Biochemical and Botanical Aspects of Allium sativum L. Sowing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description and Experimental Design
2.2. Experiment Procedure
2.3. Measurement of Traits
2.4. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Viana, J.P.G.; Pires, C.D.J.; Bajay, M.M.; Valente, S.E.D.S.; Pinheiro, J.B.; Zucchi, M.I.; Lopes, C.D.A.; Gomes, R.L.F. Do the importations of crop products affect the genetic diversity from landraces? A study case in garlic (Allium sativum L.). Genet. Resour. Crop Evol. 2020, 68, 1199–1211. [Google Scholar] [CrossRef]
- Takagi, H. Garlic (Allium sativum L.). In Onions and Allied Crops; Bresgterv, J.L., Rabinowitch, H.D., Eds.; CRC Press: Boca Raton, FL, USA, 1990; Volume 3. [Google Scholar]
- Brewster, J.L. Onions and Other Vegetable Alliums, 2nd ed.; CABI: Wallingford, UK; Cambridge, MA, USA, 2008; p. xii. [Google Scholar]
- Fritsch, R.M.; Friesen, N. Evolution, domestication and taxonomy. In Allium Crop Science: Recent Advances; CABI: Wallingford, UK; Cambridge, MA, USA, 2002; pp. 5–30. [Google Scholar] [CrossRef]
- Bayan, L.; Koulivand, P.H.; Gorji, A. Garlic: A review of potential therapeutic effects. Avicenna J. Phytomed. 2014, 4, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lanzotti, V.; Scala, F.; Bonanomi, G. Compounds from Allium species with cytotoxic and antimicrobial activity. Phytochem. Rev. 2014, 13, 769–791. [Google Scholar] [CrossRef]
- Chuacharoen, T.; Sabliov, C.M. Stability and controlled release of lutein loaded in zein nanoparticles with and without lecithin and pluronic F127 surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2016, 503, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Njimoh, D.L.; Assob, J.C.N.; Mokake, S.E.; Nyhalah, D.J.; Yinda, C.K.; Sandjon, B. Antimicrobial Activities of a Plethora of Medicinal Plant Extracts and Hydrolates against Human Pathogens and Their Potential to Reverse Antibiotic Resistance. Int. J. Microbiol. 2015, 2015, 547156. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Premoli, M.; Aria, F.; Bonini, S.A.; Maccarinelli, G.; Gianoncelli, A.; Memo, M.; Mastinu, A. Cannabimimetic plants: Are they new cannabinoidergic modulators? Planta 2019, 249, 1681–1694. [Google Scholar] [CrossRef]
- Abate, G.; Zhang, L.; Pucci, M.; Morbini, G.; Mac Sweeney, E.; Maccarinelli, G.; Ribaudo, G.; Gianoncelli, A.; Uberti, D.; Memo, M.; et al. Phytochemical Analysis and Anti-Inflammatory Activity of Different EthanolicPhyto-Extracts of Artemisia annua L. Biomolecules 2021, 11, 975. [Google Scholar] [CrossRef]
