Xylanase Production by Talaromyces amestolkiae Valuing Agroindustrial Byproducts
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Agroindustrial Byproducts Characterization
2.3. Microorganism
2.4. Xylanase Production
2.5. Cultivation Parameters Optimization
2.6. Enzymatic Activity Assay
2.7. Enzyme Characterization
2.7.1. Determination of Optimum pH and Temperature
2.7.2. Enzymatic Stability in Function of pH and Temperature
2.8. Data Analysis and Presentation
3. Results
3.1. Agroindustrial Byproducts Characterization
3.2. Xylanase Production
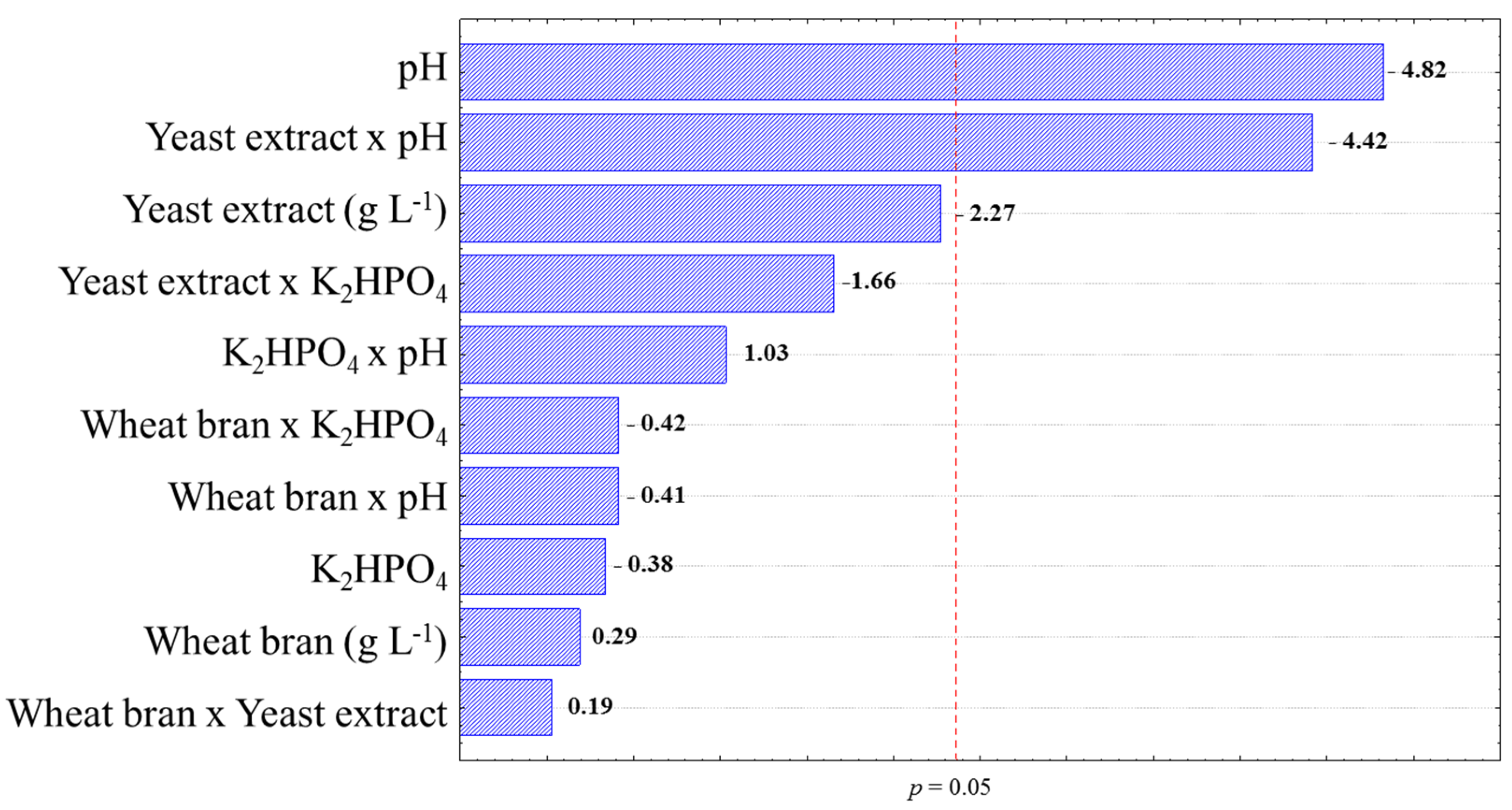
3.3. Ideal Conditions for Xylanase Production
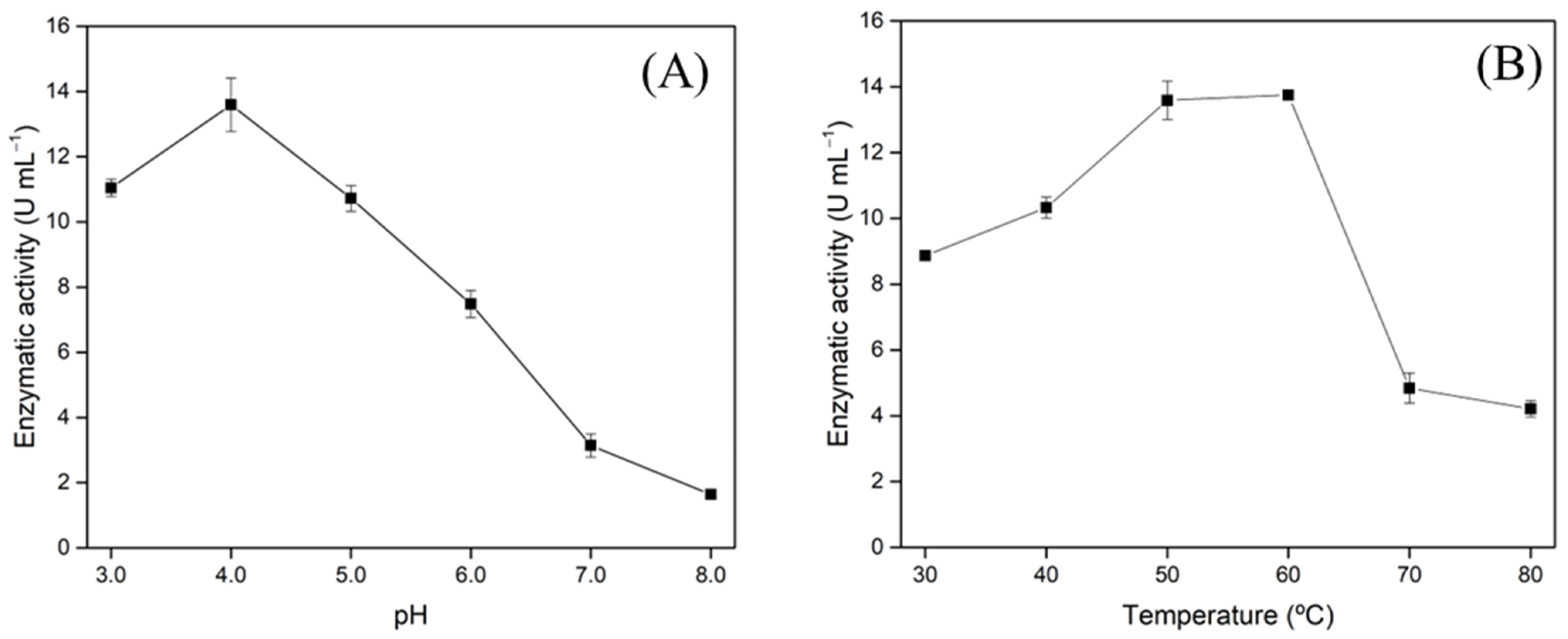
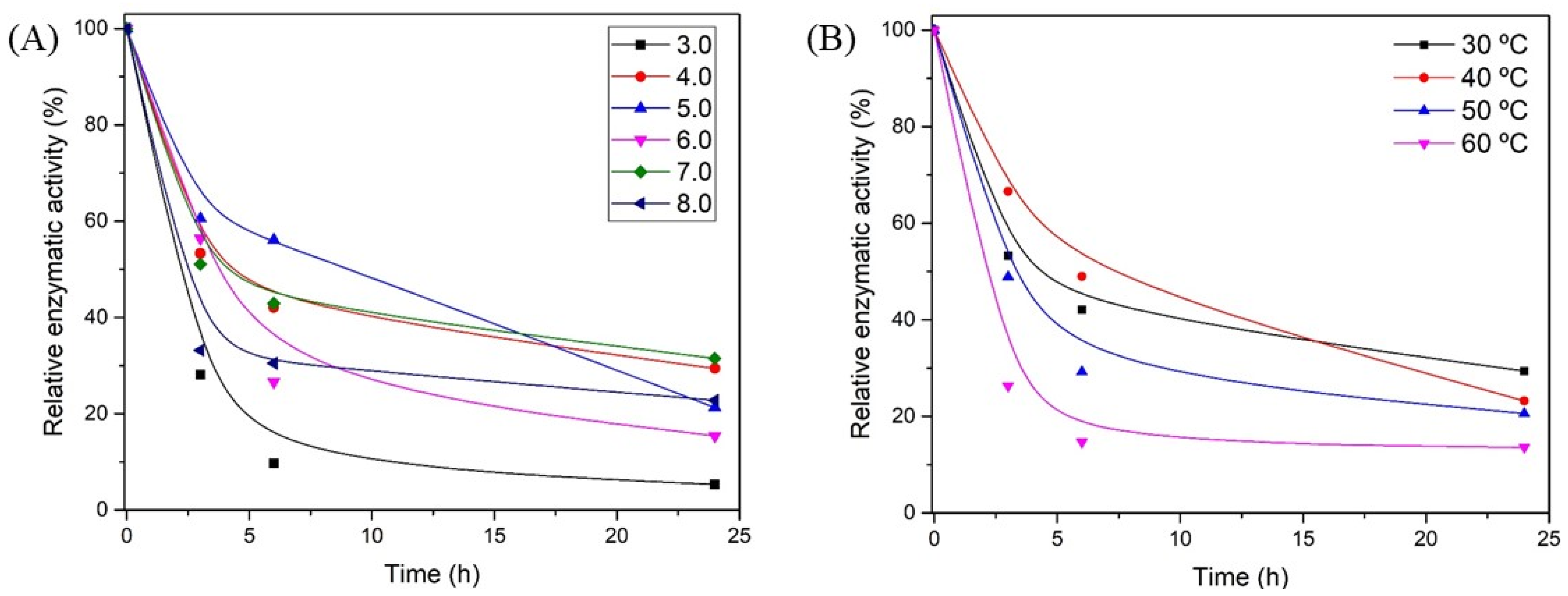
3.4. Xylanase Characterization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pellera, F.M.; Gidarakos, E. Chemical pretreatment of lignocellulosic agroindustrial waste for methane production. Waste Manag. 2018, 71, 689–703. [Google Scholar] [CrossRef]
- Araújo, D.J.C.; Machado, A.V.; Vilarinho, M.C.L.G. Availability and Suitability of Agroindustrial Residues as Feedstock for Cellulose-Based Materials: Brazil Case Study. Waste Biomass Valor 2019, 10, 2863–2878. [Google Scholar] [CrossRef]
- Bolan, N.S.; Thangarajan, R.; Seshadri, B.; Jena, U.; Das, K.C.; Wang, H.; Naidu, R. Landfills as a biorefinery to produce biomass and capture biogas. Bioresour. Technol. 2013, 135, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Iram, A.; Cekmecelioglu, D.; Demirci, A. Screening of bacterial and fungal strains for cellulase and xylanase production using distillers’ dried grains with solubles (DDGS) as the main feedstock. Biomass Conv. Bioref. 2021, 11, 1955–1964. [Google Scholar] [CrossRef]
- Tursi, A. A review on biomass: Importance, chemistry, classification, and conversion. Biofuel Res. J. 2019, 6, 962–979. [Google Scholar] [CrossRef]
- Hilpmann, G.; Becher, N.; Pahner, F.A.; Kusema, B.; Mäki-Arvela, P.; Lange, R.; Murzin, D.Y.; Salmi, T. Acid hydrolysis of xylan. Catal. Today 2016, 259, 376–380. [Google Scholar] [CrossRef]
