Industrial Biotechnology Conservation Processes: Similarities with Natural Long-Term Preservation of Biological Organisms
Abstract
1. Introduction
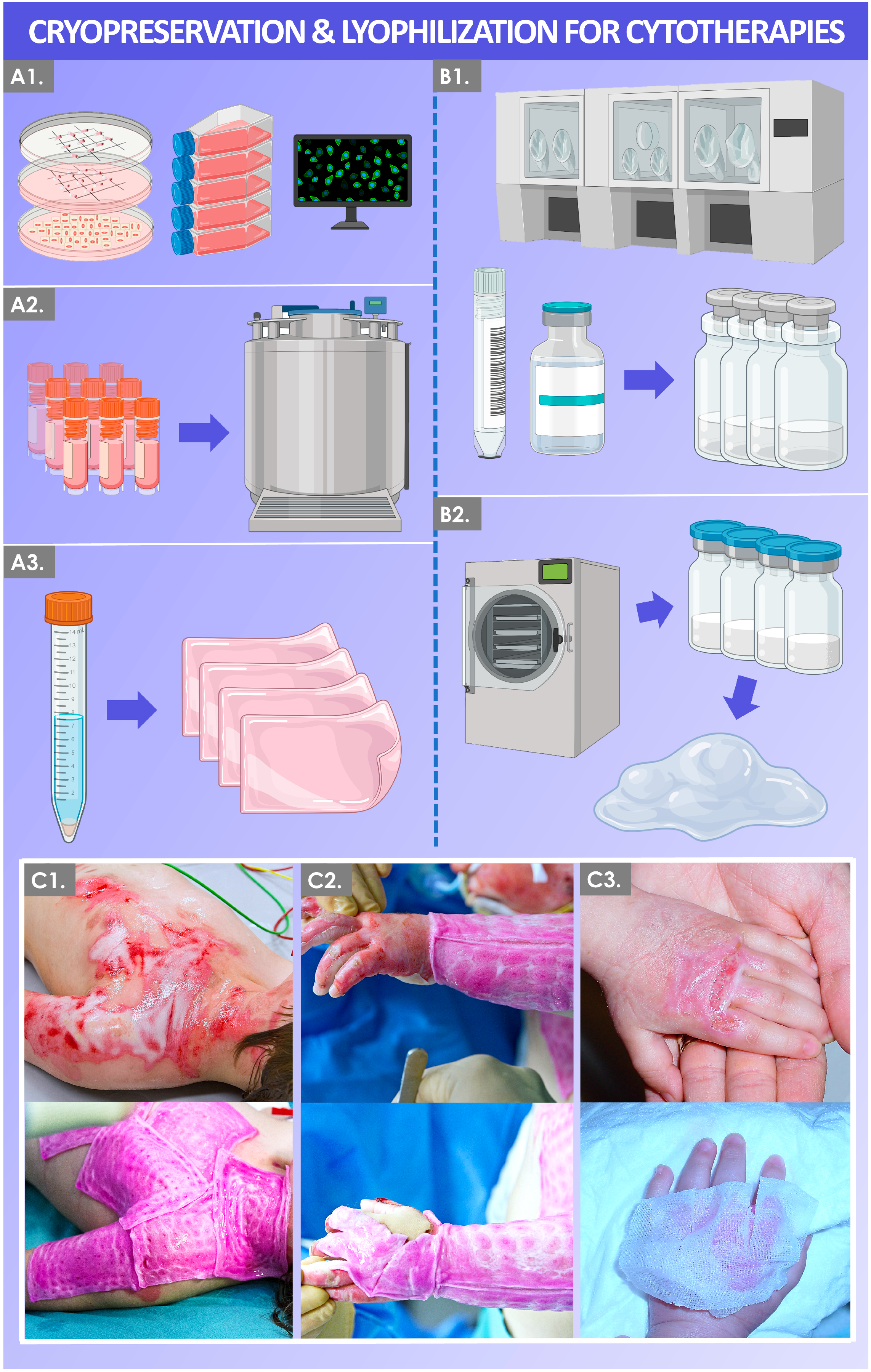
2. Cryopreservation of Biological Tissues and Cells: Principles and Application in Cytotherapies
3. Natural Forms of Environment-Mediated Cryopreservation and Freezing Tolerance
4. Lyophilization: Principles and Application for Temperature-Stabilized Cytotherapeutic Derivatives
5. Natural Cycles of Active Life and Latent Life: Animal, Plant, and Microorganism Dormancy
6. Origins and Evolution of Biological Lifeforms: Primordial Environment-Related Considerations
7. Similarities between Industrial Biotechnology Conservation Processes and Natural Long-Term Preservation of Biological Organisms
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin, W.; Russell, M.J. On the origins of cells: A hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells. Philos. Trans. R Soc. Lond. B Biol. Sci. 2003, 358, 59–85. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.; Sousa, F.; Mrnjavac, N.; Neukirchen, S.; Roettger, M.; Nelson-Sathi, S.; Martin, W. The physiology and habitat of the last universal common ancestor. Nat. Microbiol. 2016, 1, 16116. [Google Scholar] [CrossRef] [PubMed]
- Kalson, N.; Furman, D.; Zeiri, Y. Cavitation-induced synthesis of biogenic molecules on primordial Earth. ACS Cent. Sci. 2017, 3, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Pearce, B.; Pudritz, R.; Semenov, D.; Henning, T. Origin of the RNA world: The fate of nucleobases in warm little ponds. Proc. Natl. Acad. Sci. USA 2017, 114, 11327–11332. [Google Scholar] [CrossRef]
- Shen-Miller, J.; Mudgett, M.; Schopf, J.; Clarke, S.; Berger, R. Exceptional seed longevity and robust growth: Ancient sacred lotus from China. Am. J. Bot. 1995, 82, 1367–1380. [Google Scholar] [CrossRef]
- Calahan, D.; Dunham, M.; DeSevo, C.; Koshland, D. Genetic analysis of desiccation tolerance in Saccharomyces cerevisiae. Genetics 2011, 189, 507–519. [Google Scholar] [CrossRef]
- Erkut, C.; Penkov, S.; Khesbak, H.; Vorkel, D.; Verbavatz, J.M.; Fahmy, K.; Kurzchalia, T.V. Trehalose renders the dauer larva of Caenorhabditis elegans resistant to extreme desiccation. Curr. Biol. 2011, 21, 1331–1336. [Google Scholar] [CrossRef]
- Mobjerg, N.; Halberg, K.A.; Jorgensen, A.; Persson, D.; Bjorn, M.; Ramlov, H.; Kristensen, R.M. Survival in extreme environments—On the current knowledge of adaptations in tardigrades. Acta Physiol. 2011, 202, 409–420. [Google Scholar] [CrossRef]
- Yang, G.; Gilstrap, K.; Zhang, A.; Xu, L.X.; He, X. Collapse temperature of solutions important for lyopreservation of living cells at ambient temperature. Biotechnol. Bioeng. 2010, 106, 247–259. [Google Scholar]
- Chakraborty, N.; Chang, A.; Elmoazzen, H.; Menze, M.A.; Hand, S.C.; Toner, M. A spin-drying technique for lyopreservation of mammalian cells. Ann. Biomed. Eng. 2011, 39, 1582–1591. [Google Scholar] [CrossRef]