- Gharibvandi, A.; Karimmojeni, H.; Ehsanzadeh, P.; Maleki, M.R.; Mastinu, A. Weed management by allelopathic activity of Foeniculumvulgare essential oil. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2022, 1–9. [Google Scholar] [CrossRef]
- Gupta, A.K.; Dhua, S.; Sahu, P.P.; Abate, G.; Mishra, P.; Mastinu, A. Variation in Phytochemical, Antioxidant and Volatile Composition of Pomelo Fruit (Citrus grandis (L.) Osbeck) during Seasonal Growth and Development. Plants 2021, 10, 1941. [Google Scholar] [CrossRef]
- Gupta, A.K.; Rather, M.A.; Jha, A.K.; Shashank, A.; Singhal, S.; Sharma, M.; Pathak, U.; Sharma, D.; Mastinu, A. ArtocarpusLakoochaRoxb. andArtocarpusheterophyllusLam. Flowers: New Sources of Bioactive Compounds. Plants 2020, 9, 1329. [Google Scholar] [CrossRef]
- Karimmojeni, H.; Rahimian, H.; Alizadeh, H.; Yousefi, A.R.; Gonzalez-Andujar, J.L.; Mac Sweeney, E.; Mastinu, A. Competitive Ability Effects of Daturastramonium L. and Xanthium strumarium L. on the Development of Maize (Zea mays) Seeds. Plants 2021, 10, 1922. [Google Scholar] [CrossRef]
- Kumar, A.; Memo, M.; Mastinu, A. Plant behaviour: An evolutionary response to the environment? Plant Biol. 2020, 22, 961–970. [Google Scholar] [CrossRef]
- Mahdavi, A.; Moradi, P.; Mastinu, A. Variation in Terpene Profiles of Thymus vulgaris in Water Deficit Stress Response. Molecules 2020, 25, 1091. [Google Scholar] [CrossRef] [Green Version]
- Mastinu, A.; Ascrizzi, R.; Ribaudo, G.; Bonini, S.A.; Premoli, M.; Aria, F.; Maccarinelli, G.; Gianoncelli, A.; Flamini, G.; Pistelli, L.; et al. Prosocial Effects of NonpsychotropicCannabis sativa in Mice. Cannabis Cannabinoid Res. 2022, 7, 170–178. [Google Scholar] [CrossRef]
- Mastinu, A.; Bonini, S.A.; Premoli, M.; Maccarinelli, G.; Mac Sweeney, E.; Zhang, L.; Lucini, L.; Memo, M. Protective Effects of Gynostemmapentaphyllum (var. Ginpent) against Lipopolysaccharide-Induced Inflammation and Motor Alteration in Mice. Molecules 2021, 26, 570. [Google Scholar] [CrossRef]
- Noryan, M.; Hervan, I.M.; Sabouri, H.; Kojouri, F.D.; Mastinu, A. Drought Resistance Loci in Recombinant Lines of Iranian Oryza sativa L. in Germination Stage. BioTech 2021, 10, 26. [Google Scholar] [CrossRef]
- Randle, W.M.; Lancaster, J.E. Sulphur compounds in alliums in relation to flavour quality. In Allium Crop Science: Recent Advances; CABI: Wallingford, UK, 2002; pp. 329–356. [Google Scholar]
