Advances in Collagen-/Gelatin-Based Hydrogels: Rheological Properties and Applications
Abstract
1. Introduction
2. Properties of Collagen and Gelatin
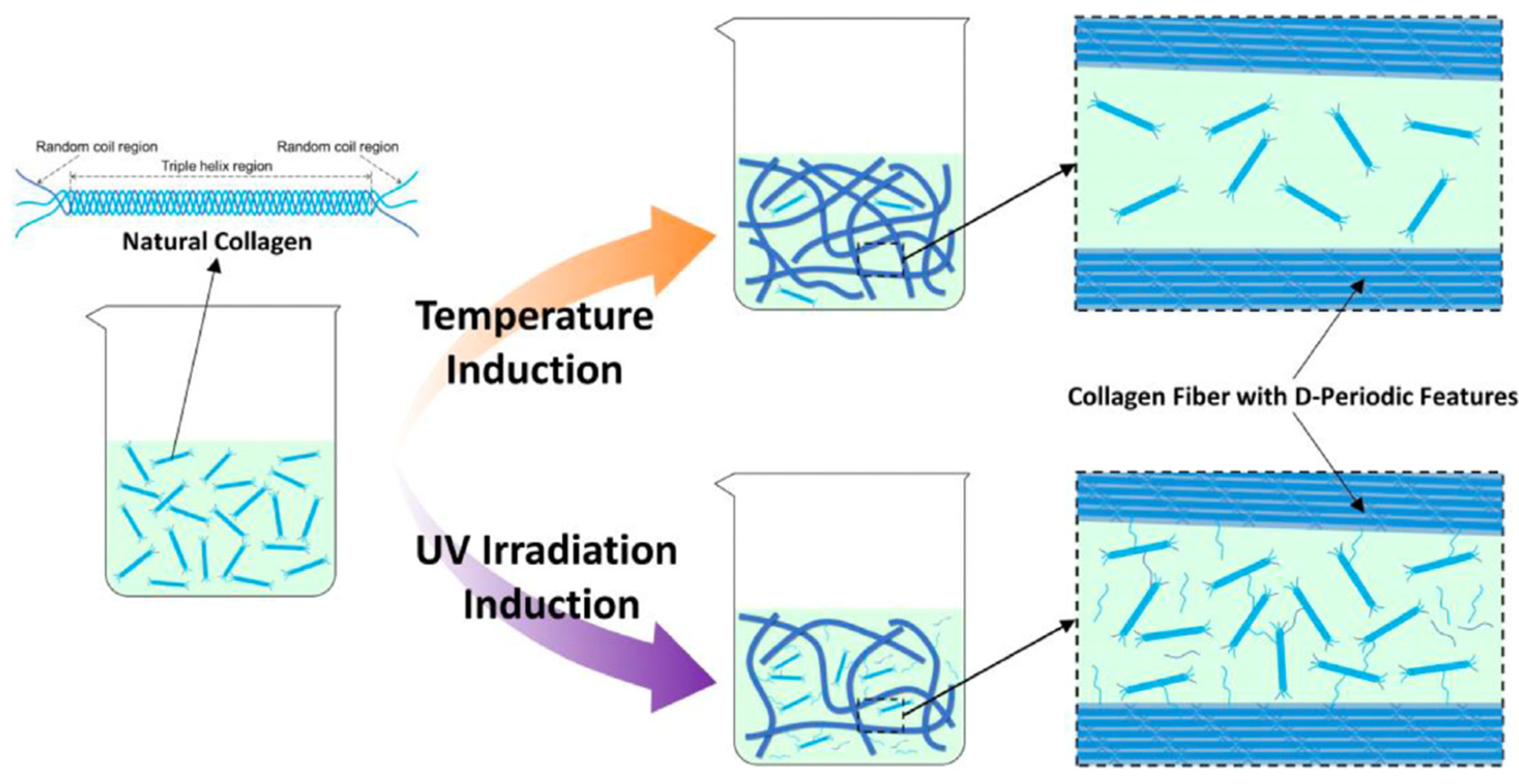
2.1. Collagen
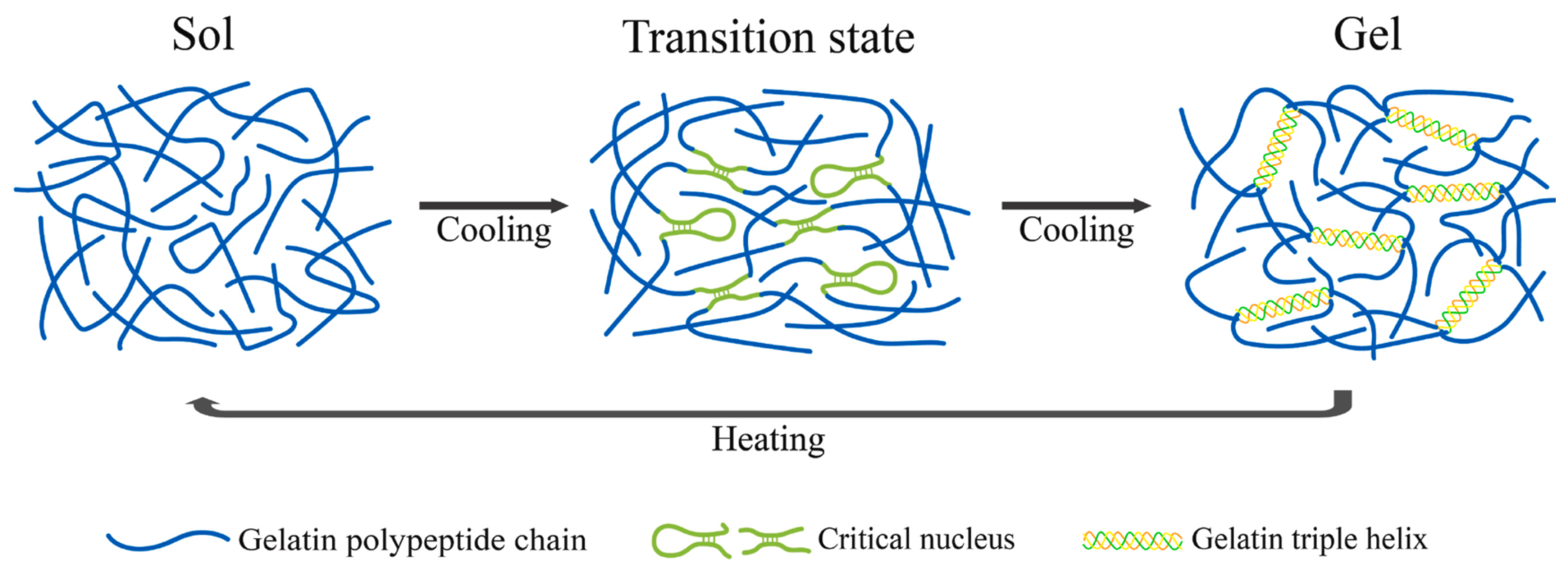
2.2. Gelatin
| Source | Molecular Weight Distribution (kDa) | Gelling Temperature (°C) | Bloom Strength (g) | Reference |
|---|---|---|---|---|
| Bovine bone gelatin | α1- 130 α2- 120 | 25.05 | 221 | [68] |
| Duck feet gelatin | β- ∼180 α1- ∼115 α2- ∼100 | 20.50 | 209.63 | [69] |
| Sheep hoof gelatin | β- 245 α1- 63–75 α2- 100–135 | 25.38 | 378.55 | [70] |
| Porcine skin gelatin | β > 180 α1- 135 α2 < 135 | 28.06 | 581.61 | [71] |
| Frog skin gelatin | β- ∼200 α1- ∼120 α2- ∼120 | 28 | 363 | [72] |
| Camel skin gelatin | β- ∼225 α1- ∼120 α2- ∼116 | 20.9–25.8 | 365.5 | [73] |
| Chicken head gelatin | β- ∼202 α- ∼113 | 27°–28 | >309 | [74] |
| Cold-water fish gelatin | β > 200 α1- 130 α2- 110 | NR | 253 | [75] |
| Dog shark skin gelatin | β-∼200 α1–116 α2–97 | 20.8 | 206 | [76] |
| Bigeye snapper skin gelatin | β-∼205 α-97 | 10 | 108 | [77] |
3. Collagen-/Gelatin-Based Hydrogels
4. Methods to Modify the Rheological Properties of Collagen/Gelatin Gels for Improved End-Use Applications
4.1. Addition of Co-Agent Molecules
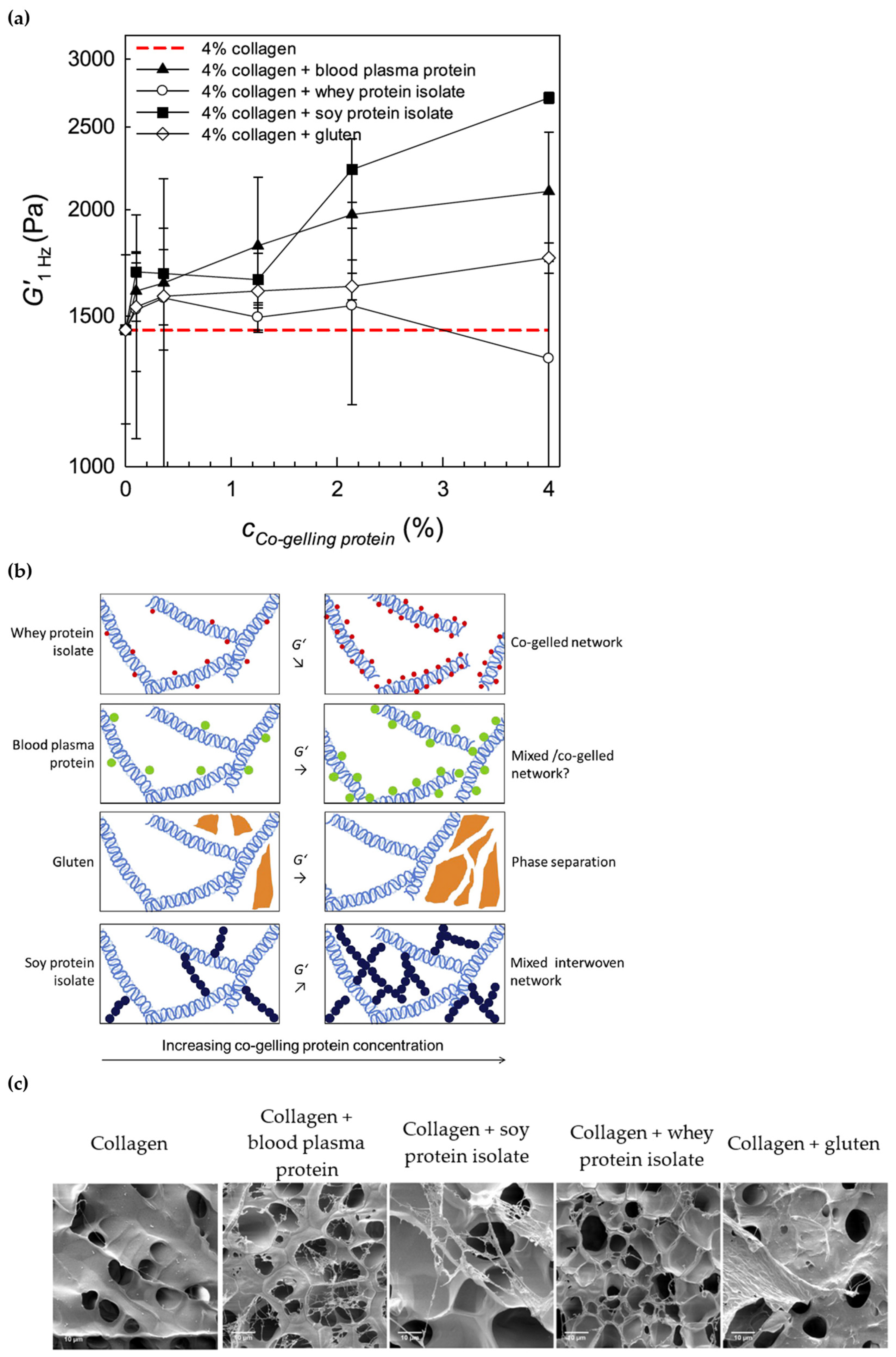
4.1.1. Rheological Properties of Collagen Gels with Added Co-Agents
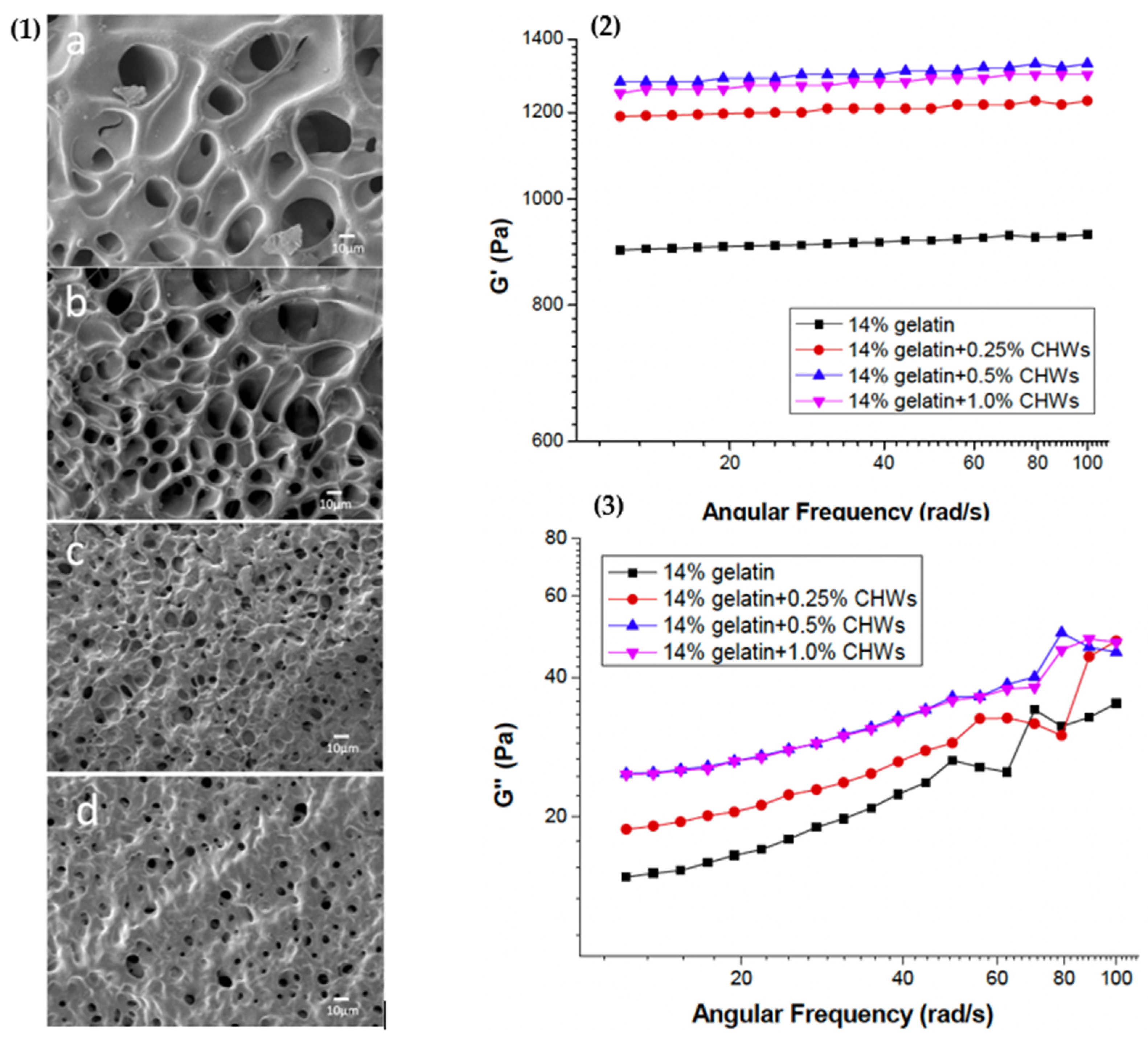
4.1.2. Rheological Properties of Gelatin Gels with Added Co-Agents
4.2. Chemical and Mechanical Treatments to Modify the Rheological Properties of Collagen and Gelatin Gels
4.2.1. Treatments to Modify the Rheological Properties of Collagen Gels
| Treatment | Samples | Parameters Applied | Suggested Optimum Values for the Treatment | Achieved Traits in the Gel |
|---|---|---|---|---|
| UV irradiation | 0.5, 1.0, 1.5% (w/w) collagen solutions | Power: 4.8 W Wavelength: 366 nm Time: 10, 20, 30, and 60 min | All treatments were found to be useful. | Increase in the viscosity of the collagen solutions led to the flexibility to work with lower collagen concentrations to form gel [150]. |
| Collagen gels with 0.5, 1.0, 2.0, and 3.0 mg/mL concentrations | Wavelength: 254–290 nm Time: 30, 60 and 120 min | 0.5 mg/mL collagen concentration and a UV irradiation treatment for 30 and 60 min were suggested. | A stabilized collagen gel surface area for cell cultivation [149]. | |
| 2 mg/mL collagen solution | Wavelength: 254 nm Intensity: 5.0 × 10−3 W/cm2 Time: 30 min for 5 times (with intervals of >60 min) | The applied protocol was successful. | UV irradiation-induced collagen gels with thermal stability, mechanical properties, and cell growth compatibility comparable to those of temperature-induced collagen gel [13]. | |
| Ultrasonication | Collagen solution with a concentration of 3 g/L | Ultrasonic power: 0, 100, 140, 180 W Time (at 30 °C): 0, 5, 15, 60 min | Manipulating power was suggested to meet the end-use needs. Increasing the treatment time did not affect the collagen self-assembly dynamics. Therefore, 5-min treatment was enough. | Viscosity of the collagen gel decreased with increasing ultrasonication power [27]. |
| 5 mg/mL of collagen solution | Ultrasonic power: 0, 50, 100, 200, 400 W Time (at 30 °C): 0, 5, 10, 15, 30, 60 min | Power value of ≤200 W for a time period of ≤15 min have been suggested as proper ultrasonic treatment parameters. | With the proper parameters used, the self-assembly rate of collagen increased, fibril diameters became more homogenous, thermal stability increased; while viscosity decreased, leading to a softer gel [139]. |
4.2.2. Treatments to Modify the Rheological Properties of Gelatin Gels
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
Abbreviations
| Col | Collagen |
| UV | Ultraviolet |
| EDC | Ethyl-3-(3-dimethylaminopropyl) carbodiimide |
| NHS | N-hydroxysuccinimide |
| TGase | Transglutaminase |
| MTGase | Microbial transglutaminase |
| BSE | Bovine spongiform encephalopathy |
| TSE | Transmissible spongiform encephalopathy |
| FMD | Foot-and-mouth disease |
| HMPC | Hydroxypropyl methylcellulose |
| AFM | Atomic force microscopy |
| SA | Sodium alginate |
| HA | Hyaluronic acid |
| CS | Chondroitin sulfate |
| SMP | Skim milk powder |
| MPC | Milk protein concentrate |
| WPI | Whey protein isolate |
| SDS | Sodium dodecyl sulfate |
| AOT | Sodium dioctyl sulfosuccinate |
| CTAB | Cetyltrimethylammonium bromide |
| L-GA | Laccase-catalyzed gallic acid |
| PPI | Pea protein isolate |
| ADA | Alginate dialdehyde |
| HPP | High pressure processing |
| STPP | Sodium tripolyphosphate |
| LAOS | Large amplitude oscillatory shear |
| FG | Fish gelatin |
| GA | Gum Arabic |
References
- Mehdi-Sefiani, H.; Chicardi, E.; Romero, A.; Perez-Puyana, V.M. Unveiling the impact of gelation temperature on the rheological and microstructural properties of type A gelatin hydrogels. Polymers 2024, 16, 1842. [Google Scholar] [CrossRef]
- Shan, P.; Wang, K.; Sun, F.; Li, Y.; Sun, L.; Li, H.; Peng, L. Humidity-adjustable functional gelatin hydrogel/ethyl cellulose bilayer films for active food packaging application. Food Chem. 2024, 439, 138202. [Google Scholar] [CrossRef]
- Yang, J.; Duan, A.; Shen, L.; Liu, Q.; Wang, F.; Liu, Y. Preparation and application of curcumin loaded with citric acid crosslinked chitosan-gelatin hydrogels. Int. J. Biol. Macromol. 2024, 264, 130801. [Google Scholar] [CrossRef]
- Nazir, S.; Wani, I.A. Stability and release properties of alginate, modified starch/basil seed gum, and gelatin-based hydrogel beads infused with basil seed (Ocimum basilicum L.) oil. ACS Food Sci. Technol. 2024, 4, 2835–2846. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakash, S.; Razeen, Z.M.A.; Kumar, R.N.; He, J.; Milky, M.G.; Renuka, R.; Sanskrithi, M.V. Enriched Characteristics of Poultry Collagen over Other Sources of Collagen and Its Extraction Methods: A Review. Int. J. Biol. Macromol. 2024, 273, 133004. [Google Scholar] [CrossRef] [PubMed]
