Adenovirus Protease: An Overlooked but Druggable Antiviral Target
Abstract
1. Introduction
2. Methodology
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection Process
2.4. Quality Appraisal
2.5. Data Synthesis
- i.
- Drug repurposing strategies—highlighting efforts to reposition existing or investigating protease inhibitors for SARS-CoV-2 Mpro or PLpro, as candidate modulators of AVP.
- ii.
- Lessons from other viral proteases—distilling mechanistic and translational insights from successful protease-targeted antiviral programs, particularly those for HIV protease, human rhinovirus 3C protease (HRV-3Cpro), hepatitis C (HCV) NS3/4A protease, and SARS-CoV-2 Mpro/PLpro, to inform AVP-directed strategies.
- iii.
- Phytochemicals as novel scaffolds—evaluating natural compounds with reported antiviral activity, screening outcomes, and structural diversity as an untapped resource for AVP inhibition.
2.6. Molecular Docking
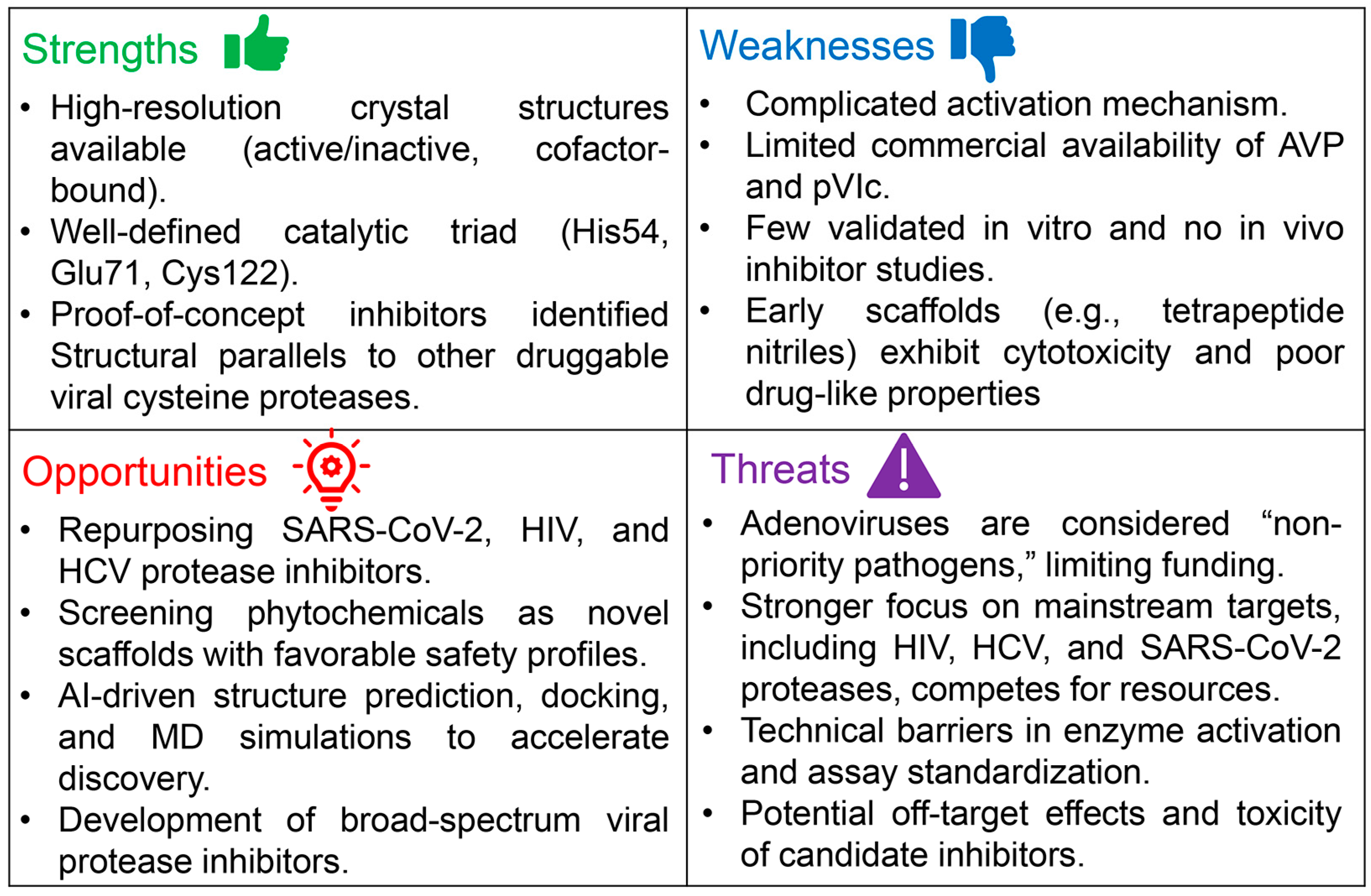
3. Why Research on Adenovirus Protease Lags Behind Other Viral Proteases
4. Adenovirus Protease: A High-Potential Target Deserving Greater Attention
4.1. Where AVP Research Stands—And Where It Needs to Go
4.2. Virtual Screening of Adenovirus Protease: Opportunities and Challenges
4.3. From Virtual Screening to the Bench: Overcoming Barriers in AVP Assay Development
- i.