- Sato, T.; Miyata, G. The nutraceutical benefit, part iv: Garlic. Nutrition 2000, 16, 787–788. [Google Scholar] [CrossRef]
- Hughes, J.; Tregova, A.; Tomsett, A.; Jones, M.; Cosstick, R.; Collin, H. Synthesis of the flavour precursor, alliin, in garlic tissue cultures. Phytochemistry 2005, 66, 187–194. [Google Scholar] [CrossRef]
- Whitaker, J.R. Development of Flavor, Odor, and Pungency in Onion and Garlic. In Advances in Food Research; Academic Press: Cambridge, MA, USA, 1976; Volume 22, pp. 73–133. [Google Scholar] [CrossRef]
- Banerjee, S.; Mukherjee, P.K.; Maulik, S. Garlic as an antioxidant: The good, the bad and the ugly. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2003, 17, 97–106. [Google Scholar] [CrossRef]
- Block, E.; Naganathan, S.; Putman, D.; Zhao, S.-H. Organosulfur chemistry of garlic and onion: Recent results. Pure Appl. Chem. 1993, 65, 625–632. [Google Scholar] [CrossRef] [Green Version]
- Jabbes, N.; Arnault, I.; Auger, J.; Dridi, B.A.M.; Hannachi, C. Agro-morphological markers and organo-sulphur compounds to assess diversity in Tunisian garlic landraces. Sci. Hortic. 2012, 148, 47–54. [Google Scholar] [CrossRef]
- Kopeć, A.; Piątkowska, E.; Leszczyńska, T.; Sikora, E. Healthy Properties of Garlic. Curr. Nutr. Food Sci. 2013, 9, 59–64. [Google Scholar] [CrossRef]
- Wall, M.M.; Corgan, J.N. Relationship between Pyruvate Analysis and Flavor Perception for Onion Pungency Determination. HortScience 1992, 27, 1029–1030. [Google Scholar] [CrossRef] [Green Version]
- Aghajanlou, F.; Mirdavoudi, H.; Shojaee, M.; Mac Sweeney, E.; Mastinu, A.; Moradi, P. Rangeland Management and Ecological Adaptation Analysis Model for AstragaluscurvirostrisBoiss. Horticulturae 2021, 7, 67. [Google Scholar] [CrossRef]
- Damania, A.B. History, Achievements, and Current Status of Genetic Resources Conservation. Agron. J. 2008, 100, 9–21. [Google Scholar] [CrossRef]
- Hirata, S.; Abdelrahman, M.; Yamauchi, N.; Shigyo, M. Diversity evaluation based on morphological, physiological and isozyme variation in genetic resources of garlic (Allium sativum L.) collected worldwide. Genes Genet. Syst. 2016, 91, 161–173. [Google Scholar] [CrossRef] [Green Version]
- Mario, P.C.; Viviana, B.V.; María I, G.A. Low Genetic Diversity Among Garlic (Allium sativum L.) Accessions Detected Using Random Amplified Polymorphic DNA (RAPD). Chil. J. Agric. Res. 2008, 68, 3–12. [Google Scholar] [CrossRef]