- Osidak, E.O.; Kalabusheva, E.P.; Alpeeva, E.V.; Belousov, S.I.; Krasheninnikov, S.V.; Grigoriev, T.E.; Sergey, P.D.; Vorotelyak, E.A.; Chermnykh, E.S. Concentrated collagen hydrogels: A new approach for developing artificial tissues. Materialia 2021, 20, 101217. [Google Scholar] [CrossRef]
- Andriakopoulou, C.E.; Zadpoor, A.A.; Grant, M.H.; Riches, P.E. Development and mechanical characterisation of self-compressed collagen gels. Mater. Sci. Eng. C 2018, 84, 243–247. [Google Scholar] [CrossRef]
- An, X.; Duan, S.; Jiang, Z.; Chen, S.; Sun, W.; Liu, X.; Sun, Z.; Li, Y.; Yan, M. Role of chlorogenic acid and procyanidin in the modification of self-assembled fibrillar gel prepared from tilapia collagen. Polym. Degrad. Stab. 2022, 206, 110177. [Google Scholar] [CrossRef]
- Tang, C.; Zhou, K.; Zhu, Y.; Zhang, W.; Xie, Y.; Wang, Z.; Zhou, H.; Yang, T.; Zhang, Q.; Xu, B. Collagen and its derivatives: From structure and properties to their applications in food industry. Food Hydrocoll. 2022, 131, 107748. [Google Scholar] [CrossRef]
- Derkach, S.R.; Voron’ko, N.G.; Kuchina, Y.A.; Kolotova, D.S. Modified fish gelatin as an alternative to mammalian gelatin in modern food technologies. Polymers 2020, 12, 3051. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.A.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Xu, C.; Wei, X.; Shu, F.; Li, X.; Wang, W.; Li, P.; Li, Y.; Li, S.; Zhang, J.; Wang, H. Induction of fiber-like aggregation and gelation of collagen by ultraviolet irradiation at low temperature. Int. J. Biol. Macromol. 2020, 153, 232–239. [Google Scholar] [CrossRef]
- Gao, Y.; Li, B.; Kong, W.; Yuan, L.; Guo, L.; Li, C.; Fan, H.; Fan, Y.; Zhang, X. Injectable and self-crosslinkable hydrogels based on collagen type II and activated chondroitin sulfate for cell delivery. Int. J. Biol. Macromol. 2018, 118, 2014–2020. [Google Scholar] [CrossRef]
- Zhu, S.; Yu, X.; Xiong, S.; Liu, R.; Gu, Z.; You, J.; Yin, T.; Hu, Y. Insights into the rheological behaviors evolution of alginate dialdehyde crosslinked collagen solutions evaluated by numerical models. Mater. Sci. Eng. C 2017, 78, 727–737. [Google Scholar] [CrossRef]
- Katarzyna, A.; Alina, S. Current methods of collagen crosslinking: Review. Int. J. Biol. Macromol. 2020, 161, 550–560. [Google Scholar] [CrossRef]
- Yang, H.; Wang, H.; Huang, M.; Cao, G.; Tao, F.; Shen, Q.; Zhou, G.; Yang, H. Repurposing fish waste into gelatin as a potential alternative for mammalian sources: A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 942–963. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Ma, L.; Fu, Y.; Chen, H.; Dai, H.; Zhu, H.; Wang, H.; Yu, Y.; Zhang, Y. Transglutaminase modified type A gelatin gel: The influence of intra-molecular and inter-molecular crosslinking on structure-properties. Food Chem. 2022, 395, 133578. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wu, D.; Ma, W.; Wu, C.; Liu, J.; Du, M. Strong fish gelatin hydrogels double crosslinked by transglutaminase and carrageenan. Food Chem. 2022, 376, 131873. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Y.; Liu, R.; Xiong, S.; Xu, Y.; Hu, Y. Effects of microbial transglutaminase on the gelling property and in vitro digestibility of fish scale gelatin from grass carp. Food Biosci. 2023, 53, 102569. [Google Scholar] [CrossRef]
- Lin, Y.; Roos, Y.H.; Miao, S. Transglutaminase crosslinked fish gelatin emulsion gels: Structure, rheology behaviors, and delivery functionality. Food Hydrocoll. 2025, 162, 111001. [Google Scholar] [CrossRef]
- Yan, M.; Jiang, X.; Wang, G.; Wang, A.; Wang, X.; Wang, X.; Zhao, X.; Xu, H.; An, X.; Li, Y. Preparation of self-assembled collagen fibrillar gel from tilapia skin and its formation in presence of acidic polysaccharides. Carbohydr. Polym. 2020, 233, 115831. [Google Scholar] [CrossRef]
- Ge, H.; Wu, Y.; Woshnak, L.L.; Mitmesser, S.H. Effects of hydrocolloids, acids and nutrients on gelatin network in gummies. Food Hydrocoll. 2021, 113, 106549. [Google Scholar] [CrossRef]
- Kaewruang, P.; Benjakul, S.; Prodpran, T. Effect of phosphorylation on gel properties of gelatin from the skin of unicorn leatherjacket. Food Hydrocoll. 2014, 35, 694–699. [Google Scholar] [CrossRef]
- Kaewruang, P.; Benjakul, S.; Prodpran, T.; Encarnacion, A.B.; Nalinanon, S. Impact of divalent salts and bovine gelatin on gel properties of phosphorylated gelatin from the skin of unicorn leatherjacket. LWT-Food Sci. Technol. 2014, 55, 477–482. [Google Scholar] [CrossRef]
- Song, J.; Hu, S.; Liu, Z.; Wang, Y.; Lei, L.; Zhao, G.; Zhou, Y. Oscillatory rheometry for elucidating the influence of non-network biopolymer aggregation on pectin-gelatin composite gels. Int. J. Biol. Macromol. 2024, 257, 128543. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, H.; Deng, M.; Wang, Z.; Zhang, J.; Wang, H.; Zhang, H. Effect of Ultrasonication on the Fibril-Formation and Gel Properties of Collagen from Grass Carp Skin. Mater. Sci. Eng. C 2016, 59, 1038–1046. [Google Scholar] [CrossRef]
- Davidenko, N.; Schuster, C.F.; Bax, D.V.; Farndale, R.W.; Hamaia, S.; Best, S.M.; Cameron, R.E. Evaluation of cell binding to collagen and gelatin: A study of the effect of 2D and 3D architecture and surface chemistry. J. Mater. Sci. Mater. Med. 2016, 27, 148. [Google Scholar] [CrossRef]
- Varela, M.S.; Palacio, M.A.; Navarro, A.S.; Yamul, D.K. Structural and functional properties and digital image texture analysis of gelatin, pectin, and carrageenan gels with honey addition. J. Texture Stud. 2023, 54, 646–658. [Google Scholar] [CrossRef]
- Ishihara, S.; Kurosawa, H.; Haga, H. Stiffness-Modulation of Collagen Gels by Genipin-Crosslinking for Cell Culture. Gels 2023, 9, 148. [Google Scholar] [CrossRef]
- Yang, Z.; Hemar, Y.; Hilliou, L.; Gilbert, E.P.; McGillivray, D.J.; Williams, M.A.; Chaieb, S. Nonlinear behavior of gelatin networks reveals a hierarchical structure. Biomacromolecules 2016, 17, 590–600. [Google Scholar] [CrossRef]
- Nitsuwat, S.; Zhang, P.; Ng, K.; Fang, Z. Fish gelatin as an alternative to mammalian gelatin for food industry: A meta-analysis. LWT-Food Sci. Technol. 2021, 141, 110899. [Google Scholar] [CrossRef]