- Oriez, V.; Peydecastaing, J.; Pontalier, P.-Y. Lignocellulosic Biomass Mild Alkaline Fractionation and Resulting Extract Purification Processes: Conditions, Yields, and Purities. Clean Technol. 2020, 2, 91–115. [Google Scholar] [CrossRef]
- De Buck, V.; Polanska, M.; Van Impe, J. Modeling biowaste biorefineries: A review. Front. Sustain. Food Syst. 2020, 4, 11. [Google Scholar] [CrossRef]
- Cuesta, S.M.; Rahman, S.A.; Furnham, N.; Thornton, J.M. The classification and evolution of enzyme function. Biophys. J. 2015, 109, 1082–1086. [Google Scholar] [CrossRef]
- Robinson, P.K. Enzymes: Principles and biotechnological applications. Essays Biochem. 2015, 59, 1. [Google Scholar] [CrossRef]
- Motta, F.L.; Andrade, C.C.P.; Santana, M.H.A.; Andrade, C.C.P.; Santana, M.H.A. A review of xylanase production by the fermentation of xylan: Classification, characterization and applications. In Sustainable Degradation of Lignocellulosic Biomass-Techniques, Applications and Commercialization; Chandel, A., Da Silva, S.S., Eds.; IntechOpen: London, UK, 2013; Volume 1, pp. 251–276. [Google Scholar]
- Bhardwaj, N.; Kumar, B.; Verma, P. A Detailed overview of xylanases: An emerging biomolecule for current and future prospective. Bioresour. Bioprocess. 2019, 6, 1–36. [Google Scholar] [CrossRef]
- Zaccarim, B.R.; de Oliveira, F.; Passarini, M.R.; Duarte, A.W.; Sette, L.D.; Jozala, A.F.; Teixeira, M.F.S.; de Carvalho Santos-Ebinuma, V. Sequencing and phylogenetic analyses of Talaromyces amestolkiae from amazon: A producer of natural colorants. Biotechnol. Progr. 2019, 35, e2684. [Google Scholar] [CrossRef] [PubMed]
- Long, C.; Liu, J.; Gan, L.; Zeng, B.; Long, M. Optimization of Xylanase Production by Trichoderma orientalis Using Corn Cobs and Wheat Bran via Statistical Strategy. Waste Biomass Valor 2019, 10, 1277–1284. [Google Scholar] [CrossRef]