- Abdelrahman, M.; Hirata, S.; Mukae, T.; Yamada, T.; Sawada, Y.; El-Syaed, M.; Yamada, Y.; Sato, M.; Hirai, M.; Shigyo, M. Comprehensive Metabolite Profiling in Genetic Resources of Garlic (Allium sativum L.) Collected from Different Geographical Regions. Molecules 2021, 26, 1415. [Google Scholar] [CrossRef]
- García-Lampasona, S.; Asprelli, P.; Burba, J.L. Genetic analysis of a garlic (Allium sativum L.) germplasm collection from Argentina. Sci. Hortic. 2012, 138, 183–189. [Google Scholar] [CrossRef]
- Jabbes, N.; Dridi, B.; Hannechi, C.; Geoffriau, E.; Le Clerc, V. Inter Simple Sequence Repeat Fingerprints for Assess Genetic Diversity of Tunisian Garlic Populations. J. Agric. Sci. 2011, 3, 77. [Google Scholar] [CrossRef]
- Bayraktar, H.; Dolar, F.S. Molecular Identification and Genetic Diversity of Fusarium species Associated with Onion Fields in Turkey. J. Phytopathol. 2010, 159, 28–34. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Shen, D.; Oiu, Y.; Song, J. Diversity evaluation of morphological traits and allicin content in garlic (Allium sativum L.) from China. Euphytica 2014, 198, 243–254. [Google Scholar] [CrossRef]
- Yoo, K.S.; Pike, L.M. Determination of background pyruvic acid concentrations in onions, Allium species, and other vegetables. Sci. Hortic. 2001, 89, 249–256. [Google Scholar] [CrossRef]
- Arnault, I.; Christidès, J.; Mandon, N.; Haffner, T.; Kahane, R.; Auger, J. High-performance ion-pair chromatography method for simultaneous analysis of alliin, deoxyalliin, allicin and dipeptide precursors in garlic products using multiple mass spectrometry and UV detection. J. Chromatogr. A 2003, 991, 69–75. [Google Scholar] [CrossRef]
- Odum, E.P.; Odom, H. Fundamentals of Ecology, 2nd ed; W.B. Saunders Company: Philadelphia, PA, USA, 1959. [Google Scholar]
- Lavelle, P.; Dugdale, R.; Scholes, R.; Berhe, A.; Carpenter, E.; Codispoti, L.; Izac, A.; Lemoalle, J.; Luizao, F.; Treguer, P. Nutrient cycling. In Ecosystems and Human Well-Being: Current State and Trends: Findings of the Condition and Trends Working Group; Island Press: Washington, DC, USA; Covelo, CA, USA; London, UK, 2005. [Google Scholar]
- Moe, S.J.; Stelzer, R.S.; Forman, M.R.; Harpole, W.S.; Daufresne, T.; Yoshida, T. Recent advances in ecological stoichiometry: Insights for population and community ecology. Oikos 2005, 109, 29–39. [Google Scholar] [CrossRef] [Green Version]
- Coolong, T.W.; Randle, W.M. Sulfur and Nitrogen Availability Interact to Affect the Flavor Biosynthetic Pathway in Onion. J. Am. Soc. Hortic. Sci. 2003, 128, 776–783. [Google Scholar] [CrossRef] [Green Version]