- Rather, J.A.; Akhter, N.; Ashraf, Q.S.; Mir, S.A.; Makroo, H.A.; Majid, D.; Barba, F.J.; Khaneghah, A.M.; Dar, B.N. A comprehensive review on gelatin: Understanding impact of the sources, extraction methods, and modifications on potential packaging applications. Food Packag. Shelf Life 2022, 34, 100945. [Google Scholar] [CrossRef]
- Samatra, M.Y.; Noor, N.Q.I.M.; Razali, U.H.M.; Bakar, J.; Shaarani, S.M. Bovidae-based gelatin: Extractions method, physicochemical and functional properties, applications, and future trends. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3153–3176. [Google Scholar] [CrossRef] [PubMed]
- Umar, S.; Umar, K.; Rahat, R.; Bhawani, S.A. Collagen/gelatin-based hydrogels for tissue engineering. In Protein-Based Nanocomposites for Tissue Engineering; Woodhead Publishing: Cambridge, UK, 2025; pp. 219–252. [Google Scholar]
- Kang, J.I.; Park, K.M. Advances in gelatin-based hydrogels for wound management. J. Mater. Chem. B 2021, 9, 1503–1520. [Google Scholar] [CrossRef] [PubMed]
- Mohanto, S.; Narayana, S.; Merai, K.P.; Kumar, J.A.; Bhunia, A.; Hani, U.; Fatease, A.A.; Gowda, B.H.J.; Nag, S.; Ahmet, M.G.; et al. Advancements in gelatin-based hydrogel systems for biomedical applications: A state-of-the-art review. Int. J. Biol. Macromol. 2023, 253, 127143. [Google Scholar] [CrossRef] [PubMed]
- Rana, D.; Desai, N.; Salave, S.; Karunakaran, B.; Giri, J.; Benival, D.; Gorantla, S.; Kommineni, N. Collagen-based hydrogels for the eye: A comprehensive review. Gels 2023, 9, 643. [Google Scholar] [CrossRef]
- Gaar, J.; Naffa, R.; Brimble, M. Enzymatic and non-enzymatic crosslinks found in collagen and elastin and their chemical synthesis. Org. Chem. Front. 2020, 7, 2789–2814. [Google Scholar] [CrossRef]
- Kirkness, M.W.; Lehmann, K.; Forde, N.R. Mechanics and structural stability of the collagen triple helix. Curr. Opin. Chem. Biol. 2019, 53, 98–105. [Google Scholar] [CrossRef]
- Gelse, K.; Pöschl, E.; Aigner, T. Collagens—Structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef]
- Fidler, A.L.; Boudko, S.P.; Rokas, A.; Hudson, B.G. The triple helix of collagens–an ancient protein structure that enabled animal multicellularity and tissue evolution. J. Cell Sci. 2018, 131, jcs203950. [Google Scholar] [CrossRef]
- Krishnamoorthi, J.; Ramasamy, P.; Shanmugam, V.; Shanmugam, A. Isolation and partial characterization of collagen from outer skin of Sepia pharaonis (Ehrenberg, 1831) from Puducherry coast. Biochem. Biophys. Rep. 2017, 10, 39–45. [Google Scholar] [CrossRef]
- Jeevithan, E.; Jingyi, Z.; Wang, N.; He, L.; Bao, B.; Wu, W. Physico-Chemical, Antioxidant and Intestinal Absorption Properties of Whale Shark Type-II Collagen Based on Its Solubility with Acid and Pepsin. Process Biochem. 2015, 50, 463–472. [Google Scholar] [CrossRef]
- Nielsen, M.J.; Karsdal, M.A. Type III collagen. In Biochemistry of Collagens, Laminins and Elastin; Academic Press: New York, NY, USA, 2016; pp. 21–30. [Google Scholar]
- Schmidt, M.M.; Dornelles, R.C.P.; Mello, R.O.; Kubota, E.H.; Mazutti, M.A.; Kempka, A.P.; Demiate, I.M. Collagen extraction process. Int. Food Res. J. 2016, 23, 913. [Google Scholar]
- Islam, J.; Mis Solval, K.E. Recent Advancements in Marine Collagen: Exploring New Sources, Processing Approaches, and Nutritional Applications. Mar. Drugs 2025, 23, 190. [Google Scholar] [CrossRef] [PubMed]
- Abedinia, A.; Nafchi, A.M.; Sharifi, M.; Ghalambor, P.; Oladzadabbasabadi, N.; Ariffin, F.; Huda, N. Poultry gelatin: Characteristics, developments, challenges, and future outlooks as a sustainable alternative for mammalian gelatin. Trends Food Sci. Technol. 2020, 104, 14–26. [Google Scholar] [CrossRef]
- Avila Rodríguez, M.I.; Rodríguez Barroso, L.G.; Sánchez, M.L. Collagen: A review on its sources and potential cosmetic applications. J. Cosmet. Dermatol. 2018, 17, 20–26. [Google Scholar] [CrossRef]
- Coppola, D.; Oliviero, M.; Vitale, G.A.; Lauritano, C.; D’Ambra, I.; Iannace, S.; de Pascale, D. Marine collagen from alternative and sustainable sources: Extraction, processing and applications. Mar. Drugs 2020, 18, 214. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kuriyama, T.; Nakagawara, R.; Aihara, M.; Hamada-Sato, N. Allergy to fish collagen: Thermostability of collagen and IgE reactivity of patients’ sera with extracts of 11 species of bony and cartilaginous fish. Allergol. Int. 2016, 65, 450–458. [Google Scholar] [CrossRef]
- Ata, O.; Bakar, B.; Turkoz, B.K.; Kumcuoglu, S.; Aydogdu, Y.; Gumustas, B.; Doganay, G.D.; Basturk, E.; Tavman, S. Structural and molecular characterization of collagen-type I extracted from lamb feet. J. Food Sci. 2024, 89, 330–341. [Google Scholar] [CrossRef]
- Kaewbangkerd, K.; Hamzeh, A.; Yongsawatdigul, J. Ultrasound-assisted extraction of collagen from broiler chicken trachea and its biochemical characterization. Ultrason. Sonochem. 2023, 95, 106372. [Google Scholar] [CrossRef]
- Petcharat, T.; Benjakul, S.; Karnjanapratum, S.; Nalinanon, S. Ultrasound-assisted extraction of collagen from clown featherback (Chitala ornata) skin: Yield and molecular characteristics. J. Sci. Food Agric. 2021, 101, 648–658. [Google Scholar] [CrossRef]
- Akram, A.N.; Zhang, C. Effect of ultrasonication on the yield, functional and physicochemical characteristics of collagen-II from chicken sternal cartilage. Food Chem. 2020, 307, 125544. [Google Scholar] [CrossRef]
- Sousa, R.O.; Martins, E.; Carvalho, D.N.; Alves, A.L.; Oliveira, C.; Duarte, A.R.C.; Silva, T.H.; Reis, R.L. Collagen from Atlantic cod (Gadus morhua) skins extracted using CO2 acidified water with potential application in healthcare. J. Polym. Res. 2020, 27, 73. [Google Scholar] [CrossRef]
- Asaduzzaman, A.K.M.; Getachew, A.T.; Cho, Y.J.; Park, J.S.; Haq, M.; Chun, B.S. Characterization of pepsin-solubilised collagen recovered from mackerel (Scomber japonicus) bone and skin using subcritical water hydrolysis. Int. J. Biol. Macromol. 2020, 148, 1290–1297. [Google Scholar] [CrossRef]