- Laurent, A.; Hirt-Burri, N.; Scaletta, C.; Michetti, M.; de Buys Roessingh, A.S.; Raffoul, W.; Applegate, L.A. Holistic approach of Swiss fetal progenitor cell banking: Optimizing safe and sustainable substrates for regenerative medicine and biotechnology. Front. Bioeng. Biotechnol. 2020, 23, 557758. [Google Scholar] [CrossRef]
- Laurent, A.; Scaletta, C.; Abdel-Sayed, P.; Michetti, M.; Flahaut, M.; Simon, J.P.; Roessingh, A.B.; Raffoul, W.; Hirt-Burri, N.; Applegate, L.A. Optimized manufacture of lyophilized dermal fibroblasts for next-generation off-the-shelf progenitor biological bandages in topical post-burn regenerative medicine. Biomedicines 2021, 9, 1072. [Google Scholar] [CrossRef]
- Strong, D.M. The US Navy Tissue Bank: 50 years on the cutting edge. Cell Tissue Bank 2000, 1, 9–16. [Google Scholar] [CrossRef]
- Gresham, R.B. Freeze-drying of human tissue for clinical use. Cryobiology 1964, 1, 150–156. [Google Scholar] [CrossRef]
- Laurent, A.; Scaletta, C.; Hirt-Burri, N.; Raffoul, W.; de Buys Roessingh, A.S.; Applegate, L.A. Swiss fetal transplantation program and nonenzymatically isolated primary progenitor cell types for regenerative medicine. In Stem Cells and Good Manufacturing Practices: Methods and Protocols; Kursad, T., Ed.; Springer: New York, NY, USA, 2020. [Google Scholar]
- Massie, I.; Selden, C.; Hodgson, H.; Fuller, B.; Gibbons, S.; Morris, G.J. GMP cryopreservation of large volumes of cells for regenerative medicine: Active control of the freezing process. Tissue Eng. Part C 2014, 20, 693–702. [Google Scholar] [CrossRef]
- Davis, K.E.; Killeen, A.L.; Farrar, D.; Raspovic, K.M.; Berriman-Rozen, Z.D.; Malone, M.; Lavery, L.A. Lyopreserved amniotic membrane is cellularly and clinically similar to cryopreserved construct for treating foot ulcers. Int. Wound. J. 2020, 17, 1893–1901. [Google Scholar] [CrossRef]
- Nowak, D.; Jakubczyk, E. The freeze-drying of foods-The characteristic of the process course and the effect of its parameters on the physical properties of food materials. Foods 2020, 9, 1488. [Google Scholar] [CrossRef]
- Hohlfeld, J.; de Buys Roessingh, A.S.; Hirt-Burri, N.; Chaubert, P.; Gerber, S.; Scaletta, C.; Hohlfeld, P.; Applegate, L.A. Tissue engineered fetal skin constructs for pediatric burns. Lancet 2005, 366, 840–842. [Google Scholar] [CrossRef]
- De Buys Roessingh, A.S.; Hohlfeld, J.; Scaletta, C.; Hirt-Burri, N.; Gerber, S.; Hohlfeld, P.; Gebbers, J.O.; Applegate, L.A. Development, characterization, and use of a fetal skin cell bank for tissue engineering in wound healing. Cell Transplant. 2006, 15, 823–834. [Google Scholar] [CrossRef]
- Al-Dourobi, K.; Laurent, A.; Deghayli, L.; Flahaut, M.; Abdel-Sayed, P.; Scaletta, C.; Michetti, M.; Waselle, L.; Simon, J.P.; Ezzi, O.E.; et al. Retrospective evaluation of progenitor biological bandage use: A complementary and safe therapeutic management option for prevention of hypertrophic scarring in pediatric burn care. Pharmaceuticals 2021, 14, 201. [Google Scholar] [CrossRef]
