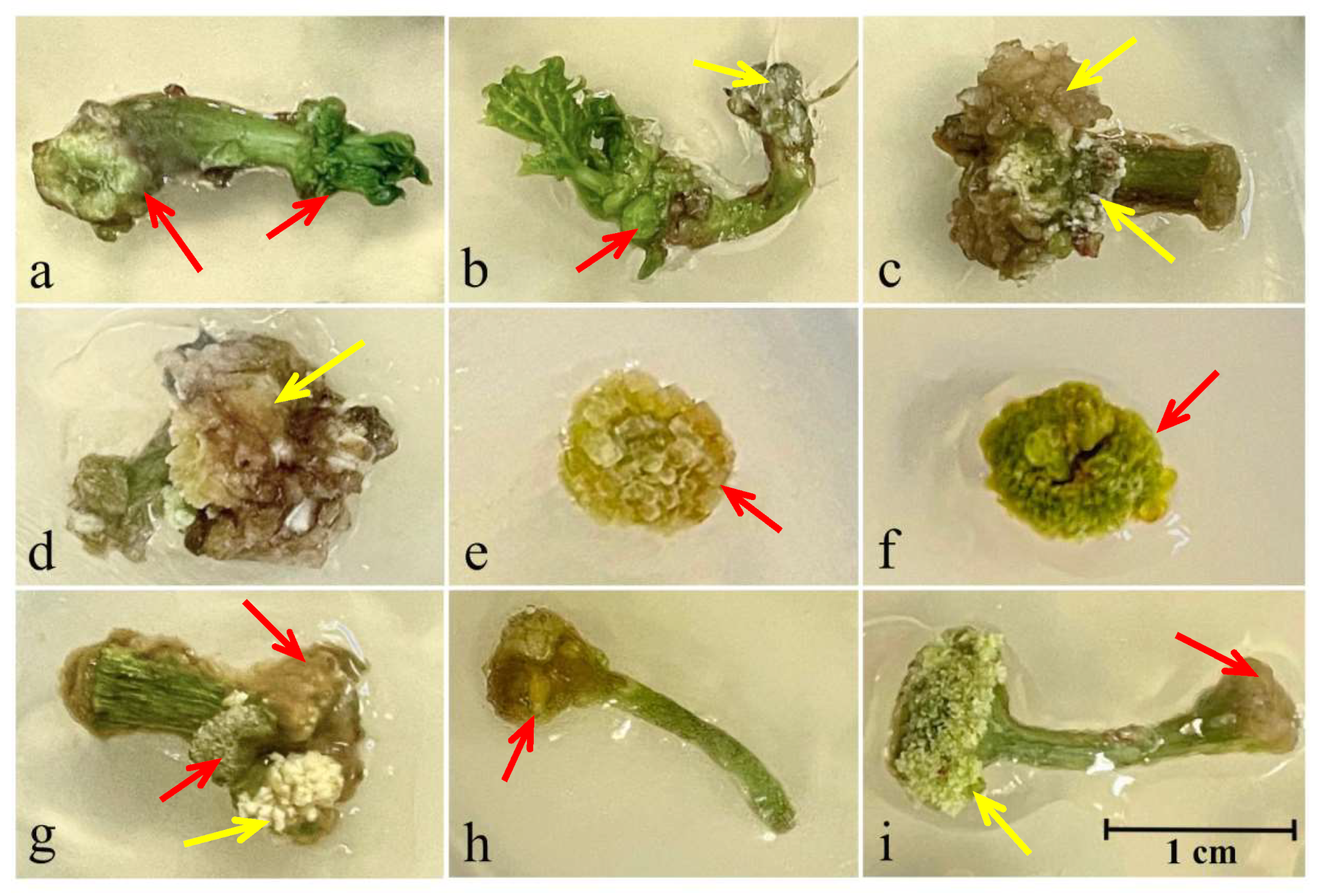
3.2.1. Morphological Characteristics of Callus Tissue
3.2.2. Callus Formation Efficiency
3.3. Effects of Explant Source and Culture Medium Composition on In Vitro Induction of Somatic Shoot Organogenesis
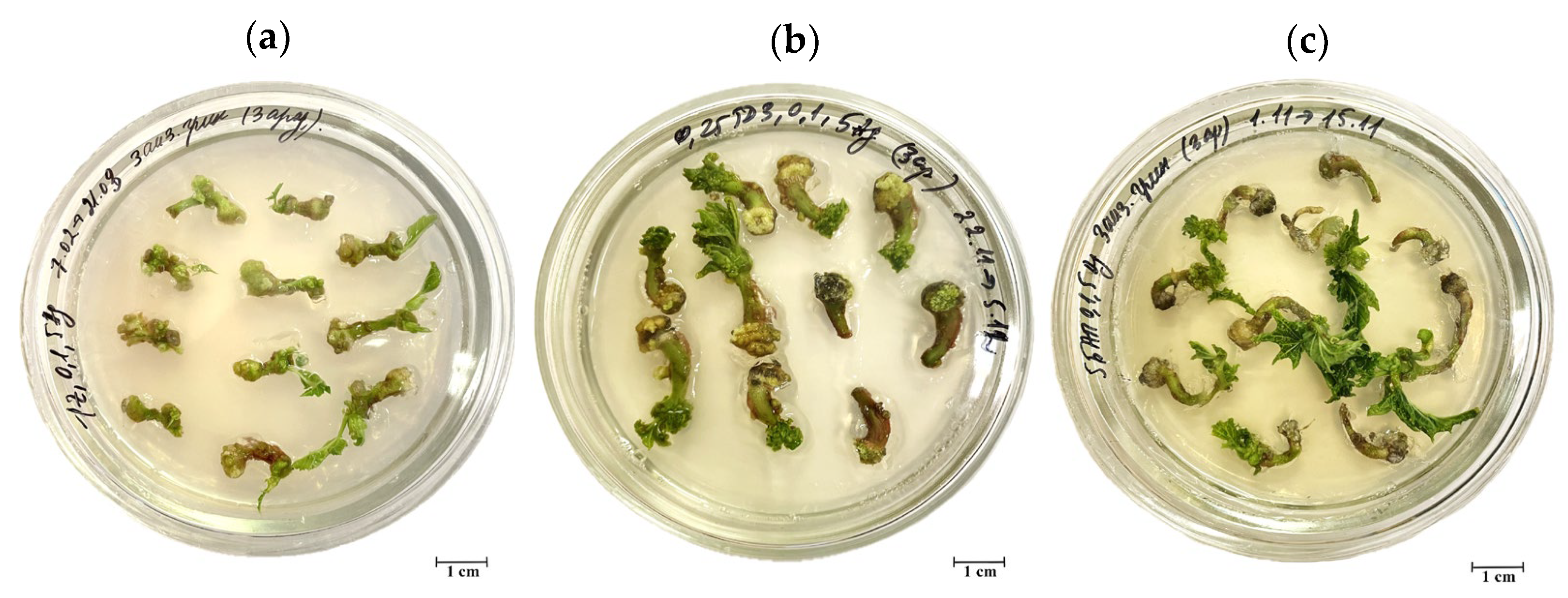
3.4. In Vitro Rooting of Regenerants and Plantlets Adaptation to Ex Vitro Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization (FAO). World’s Castor Bean Production. Available online: http://www.fao.org/faostat/en/#data/QCL (accessed on 15 March 2024).
- Patel, R.; Menon, J.; Kumar, S.; Nóbrega, M.; Patel, D.A.; Sakure, A.A.; Vaja, M.B. Modern Day Breeding Approaches for Improvement of Castor. Heliyon 2024, 10, e27048. [Google Scholar] [CrossRef] [PubMed]
- Krat-Kravchenko, E.A.; Luchkina, T.N.; Zbrailova, L.P. Cultivation of Castor in the Conditions of the Rostov Region. Maslichnyye Kul’tury 2022, 4, 96–101. (In Russian) [Google Scholar] [CrossRef]
- Patel, V.R.; Dumancas, G.G.; Viswanath, L.C.K.; Maples, R.; Subong, B.J.J. Castor Oil: Properties, Uses, and Optimization of Processing Parameters in Commercial Production. Lipid Insights 2016, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nitbani, F.O.; Tjitda, P.J.P.; Wogo, H.E.; Detha, A.I.R. Preparation of Ricinoleic Acid from Castor Oil: A review. J. Oleo Sci. 2022, 71, 781–793. [Google Scholar] [CrossRef] [PubMed]
- Polito, L.; Bortolotti, M.; Battelli, M.G.; Calafato, G.; Bolognesi, A. Ricin: An Ancient Story for a Timeless Plant Toxin. Toxins 2019, 11, 324. [Google Scholar] [CrossRef] [PubMed]
- Ramanjaneyulu, A.V.; Anudradha, G.; Ramana, M.V.; Reddy, A.V.V.; Gopal, N.M. Multifarious Uses of Castor (Ricinus communis L.). Int. J. Econ. Plants 2017, 4, 170–176. [Google Scholar]