- Pan, Z.; Ge, B.; Wei, M.; Elango, J.; Wu, W. Isolation and biochemical properties of type II collagen from blue shark (Prionace glauca) cartilage. Mar. Drugs 2023, 21, 260. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, L.; Wu, S.; Chen, J.; Lin, H. Structural, functional, rheological, and biological properties of the swim bladder collagen extracted from grass carp (Ctenopharyngodon idella). LWT-Food Sci. Technol. 2022, 153, 112518. [Google Scholar] [CrossRef]
- Sukkon, P.; Ali, A.M.M.; Nalinanon, S.; Kishimura, H.; Takeungwongtrakul, S. Characterization of acid soluble collagen from the skin of snakeskin gourami (Trichogaster pectoralis). Carpath. J. Food Sci. Technol. 2020, 12, 75–87. [Google Scholar]
- Indriani, S.; Benjakul, S.; Kishimura, H.; Karnjanapratum, S.; Nalinanon, S. Impact of extraction condition on the yield and molecular characteristics of collagen from Asian bullfrog (Rana tigerina) skin. LWT-Food Sci. Technol. 2022, 162, 113439. [Google Scholar] [CrossRef]
- Xu, X.; Wang, D.; Li, J.; Zeng, X.; Zhang, Z.; Zhu, J.; Liu, G.; Zhang, J.; Liang, L.; Liu, X.; et al. Collagen hydrolysates from deer tendon: Preparation assisted with different ultrasound pretreatment times and promotion in MC3T3-E1 cell proliferation and antioxidant activities. Process Biochem. 2023, 133, 228–240. [Google Scholar] [CrossRef]
- Lai, C.S.; Tu, C.W.; Kuo, H.C.; Sun, P.P.; Tsai, M.L. Type II collagen from cartilage of Acipenser baerii promotes wound healing in human dermal fibroblasts and in mouse skin. Mar. Drugs 2020, 18, 511. [Google Scholar] [CrossRef]
- Saallah, S.; Roslan, J.; Julius, F.S.; Saallah, S.; Mohamad Razali, U.H.; Pindi, W.; Sulaiman, M.R.; Pa’ee, K.F.; Mustapa Kamal, S.M. Comparative study of the yield and physicochemical properties of collagen from sea cucumber (Holothuria scabra), obtained through dialysis and the ultrafiltration membrane. Molecules 2021, 26, 2564. [Google Scholar] [CrossRef]
- Kleinnijenhuis, A.J.; Van Holthoon, F.L.; Herregods, G. Validation and theoretical justification of an LC-MS method for the animal species specific detection of gelatin. Food Chem. 2018, 243, 461–467. [Google Scholar] [CrossRef]
- Dille, M.J.; Haug, I.J.; Draget, K.I. Gelatin and collagen. In Handbook of Hydrocolloids; Woodhead Publishing: Cambridge, UK, 2021; pp. 1073–1097. [Google Scholar]
- Badii, F.; Howell, N.K. Fish gelatin: Structure, gelling properties and interaction with egg albumen proteins. Food Hydrocoll. 2006, 20, 630–640. [Google Scholar] [CrossRef]
- Cao, S.; Wang, Y.; Xing, L.; Zhang, W.; Zhou, G. Structure and physical properties of gelatin from bovine bone collagen influenced by acid pretreatment and pepsin. Food Bioprod. Process. 2020, 121, 213–223. [Google Scholar] [CrossRef]
- Kuan, Y.H.; Nafchi, A.M.; Huda, N.; Ariffin, F.; Karim, A.A. Comparison of physicochemical and functional properties of duck feet and bovine gelatins. J. Sci. Food Agric. 2017, 97, 1663–1671. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, Z.; Yan, Q.; Cao, D.; Yuan, R.; Wang, J.; Lu, S. Effect of low-frequency ultrasound-assisted acid extraction on gel properties and structural characterization of sheep’s hoof gelatin. Int. J. Biol. Macromol. 2024, 271, 132701. [Google Scholar] [CrossRef] [PubMed]
- Sha, X.M.; Zhang, L.J.; Chen, W.M.; Wang, G.Y.; Li, J.L.; Hu, Z.Z.; Tu, Z.C. Characteristic tryptic peptides and gelling properties of porcine skin gelatin affected by thermal action. Int. J. Food Sci. Technol. 2022, 57, 1573–1586. [Google Scholar] [CrossRef]
- Tümerkan, E.T.A.; Cansu, Ü.; Boran, G.; Mac Regenstein, J.; Özoğul, F. Physiochemical and functional properties of gelatin obtained from tuna, frog and chicken skins. Food Chem. 2019, 287, 273–279. [Google Scholar] [CrossRef]
- Fawale, S.O.; Abuibaid, A.; Hamed, F.; Kittiphattanabawon, P.; Maqsood, S. Molecular, structural, and rheological characterization of camel skin gelatin extracted using different pretreatment conditions. Foods 2021, 10, 1563. [Google Scholar] [CrossRef]
- Ee, S.C.; Bakar, J.; Saari, N.; Abas, F.; Ismail, A. Rheological and molecular properties of chicken head gelatin as affected by combined temperature and time using warm water rendering. Int. J. Food Prop. 2021, 24, 1495–1509. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, J.; Zhu, M.; Yang, H.; Liu, J.; Liu, Y. Study of physicochemical and gelation properties of fish gelatin from different sources. Appl. Sci. 2023, 13, 5337. [Google Scholar] [CrossRef]
- Shyni, K.; Hema, G.S.; Ninan, G.; Mathew, S.; Joshy, C.G.; Lakshmanan, P.T. Isolation and characterization of gelatin from the skins of skipjack tuna (Katsuwonus pelamis), dog shark (Scoliodon sorrakowah), and rohu (Labeo rohita). Food Hydrocoll. 2014, 39, 68–76. [Google Scholar] [CrossRef]
- Binsi, P.K.; Shamasundar, B.A.; Dileep, A.O.; Badii, F.; Howell, N.K. Rheological and functional properties of gelatin from the skin of Bigeye snapper (Priacanthus hamrur) fish: Influence of gelatin on the gel-forming ability of fish mince. Food Hydrocoll. 2009, 23, 132–145. [Google Scholar] [CrossRef]
- Giannetti, G.; Matsumura, F.; Caporaletti, F.; Micha, D.; Koenderink, G.H.; Ilie, I.M.; Bonn, M.; Woutersen, S.; Giubertoni, G. Water and Collagen: A Mystery Yet to Unfold. Biomacromolecules 2025, 26, 2784–2799. [Google Scholar] [CrossRef]
- Pawelec, K.M.; Best, S.M.; Cameron, R. Collagen: A network for regenerative medicine. J. Mater. Chem. B 2016, 4, 6484–6496. [Google Scholar] [CrossRef] [PubMed]
- Drzewiecki, K.E.; Parmar, A.S.; Gaudet, I.D.; Branch, J.R.; Pike, D.H.; Nanda, V.; Shreiber, D.I. Methacrylation induces rapid, temperature-dependent, reversible self-assembly of type-I collagen. Langmuir 2014, 30, 11204–11211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shen, L.; Cheng, Y.; Li, G. Stable and biocompatible fibrillar hydrogels based on the self-crosslinking between collagen and oxidized chondroitin sulfate. Polym. Degrad. Stab. 2021, 193, 109742. [Google Scholar] [CrossRef]
- Kilmer, C.E.; Walimbe, T.; Panitch, A.; Liu, J.C. Incorporation of a collagen-binding chondroitin sulfate molecule to a collagen type I and II blend hydrogel for cartilage tissue engineering. ACS Biomater. Sci. Eng. 2022, 8, 1247–1257. [Google Scholar] [CrossRef]