- Sakthiselvan, P.; Naveena, B.; Partha, N. Molecular characterization of a Xylanase-producing fungus isolated from fouled soil. Braz. J. Microbiol. 2014, 45, 1293–1302. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Oliveira, F.; Ferreira, L.C.; Neto, Á.B.; Teixeira, M.F.S.; Ebinuma, V.D.C.S. Biosynthesis of natural colorant by Talaromyces amestolkiae: Mycelium accumulation and colorant formation in incubator shaker and in bioreactor. Biochem. Eng. J. 2020, 161, 107694. [Google Scholar] [CrossRef]
- De Oliveira, F.; Rocha, I.L.; Pinto, D.C.G.A.; Ventura, S.P.; Dos Santos, A.G.; Crevelin, E.J.; Ebinuma, V.D.C.S. Identification of azaphilone derivatives of Monascus colorants from Talaromyces amestolkiae and their halochromic properties. Food Chem. 2022, 372, 131214. [Google Scholar] [CrossRef]
- Prieto, A.; de Eugenio, L.; Méndez-Líter, J.A.; Nieto-Domínguez, M.; Murgiondo, C.; Barriuso, J.; Bejarano-Muñoz, L.; Jesús Martínez, M. Fungal glycosyl hydrolases for sustainable plant biomass valorization: Talaromyces amestolkiae as a model fungus. Int. Microbiol. 2021, 24, 545–558. [Google Scholar] [CrossRef]
- Romdhane, I.B.B.; Achouri, I.M.; Belghith, H. Improvement of Highly Thermostable Xylanases Production by Talaromyces thermophilus for the Agro-industrials Residue Hydrolysis. Appl. Biochem. Biotechnol. 2010, 162, 1635–1646. [Google Scholar] [CrossRef]
- Singh, R.D.; Banerjee, J.; Arora, A. Prebiotic potential of oligosaccharides: A focus on xylan derived oligosaccharides. Bioact. Carbohydr. Diet. Fibre 2015, 5, 19–30. [Google Scholar] [CrossRef]
- Abdul Manan, M.; Webb, C. Estimating fungal growth in submerged fermentation in the presence of solid particles based on colour development. Biotechnol. Biotechnol. Equip. 2018, 32, 618–627. [Google Scholar] [CrossRef]
- Paz-Cedeño, F.R. Enzymatic Hydrolysis of the Polysaccharide Fraction of the Residue from the Carrageenan Processing of Kappaphycus alvarezii. Ph.D. Thesis, Universidade Estadual Paulista—UNESP, Araraquara, SP, Brazil, 2017. [Google Scholar]
- Masarin, F.; Gurpilhares, D.B.; Baffa, D.C. Chemical composition and enzymatic digestibility of sugarcane clones selected for varied lignin content. Biotechnol. Biofuels 2011, 4, 55. [Google Scholar] [CrossRef] [PubMed]