- Osorio-González, C.S.; Gómez-Falcon, N.; Sandoval-Salas, F.; Saini, R.; Brar, S.K.; Ramírez, A.A. Production of Biodiesel from Castor Oil: A review. Energies 2020, 13, 2467. [Google Scholar] [CrossRef]
- Keera, S.T.; El Sabagh, S.M.; Taman, A.R. Castor Oil Biodiesel Production and Optimization. Egypt J. Pet. 2018, 27, 979–984. [Google Scholar] [CrossRef]
- Faria Filho, D.E.D.; Dias, A.N.; Carneiro, W.A.; Bueno, C.F.D.; Matos Júnior, J.B.; Veloso, A.L.C.; Rodrigues, P.A. Detoxified Castor Seed Cake for Broilers. Braz. J. Poult. Sci. 2016, 18, 69–72. [Google Scholar] [CrossRef]
- Cheikhyoussef, N.; Cheikhyoussef, A. Bioactive Phytochemicals from Castor (Ricinus communis Linneo) Seed Oil Processing By-products. In Bioactive Phytochemicals from Vegetable Oil and Oilseed Processing By-Products; Ramadan Hassanien, M.F., Ed.; Reference Series in Phytochemistry; Springer: Cham, Switzerland, 2023; pp. 1–20. ISBN 978-3-030-63961-7. [Google Scholar]
- Balogun, J.K.; Auta, J.; Abdullahi, S.A.; Agboola, O.E. Potentials of Castor Seed Meal (Ricinus communis L.) as Feed Ingredient for Oreochromis niloticus. In Proceedings of the 19th Annual Conference Fisheries Society Nigeria, Ilorin, Nigeria, 29 November–3 December 2004; pp. 838–843. [Google Scholar]
- Moshiri, M.; Hamid, F.; Etemad, L. Ricin Toxicity: Clinical and Molecular Aspects. Rep. Biochem. Mol. Biol. 2016, 4, 60–65. [Google Scholar] [PubMed]
- Yeboah, A.; Ying, S.; Lu, J.; Xie, Y.; Amoanimaa-Dede, H.; Boateng, K.G.A.; Chen, M.; Yin, X. Castor oil (Ricinus communis): A review on the chemical composition and physicochemical properties. Food Sci. Technol. 2020, 41, 399–413. [Google Scholar] [CrossRef]