- Menezes, M.D.L.L.R.; Ribeiro, H.L.; Flávia de Oliveira, M.; de Andrade Feitosa, J.P. Optimization of the collagen extraction from Nile tilapia skin (Oreochromis niloticus) and its hydrogel with hyaluronic acid. Colloids Surf. B Biointerfaces 2020, 189, 110852. [Google Scholar] [CrossRef]
- Yan, M.; An, X.; Duan, S.; Jiang, Z.; Liu, X.; Zhao, X.; Li, Y. A comparative study on crosslinking of fibrillar gel prepared by tilapia collagen and hyaluronic acid with EDC/NHS and genipin. Int. J. Biol. Macromol. 2022, 213, 639–650. [Google Scholar] [CrossRef]
- Kong, W.; Gao, Y.; Liu, Q.; Dong, L.; Guo, L.; Fan, H.; Fan, Y.; Zhang, X. The effects of chemical crosslinking manners on the physical properties and biocompatibility of collagen type I/hyaluronic acid composite hydrogels. Int. J. Biol. Macromol. 2020, 160, 1201–1211. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Q.; Kong, W.; Wang, J.; He, L.; Guo, L.; Lin, H.; Fan, H.; Fan, Y.; Zhang, X. Activated hyaluronic acid/collagen composite hydrogel with tunable physical properties and improved biological properties. Int. J. Biol. Macromol. 2020, 164, 2186–2196. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhai, Y.N.; Xu, J.P.; Zhu, X.Y.; Yang, H.R.; Che, H.J.; Liu, C.K.; Qu, J.B. An injectable collagen peptide-based hydrogel with desirable antibacterial, self-healing and wound-healing properties based on multiple-dynamic crosslinking. Int. J. Biol. Macromol. 2024, 259, 129006. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Cid, P.; Alonso-González, M.; Jiménez-Rosado, M.; Benhnia, M.R.E.I.; Ruiz-Mateos, E.; Ostos, F.J.; Romero, A.; Perez-Puyana, V.M. Effect of different crosslinking agents on hybrid chitosan/collagen hydrogels for potential tissue engineering applications. Int. J. Biol. Macromol. 2024, 263, 129858. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Portalatin, N.; Alfonso-García, A.; Liu, J.C.; Marcu, L.; Panitch, A. Physical, biomechanical, and optical characterization of collagen and elastin blend hydrogels. Ann. Biomed. Eng. 2020, 48, 2924–2935. [Google Scholar] [CrossRef]
- Amaya-Chantaca, N.J.; Caldera-Villalobos, M.; Claudio-Rizo, J.A.; Flores-Guía, T.E.; Becerra-Rodríguez, J.J.; Soriano-Corral, F.; Herrera-Guerrero, A. Semi-IPN hydrogels of collagen and gum arabic with antibacterial capacity and controlled release of drugs for potential application in wound healing. Prog. Biomater. 2023, 12, 25–40. [Google Scholar] [CrossRef]
- Chen, K.; Sivaraj, D.; Davitt, M.F.; Leeolou, M.C.; Henn, D.; Steele, S.R.; Huskins, S.L.; Trotsyuk, A.A.; Kussie, H.C.; Greco, A.H.; et al. Pullulan-Collagen hydrogel wound dressing promotes dermal remodelling and wound healing compared to commercially available collagen dressings. Wound Repair Regen. 2022, 30, 397–408. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Li, J.; Zhang, N.; Xu, B.; Li, Y.; Ding, N.; Ge, B. Self-assembly and crosslinking preparation of tilapia-skin-derived collagen/alginate hydrogels for efficient wound repairing. Polym. Eng. Sci. 2024, 64, 2146–2156. [Google Scholar] [CrossRef]
- Zhang, L.; Li, K.; Xiao, W.; Zheng, L.; Xiao, Y.; Fan, H.; Zhang, X. Preparation of collagen–chondroitin sulfate–hyaluronic acid hybrid hydrogel scaffolds and cell compatibility in vitro. Carbohydr. Polym. 2011, 84, 118–125. [Google Scholar] [CrossRef]
- Goodarzi, H.; Jadidi, K.; Pourmotabed, S.; Sharifi, E.; Aghamollaei, H. Preparation and in vitro characterization of crosslinked collagen–gelatin hydrogel using EDC/NHS for corneal tissue engineering applications. Int. J. Biol. Macromol. 2019, 126, 620–632. [Google Scholar] [CrossRef]
- Zou, J.; Wang, L.; Sun, G. Sustainable and reusable gelatin-based hydrogel “jelly ice cubes” as food coolant. II: Ideal freeze–thaw conditions. ACS Sustain. Chem. Eng. 2021, 9, 15365–15374. [Google Scholar] [CrossRef]
- Munawaroh, H.S.H.; Pratiwi, R.N.; Gumilar, G.G.; Aisyah, S.; Rohilah, S.; Nurjanah, A.; Ningrum, A.; Susanto, E.; Pratiwi, A.; Arindita, N.P.Y.; et al. Synthesis, modification and application of fish skin gelatin-based hydrogel as sustainable and versatile bioresource of antidiabetic peptide. Int. J. Biol. Macromol. 2023, 231, 123248. [Google Scholar] [CrossRef]
- Ponsubha, S.; Jaiswal, A.K. Effect of interpolymer complex formation between chondroitin sulfate and chitosan-gelatin hydrogel on physico-chemical and rheological properties. Carbohydr. Polym. 2020, 238, 116179. [Google Scholar]
- Moshayedi, S.; Sarpoolaky, H.; Khavandi, A. Fabrication, swelling behavior, and water absorption kinetics of genipin-crosslinked gelatin–chitosan hydrogels. Polym. Eng. Sci. 2021, 61, 3094–3103. [Google Scholar] [CrossRef]
- Peng, Z.; Peng, Z.; Shen, Y. Fabrication and properties of gelatin/chitosan composite hydrogel. Polym.-Plast. Technol. Eng. 2011, 50, 1160–1164. [Google Scholar] [CrossRef]
- Yang, C.; Xu, L.; Zhou, Y.; Zhang, X.; Huang, X.; Wang, M.; Han, Y.; Zhai, M.; Wei, S.; Li, J. A green fabrication approach of gelatin/CM-chitosan hybrid hydrogel for wound healing. Carbohydr. Polym. 2010, 82, 1297–1305. [Google Scholar] [CrossRef]
- Da Silva, M.A.; Bode, F.; Drake, A.F.; Goldoni, S.; Stevens, M.M.; Dreiss, C.A. Enzymatically cross-linked gelatin/chitosan hydrogels: Tuning gel properties and cellular response. Macromol. Biosci. 2014, 14, 817–830. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, R.; Wang, D.; Sun, Z.; Liu, F.; Zhang, D.; Wang, D. Development of a food packaging antibacterial hydrogel based on gelatin, chitosan, and 3-phenyllactic acid for the shelf-life extension of chilled chicken. Food Hydrocoll. 2022, 127, 107546. [Google Scholar] [CrossRef]
- Li, C.; Yang, Y.; Zhang, R.; Wang, J.; Zhong, S.; Cui, X. Chitosan-gelatin composite hydrogel antibacterial film for food packaging. Int. J. Biol. Macromol. 2025, 285, 138330. [Google Scholar] [CrossRef]
- Du, M.; Zhao, Y.; Zhang, Y.; Sun, S.; Fang, Y. Fabrication of agarose/fish gelatin double-network hydrogels with high strength and toughness for the development of artificial beef tendons. Food Funct. 2022, 13, 6975–6986. [Google Scholar] [CrossRef]