- Bailey, M.J.; Biely, P.; Poutanen, K. Interlaboratory testing of methods for assay of xylanase activity. J. Biotechnol. 1992, 23, 257–270. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- McIlvaine, T.C. A buffer solution for colorimetric comparison. J. Biol. Chem. 1921, 49, 183–186. [Google Scholar] [CrossRef]
- Li, M.; Liao, X.; Zhang, D.; Du, G.; Chen, J. Yeast extract promotes cell growth and induces production of polyvinyl alcohol-degrading enzymes. Enzyme Res. 2011, 2011, 179819. [Google Scholar] [CrossRef]
- de Oliveira, F.; Pedrolli, D.B.; Teixeira, M.F.S.; Santos-Ebinuma, V.C. Water-soluble fluorescent red colorant production by Talaromyces amestolkiae. Appl. Microbiol. Biotechnol. 2019, 103, 6529–6541. [Google Scholar] [CrossRef]
- Michelin, M.; Polizeli, M.L.T.M.; Ruzene, D.S.; Silva, D.P.; Vicente, A.A.; Jorge, J.A.; Terenzi, H.F.; Teixeira, J.A. Xylanase and β-Xylosidase Production by Aspergillus ochraceus: New Perspectives for the Application of Wheat Straw Autohydrolysis Liquor. Appl. Biochem. Biotechnol. 2012, 166, 336–347. [Google Scholar] [CrossRef]
- Survase, S.A.; Nimbalkar, P.; Jurgens, G.; Granstroöm, T.; Chavan, P.; Bankar, S.B. Efficient strategy to alleviate the inhibitory effect of lignin-derived compounds for enhanced butanol production. ACS Sustain. Chem. Eng. 2020, 9, 1172–1179. [Google Scholar] [CrossRef]
- Schmatz, A.A.; Tyhoda, L.; Brienzo, M. Sugarcane biomass conversion influenced by lignin. Biofuels Bioprod. Biorefin. 2020, 14, 469–480. [Google Scholar] [CrossRef]
- Ahmad, Z.; Sadiq Butt, M.; Riaz, M. Partial Purification and Charactrization of Xylanase produced from Aspergillus niger using Wheat Bran. Pak. J. Agric. Sci. 2013, 50, 433–437. [Google Scholar]
- Raj, K.C.; Chandra, T.S. Purification and characterization of xylanase from alkali-tolerant Aspergillus fischeri Fxn1. FEMS Microbiol. Lett. 1996, 145, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Anthony, T.; Raj, K.C.; Rajendran, A.; Gunasekaran, P. High molecular weight cellulase-free xylanase from alkali-tolerant Aspergillus fumigatus AR1. Enzyme Microb. Technol. 2003, 32, 647–654. [Google Scholar] [CrossRef]