- Chyba, C.; Sagan, C. Endogenous production, exogenous delivery and impact-shock synthesis of organic molecules: An inventory for the origins of life. Nature 1992, 355, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Whaley, D.; Damyar, K.; Witek, R.P.; Mendoza, A.; Alexander, M.; Lakey, J.R. Cryopreservation: An overview of principles and cell-specific considerations. Cell Transpl. 2021, 30, 963689721999617. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, A.; De Francesco, F.; Tirino, V.; Ferraro, G.A.; Desiderio, V.; Paino, F.; Pirozzi, G.; D’Andrea, F.; Papaccio, G. A new method for cryopreserving adipose-derived stem cells: An attractive and suitable large-scale and long-term cell banking technology. Tissue Eng. Part C 2009, 15, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Hanna, J.; Hubel, A. Preservation of stem cells. Organogenesis 2009, 5, 134–137. [Google Scholar] [CrossRef]
- Deller, R.C.; Vatish, M.; Mitchell, D.A.; Gibson, M.I. Glycerol-free cryopreservation of red blood cells enabled by ice-recrystallization-inhibiting polymers. ACS Biomat. Sci. Eng. 2015, 1, 789–794. [Google Scholar] [CrossRef]
- Yanaga, H.; Udoh, Y.; Yamamoto, M.; Yoshii, S.; Mori, S.; Yamauchi, T.; Kiyokawa, K.; Koga, M.; Yanaga, K. Cryopreserved cultured epithelial allografts for pediatric deep partial dermal burns: Early wound closure and suppression of scarring. Regen. 2017, 6, 74–82. [Google Scholar] [CrossRef]
- Thirumala, S.; Goebel, W.S.; Woods, E.J. Clinical grade adult stem cell banking. Organogenesis 2009, 5, 143–154. [Google Scholar] [CrossRef]
- Hunt, C.J. Cryopreservation of human stem cells for clinical application: A review. Transfus. Med. Hemother. 2011, 38, 107–123. [Google Scholar] [CrossRef]
- Li, Y.; Ma, T. Bioprocessing of cryopreservation for large-scale banking of human pluripotent stem cells. Biores Open Access 2012, 1, 205–214. [Google Scholar] [CrossRef]
- Shu, W.; Wang, Y.; Zhang, X.; Li, C.; Le, H.; Chang, F. Functional hydrogel dressings for treatment of burn wounds. Front. Bioeng. Biotechnol. 2021, 9, 788461. [Google Scholar] [CrossRef]
- Verbeken, G.; Draye, J.; Fauconnier, A.; Vanlaere, I.; Huys, I.; De Corte, P.; Verween, G.; Pascual, B.; Delmotte, N.; Pierlot, A.; et al. The magistral preparation of advanced therapy medicinal products (ATMPs). J. Surg. Pract. 2020, 2, 16. [Google Scholar]
- Verbeken, G.; Verween, G.; De Vos, D.; Pascual, B.; De Corte, P.; Richters, C.; De Coninck, A.; Roseeuw, D.; Ectors, N.; Rose, T.; et al. Glycerol treatment as recovery procedure for cryopreserved human skin allografts positive for bacteria and fungi. Cell Tissue Bank 2012, 13, 1–7. [Google Scholar] [CrossRef]
- Navrátilová, Z.; Slonková, V.; Semrádová, V.; Adler, J. Cryopreserved and lyophilized cultured epidermal allografts in the treatment of leg ulcers: A pilot study. J. Eur. Acad. Derm. Venerol. 2004, 18, 173–179. [Google Scholar] [CrossRef]