- Bostancı, N.S.; Büyüksungur, S.; Hasirci, N.; Tezcaner, A. pH responsive release of curcumin from photocrosslinked pectin/gelatin hydrogel wound dressings. Biomater Adv. 2022, 134, 112717. [Google Scholar] [CrossRef] [PubMed]
- Makarova, A.O.; Derkach, S.R.; Kadyirov, A.I.; Ziganshina, S.A.; Kazantseva, M.A.; Zueva, O.S.; Gubaidullin, A.T.; Zuev, Y.F. Supramolecular structure and mechanical performance of κ-carrageenan–gelatin gel. Polymers 2022, 14, 4347. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Xu, J.; Zhang, R.; Lin, J.; Zhou, M.; Qin, X.; Wang, K.; Zhou, Y.; Zhu, Q.; Jin, Y.; et al. Development of multi-crosslinking, rapid curing, and easy cleaning, edible hydrogels for meat preservation. Food Hydrocoll. 2024, 155, 110186. [Google Scholar] [CrossRef]
- Serafin, A.; Culebras, M.; Collins, M.N. Synthesis and evaluation of alginate, gelatin, and hyaluronic acid hybrid hydrogels for tissue engineering applications. Int. J. Biol. Macromol. 2023, 233, 123438. [Google Scholar] [CrossRef]
- Kim, Y.M.; Lee, K.; Lee, Y.; Yang, K.; Choe, D.; Roh, Y.H. Thermoresponsive semi-interpenetrating gelatin-alginate networks for encapsulation and controlled release of scent molecules. Int. J. Biol. Macromol. 2022, 208, 1096–1105. [Google Scholar] [CrossRef]
- Van Nieuwenhove, I.; Salamon, A.; Peters, K.; Graulus, G.J.; Martins, J.C.; Frankel, D.; Kersemans, K.; De Vos, F.; Van Vlierberghe, S.; Dubruel, P. Gelatin-and starch-based hydrogels. Part A: Hydrogel development, characterization and coating. Carbohydr. Polym. 2016, 152, 129–139. [Google Scholar] [CrossRef]
- Yan, J.; Li, S.; Chen, G.; Ma, C.; McClements, D.J.; Liu, X.; Liu, F. Formation, physicochemical properties, and comparison of heat- and enzyme-induced whey protein-gelatin composite hydrogels. Food Hydrocoll. 2023, 137, 108384. [Google Scholar] [CrossRef]
- Cui, T.; Wu, Y.; Ni, C.; Sun, Y.; Cheng, J. Rheology and texture analysis of gelatin/dialdehyde starch hydrogel carriers for curcumin controlled release. Carbohydr. Polym. 2022, 283, 119154. [Google Scholar] [CrossRef]
- Nicoleti, J.F.; Telis, V.R.N. Viscoelastic and thermal properties of collagen–xanthan gum and collagen–maltodextrin suspensions during heating and cooling. Food Biophys. 2009, 4, 135–146. [Google Scholar] [CrossRef]
- Duvarci, O.C.; Yazar, G.; Dogan, H.; Kokini, J.L. Linear and non-linear rheological properties of foods. In Handbook of Food Engineering; Heldman, D.R., Lund, D.B., Sabliov, C., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 1–152. [Google Scholar]
- Ding, C.; Zhang, M.; Tian, H.; Li, G. Effect of hydroxypropyl methylcellulose on collagen fibril formation in vitro. Int. J. Biol. Macromol. 2013, 52, 319–326. [Google Scholar] [CrossRef]
- Sánchez-Cid, P.; Jiménez-Rosado, M.; Rubio-Valle, J.F.; Romero, A.; Ostos, F.J.; Rafii-El-Idrissi Benhnia, M.; Perez-Puyana, V. Biocompatible and thermoresistant hydrogels based on collagen and chitosan. Polymers 2022, 14, 272. [Google Scholar] [CrossRef]
- Bao, Z.; Sun, Y.; Rai, K.; Peng, X.; Wang, S.; Nian, R.; Xian, M. The promising indicators of the thermal and mechanical properties of collagen from bass and tilapia: Synergistic effects of hydroxyproline and cysteine. Biomater. Sci. 2018, 6, 3042–3052. [Google Scholar] [CrossRef] [PubMed]
- Oechsle, A.M.; Häupler, M.; Gibis, M.; Kohlus, R.; Weiss, J. Modulation of the rheological properties and microstructure of collagen by addition of co-gelling proteins. Food Hydrocoll. 2015, 49, 118–126. [Google Scholar] [CrossRef]
- Derkach, S.R.; Voron’ko, N.G.; Sokolan, N.I. The rheology of hydrogels based on chitosan–gelatin (bio) polyelectrolyte complexes. J. Dispers. Sci. Technol. 2017, 38, 1427–1434. [Google Scholar] [CrossRef]
- Sow, L.C.; Chong, J.M.N.; Liao, Q.X.; Yang, H. Effects of κ-carrageenan on the structure and rheological properties of fish gelatin. J. Food Eng. 2018, 239, 92–103. [Google Scholar] [CrossRef]
- Warner, E.L.; Norton, I.T.; Mills, T.B. Comparing the viscoelastic properties of gelatin and different concentrations of kappa-carrageenan mixtures for additive manufacturing applications. J. Food Eng. 2019, 246, 58–66. [Google Scholar] [CrossRef]
- de Alcântara, M.G.; de Freitas Ortega, N.; Souza, C.J.F.; Garcia-Rojas, E.E. Electrostatic hydrogels formed by gelatin and carrageenan induced by acidification: Rheological and structural characterization. Food Struct. 2020, 24, 100137. [Google Scholar] [CrossRef]
- Chen, H.; Wu, D.; Ma, W.; Wu, C.; Tian, Y.; Wang, S.; Du, M. Strong fish gelatin hydrogels enhanced by carrageenan and potassium sulfate. Food Hydrocoll. 2021, 119, 106841. [Google Scholar] [CrossRef]
- Tong, L.; Kang, X.; Fang, Q.; Yang, W.; Cen, S.; Lou, Q.; Huang, T. Rheological properties and interactions of fish gelatin–κ-carrageenan polyelectrolyte hydrogels: The effects of salt. J. Texture Stud. 2022, 53, 122–132. [Google Scholar] [CrossRef]
- Derkach, S.R.; Kuchina, Y.A.; Kolotova, D.S.; Voron’ko, N.G. Polyelectrolyte polysaccharide–gelatin complexes: Rheology and structure. Polymers 2020, 12, 266. [Google Scholar] [CrossRef]
- Ata, O.; Yazar, G.; Tavman, S.; Kokini, J.L. Linear and nonlinear rheological properties of gelatin-chitosan hydrogels: Evaluation of crosslinker concentration and temperature effects. Food Hydrocoll. 2025, 163, 111130. [Google Scholar] [CrossRef]
- Ge, S.; Liu, Q.; Li, M.; Liu, J.; Lu, H.; Li, F.; Zhang, S.; Sun, Q.; Xiong, L. Enhanced mechanical properties and gelling ability of gelatin hydrogels reinforced with chitin whiskers. Food Hydrocoll. 2018, 75, 1–12. [Google Scholar] [CrossRef]
- Lara-Espinoza, C.; Carvajal-Millán, E.; Balandrán-Quintana, R.; López-Franco, Y.; Rascón-Chu, A. Pectin and pectin-based composite materials: Beyond food texture. Molecules 2018, 23, 942. [Google Scholar] [CrossRef] [PubMed]
- Hyun, K.; Kim, S.H.; Ahn, K.H.; Lee, S.J. Large amplitude oscillatory shear as a way to classify the complex fluids. J. Non-Newton. Fluid Mech. 2002, 107, 51–65. [Google Scholar] [CrossRef]