- Ravichandra, K.; Yaswanth, V.V.N.; Nikhila, B.; Ahmad, J.; Srinivasa Rao, P.; Uma, A.; Ravindrababu, V.; Prakasham, R.S. Xylanase production by isolated fungal strain, Aspergillus fumigatus RSP-8 (MTCC 12039): Impact of agro-industrial material as substrate. Sugar Tech 2016, 18, 29–38. [Google Scholar] [CrossRef][Green Version]
- Kang, M.K.; Maeng, P.J.; Rhee, Y.H. Purification and characterization of two xylanases from alkalophilic Cephalosporium sp. strain RYM-202. Appl. Environ. Microbiol. 1996, 62, 3480–3482. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Zhao, H.; Xu, Y.; Cui, F. Statistical optimization of xylanase production from new isolated Penicillium oxalicum ZH-30 in submerged fermentation. Biochem. Eng. J. 2007, 34, 82–86. [Google Scholar] [CrossRef]
- Knob, A.; Carmona, E.C. Xylanase production by Penicillium sclerotiorum and its characterization. World Appl. Sci. J. 2008, 4, 277–283. [Google Scholar]
- Yoshioka, H.; Chavanich, S.; Nilubol, N.; Hayashida, S. Production and characterization of thermostable xylanase from Talaromyces byssochlamydoides YH-50. Agric. Biol. Chem. 1981, 45, 579–586. [Google Scholar] [CrossRef]
- Waters, D.M.; Murray, P.G.; Ryan, L.A.; Arendt, E.K.; Tuohy, M.G. Talaromyces emersonii thermostable enzyme systems and their applications in wheat baking systems. J. Agric. Food Chem. 2010, 58, 7415–7422. [Google Scholar] [CrossRef]
- Orencio-Trejo, M.; Torres-Granados, J.; Rangel-Lara, A.; Beltrán-Guerrero, E.; García-Aguilar, S.; Moss-Acosta, C.; Valenzuela-Soto, H.; De la Torre-Zavala, S.; Gastelum-Arellanez, A.; Martinez, A.; et al. Cellulase and xylanase production by the Mexican strain Talaromyces stollii LV186 and its application in the saccharification of pretreated corn and sorghum stover. BioEnergy Res. 2016, 9, 1034–1045. [Google Scholar] [CrossRef]
- Maalej, I.; Belhaj, I.; Masmoudi, N.F.; Belghith, H. Highly thermostable xylanase of the thermophilic fungus Talaromyces thermophilus: Purification and characterization. Appl. Biochem. Biotechnol. 2009, 158, 200–212. [Google Scholar] [CrossRef]