- The target enzyme in a pure and active form.
- ii.
- A robust enzymatic assay, ideally adaptable to a high-throughput screening (HTS) format.
- iii.
- The test compounds in sufficiently pure form.
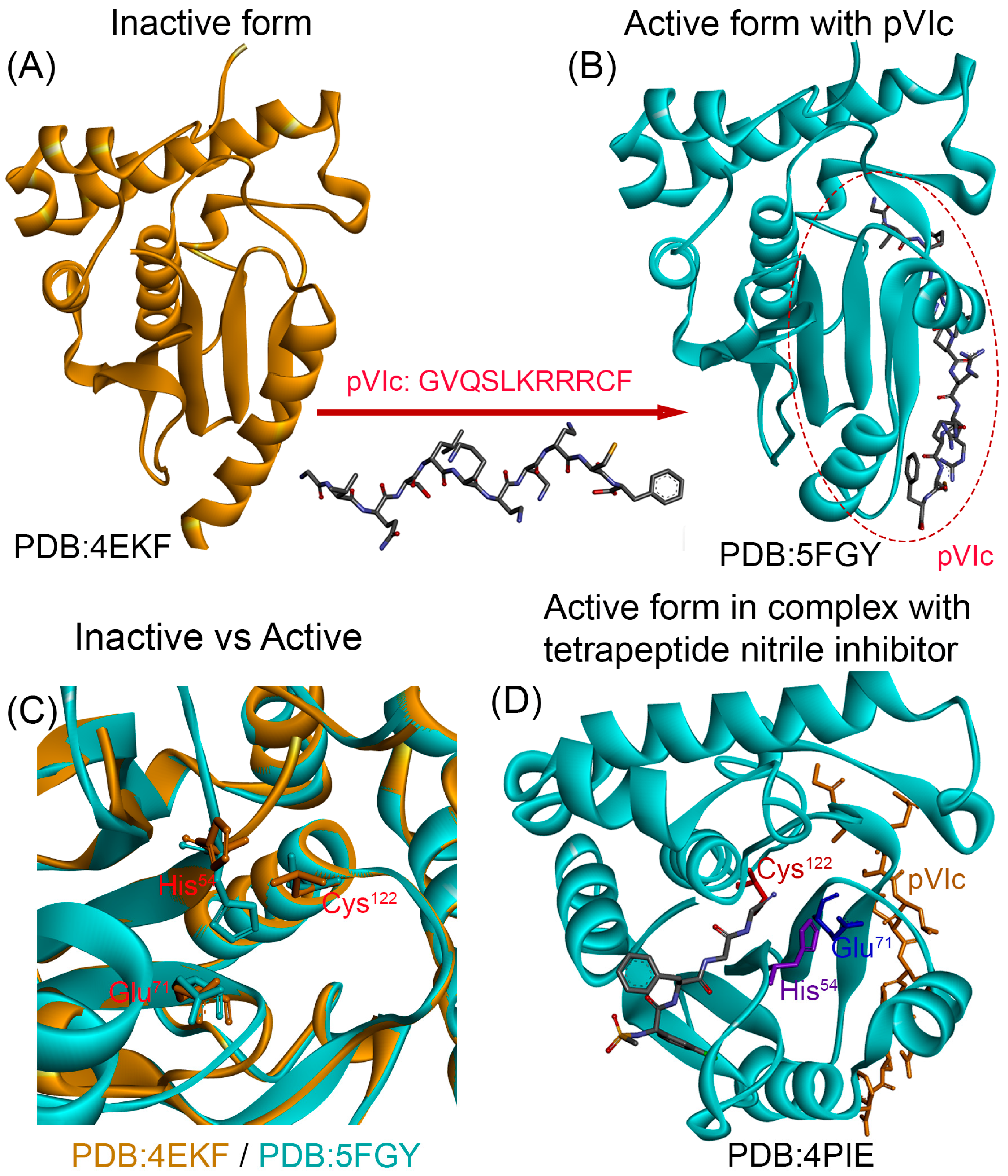
4.3.1. Recombinant AVP and Its Cofactor pVIc: Progress and Limitations
4.3.2. Measuring AVP Activity: Current Assays and Their Limitations
4.3.3. Availability and Purity of Test Compounds
5. Learning from Successful Viral Protease Programs: A Roadmap for AVP Development
- i.