- Jang, H.; Kim, Y.H.; Kim, M.K.; Lee, K.H.; Jeon, S. Wound-healing potential of Cultured Epidermal Sheets is unaltered after lyophilization: A preclinical study in comparison to cryopreserved CES. BioMed Res. Int. 2013, 2013, 907209. [Google Scholar] [CrossRef][Green Version]
- Cottle, C.; Porter, A.P.; Lipat, A.; Turner-Lyles, C.; Nguyen, J.; Moll, G.; Chinnadurai, R. Impact of cryopreservation and freeze-thawing on therapeutic properties of mesenchymal stromal/stem cells and other common cellular therapeutics. Curr. Stem Cell Rep. 2022, 8, 72–92. [Google Scholar] [CrossRef]
- Thirumala, S.; Gimble, J.M.; Devireddy, R.V. Evaluation of methylcellulose and dimethyl sulfoxide as the cryoprotectants in a serum-free freezing media for cryopreservation of adipose-derived adult stem cells. Stem Cells Dev. 2010, 19, 513–522. [Google Scholar] [CrossRef]
- Liu, G.; Zhou, H.; Li, Y.; Li, G.; Cui, L.; Liu, W.; Cao, Y. Evaluation of the viability and osteogenic differentiation of cryopreserved human adipose-derived stem cells. Cryobiology 2008, 57, 18–24. [Google Scholar] [CrossRef]
- Thirumala, S.; Wu, X.; Gimble, J.M.; Devireddy, R.V. Evaluation of polyvinylpyrrolidone as a cryoprotectant for adipose tissue-derived adult stem cells. Tissue Eng. Part C 2010, 16, 783–792. [Google Scholar] [CrossRef]
- Zeisberger, S.M.; Schulz, J.C.; Mairhofer, M.; Ponsaerts, P.; Wouters, G.; Doerr, D.; Katsen-Globa, A.; Ehrbar, M.; Hescheler, J.; Hoerstrup, S.P.; et al. Biological and physicochemical characterization of a serum- and xeno-free chemically defined cryopreservation procedure for adult human progenitor cells. Cell Transpl. 2011, 20, 1241–1257. [Google Scholar] [CrossRef]
- Taylor, M.J.; Weegman, B.P.; Baicu, S.C.; Giwa, S.E. New approaches to cryopreservation of cells, tissues, and organs. Transfus. Med. Hemother. 2019, 46, 197–215. [Google Scholar] [CrossRef]
- Fahy, G.M.; Wowk, B.; Wu, J.; Phan, J.; Rasch, C.; Chang, A.; Zendejas, E. Cryopreservation of organs by vitrification: Perspectives and recent advances. Cryobiology 2004, 48, 157–178. [Google Scholar] [CrossRef] [PubMed]
- Jang, T.H.; Park, S.C.; Yang, J.H.; Kim, J.Y.; Seok, J.H.; Park, U.S.; Choi, C.W.; Lee, S.R.; Han, J. Cryopreservation and its clinical applications. Integr. Med. Res. 2017, 6, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Laurent, A.; Abdel-Sayed, P.; Hirt-Burri, N.; Scaletta, C.; Michetti, M.; de Buys Roessingh, A.; Raffoul, W.; Applegate, L.A. Evolution of diploid progenitor lung cell applications: From optimized biotechnological substrates to potential active pharmaceutical ingredients in respiratory tract regenerative medicine. Cells 2021, 10, 2526. [Google Scholar] [CrossRef] [PubMed]
- Rozati, H.; Handley, T.; Jayasena, C.N. Process and pitfalls of sperm cryopreservation. J. Clin. Med. 2017, 6, 89. [Google Scholar] [CrossRef]
- Pai, H.D.; Baid, R.; Palshetkar, N.P.; Pai, A.; Pai, R.D.; Palshetkar, R. Oocyte cryopreservation—Current scenario and future perspectives: A narrative review. J. Hum. Reprod. Sci. 2021, 14, 340–349. [Google Scholar]