- Bedade, D.; Berezina, O.; Singhal, R.; Deska, J.; Shamekh, S. Extracellular xylanase production from a new xylanase producer Tuber maculatum mycelium under submerged fermentation and its characterization. Biocatal. Agric. Biotechnol. 2017, 11, 288–293. [Google Scholar] [CrossRef]
- Walia, A.; Guleria, S.; Mehta, P.; Chauhan, A.; Parkash, J. Microbial xylanases and their industrial application in pulp and paper biobleaching: A review. 3 Biotech 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Prasetyo, J.; Sumita, S.; Okuda, N.; Park, E.Y. Response of cellulase activity in pH-controlled cultures of the filamentous fungus Acremonium cellulolyticus. Appl. Biochem. Biotechnol. 2010, 162, 52–61. [Google Scholar] [CrossRef] [PubMed]


| Component (% wt.) | Wheat Bran | Rice Bran | Citrus Pulp | Peanut Skin | Peanut Shell |
|---|---|---|---|---|---|
| Cellulose | 32.7 | 25.4 | 33.3 | 5.0 | 20.9 |
| Xylan | 14.6 | 6.5 | 10.3 | 1.1 | 9.8 |
| Lignin | 8.1 | 24.6 | 7.5 | 14.5 | 43.6 |
| Extractives | 14.0 | 26.3 | 24.5 | 42.1 | 3.6 |
| Oil | 22.6 | 10.2 | 9.9 | 35.3 | 20.1 |
| Total | 92 | 93 | 85.5 | 98 | 98 |
| Run | Wheat Bran (g·L−1) | Yeast Extract (g·L−1) | K2HPO4 (g·L−1) | pH | Enzyme Activity (U·mL−1) |
|---|---|---|---|---|---|
| 1 | 10 | 0 | 1 | 5 | 1.34 ± 0.16 |
| 2 | 30 | 0 | 1 | 5 | 2.57 ± 0.37 |
| 3 | 10 | 5 | 1 | 5 | 8.67 ± 0.26 |
| 4 | 30 | 5 | 1 | 5 | 8.33 ± 0.70 |
| 5 | 10 | 0 | 5 | 5 | 2.48 ± 0.25 |
| 6 | 30 | 0 | 5 | 5 | 2.80 ± 0.21 |
| 7 | 10 | 5 | 5 | 5 | 5.55 ± 1.02 |
| 8 | 30 | 5 | 5 | 5 | 6.09 ± 0.51 |
| 9 | 10 | 0 | 1 | 9 | 1.16 ± 0.07 |
| 10 | 30 | 0 | 1 | 9 | 1.61 ± 0.14 |
| 11 | 10 | 5 | 1 | 9 | 0.35 ± 0.02 |
| 12 | 30 | 5 | 1 | 9 | 0.76 ± 0.11 |
| 13 | 10 | 0 | 5 | 9 | 3.38 ± 0.32 |
| 14 | 30 | 0 | 5 | 9 | 1.85 ± 0.03 |
| 15 | 10 | 5 | 5 | 9 | 0.32 ± 0.03 |
| 16 | 30 | 5 | 5 | 9 | 0.35 ± 0.02 |
| 17–20 * | 20 | 2.5 | 3 | 7 | 13.02 ± 0.88 |
| Microorganism | Main Carbon Source | Enzymatic Activity (U·mL−1) | Molecular Weight (kDa) | Optimum pH | Optimum T (°C) | References |
|---|---|---|---|---|---|---|