- The HIV protease program is a landmark example of success in antiviral drug development. In the 1980s, scientists identified the viral protease, and by 1995 the first inhibitor—saquinavir—received approval from the FDA, followed by authorization from the European Medicines Agency (EMA) in 1996 under the trade name Invirase (Hoffmann-La Roche) [67]. This achievement was driven by meticulous structure-based drug design, robust assay development, and substantial industry investment. The impact extended far beyond HIV: it not only revolutionized HIV therapy but also established a blueprint for viral protease drug discovery, informing strategies later applied to COVID-19 and even cancer [68].
- ii.
- The case of HCV offers another pivotal lesson in protease-targeted drug design. Its NS3/4A protease is a serine protease that depends on the NS4A cofactor for proper function [69,70]. NS4A acts as a molecular tether, anchoring NS3 to the cellular membrane and stabilizing its active conformation [71]—an arrangement reminiscent of the activation requirements of AVP. Despite this structural complexity, researchers successfully developed potent NS3/4A inhibitors by (i) mapping the protease–cofactor interface to define how NS4A modulates activity, (ii) leveraging structure-based design to create molecules that bind effectively to the active site, and (iii) advancing pan-genotypic inhibitors such as grazoprevir, glecaprevir, and voxilaprevir, which retained efficacy across diverse HCV strains and resistance-associated substitutions [72]. When combined with NS5A and NS5B inhibitors, these agents produced sustained virological response (SVR) rates above 95%, even in patients with cirrhosis or prior treatment failure [73]. The success of HCV protease inhibitors highlights a critical principle: cofactor dependency is not an insurmountable barrier but a design challenge that can be overcome with molecular insight and strategic targeting,
- iii.
- The COVID-19 pandemic demonstrated what happens when decades of protease knowledge are combined with global collaboration. The SARS-CoV-Mpro program showed unprecedented speed: in less than two years, nirmatrelvir (part of Paxlovid) moved from concept to approval [74]. That timeline was not achieved by luck—it was the payoff from years of learning how to target viral proteases efficiently and systematically. Since early 2020, proteases like the Mpro and PLpro have been at the center of SARS-CoV-2 antiviral strategies. High-resolution crystal structures of both enzymes were rapidly published [75,76], and their roles in viral polyprotein processing made them ideal therapeutic targets [77]. Among these, Mpro has emerged as especially druggable, owing to its conserved active site and unique substrate specificity—favoring glutamine at the P1 position—a feature not shared with human proteases [78]. This has enabled the design of highly selective inhibitors with minimal off-target effects.
6. Drug Repurposing: A Strategic Path Toward AVP Inhibition
7. Phytochemicals: An Unexplored Source of Potential AVP Inhibitors
8. Perspectives and Recommendations
- i.
- Establish standardized protocols for recombinant AVP soluble expression. This step is among the most straightforward in the drug discovery pipeline, as cloning and expression technologies are now routine. Several companies, including GenScript, offer artificial gene synthesis directly into expression vectors, with options for codon optimization to enhance expression in both prokaryotic and eukaryotic systems. For AVP, we strongly recommend the use of pGEX vectors, which enable expression of the protein as a GST-tagged fusion. Importantly, the pGEX-6P series encodes a cleavage site for the HRV 3C pro, and the corresponding GST-HRV-3Cpro (commercially available as PreScission Protease) facilitates on-column tag removal and simultaneous purification of the target protein. This strategy has been successfully employed for the production of numerous proteins, including TNF [131], and RANKL [132]. As discussed earlier, soluble expression conditions can be further optimized using DoE approaches, which allow rapid small-scale optimization of parameters. In our experience, DoE enables identification of conditions that maximize soluble enzyme yield within just two days of experimentation [130,133]. Regarding the second essential component for AVP activation, the pVIc peptide, although it is not broadly available as a catalog item, several studies have obtained it through custom peptide synthesis from specialized vendors. Protocols describing AVP activation with pVIc are available in the literature; however, these methods can be further refined and optimized using DoE to maximize reproducibility and efficiency.
- ii.