- Anandan, S.; Kumar, G.A.; Ghosh, J.; Ramachandra, K.S. Effect of Different Physical and Chemical Treatments on Detoxification of Ricin in Castor Cake. Japan J. Forensic Sci. Tech. 2005, 120, 159–168. [Google Scholar] [CrossRef]
- Sousa, N.L.; Cabral, G.B.; Vieira, P.M.; Baldoni, A.B.; Aragão, F.J. Bio- detoxification of Ricin in Castor Bean (Ricinus communis L.) Seeds. Sci. Rep. 2017, 7, 15385. [Google Scholar] [CrossRef] [PubMed]
- Landoni, M.; Bertagnon, G.; Ghidoli, M.; Cassani, E.; Adani, F.; Pilu, R. Opportunities and Challenges of Castor Bean (Ricinus communis L.) Genetic Improvement. Agronomy 2023, 13, 2076. [Google Scholar] [CrossRef]
- Gil-Correal, A.; Restrepo-Osorio, C.; Álvarez, J.C.; Villanueva-Mejía, D.F. Direct In Vitro Regeneration of Castor Bean Plants (Ricinus communis) Using Epicotyls. Biosci. J. 2019, 35, 347–355. [Google Scholar] [CrossRef]
- Zhang, J.X.; Wang, X.Y.; Feng, Z.Z.; Geng, X.J.; Mu, S.M.; Huo, H.Y.; Huan, T.O.; Li, M.Z.; Yi, L.I.; Yue, C.H.; et al. In Vitro Establishment of a Highly Effective Method of Castor Bean (Ricinus communis L.) Regeneration Using Shoot Explants. J. Integr. Agric. 2016, 15, 1417–1422. [Google Scholar] [CrossRef]
- Ahn, Y.J.; Vang, L.; McKeon, T.A.; Chen, G.Q. High-frequency Plant Regeneration Through Adventitious Shoot Formation in Castor (Ricinus communis L.). In Vitro Cell. Dev. Biol. Plant 2007, 43, 9–15. [Google Scholar] [CrossRef]
- Zalavadiya, V.K.; Mehta, D.R.; Javia, R.M.; Padhiyar, S.M.; Madariya, R.B. Somatic Organogenesis and Plant Regeneration in Castor (Ricinus communis L.). Asian J. Biol. Sci. 2014, 9, 43–52. [Google Scholar]
- Ganesh Kumari, K.; Ganesan, M.; Jayabalan, N. Somatic Organogenesis and Plant Regeneration in Ricinus communis. Biol. Plant. 2008, 52, 17–25. [Google Scholar] [CrossRef]