- François, M.; Copland, I.B.; Yuan, S.; Romieu-Mourez, R.; Waller, E.K.; Galipeau, J. Cryopreserved mesenchymal stromal cells display impaired immunosuppressive properties as a result of heat-shock response and impaired interferon-γ licensing. Cytotherapy 2012, 14, 147–152. [Google Scholar] [CrossRef]
- Lemler, J.; Harris, S.B.; Platt, C.; Huffman, T.M. The arrest of biological time as a bridge to engineered negligible senescence. Ann. N. Y. Acad. Sci. 2004, 1019, 559–563. [Google Scholar] [CrossRef]
- Santos, J.L.; Ebert, D. Trehalose provisioning in Daphnia resting stages reflects local adaptation to the harshness of diapause conditions. Biol. Let. 2022, 18, 20210615. [Google Scholar] [CrossRef]
- Gladyshev, E.; Meselson, M. Extreme resistance of bdelloid rotifers to ionizing radiation. Proc. Natl. Acad. Sci. USA 2008, 105, 5139–5144. [Google Scholar] [CrossRef]
- Shmakova, L.; Malavin, S.; Iakovenko, N.; Vishnivetskaya, T.; Shain, D.; Plewka, M.; Rivkina, E. A living bdelloid rotifer from 24,000-year-old Arctic permafrost. Curr. Biol. 2021, 31, R712–R713. [Google Scholar] [CrossRef]
- Ricci, C.; Fontaneto, D. The importance of being a bdelloid: Ecological and evolutionary consequences of dormancy. Ital. J. Zool. 2009, 76, 240–249. [Google Scholar] [CrossRef][Green Version]
- Storey, J.M.; Wu, S.; Storey, K.B. Mitochondria and the frozen frog. Antioxidants 2021, 10, 543. [Google Scholar] [CrossRef]
- Wen, X.; Wang, S.; Duman, J.G.; Arifin, J.F.; Juwita, V.; Goddard, W.A., III; Rios, A.; Liu, F.; Kim, S.K.; Abrol, R.; et al. Antifreeze proteins govern the precipitation of trehalose in a freezing-avoiding insect at low temperature. Proc. Natl. Acad. Sci. USA 2016, 113, 6683–6688. [Google Scholar] [CrossRef]
- Gupta, R.; Deswal, R. Antifreeze proteins enable plants to survive in freezing conditions. J. Biosci. 2014, 39, 931–944. [Google Scholar] [CrossRef]
- Shatilovich, A.V.; Tchesunov, A.V.; Neretina, T.V.; Grabarnik, I.P.; Gubin, S.V.; Vishnivetskaya, T.A.; Onstott, T.C.; Rivkina, E.M. Viable nematodes from late pleistocene permafrost of the Kolyma river lowland. Dokl. Biol. Sci. 2018, 480, 100–102. [Google Scholar] [CrossRef]
- Ehlers, S.; Schroeder, R.; Friess, W. Process optimization and transfer of freeze-drying in nested vial systems. Eur. J. Pharm. Biopharm. 2021, 159, 143–150. [Google Scholar] [CrossRef]
- Emami, F.; Vatanara, A.; Park, E.J.; Na, D.H. Drying technologies for the stability and bioavailability of biopharmaceuticals. Pharmaceutics 2018, 10, 131. [Google Scholar] [CrossRef]
- Kawasaki, H.; Shimanouchi, T.; Kimura, Y. Recent development of optimization of lyophilization process. J. Chem. 2019, 2019, 9502856. [Google Scholar] [CrossRef]
- Wang, B.; Pikal, M.J. Stabilization of lyophilized pharmaceuticals by process optimization: Challenges and opportunities. Am. Pharma. Rev. 2012. Available online: https://www.americanpharmaceuticalreview.com/Featured-Articles/122325-Stabilization-of-Lyophilized-Pharmaceuticals-by-Process-Optimization-Challenges-and-Opportunities/ (accessed on 31 January 2023).