- Bayati, P.; Karimmojeni, H.; Razmjoo, J.; Pucci, M.; Abate, G.; Baldwin, T.C.; Mastinu, A. Physiological, Biochemical, and Agronomic Trait Responses of Nigella sativa Genotypes to Water Stress. Horticulturae 2022, 8, 193. [Google Scholar] [CrossRef]
- Biareh, V.; Shekari, F.; Sayfzadeh, S.; Zakerin, H.; Hadidi, E.; Beltrão, J.G.T.; Mastinu, A. Physiological and Qualitative Response of Cucurbitapepo L. to Salicylic Acid under Controlled Water Stress Conditions. Horticulturae 2022, 8, 79. [Google Scholar] [CrossRef]
- Chaichi, M.; Nemati, A.; Dadrasi, A.; Heydari, M.; Hassanisaadi, M.; Yousefi, A.R.; Baldwin, T.C.; Mastinu, A. Germination of Triticumaestivum L.: Effects of Soil–Seed Interaction on the Growth of Seedlings. Soil Syst. 2022, 6, 37. [Google Scholar] [CrossRef]
- Karimmojeni, H.; Rezaei, M.; Tseng, T.-M.; Mastinu, A. Effects of Metribuzin Herbicide on Some Morpho-Physiological Characteristics of Two Echinacea Species. Horticulturae 2022, 8, 169. [Google Scholar] [CrossRef]
- Moradi, P.; Aghajanloo, F.; Moosavi, A.; Monfared, H.; Khalafi, J.; Taghiloo, M.; Khoshzaman, T.; Shojaee, M.; Mastinu, A. Anthropic Effects on the Biodiversity of the Habitats of Ferula gummosa. Sustainability 2021, 13, 7874. [Google Scholar] [CrossRef]
- Naservafaei, S.; Sohrabi, Y.; Moradi, P.; Mac Sweeney, E.; Mastinu, A. Biological Response of Lallemantiaiberica to Brassinolide Treatment under Different Watering Conditions. Plants 2021, 10, 496. [Google Scholar] [CrossRef]
- Rad, S.V.; Valadabadi, S.A.R.; Pouryousef, M.; Saifzadeh, S.; Zakrin, H.R.; Mastinu, A. Quantitative and Qualitative Evaluation of Sorghum bicolor L. under Intercropping with Legumes and Different Weed Control Methods. Horticulturae 2020, 6, 78. [Google Scholar] [CrossRef]
- Reza Yousefi, A.; Rashidi, S.; Moradi, P.; Mastinu, A. Germination and Seedling Growth Responses of Zygophyllumfabago, Salsola kali L. and Atriplexcanescens to PEG-Induced Drought Stress. Environments 2020, 7, 107. [Google Scholar] [CrossRef]
- Yousefvand, P.; Sohrabi, Y.; Heidari, G.; Weisany, W.; Mastinu, A. Salicylic Acid Stimulates Defense Systems in Allium hirtifolium Grown under Water Deficit Stress. Molecules 2022, 27, 3083. [Google Scholar] [CrossRef]
- Zangani, E.; Afsahi, K.; Shekari, F.; Mac Sweeney, E.; Mastinu, A. Nitrogen and Phosphorus Addition to Soil Improves Seed Yield, Foliar Stomatal Conductance, and the Photosynthetic Response of Rapeseed (Brassica napus L.). Agriculture 2021, 11, 483. [Google Scholar] [CrossRef]