- Alexandrov, O.S.; Petrov, N.R.; Varlamova, N.V.; Khaliluev, M.R. An Optimized Protocol for In Vitro Indirect Shoot Organogenesis of Impala Bronzovaya and Zanzibar Green Ricinus communis L. Varieties. Horticulturae 2021, 7, 105. [Google Scholar] [CrossRef]
- Figueroa-Varela, P.; Susunaga-Gómez, D.; Restrepo-Osorio, C.; Harms, C.; Villanueva-Mejía, D. An Efficient Method for Protoplast-mediated Production of Transformed Castor Bean (Ricinus communis) Lines. BMC Res. Not. 2023, 16, 140. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.K.; Rao, G.P.; Bahadur, B. In Vitro Morphogenesis from Seedling Explants and Callus Cultures of Castor (Ricinus communis L.). Phytomorphology 1987, 37, 337–340. [Google Scholar]
- Ahn, Y.J.; Chen, G.Q. In Vitro Regeneration of Castor (Ricinus communis L.) Using Cotyledon Explants. HortScience 2008, 43, 215–219. [Google Scholar] [CrossRef]
- Danso, K.E.; Afful, N.T.; Annor, C.; Amoatey, H.M. In Vitro Regeneration of Ricinus communis L. and Jatropha curcas L. for Biofuel Production. Biotechnology 2011, 10, 400–407. [Google Scholar] [CrossRef]
- Louis, G.C.; Okafor, C.U.; Okezie, C.E.A. Nutrient Requirements for In Vitro Propagation of Ricinus communis L. Mature Zygotic Embryos. Int. J. Agric. Res. 2018, 13, 19–25. [Google Scholar] [CrossRef]
- Nahar, K.; Borna, R.S. In Vitro Propagation from Shoot Tip Explants of Castor Oil Plant (Ricinus communis L.): A Bioenergy Plant. Can. J. Sci. Ind. Res. 2012, 3, 354–355. [Google Scholar]