- Establish robust HTS assays to monitor AVP activity. This area requires particular attention, as currently only fluorogenic substrates have been reported for monitoring AVP activity. Fluorescence-based assays can be affected by background signals and by the intrinsic fluorescence or quenching properties of candidate inhibitors. To overcome these limitations, we strongly recommend the development of non-fluorescent substrates, such as those labeled with p-nitroaniline (pNA). Adenovirus protease recognizes consensus sequences (M/I/L)XGX-G and (M/I/L)XGG-X [134]. Accordingly, synthetic substrates such as Leu-Arg-Gly-Gly-pNA can be designed, in which pNA is conjugated to the C-terminus of the peptide and released upon cleavage. The liberated pNA produces a yellow color that can be quantitatively measured at 405 nm using a spectrophotometer or plate reader. Such pNA-based substrates have been successfully used to establish HTS-compatible assays, including in our group’s work on HRV-3Cpro, where the assay was validated and proven effective in distinguishing true inhibitors from false positives [124,128]. In addition to optimizing enzymatic conditions, it is essential to implement rigorous controls. Negative controls without enzyme are required to account for spontaneous substrate hydrolysis, while positive controls with active AVP ensure the assay is functioning correctly. Including unrelated proteases as additional controls can further validate substrate specificity and exclude non-specific cleavage.
- iii.
- Leverage drug repurposing strategies. The in silico screening results presented in this work, together with our previous studies, highlight the potential of repurposing established viral protease inhibitors as modulators of AVP. In particular, the extensive compound libraries developed over the past five years for the SARS-CoV-2 Mpro and PLpro represent a valuable resource that can be systematically screened for AVP activity. Similarly, inhibitors originally designed for other well-characterized viral proteases, including HIV and HCV proteases, should be evaluated for cross-reactivity with AVP. This strategy offers clear advantages over de novo drug discovery, as it can significantly reduce both the time and cost associated with developing new antivirals. It should be noted that while molecular docking provides a rapid and cost-effective approach to screen large compound libraries and generate testable hypotheses, it also has inherent limitations. Docking can identify plausible binding modes and prioritize candidates, which is particularly valuable when experimental data are limited, as in the case of AVP. However, docking relies on simplified scoring functions that may not fully account for solvation, entropic effects, or protein flexibility, and predictions can vary depending on the structural model used. Therefore, docking results should be viewed as a starting point for hypothesis generation rather than definitive evidence of binding. Integrating docking with biochemical assays, structural biology, and medicinal chemistry will be essential to validate and optimize repurposed inhibitors against AVP.
- iv.
- Encourage multi-viral protease inhibitor development. Building on the experience gained with protease inhibitors for SARS-CoV-2, HIV, and HCV, as well as findings from our earlier work [35], there is clear potential to develop broad-spectrum protease inhibitors that target conserved catalytic mechanisms across different viruses. Such agents could be particularly valuable in the context of co-infections, for example, AVP and HIV, which are associated with especially severe and life-threatening outcomes. Expanding efforts toward multi-viral inhibitor development would not only enhance the therapeutic relevance of AVP research but also contribute to more versatile antiviral strategies.
- v.
- Explore phytochemicals as structurally diverse scaffolds. As discussed above, phytochemicals represent an excellent resource for the discovery of inhibitors targeting AVP and other viral proteases. In this work, we provide supporting evidence that compounds such as apigenin, camptothecin, and piperine can bind to the AVP active site while also exhibiting favorable in silico ADME/Tox profiles. However, to fully exploit this potential, larger-scale screening efforts are required. Expanding beyond the limited panel of 50 phytochemicals examined here, systematic exploration of broader phytochemical libraries—supported by virtual screening, ADME/Tox profiling, and subsequent biochemical validation—will be essential to enlarge the chemical space of candidate inhibitors and identify promising leads.
- vi.
- Integrate AI and molecular modeling approaches. In addition to drug repurposing and phytochemical screening, AI and machine learning (ML) approaches represent powerful tools to accelerate AVP drug discovery. These computational strategies can be applied to identify and prioritize novel scaffolds by mining large chemical libraries, predicting ligand–protein interactions, and optimizing lead compounds with improved pharmacological properties. Moreover, molecular docking combined with MD simulations can provide mechanistic insights into inhibitor binding, reveal conformational flexibility of the AVP active site, and guide the rational design of next-generation inhibitors. Recent advances in generative AI and deep learning frameworks further enable de novo design of small molecules tailored to the structural features of AVP. Integrating these approaches into AVP research pipelines could significantly shorten discovery timelines, reduce costs, and improve the likelihood of identifying inhibitors with both potency and drug-like properties.
- vii.