- Willis, C.G.; Baskin, C.C.; Baskin, J.M.; Auld, J.R.; Venable, D.L.; Cavender-Bares, J.; Donohue, K.; de Casas, R.R. The evolution of seed dormancy: Environmental cues, evolutionary hubs, and diversification of the seed plants. New Phytol. 2014, 203, 300–309. [Google Scholar] [CrossRef]
- Donohue, K. Completing the cycle: Maternal effects as the missing link in plant life histories. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1059–1074. [Google Scholar] [CrossRef] [Green Version]
- Richards, C.L.; Bossdorf, O.; Muth, N.Z.; Gurevitch, J.; Pigliucci, M. Jack of all trades, master of some? On the role of phenotypic plasticity in plant invasions. Ecol. Lett. 2006, 9, 981–993. [Google Scholar] [CrossRef] [Green Version]
- Colautti, R.I.; Barrett, S.C.H. Natural Selection and Genetic Constraints on Flowering Phenology in an Invasive Plant. Int. J. Plant Sci. 2010, 171, 960–971. [Google Scholar] [CrossRef] [Green Version]
- Wilczek, A.M.; Cooper, M.D.; Korves, T.M.; Schmitt, J. Lagging adaptation to warming climate in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2014, 111, 7906–7913. [Google Scholar] [CrossRef] [Green Version]
- Alba, C.; Moravcová, L.; Pyšek, P. Geographic structuring and transgenerational maternal effects shape germination in native, but not introduced, populations of a widespread plant invader. Am. J. Bot. 2016, 103, 837–844. [Google Scholar] [CrossRef] [Green Version]
- Tisneé, S.; Reymond, M.; Vile, D.; Fabre, J.; Dauzat, M.; Koornneef, M.; Granier, C. Combined Genetic and Modeling Approaches Reveal That Epidermal Cell Area and Number in Leaves Are Controlled by Leaf and Plant Developmental Processes in Arabidopsis. Plant Physiol. 2008, 148, 1117–1127. [Google Scholar] [CrossRef] [Green Version]
- Sebnie, W.; Mengesha, M.; Girmay, G.; Feyisa, T. Response of garlic (Allium sativum L.) to nitrogen and phosphorus under irrigation in Lasta district of Amhara Region, Ethiopia. Cogent Food Agric. 2018, 4, 1532862. [Google Scholar] [CrossRef]
- Mahajan, S.; Tuteja, N. Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys. 2005, 444, 139–158. [Google Scholar] [CrossRef]
- Westgate, M.E.; Schussler, J.R.; Reicosky, D.C.; Brenner, M.L. Effect of Water Deficits on Seed Development in Soybean. Plant Physiol. 1989, 91, 980–985. [Google Scholar] [CrossRef]
- Germ, M.; Kreft, I.; Stibilj, V.; Urbanc-Berčič, O. Combined effects of selenium and drought on photosynthesis and mitochondrial respiration in potato. Plant Physiol. Biochem. 2007, 45, 162–167. [Google Scholar] [CrossRef]