- Raldugina, G.N.; Hoang, T.Z.; Ngoc, H.B.; Karpichev, I.V. An Increased Proportion of Transgenic Plants in the Progeny of Rapeseed (Brassica napus L.) Transformants. Vavilov J. Genet. Breed. 2021, 25, 147. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bioassays with Tobacco Tissue Culture. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Sujatha, M.; Reddy, T.P.; Mahasi, M.J. Role of Biotechnological Interventions in the Improvement of Castor (Ricinus communis L.) and Jatropha curcas L. Biotech. Adv. 2008, 26, 424–435. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Liu, K.; Chen, J.; Li, X.; Chen, J.; Hu, H.; Yang, S.; Xue, Y. In vitro Grafting Procedure for Facilitating Growth of Separated Shoots in Castor. Agron. J. 2021, 113, 878–885. [Google Scholar] [CrossRef]
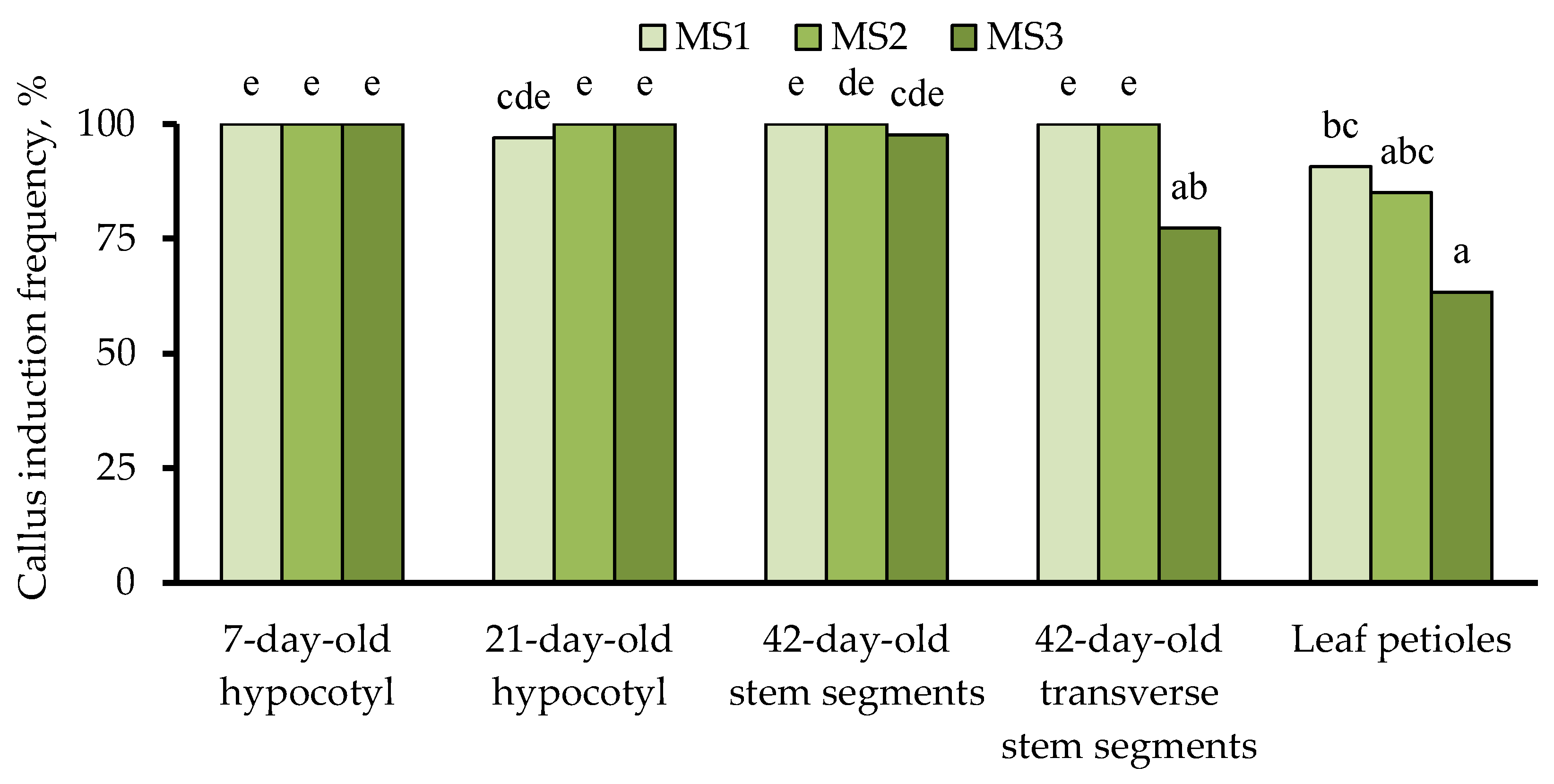
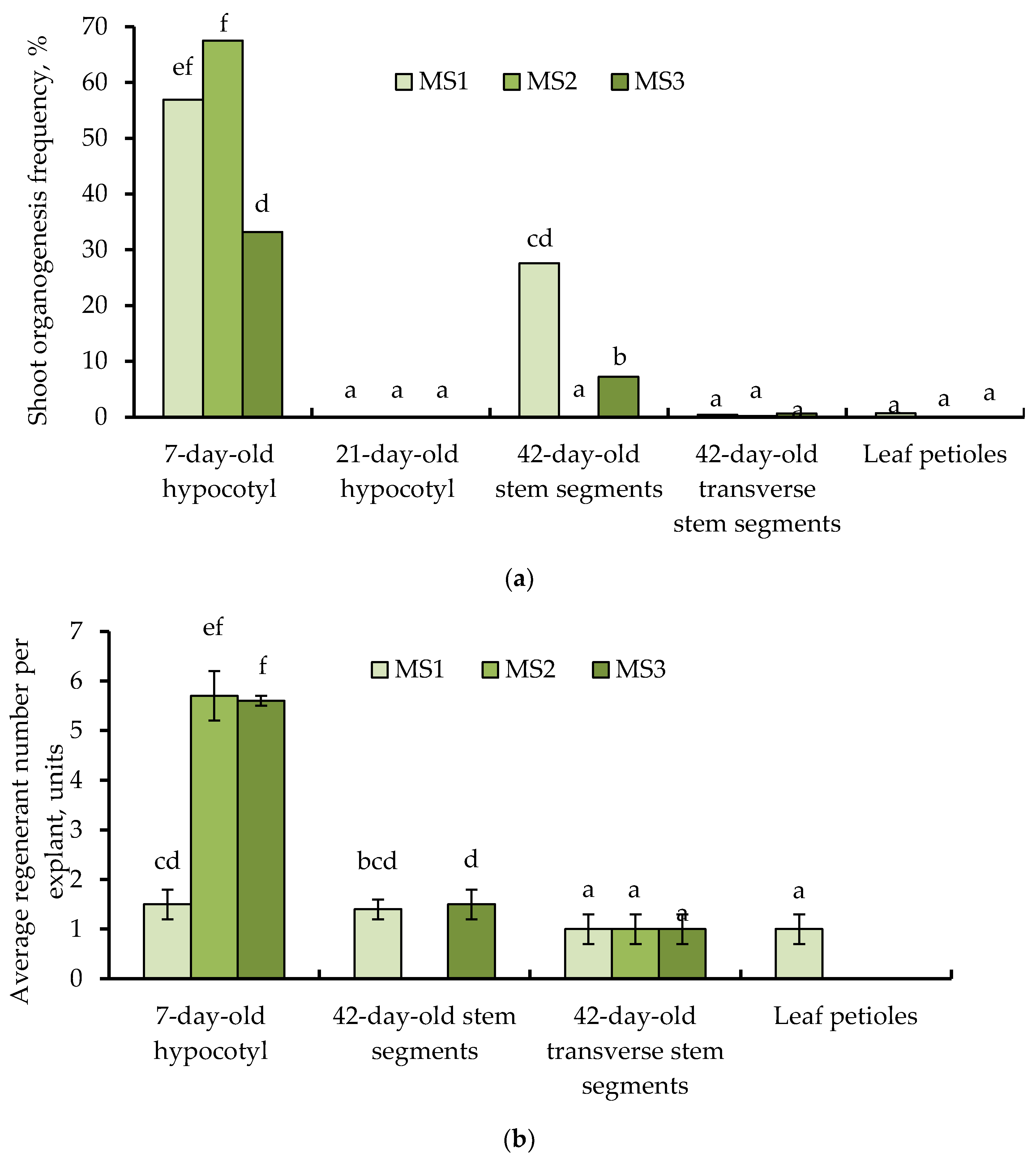
| Explant Type | Number of Explants (Units) | Explant Viability (%) | |
|---|---|---|---|
| Total | Necrotic | ||
| MS1 (1 mg/L zeatin + 0.1 mg/L IAA + 5 mg/L AgNO3) | |||
| 7-day-old hypocotyl fragments | 51 | 0 | 100 e |
| 21-day-old hypocotyl fragments | 216 | 18 | 91.7 cde |
| 42-day-old nodal segments of stem | 54 | 0 | 100 e |
| 42-day-old transverse nodal segments of stem | 162 | 0 | 100 e |
| Petioles of first true leaves | 288 | 53 | 81.6 bc |
| MS2 (0.25 mg/L TDZ + 0.1 mg/L IAA + 5 mg/L AgNO3) | |||
| 7-day-old hypocotyl fragments | 66 | 0 | 100 e |
| 21-day-old hypocotyl fragments | 78 | 0 | 100 e |
| 42-day-old nodal segments of stem | 30 | 0 | 100 e |
| 42-day-old transverse nodal segments of stem | 144 | 0 | 100 e |
| Petioles of first true leaves | 78 | 13 | 83.3 abc |
| MS3 (5 mg/L 6-BAP + 0.1 mg/L IAA + 5 mg/L AgNO3) | |||
| 7-day-old hypocotyl fragments | 90 | 0 | 100 e |
| 21-day-old hypocotyl fragments | 30 | 0 | 100 e |
| 42-day-old nodal segments of stem | 90 | 6 | 93.3 cde |