- Target the AVP–pVIc -DNA interaction. Because AVP activity strictly depends on the presence of both the pVIc peptide and viral DNA, disrupting the AVP–pVIc-DNA complex represents a promising alternative strategy for inhibitor development. Identifying compounds that interfere with pVIc interaction could provide novel avenues for modulating AVP activity beyond active-site inhibition
- viii.
- Increase prioritization and funding for AVP research: Greater investment is needed to overcome the perception of adenoviruses as “low-priority pathogens.” Enhanced prioritization and targeted funding would stimulate translational studies, support the development of standardized research tools, and accelerate progress toward clinically relevant AVP inhibitors.
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lynch, J.P., 3rd; Kajon, A.E. Adenovirus: Epidemiology, global spread of novel serotypes, and advances in treatment and prevention. Semin. Respir. Crit. Care Med. 2016, 37, 586–602. [Google Scholar] [CrossRef]
- Crenshaw, B.J.; Jones, L.B.; Bell, C.R.; Kumar, S.; Matthews, Q.L. Perspective on adenoviruses: Epidemiology, pathogenicity, and gene therapy. Biomedicines 2019, 7, 61. [Google Scholar] [CrossRef]
- Lion, T. Adenovirus infections in immunocompetent and immunocompromised patients. Clin. Microbiol. Rev. 2014, 27, 441–462. [Google Scholar] [CrossRef]
- Kadhim Jwaziri, A.; Karbalaie Niya, M.H.; Khales, P.; Kachooei, A.; Sabaei, M.; Rahmani Fard, S.; Tavakoli, A. Molecular prevalence and genotype distribution of human adenovirus in Iranian children with gastroenteritis. Fetal Pediatr. Pathol. 2023, 42, 901–913. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, S.; Jiang, H.; Lyu, X.; Wang, Y.; Dong, T.; Li, Y. Rapid genotype recognition of human adenovirus based on surface-enhanced Raman scattering combined with machine learning. Sens. Actuators B Chem. 2024, 400, 134873. [Google Scholar] [CrossRef]
- Kulanayake, S.; Tikoo, S.K. Adenovirus core proteins: Structure and function. Viruses 2021, 13, 388. [Google Scholar] [CrossRef]
- Lindemans, C.A.; Leen, A.M.; Boelens, J.J. How I treat adenovirus in hematopoietic stem cell transplant recipients. Blood 2010, 116, 5476–5485. [Google Scholar] [CrossRef] [PubMed]
- Matthes-Martin, S.; Boztug, H.; Lion, T. Diagnosis and treatment of adenovirus infection in immunocompromised patients. Expert Rev. Anti-Infect. Ther. 2013, 11, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Matthes-Martin, S.; Feuchtinger, T.; Shaw, P.J.; Engelhard, D.; Hirsch, H.H.; Cordonnier, C.; Ljungman, P. European guidelines for diagnosis and treatment of adenovirus infection in leukemia and stem cell transplantation: Summary of ECIL-4 (2011). Transpl. Infect Dis. 2012, 14, 555–563. [Google Scholar] [CrossRef]
- Zhu, G.; Xu, D.; Zhang, Y.; Wang, T.; Zhang, L.; Gu, W.; Shen, M. Epidemiological characteristics of four common respiratory viral infections in children. Virol. J. 2021, 18, 10. [Google Scholar] [CrossRef]
- Ison, M.G. Adenovirus infections in transplant recipients. Clin. Infect. Dis. 2006, 43, 331–339. [Google Scholar] [CrossRef]
- D’Angelo, L.J.; Hierholzer, J.C.; Keenlyside, R.A.; Anderson, L.J.; Martone, W.J. Pharyngoconjunctival fever caused by adenovirus type 4: Report of a swimming pool-related outbreak with recovery of virus from pool water. J. Infect. Dis. 1979, 140, 42–47. [Google Scholar] [CrossRef]
- Osborne, C.M.; Montano, A.C.; Robinson, C.C.; Schultz-Cherry, S.; Dominguez, S.R. Viral gastroenteritis in children in Colorado 2006–2009. J. Med. Virol. 2015, 87, 931–939. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, J.; Lan, W.; Quan, L.; Ou, J.; Zhao, W.; Wu, J.; Woo, P.C.Y.; Seto, D.; Zhang, Q. Molecular typing and rapid identification of human adenoviruses associated with respiratory diseases using universal PCR and sequencing primers for the three major capsid genes: Penton base, hexon, and fiber. Front. Microbiol. 2022, 13, 911694. [Google Scholar] [CrossRef]