- Biswas, G.R.; Majee, S. Challenges to optimisation and scale-up of the process of lyophilisation of pharmaceuticals. World J. Pharm. Res. 2015, 4, 371–386. [Google Scholar]
- Tang, X.; Pikal, M.J. Design of freeze-drying processes for pharmaceuticals: Practical advice. Pharm. Res. 2004, 21, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Jameel, F.; Alexeenko, A.; Bhambhani, A.; Sacha, G.; Zhu, T.; Tchessalov, S.; Kumar, L.; Sharma, P.; Moussa, E.; Iyer, L.; et al. Recommended best practices for lyophilization validation-2021 Part I: Process design and modeling. AAPS PharmSciTech 2021, 22, 221. [Google Scholar] [CrossRef] [PubMed]
- Soejima, K.; Shimoda, K.; Kashimura, T.; Yamaki, T.; Kono, T.; Sakurai, H.; Nakazawa, H. Wound dressing material containing lyophilized allogeneic cultured cells. Cryobiology 2013, 66, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Laurent, A.; Porcello, A.; Fernandez, P.G.; Jeannerat, A.; Peneveyre, C.; Abdel-Sayed, P.; Scaletta, C.; Hirt-Burri, N.; Michetti, M.; de Buys Roessingh, A.; et al. Combination of hyaluronan and lyophilized progenitor cell derivatives: Stabilization of functional hydrogel products for therapeutic management of tendinous tissue disorders. Pharmaceutics 2021, 13, 2196. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, B.; Ling, P.; Zhang, T. Trehalose and hyaluronic acid coordinately stabilized freeze-dried pancreatic kininogenase. Eur. J. Pharm. Biopharm. 2007, 65, 18–25. [Google Scholar] [CrossRef]
- Gatlin, L.A.; Nail, S.L. Protein purification process engineering. Freeze drying: A practical overview. Bioprocess Technol. 1994, 18, 317–367. [Google Scholar]
- Puhlev, I.; Guo, N.; Brown, D.R.; Levine, F. Desiccation tolerance in human cells. Cryobiology 2001, 42, 207–217. [Google Scholar] [CrossRef]
- Duinslaeger, L.; Verbeken, G.; Reper, P.; Delaey, B.; Vanhalle, S.; Vanderkelen, A. Lyophilized keratinocyte cell lysates contain multiple mitogenic activities and stimulate closure of meshed skin autograft-covered burn wounds with efficiency similar to that of fresh allogeneic keratinocyte cultures. Plast. Reconstr. Surg. 1996, 98, 110–117. [Google Scholar] [CrossRef]
- El Baradie, K.; Nouh, M.; O’Brien, F., III; Liu, Y.; Fulzele, S.; Eroglu, A.; Hamrick, M.W. Freeze-dried extracellular vesicles from adipose-derived stem cells prevent hypoxia-induced muscle cell injury. Front. Cell Dev. Biol. 2020, 8, 181. [Google Scholar] [CrossRef]
- Laurent, A.; Porcello, A.; Jeannerat, A.; Peneveyre, C.; Coeur, A.; Abdel-Sayed, P.; Scaletta, C.; Michetti, M.; De Buys Roessingh, A.; Jordan, O.; et al. Lyophilized progenitor tenocyte extracts: Sterilizable cytotherapeutic derivatives with antioxidant properties and hyaluronan hydrogel functionalization effects. Antioxidants 2023, 12, 163. [Google Scholar] [CrossRef]
- Peng, Y.; Xuan, M.; Zou, J.; Liu, H.; Zhuo, Z.; Wan, Y.; Cheng, B. Freeze-dried rat bone marrow mesenchymal stem cell paracrine factors: A simplified novel material for skin wound therapy. Tissue Eng. Part A 2015, 21, 1036–1046. [Google Scholar] [CrossRef]
- Bronshtein, V.; Watts, D.M.; Monath, T.P. Stabilization of live biologicals at ambient temperatures using preservation by vaporization. Cryobiology 2008, 57, 334. [Google Scholar] [CrossRef]
- Depaz, R.A.; Pansare, S.; Patel, S.M. Freeze-drying above the glass transition temperature in amorphous protein formulations while maintaining product quality and improving process efficiency. J. Pharm. Sci. 2015, 105, 40–49. [Google Scholar] [CrossRef]
- Jangle, R.D.; Pisal, S.S. Vacuum foam drying: An alternative to lyophilization for biomolecule preservation. Ind. J. Pharm. Sci. 2012, 74, 91–100. [Google Scholar] [CrossRef]
- Nonogaki, H. Seed dormancy and germination-emerging mechanisms and new hypotheses. Front. Plant Sci. 2014, 5, 233. [Google Scholar] [CrossRef]
- Jones, S.; Lennon, J. Dormancy contributes to the maintenance of microbial diversity. Proc. Natl. Acad. Sci. USA 2010, 107, 5881–5886. [Google Scholar] [CrossRef]