| 42-day-old transverse nodal segments of stem | 60 | 15 | 75.0 ab |
| Petioles of first true leaves | 30 | 11 | 63.3 a |
| Explant Type | Morphological Characteristics of Callus Tissue | Morphogenetic Response c | ||
|---|---|---|---|---|
| Color a | Consistency b | Site of Callus Formation | ||
| MS1 (1 mg/L zeatin + 0.1 mg/L IAA + 5 mg/L AgNO3) | ||||
| 7-day-old hypocotyl fragments | G | D | Cut from the shoot meristem side | + |
| B, W | F | Cut from the root meristem side | – | |
| 21-day-old hypocotyl fragments | Y-B | F | At both ends of the cut | – |
| 42-day-old nodal segments of stem | Y | D | At both ends of the cut | + |
| 42-day-old transverse nodal segments of stem | Y-B | D | Whole explant | + |
| Petioles of first true leaves | LB | D | At both ends of the cut | + |
| MS2 (0.25 mg/L TDZ + 0.1 mg/L IAA + 5 mg/L AgNO3) | ||||
| 7-day-old hypocotyl fragments | G | D | Cut from the shoot meristem side | + |
| LG | D | Cut from the root meristem side | – | |
| 21-day-old hypocotyl fragments | B, W | F | At both ends of the cut | – |
| 42-day-old nodal segments of stem | Y-B | D | At both ends of the cut | – |
| 42-day-old transverse nodal segments of stem | G | D | Whole explant | + |
| Petioles of first true leaves | B | D | Cut from the leave blade side | – |
| LG | F | Cut from the stem side | – | |
| MS3 (5 mg/L 6-BAP + 0.1 mg/L IAA + 5 mg/L AgNO3) | ||||
| 7-day-old hypocotyl fragments | G | D | Cut from the shoot meristem side | + |
| B, W | F | Cut from the root meristem side | – | |
| 21-day-old hypocotyl fragments | B, W | F | At both ends of the cut | – |
| 42-day-old nodal segments of stem | G, B | D | At both ends of the cut | + |
| 42-day-old transverse nodal segments of stem | Y-B | D | Whole explant | + |