- Khanal, S.; Ghimire, P.; Dhamoon, A.S. The repertoire of adenovirus in human disease: The innocuous to the deadly. Biomedicines 2018, 6, 30. [Google Scholar] [CrossRef]
- Narsana, N.; Ha, D.; Ho, D.Y. Treating adenovirus infection in transplant populations: Therapeutic options beyond Cidofovir? Viruses 2025, 17, 599. [Google Scholar] [CrossRef]
- Riggsbee, D.L.; Alali, M.; Kussin, M.L. Cidofovir for viral infections in immunocompromised children: Guidance on dosing, safety, efficacy, and a review of the literature. Ann. Pharmacother. 2024, 58, 286–304. [Google Scholar] [CrossRef] [PubMed]
- Grimley, M.; Maron, G.; Gomez, C.A.; Prasad, V.K.; Dara, J.; Papanicolaou, G.A.; Fukushima, K.; Wynn, R.F.; Boeckh, M. Preliminary results of a phase 2a clinical trial to evaluate safety, tolerability and antiviral activity of intravenous Brincidofovir (BCV IV) in immunocompromised patients with adenovirus infection. Blood 2023, 142, 112. [Google Scholar] [CrossRef]
- Zang, J.; Xing, Y.; Zhang, H.; Wu, Y.; Ya, X.; Shen, Q.; Dong, Z. Molecular characteristics of human adenovirus isolated from the 2024 influenza-like illness outbreaks in Suzhou City, China. Virol. J. 2025, 22, 243. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; McGrath, W.J.; Sweet, R.M.; Mangel, W.F. Crystal structure of the human adenovirus proteinase with its 11 amino acid cofactor. EMBO J. 1996, 15, 1778–1783. [Google Scholar] [CrossRef]
- Mangel, W.F.; Toledo, D.L.; Brown, M.T.; Martin, J.H.; McGrath, W.J. Characterization of three components of human adenovirus proteinase activity in vitro. J. Biol. Chem. 1996, 271, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.T.; McGrath, W.J.; Toledo, D.L.; Mangel, W.F. Different modes of inhibition of human adenovirus proteinase, probably a cysteine proteinase, by bovine pancreatic trypsin inhibitor. FEBS Lett. 1996, 388, 233–237. [Google Scholar] [CrossRef]
- Benevento, M.; Di Palma, S.; Snijder, J.; Moyer, C.L.; Reddy, V.S.; Nemerow, G.R.; Heck, A.J.R. Adenovirus composition, proteolysis, and disassembly studied by in-depth qualitative and quantitative proteomics. J. Biol. Chem. 2014, 289, 11421–11430. [Google Scholar] [CrossRef] [PubMed]
- Greber, U.F.; Webster, P.; Weber, J.; Helenius, A. The role of the adenovirus protease on virus entry into cells. EMBO J. 1996, 15, 1766–1777. [Google Scholar] [CrossRef]
- McGrath, W.J.; Baniecki, M.L.; Li, C.; McWhirter, S.M.; Brown, M.T.; Toledo, D.L.; Mangel, W.F. Human adenovirus proteinase: DNA binding and stimulation of proteinase activity by DNA. Biochemistry 2001, 40, 13237–13245. [Google Scholar] [CrossRef]
- Webster, A.; Hay, R.T.; Kemp, G. The adenovirus protease is activated by a virus-coded disulphide-linked peptide. Cell 1993, 72, 97–104. [Google Scholar] [CrossRef]
- Mangel, W.F.; McGrath, W.J.; Toledo, D.L.; Anderson, C.W. Viral DNA and a viral peptide can act as cofactors of adenovirus virion proteinase activity. Nature 1993, 361, 274–275. [Google Scholar] [CrossRef]
- Blainey, P.C.; Graziano, V.; Pérez-Berná, A.J.; McGrath, W.J.; Flint, S.J.; San Martín, C.; Xie, X.S.; Mangel, W.F. Regulation of a viral proteinase by a peptide and DNA in one-dimensional space: IV. Viral proteinase slides along DNA to locate and process its substrates. J. Biol. Chem. 2013, 288, 2092–2102. [Google Scholar] [CrossRef]
- Mangel, W.F.; McGrath, W.J.; Xiong, K.; Graziano, V.; Blainey, P.C. Molecular sled is an eleven-amino acid vehicle facilitating biochemical interactions via sliding components along DNA. Nat. Commun. 2016, 7, 10202. [Google Scholar] [CrossRef]