- Evans, A.L.; Singh, N.J.; Friebe, A.; Arnemo, J.M.; Laske, T.G.; Fröbert, O.; Swenson, J.E.; Blanc, S. Drivers of hibernation in the brown bear. Front. Zool. 2016, 13, 7. [Google Scholar] [CrossRef]
- Delaney, R.G.; Lahiri, S.; Fishman, A.P. Aestivation of the African lungfish Protopterus aethiopicus: Cardiovascular and respiratory functions. J. Exp. Biol. 1974, 61, 111–128. [Google Scholar] [CrossRef]
- Chun, T.W.; Fauci, A.S. Latent reservoirs of HIV: Obstacles to the eradication of virus. Proc. Natl. Acad. Sci. USA 1999, 96, 10958–10961. [Google Scholar] [CrossRef]
- Thellman, N.M.; Triezenberg, S.J. Herpes Simplex Virus establishment, maintenance, and reactivation: In vitro modeling of latency. Pathogens 2017, 6, 28. [Google Scholar] [CrossRef]
- Somme, L.; Meier, T. Cold tolerance in Tardigrada from Dronning Maud Land, Antarctica. Polar Biol. 1995, 15, 221–224. [Google Scholar] [CrossRef]
- Rebecchi, L.; Altiero, T.; Guidetti, R.; Cesari, M.; Bertolani, R.; Negroni, M.; Rizzo, A.M. Tardigrade Resistance to Space Effects: First results of experiments on the LIFE-TARSE mission on FOTON-M3 (September 2007). Astrobiology 2009, 9, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Orgel, L.E. Prebiotic adenine revisited: Eutectics and photochemistry. Orig. Life Evol. Biosph. 2004, 34, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Chen, I. The emergence of cells during the origin of life. Science 2006, 314, 1558–1559. [Google Scholar] [CrossRef]
- Wickramasinghe, C. Bacterial morphologies supporting cometary panspermia: A reappraisal. Int. J. Astrobiol. 2011, 10, 25–30. [Google Scholar] [CrossRef]
- Fajardo-Cavazos, P.; Link, L.; Melosh, H.J.; Nicholson, W.L. Bacillus subtilis spores on artificial meteorites survive hypervelocity atmospheric entry: Implications for lithopanspermia. Astrobiology 2005, 5, 726–736. [Google Scholar] [CrossRef]
- Krasnokutski, S.A.; Chuang, K.J.; Jäger, C.; Ueberschaar, N.; Henning, T. A pathway to peptides in space through the condensation of atomic carbon. Nat. Astron. 2022, 6, 381–386. [Google Scholar] [CrossRef]
- Parker, E.T.; Cleaves, H.J.; Dworkin, J.P.; Glavin, D.P.; Callahan, M.; Aubrey, A.; Lazcano, A.; Bada, J.L. Primordial synthesis of amines and amino acids in a 1958 Miller H2S-rich spark discharge experiment. Proc. Natl. Acad. Sci. USA 2011, 108, 5526–5531. [Google Scholar] [CrossRef]
- Wacey, D.; Kilburn, M.; Saunders, M.; Cliff, J.; Brasier, M. Microfossils of sulphur-metabolizing cells in 3.4-billion-year-old rocks of Western Australia. Nat. Geosci. 2011, 4, 698–702. [Google Scholar] [CrossRef]
- Goncharuk, V.V.; Zui, O.V. Water and carbon dioxide as the main precursors of organic matter on Earth and in space. J. Water Chem. Technol. 2015, 37, 2–3. [Google Scholar] [CrossRef]
- Oba, Y.; Takano, Y.; Furukawa, Y.; Koga, T.; Glavin, D.; Dworkin, J.; Naraoka, H. Identifying the wide diversity of extraterrestrial purine and pyrimidine nucleobases in carbonaceous meteorites. Nat. Commun. 2022, 13, 2008. [Google Scholar] [CrossRef]
- Martins, Z. Organic chemistry of carbonaceous meteorites. Elements 2011, 7, 35–40. [Google Scholar] [CrossRef]
- Furukawa, Y.; Chikaraishi, Y.; Ohkouchi, N.; Nakamura, T. Extraterrestrial ribose and other sugars in primitive meteorites. Proc. Natl. Acad. Sci. USA 2019, 116, 24440–24445. [Google Scholar] [CrossRef]
- Cano, R.J.; Borucki, M.K. Revival and identification of bacterial spores in 25- to 40-million-year-old Dominican amber. Science 1995, 268, 1060–1064. [Google Scholar] [CrossRef]
- Simpson, D.J.; Olova, N.N.; Chandra, T. Cellular reprogramming and epigenetic rejuvenation. Clin. Epigen. 2021, 13, 170. [Google Scholar] [CrossRef]
- Hann, S.Y.; Cui, H.; Esworthy, T.; Miao, S.; Zhou, X.; Lee, S.; Fisher, J.P.; Zhang, L.G. Recent advances in 3D printing: Vascular network for tissue and organ regeneration. Transl. Res. 2019, 211, 46–63. [Google Scholar] [CrossRef]
| Cryopreservation Process Phases | Involved Methods & Operator Manipulations | Physical Processes & Techniques | Risks & Possible Damage to the Cells |
|---|---|---|---|