| Petioles of first true leaves | LB | D | Cut from the stem side | – |
| Source of Variation | ss | df | ms | F05 | F |
|---|---|---|---|---|---|
| Total | 9251.9 | 44 | – | – | – |
| Variants | 6489.3 | 14 | 463.5 | 2.01 | 5.03 * |
| Factors | |||||
| A (culture medium) | 972.8 | 2 | 486.4 | 3.32 | 5.28 * |
| B (explant) | 3914.1 | 4 | 978.5 | 2.69 | 10.63 * |
| Interaction AB | 1602.4 | 8 | 200.3 | 2.27 | 2.18 |
| Error | 2762.6 | 30 | 92.1 | – | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demidenko, D.V.; Varlamova, N.V.; Soboleva, T.M.; Shitikova, A.V.; Khaliluev, M.R. An Efficient and Rapid Protocol for Somatic Shoot Organogenesis from Juvenile Hypocotyl-Derived Callus of Castor Bean cv. Zanzibar Green. BioTech 2024, 13, 25. https://doi.org/10.3390/biotech13030025
Demidenko DV, Varlamova NV, Soboleva TM, Shitikova AV, Khaliluev MR. An Efficient and Rapid Protocol for Somatic Shoot Organogenesis from Juvenile Hypocotyl-Derived Callus of Castor Bean cv. Zanzibar Green. BioTech. 2024; 13(3):25. https://doi.org/10.3390/biotech13030025
Chicago/Turabian StyleDemidenko, Danaya V., Nataliya V. Varlamova, Taisiya M. Soboleva, Aleksandra V. Shitikova, and Marat R. Khaliluev. 2024. "An Efficient and Rapid Protocol for Somatic Shoot Organogenesis from Juvenile Hypocotyl-Derived Callus of Castor Bean cv. Zanzibar Green" BioTech 13, no. 3: 25. https://doi.org/10.3390/biotech13030025
APA StyleDemidenko, D. V., Varlamova, N. V., Soboleva, T. M., Shitikova, A. V., & Khaliluev, M. R. (2024). An Efficient and Rapid Protocol for Somatic Shoot Organogenesis from Juvenile Hypocotyl-Derived Callus of Castor Bean cv. Zanzibar Green. BioTech, 13(3), 25. https://doi.org/10.3390/biotech13030025