- Yang, Y.L.; Zhou, G.H.; Xu, X.L.; Wang, Y. Rheological properties of myosin–gelatin mixtures. J. Food Sci. 2007, 72, C270–C275. [Google Scholar] [CrossRef]
- Pang, Z.; Deeth, H.; Sopade, P.; Sharma, R.; Bansal, N. Rheology, texture and microstructure of gelatin gels with and without milk proteins. Food Hydrocoll. 2014, 35, 484–493. [Google Scholar] [CrossRef]
- He, L.; Li, S.; Xu, C.; Wei, B.; Zhang, J.; Xu, Y.; Zhu, B.; Cao, Y.; Wu, X.; Xiong, Z.; et al. A new method of gelatin modified collagen and viscoelastic study of gelatin-collagen composite hydrogel. Macromol. Res. 2020, 28, 861–868. [Google Scholar] [CrossRef]
- Howe, A.M.; Wilkins, A.G.; Goodwin, J.W. The interactions between gelatin and surfactants—A rheological study. J. Photogr. Sci. 1992, 40, 234–243. [Google Scholar] [CrossRef]
- Żamojć, K.; Wyrzykowski, D.; Chmurzyński, L. On the effect of pH, temperature, and surfactant structure on bovine serum albumin–cationic/anionic/nonionic surfactants interactions in cacodylate buffer–fluorescence quenching studies supported by UV spectrophotometry and CD spectroscopy. Int. J. Mol. Sci. 2021, 23, 41. [Google Scholar] [CrossRef]
- Greener, J.; Contestable, B.A.; Bale, M.D. Interaction of anionic surfactants with gelatin: Viscosity effects. Macromolecules 1987, 20, 2490–2498. [Google Scholar] [CrossRef]
- Gravelle, A.J.; Marangoni, A.G. The influence of network architecture on the large deformation and fracture behavior of emulsion-filled gelatin gels. Food Struct. 2021, 29, 100193. [Google Scholar] [CrossRef]
- Bot, A.; van Amerongen, I.A.; Groot, R.D.; Hoekstra, N.L.; Agterof, W.G.M. Large deformation rheology of gelatin gels. Polym. Gels Networks 1996, 4, 189–227. [Google Scholar] [CrossRef]
- Ju, H.Y.; Liu, X.Y.; Zhang, G.; Liu, D.Z.; Yang, Y.S. Comparison of the structural characteristics of native collagen fibrils derived from bovine tendons using two different methods: Modified acid-solubilized and pepsin-aided extraction. Materials 2020, 13, 358. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, H.; Wang, K.; Qi, L.; Guo, Y.; Zhang, C.; Xu, Y. Impact of ultrasonication on the self-assembly behavior and gel properties of bovine bone collagen I. Molecules 2023, 28, 3096. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, K.; Li, G. Interactions of collagen molecules in the presence of N-hydroxysuccinimide activated adipic acid (NHS-AA) as a crosslinking agent. Int. J. Biol. Macromol. 2011, 49, 847–854. [Google Scholar] [CrossRef]
- Skopinska-Wisniewska, J.; Tuszynska, M.; Olewnik-Kruszkowska, E. Comparative study of gelatin hydrogels modified by various crosslinking agents. Materials 2021, 14, 396. [Google Scholar] [CrossRef]
- Zhang, M.; Li, J.; Ding, C.; Liu, W.; Li, G. The rheological and structural properties of fish collagen crosslinked by N-hydroxysuccinimide activated adipic acid. Food Hydrocoll. 2013, 30, 504–511. [Google Scholar] [CrossRef]
- Ashokan, B.K.; Kokini, J.L. Determination of the WLF constants of cooked soy flour and their dependence on the extent of cooking. Rheol. Acta 2005, 45, 192–201. [Google Scholar] [CrossRef]
- Tang, P.; Zheng, T.; Shen, L.; Li, G. Properties of bovine type I collagen hydrogels crosslinked with laccase-catalyzed gallic acid. Polym. Degrad. Stab. 2021, 189, 109614. [Google Scholar] [CrossRef]
- Eslami, P.; Haritos, V.; Crawford, S.; van‘t Hag, L. Enhancing rheological and textural properties of pea protein-collagen gels via transglutaminase crosslinking. Food Struct. 2025, 43, 100409. [Google Scholar] [CrossRef]
- Schlangen, M.; Ribberink, M.A.; Dinani, S.T.; Sagis, L.M.; van der Goot, A.J. Mechanical and rheological effects of transglutaminase treatment on dense plant protein blends. Food Hydrocoll. 2023, 136, 108261. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Liu, X.; Yang, L.; Liu, H.; He, Y. Characterization of enzymatic crosslinking soy protein isolate xerogels and its shape memory effect induced by ethylcellulose. Food Chem. 2023, 412, 135564. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Cid, P.; Jiménez-Rosado, M.; Perez-Puyana, V.; Guerrero, A.; Romero, A. Rheological and microstructural evaluation of collagen-based scaffolds crosslinked with fructose. Polymers 2021, 13, 632. [Google Scholar] [CrossRef] [PubMed]
- Nashchekina, Y.A.; Trusova, N.A.; Nikonov, P.O.; Nashchekin, A.V.; Mikhailova, N.A. The stability of a collagen gel after UV irradiation. Biophysics 2023, 68, 190–194. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Haraguchi, R.; Aoki, S.; Oishi, Y.; Narita, T. Effect of UV Irradiation of Pre-Gel Solutions on the Formation of Collagen Gel Tubes. Gels 2023, 9, 458. [Google Scholar] [CrossRef]
- Mollenkopf, P.; Kochanowski, J.A.; Ren, Y.; Vining, K.H.; Janmey, P.A.; Purohit, P.K. Poroelasticity and permeability of fibrous polymer networks under compression. Soft Matter 2025, 21, 2400–2412. [Google Scholar] [CrossRef]
- Cacheux, J.; Ordonez-Miranda, J.; Bancaud, A.; Jalabert, L.; Alcaide, D.; Nomura, M.; Matsunaga, Y.T. Asymmetry of tensile versus compressive elasticity and permeability contributes to the regulation of exchanges in collagen gels. Sci. Adv. 2023, 9, eadf9775. [Google Scholar] [CrossRef]
- Devi, A.F.; Buckow, R.; Hemar, Y.; Kasapis, S. Modification of the structural and rheological properties of whey protein/gelatin mixtures through high pressure processing. Food Chem. 2014, 156, 243–249. [Google Scholar] [CrossRef]
- Walkenström, P.; Hermansson, A.M. High-pressure treated mixed gels of gelatin and whey proteins. Food Hydrocoll. 1997, 11, 195–208. [Google Scholar] [CrossRef]
- Kulisiewicz, L.; Delgado, A. Network structure of gelatin gel at high pressure determined by rheological measurements. High Press. Res. 2009, 29, 67–71. [Google Scholar] [CrossRef]
- Huang, T.; Tu, Z.C.; Shangguan, X.; Wang, H.; Sha, X.; Bansal, N. Rheological behavior, emulsifying properties and structural characterization of phosphorylated fish gelatin. Food Chem. 2018, 246, 428–436. [Google Scholar] [CrossRef]
- Lin, Y.; Du, H.; Roos, Y.; Miao, S. Binary complexes of whey protein fibers/isolates and fish gelatins for emulsion stabilization. Food Hydrocoll. 2023, 143, 108880. [Google Scholar] [CrossRef]