- Mangel, W.F.; San Martín, C. Structure, function and dynamics in adenovirus maturation. Viruses 2014, 6, 4536–4570. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Berná, A.J.; Mangel, W.F.; McGrath, W.J.; Graziano, V.; Flint, J.; San Martín, C. Processing of the l1 52/55k protein by the adenovirus protease: A new substrate and new insights into virion maturation. J. Virol. 2014, 88, 1513–1524. [Google Scholar] [CrossRef] [PubMed]
- Diouri, M.; Keyvani-Amineh, H.; Geoghegan, K.F.; Weber, J.M. Cleavage efficiency by adenovirus protease is site-dependent. J. Biol. Chem. 1996, 271, 32511–32514. [Google Scholar] [CrossRef]
- Webster, A.; Russell, S.; Talbot, P.; Russell, W.C.; Kemp, G.D. Characterization of the adenovirus proteinase: Substrate specificity. J. Gen. Virol. 1989, 70, 3225–3234. [Google Scholar] [CrossRef]
- Brown, M.T.; McBride, K.M.; Baniecki, M.L.; Reich, N.C.; Marriott, G.; Mangel, W.F. Actin can act as a cofactor for a viral proteinase in the cleavage of the cytoskeleton. J. Biol. Chem. 2002, 277, 46298–46303. [Google Scholar] [CrossRef]
- Papaneophytou, C. Breaking the chain: Protease inhibitors as game changers in respiratory viruses management. Int. J. Mol. Sci. 2024, 25, 8105. [Google Scholar] [CrossRef] [PubMed]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A scale for the quality assessment of narrative review articles. Res. Integr. Peer. Rev. 2019, 4, 5. [Google Scholar] [CrossRef]
- Mac Sweeney, A.; Grosche, P.; Ellis, D.; Combrink, K.; Erbel, P.; Hughes, N.; Sirockin, F.; Melkko, S.; Bernardi, A.; Ramage, P.; et al. Discovery and structure-based optimization of adenain inhibitors. ACS Med. Chem. Lett. 2014, 5, 937–941. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera-a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Theerawatanasirikul, S.; Lekcharoensuk, P. Virtual screening of catural compounds targeting proteases of coronaviruses and picornaviruses. In In Silico Modeling of Drugs Against Coronaviruses: Computational Tools and Protocols; Roy, K., Ed.; Springer: New York, NY, USA, 2021; pp. 661–681. [Google Scholar]
- Dallakyan, S.; Olson, A.J. Small-molecule library screening by docking with PyRx. In Chemical Biology: Methods and Protocols; Hempel, J.E., Williams, C.H., Hong, C.C., Eds.; Springer: New York, NY, USA, 2015; pp. 243–250. [Google Scholar]
- Liu, M.; Jiang, L.; Cao, W.; Wu, J.; Chen, X. Identification of inhibitors and drug targets for human adenovirus infections. Viruses 2022, 14, 959. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Shan, R.; Zhao, L.; Bai, Y.; Feng, L. Paxlovid for the treatment of COVID-19: A systematic review and meta-analysis. J. Infect. Dev. Ctries. 2024, 18, 1169–1178. [Google Scholar] [CrossRef]
- Borges, P.H.O.; Ferreira, S.B.; Silva, F.P., Jr. Recent advances on targeting proteases for antiviral development. Viruses 2024, 16, 366. [Google Scholar] [CrossRef]
- Dodge, M.J.; MacNeil, K.M.; Tessier, T.M.; Weinberg, J.B.; Mymryk, J.S. Emerging antiviral therapeutics for human adenovirus infection: Recent developments and novel strategies. Antiviral. Res. 2021, 188, 105034. [Google Scholar] [CrossRef] [PubMed]
- Araújo, N.M.; Rubio, I.G.S.; Toneto, N.P.A.; Morale, M.G.; Tamura, R.E. The use of adenoviral vectors in gene therapy and vaccine approaches. Genet. Mol. Biol. 2022, 45, e20220079. [Google Scholar] [CrossRef] [PubMed]
- Muravyeva, A.; Smirnikhina, S. Adenoviral vectors for gene therapy of hereditary diseases. Biology 2024, 13, 1052. [Google Scholar] [CrossRef]
- McGrath, W.J.; Graziano, V.; Zabrocka, K.; Mangel, W.F. First generation inhibitors of the adenovirus proteinase. FEBS Lett. 2013, 587, 2332–2339. [Google Scholar] [CrossRef]