- Schwimmer, S.; Weston, W.J. Onion Flavor and Odor, Enzymatic Development of Pyruvic Acid in Onion as a Measure of Pungency. J. Agric. Food Chem. 1961, 9, 301–304. [Google Scholar] [CrossRef]
- Freeman, G.G.; Mossadeghi, N. Effect of sulphate nutrition on flavour components of onion (Allium cepa). J. Sci. Food Agric. 1970, 21, 610–615. [Google Scholar] [CrossRef]
- Fenwick, G.R.; Hanley, A.B.; Whitaker, J.R. The genusallium—Part 1. C R C Crit. Rev. Food Sci. Nutr. 1985, 22, 199–271. [Google Scholar] [CrossRef]
- Mahmood, N.; Muazzam, M.A.; Ahmad, M.; Hussain, S.; Javed, W. Phytochemistry of Allium cepa L. (Onion): Its Nutritional and Pharmacological Importance. Sci. Inq. Rev. 2021, 5, 41–49. [Google Scholar] [CrossRef]
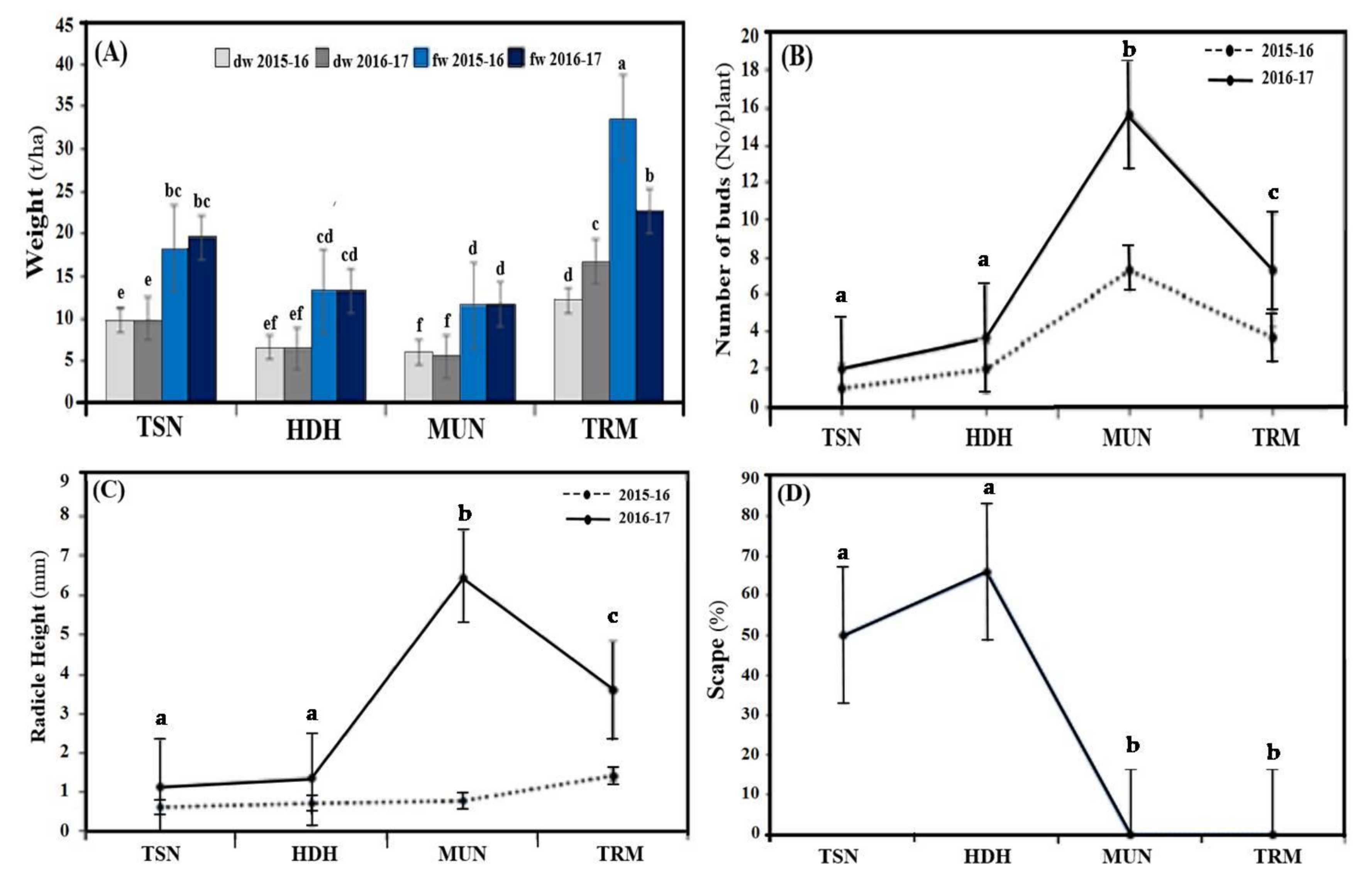
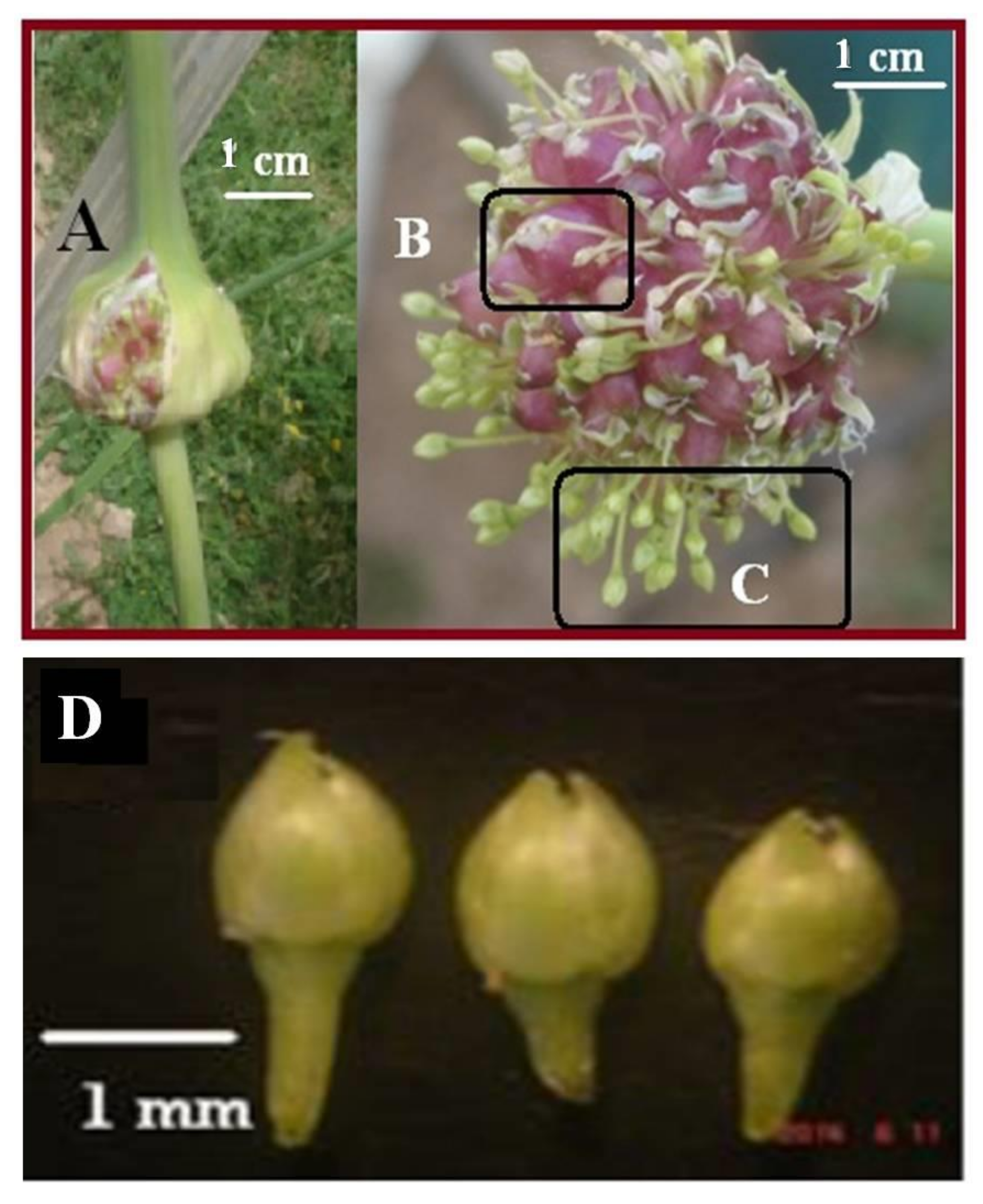
| Specifications | Depth (cm) | pH | EC * (dS/m) | Texture | N (%) | K (mg/kg) | P (mg/kg) |
|---|---|---|---|---|---|---|---|
| 2015–16 | 10–50 | 7 | 0.9 | loam | 0.5 | 301.8 | 20.33 |
| 2016–17 | 10–50 | 7.3 | 1.3 | loam | 0.81 | 119.7 | 16.8 |
| Germination Rate | Plant Height | Stem Diameter | N° of Fresh Leaves | Pyruvic Acid | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (DAP *) | (cm) | (mm) | (n/plant) | (µm/gfw) | ||||||
| 2016 | 2017 | 2016 | 2017 | 2016 | 2017 | 2016 | 2017 | 2016 | 2017 | |
| TSN | 65.6 a | 63 a | 55 b | 56 b | 1.4 a | 1.4 b | 4.3 b | 5 b,c | 74 b | 70 c |
| HDH | 62.3 a.b | 61 a | 63.3 a | 65 a | 1.06 b | 1.2c | 4.3 b | 4.6 c | 71 c | 69 c |
| MUN | 60 b | 62 a | 53.3 b | 54 b | 1.5 a | 1.5 a.b | 5.6 a | 6 a,b | 73.6 b | 73 b |
| TRM | 43.3 c | 40 b | 50.6 b | 49 b | 1.6 a | 1.7 a | 5.6 a | 6.3 a | 79.3 a | 78 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ammarellou, A.; Yousefi, A.R.; Heydari, M.; Uberti, D.; Mastinu, A. Biochemical and Botanical Aspects of Allium sativum L. Sowing. BioTech 2022, 11, 16. https://doi.org/10.3390/biotech11020016
Ammarellou A, Yousefi AR, Heydari M, Uberti D, Mastinu A. Biochemical and Botanical Aspects of Allium sativum L. Sowing. BioTech. 2022; 11(2):16. https://doi.org/10.3390/biotech11020016
Chicago/Turabian StyleAmmarellou, Ali, Ali Reza Yousefi, Moslem Heydari, Daniela Uberti, and Andrea Mastinu. 2022. "Biochemical and Botanical Aspects of Allium sativum L. Sowing" BioTech 11, no. 2: 16. https://doi.org/10.3390/biotech11020016
APA StyleAmmarellou, A., Yousefi, A. R., Heydari, M., Uberti, D., & Mastinu, A. (2022). Biochemical and Botanical Aspects of Allium sativum L. Sowing. BioTech, 11(2), 16. https://doi.org/10.3390/biotech11020016