- Xu, J.; Yang, L.; Nie, Y.; Yang, M.; Wu, W.; Wang, Z.; Wang, X.; Zhong, J. Effect of transglutaminase crosslinking on the structural, physicochemical, functional, and emulsion stabilization properties of three types of gelatins. LWT–Food Sci. Technol. 2022, 163, 113543. [Google Scholar] [CrossRef]
- Grover, C.N.; Cameron, R.E.; Best, S.M. Investigating the morphological, mechanical and degradation properties of scaffolds comprising collagen, gelatin and elastin for use in soft tissue engineering. J. Mech. Behav. Biomed. Mater. 2012, 10, 62–74. [Google Scholar] [CrossRef]
- Kirchmajer, D.M.; Watson, C.A.; Ranson, M.; Panhuis, M.I.H. Gelapin, a degradable genipin cross-linked gelatin hydrogel. RSC Adv. 2013, 3, 1073–1081. [Google Scholar] [CrossRef]
- Yao, C.H.; Liu, B.S.; Chang, C.J.; Hsu, S.H.; Chen, Y.S. Preparation of networks of gelatin and genipin as degradable biomaterials. Mater. Chem. Phys. 2004, 83, 204–208. [Google Scholar] [CrossRef]
- Boudet, C.; Iliopoulos, I.; Poncelet, O.; Cloitre, M. Control of the chemical cross-linking of gelatin by a thermosensitive polymer: Example of switchable reactivity. Biomacromolecules 2005, 6, 3073–3078. [Google Scholar] [CrossRef] [PubMed]
- Gheysoori, P.; Paydayesh, A.; Jafari, M.; Peidayesh, H. Thermoresponsive nanocomposite hydrogels based on Gelatin/poly (N–isopropylacrylamide)(PNIPAM) for controlled drug delivery. Eur. Polym. J. 2023, 186, 111846. [Google Scholar] [CrossRef]
- Kopač, T. Mathematical model for characterization of temperature-responsive polymers: A study on the rheological behavior of gelatin and poly (N-isopropylacrylamide). Polym. Test. 2024, 133, 108402. [Google Scholar] [CrossRef]
- Samimi Gharaie, S.; Dabiri, S.M.H.; Akbari, M. Smart shear-thinning hydrogels as injectable drug delivery systems. Polymers 2018, 10, 1317. [Google Scholar] [CrossRef]
- Anvari, M.; Joyner, H.S. Effect of fish gelatin and gum arabic interactions on concentrated emulsion large amplitude oscillatory shear behavior and tribological properties. Food Hydrocoll. 2018, 79, 518–525. [Google Scholar] [CrossRef]


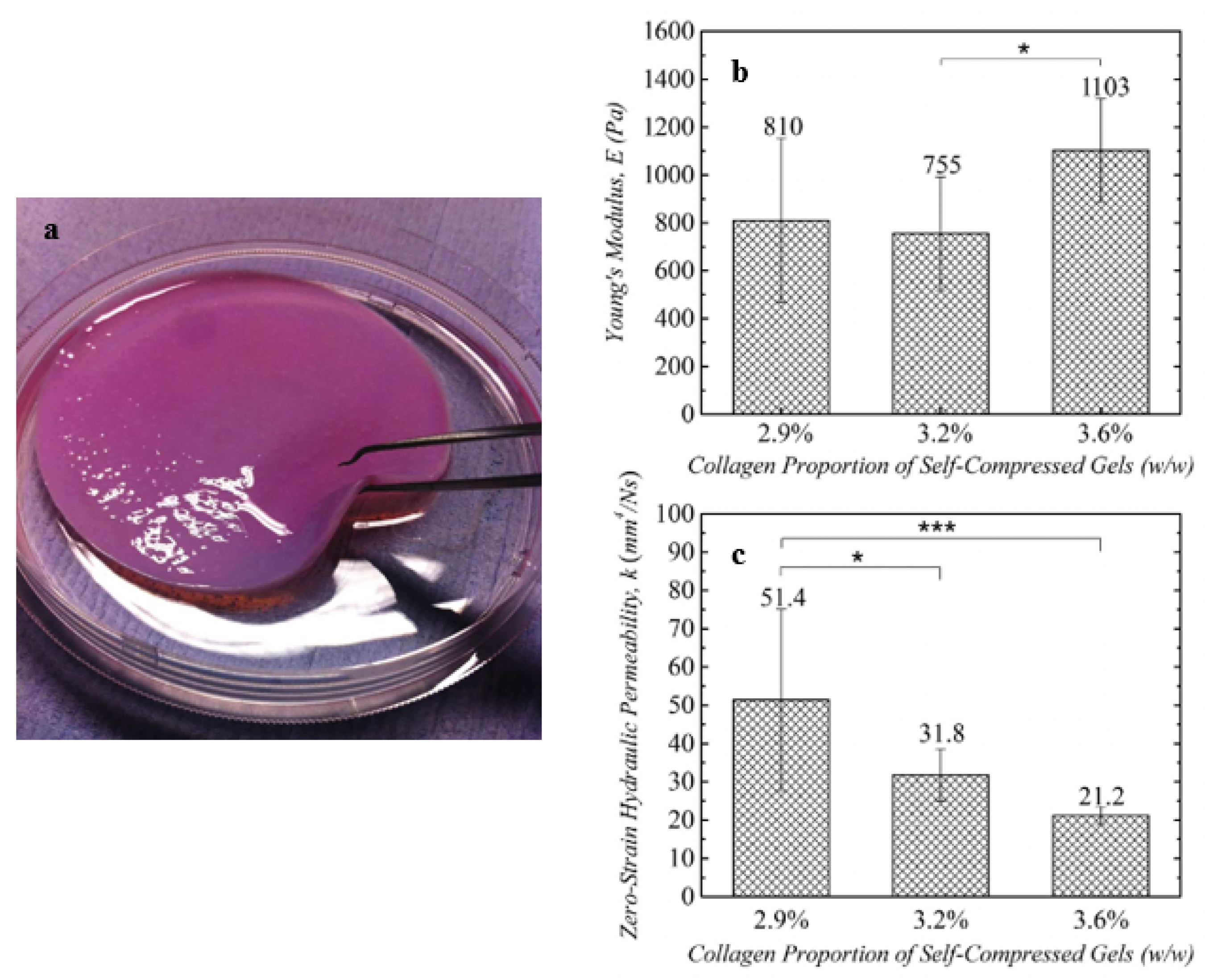
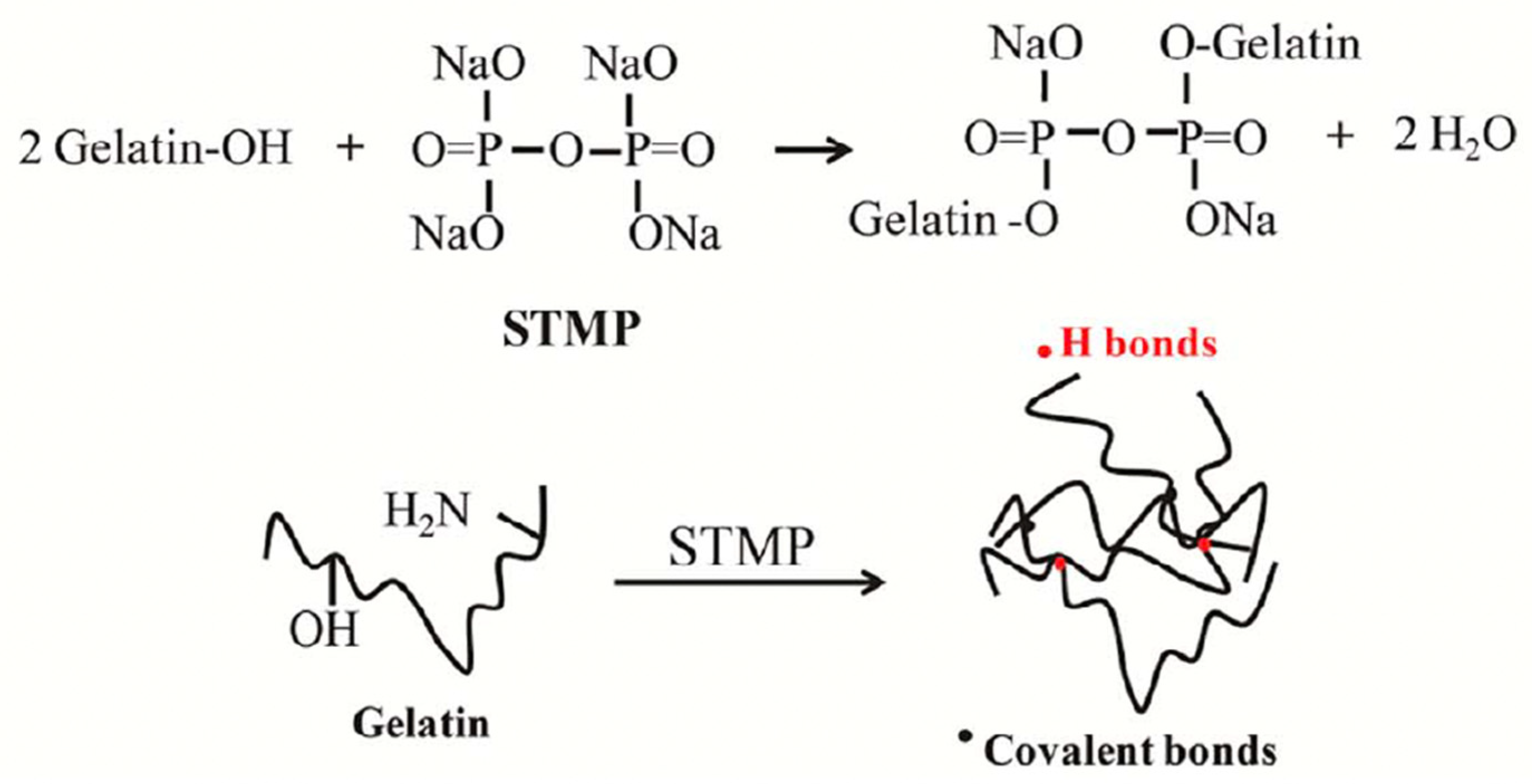
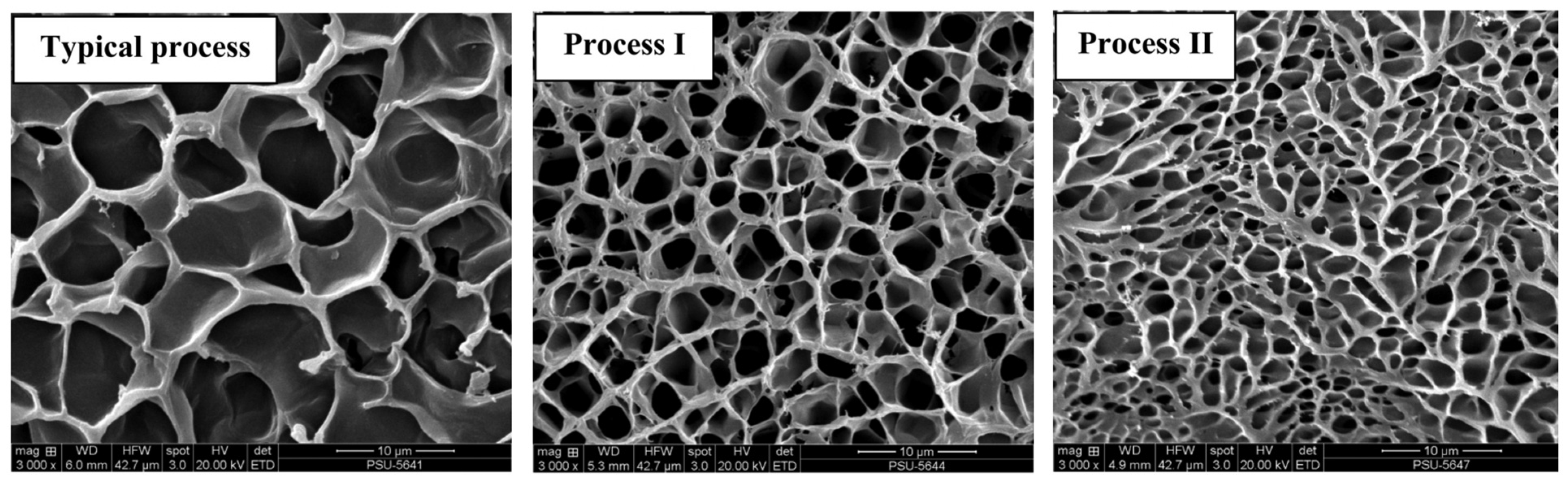


| Source | Collagen Type | Extraction Procedure | Reference |
|---|---|---|---|
| Lamb feet | I | pepsin hydrolysis + ultrasound-assisted extraction pepsin hydrolysis + ultrasound-assisted extraction acetic acid hydrolysis + ultrasound-assisted extraction acetic acid hydrolysis + ultrasound-assisted extraction supercritical fluid extraction subcritical water hydrolysis acetic acid- pepsin hydrolysis acetic acid hydrolysis acetic acid hydrolysis acetic acid-pepsin hydrolysis acetic acid hydrolysis | [52] |
| Broiler chicken trachea | I | [53] | |
| Clown featherback (Chitala ornata) skin | I | [54] | |
| Chicken sternal cartilage | II | [55] | |
| Atlantic cod (Gadus morhua) skin | I | [56] | |
| Mackerel (Scomber japonicus) bone | I | [57] | |
| Blue Shark (Prionace glauca) cartilage | II | [58] | |
| Grass carp (Ctenopharyngodon idella) swim bladder | I | [59] | |
| Snakeskin Bullfrog skin | I I | [60] [61] | |
| Deer tendon | I | [62] | |
| Sturgeon (Acipenser baerii) cartilage | II | HCl-pepsin hydrolysis | [63] |
| Sea Cucumber (Holothuria scabra) | I | ultrafiltration membrane | [64] |
| Collagen/Gelatin | Co-Agents | Application | Reference |
|---|---|---|---|
| collagen type II | chondroitin sulfate | cell delivery | [14] |
| collagen type I | chondroitin sulfate | tissue engineering | [81,82] |
| collagen type I | hyaluronic acid | cell encapsulation biomaterial tissue engineering | [83] [84] [85,86] |
| collagen peptide | dextran | wound healing | [87] |
| collagen type I | chitosan | tissue engineering | [88] |
| collagen type I | elastin | tissue engineering | [89] |
| collagen type I | gum arabic | biomedical field | [90] |
| collagen type I | pullulan | wound dressing | [91] |
| collagen type I | sodium alginate | wound healing | [92] |
| collagen type I | chondroitin sulfate/hyaluronic acid chondroitin sulfate/hyaluronic acid/sodium alginate | tissue engineering biomaterial | [93] [22] |
| collagen-gelatin | - | corneal tissue engineering | [94] |
| gelatin | - | food coolant antidiabetic peptide | [95] [96] |
| gelatin | ethyl cellulose | food packaging | [2] |
| gelatin | chitosan/chondroitin sulfate | biomaterial | [97] |
| gelatin | chitosan | drug delivery skin tissue engineering wound healing tissue repair | [3,98], [99] [100] [101] |
| gelatin | chitosan/3-phenyllactic acid chitosan/lysine | food packaging food packaging | [102] [103] |
| gelatin | agarose | artificial beef tendons | [104] |
| gelatin | pectin | wound dressings | [105] |
| gelatin | κ-carrageenan | biomedicine/biotechnology meat preservation jelly foods | [106] [107] [19] |
| gelatin | alginate | tissue engineering encapsulation and controlled release of scent molecules | [108] [109] |
| gelatin | starch | tissue regeneration | [110] |
| gelatin | whey protein | food industry | [111] |
| gelatin | dialdehyde starch | curcumin controlled release | [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ata, O.; Kokini, J.L.; Tavman, S.; Yazar, G. Advances in Collagen-/Gelatin-Based Hydrogels: Rheological Properties and Applications. Macromol 2025, 5, 55. https://doi.org/10.3390/macromol5040055
Ata O, Kokini JL, Tavman S, Yazar G. Advances in Collagen-/Gelatin-Based Hydrogels: Rheological Properties and Applications. Macromol. 2025; 5(4):55. https://doi.org/10.3390/macromol5040055
Chicago/Turabian StyleAta, Ozge, Jozef L. Kokini, Sebnem Tavman, and Gamze Yazar. 2025. "Advances in Collagen-/Gelatin-Based Hydrogels: Rheological Properties and Applications" Macromol 5, no. 4: 55. https://doi.org/10.3390/macromol5040055
APA StyleAta, O., Kokini, J. L., Tavman, S., & Yazar, G. (2025). Advances in Collagen-/Gelatin-Based Hydrogels: Rheological Properties and Applications. Macromol, 5(4), 55. https://doi.org/10.3390/macromol5040055