| Aspergillus niger | Wheat bran | 24 | 30 | 7.5 | 60 | [32] |
| Aspergillus fischeri Fxn1 | Wheat bran | ND | ND | 6.0 | 60 | [33] |
| Aspergillus fumigatus AR1 | Wheat bran | 17.5 | 212–253 | 6–6.5 | 60 | [34] |
| Aspergillus fumigatus RSP-8 (MTCC 12039) | Wheat bran | ≈22 | ND | ND | ND | [35] |
| Cephalosporium sp. | Wheat bran | ND | 35 | 7.5 | 50 | [36] |
| Penicillium oxalicum ZH-30 | Wheat bran | 14.5 | ND | ND | 50–60 | [37] |
| Penicillium sclerotiorum | Oat spelts xylan | 7.8 | ND | 4.5 | 50 | [38] |
| Talaromyces byssochlamydoides YH-50 | Wheat bran | ND | ND | 5.5 | 70 | [39] |
| Talaromyces emersonii (IMI 392299) | Wheat bran | ND | ND | 4.5–5.0 | 70 | [40] |
| Talaromyces stollii LV186 | Corn stover | 7.5 | ND | ND | ND | [41] |
| Talaromyces thermophilus | Oat spelts xylan; Wheat bran; rabbit food | ND | 25 | 7.0–8.0 | 75–80 | [42] |
| Tuber maculatum | Beechwood xylan | 13.15 | ND | 5.0 | 50 | [43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbieri, G.S.; Bento, H.B.S.; de Oliveira, F.; Picheli, F.P.; Dias, L.M.; Masarin, F.; Santos-Ebinuma, V.C. Xylanase Production by Talaromyces amestolkiae Valuing Agroindustrial Byproducts. BioTech 2022, 11, 15. https://doi.org/10.3390/biotech11020015
Barbieri GS, Bento HBS, de Oliveira F, Picheli FP, Dias LM, Masarin F, Santos-Ebinuma VC. Xylanase Production by Talaromyces amestolkiae Valuing Agroindustrial Byproducts. BioTech. 2022; 11(2):15. https://doi.org/10.3390/biotech11020015
Chicago/Turabian StyleBarbieri, Giórgia S., Heitor B. S. Bento, Fernanda de Oliveira, Flávio P. Picheli, Lídia M. Dias, Fernando Masarin, and Valéria C. Santos-Ebinuma. 2022. "Xylanase Production by Talaromyces amestolkiae Valuing Agroindustrial Byproducts" BioTech 11, no. 2: 15. https://doi.org/10.3390/biotech11020015
APA StyleBarbieri, G. S., Bento, H. B. S., de Oliveira, F., Picheli, F. P., Dias, L. M., Masarin, F., & Santos-Ebinuma, V. C. (2022). Xylanase Production by Talaromyces amestolkiae Valuing Agroindustrial Byproducts. BioTech, 11(2), 15. https://doi.org/10.3390/biotech11020015