| Formulation adaptation, solvent addition, bulk cell suspension dilution & dispensing in vials 1 | Homogenization of samples in cell cryopreservation medium; vial filling | Direct cytotoxicity of cryopreservation medium; mechanical damage to the cells during processing |
| Controlled-rate or flash lowering of sample temperature down to –80 °C or –196 °C 2 | Sample water phase transition from liquid to solid, in crystalline and/or amorphous physical state 3 | Formation of ice crystals incurring mechanical damages to the cells; solution effects or pH changes causing cytotoxicity |
| Immersion in the vapor phase or liquid phase of liquid nitrogen and steady storage temperature maintenance | Cooling of the frozen samples down to cryogenic temperatures | Non-reversible slowing or arrest of cellular metabolic processes; undesired thawing due to equipment malfunction |
| Retrieval of frozen samples from storage and rapid thawing at 37 °C 4 | Sample water phase transition from solid to liquid | Slow sample warming and thawing causing mechanical stress to the cells or chemical cytotoxicity; solution effects or pH changes causing cytotoxicity |
| Lyophilization Process Phases | Involved Methods & Manipulations | Physical Processes & Techniques | Risks & Possible Damage to the Cells |
|---|---|---|---|
| Sample concentration, formulation adaptation, solvent removal, surface area optimization | Dialysis, filtration, evaporation, extraction, homogenization of the sample in lyophilization medium | Mechanical or thermal damage; chemical cytotoxicity |
| Dispensing of the sample wet bulk in trays, recipients, or vials 1 | Liquid dispensing and homogenization of the sample | Mechanical damage to the cells |
| Slow controlled-rate cooling or rapid freezing below the triple point and eutectic point of the sample | Sample water phase transition from liquid to solid, creation of a crystalline and/or amorphous solid phase | Formation of ice crystals incurring mechanical damage to the cells; solution effects or pH changes causing cytotoxicity |
| Temperature cycling | Reorganization of water in the solid phase | Formation of ice crystals incurring mechanical damage to the cells |
| Physical transfer of the batch to the freeze-dryer shelves 2 | NA | NA |
| Low-temperature energy provision by conduction, convection, or radiation | Sample unbound water phase transition from solid to gaseous state (sublimation) | Meltback or collapse of the sample; physical damage to the cells |
| High-temperature energy provision by conduction, convection, or radiation | Sample bound water desorption | Too much water removal; collapse of whole sample or of the cells |
| Physical transfer of the sample batch from the freeze-dryer shelves 3 | NA | NA |
| Solid sample dry bulk processing or powder handling | Homogenization of the dry bulk | Mechanical damage to the cells |
| Transfer of the batch to a defined storage environment | NA | Degradation due to inappropriate conditioning or storage |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laurent, A.; Scaletta, C.; Abdel-Sayed, P.; Raffoul, W.; Hirt-Burri, N.; Applegate, L.A. Industrial Biotechnology Conservation Processes: Similarities with Natural Long-Term Preservation of Biological Organisms. BioTech 2023, 12, 15. https://doi.org/10.3390/biotech12010015
Laurent A, Scaletta C, Abdel-Sayed P, Raffoul W, Hirt-Burri N, Applegate LA. Industrial Biotechnology Conservation Processes: Similarities with Natural Long-Term Preservation of Biological Organisms. BioTech. 2023; 12(1):15. https://doi.org/10.3390/biotech12010015
Chicago/Turabian StyleLaurent, Alexis, Corinne Scaletta, Philippe Abdel-Sayed, Wassim Raffoul, Nathalie Hirt-Burri, and Lee Ann Applegate. 2023. "Industrial Biotechnology Conservation Processes: Similarities with Natural Long-Term Preservation of Biological Organisms" BioTech 12, no. 1: 15. https://doi.org/10.3390/biotech12010015
APA StyleLaurent, A., Scaletta, C., Abdel-Sayed, P., Raffoul, W., Hirt-Burri, N., & Applegate, L. A. (2023). Industrial Biotechnology Conservation Processes: Similarities with Natural Long-Term Preservation of Biological Organisms. BioTech, 12(1), 15. https://doi.org/10.3390/biotech12010015