- Grosche, P.; Sirockin, F.; Mac Sweeney, A.; Ramage, P.; Erbel, P.; Melkko, S.; Bernardi, A.; Hughes, N.; Ellis, D.; Combrink, K.D.; et al. Structure-based design and optimization of potent inhibitors of the adenoviral protease. Bioorg. Med. Chem. Lett. 2015, 25, 438–443. [Google Scholar] [CrossRef]
- Mali, S.N.; Pandey, A. Multiple QSAR and molecular modelling for identification of potent human adenovirus inhibitors. J. Indian Chem. Soc. 2021, 98, 100082. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The protein data bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Tunyasuvunakool, K.; Adler, J.; Wu, Z.; Green, T.; Zielinski, M.; Žídek, A.; Bridgland, A.; Cowie, A.; Meyer, C.; Laydon, A.; et al. Highly accurate protein structure prediction for the human proteome. Nature 2021, 596, 590–596. [Google Scholar] [CrossRef]
- Anderson, C.W. The proteinase polypeptide of adenovirus serotype 2 virions. Virology 1990, 177, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Grierson, A.W.; Nicholson, R.; Talbot, P.; Webster, A.; Kemp, G. The protease of adenovirus serotype 2 requires cysteine residues for both activation and catalysis. J. Gen. Virol. 1994, 75, 2761–2764. [Google Scholar] [CrossRef] [PubMed]
- Graziano, V.; Luo, G.; Blainey, P.C.; Pérez-Berná, A.J.; McGrath, W.J.; Flint, S.J.; San Martín, C.; Xie, X.S.; Mangel, W.F. Regulation of a viral proteinase by a peptide and DNA in one-dimensional space: II. Adenovirus proteinase is activated in an unusual one-dimensional biochemical reaction. J. Biol. Chem. 2013, 288, 2068–2080. [Google Scholar] [CrossRef]
- Graziano, V.; McGrath, W.J.; Suomalainen, M.; Greber, U.F.; Freimuth, P.; Blainey, P.C.; Luo, G.; Xie, X.S.; Mangel, W.F. Regulation of a viral proteinase by a peptide and DNA in one-dimensional space: I. Binding to DNA and to hexon of the precursor to protein VI, pVI, of human adenovirus. J. Biol. Chem. 2013, 288, 2059–2067. [Google Scholar] [CrossRef] [PubMed]
- McGrath, W.J.; Abola, A.P.; Toledo, D.L.; Brown, M.T.; Mangel, W.F. Characterization of human adenovirus proteinase activity in disrupted virus particles. Virology 1996, 217, 131–138. [Google Scholar] [CrossRef]
- Weber, J.M. Synthesis and Assay of Recombinant Adenovirus Protease. In Adenovirus Methods and Protocols: Volume 2: Ad Proteins, RNA Lifecycle, Host Interactions, and Phylogenetics; Wold, W.S.M., Tollefson, A.E., Eds.; Humana Press: Totowa, NJ, USA, 2007; pp. 251–255. [Google Scholar]
- Puente, X.S.; Sánchez, L.M.; Gutiérrez-Fernández, A.; Velasco, G.; López-Otín, C. A genomic view of the complexity of mammalian proteolytic systems. Biochem. Soc. Trans. 2005, 33, 331–334. [Google Scholar] [CrossRef]
- Verhelst, S.H.L. Intramembrane proteases as drug targets. FEBS J. 2017, 284, 1489–1502. [Google Scholar] [CrossRef]
- Smith, C.G.; Vane, J.R. The discovery of captopril. FASEB J. 2003, 17, 788–789. [Google Scholar] [CrossRef]
- Turk, B. Targeting proteases: Successes, failures and future prospects. Nat. Rev. Drug Discov. 2006, 5, 785–799. [Google Scholar] [CrossRef] [PubMed]
- Ghahremanpour, M.M.; Tirado-Rives, J.; Deshmukh, M.; Ippolito, J.A.; Zhang, C.H.; Cabeza de Vaca, I.; Liosi, M.E.; Anderson, K.S.; Jorgensen, W.L. Identification of 14 known drugs as inhibitors of the main protease of SARS-CoV-2. ACS Med. Chem. Lett. 2020, 11, 2526–2533. [Google Scholar] [CrossRef]
- Scott, C.J.; Taggart, C.C. Biologic protease inhibitors as novel therapeutic agents. Biochimie 2010, 92, 1681–1688. [Google Scholar] [CrossRef]
- Łupina, K.; Nowak, K.; Lorek, D.; Nowak, A.; Romac, A.; Głowacka, E.; Janczura, J. Pharmacological advances in HIV treatment: From ART to long-acting injectable therapies. Arch. Virol. 2025, 170, 195. [Google Scholar] [CrossRef]
- James, J.S. Saquinavir (Invirase): First protease inhibitor approved--reimbursement, information hotline numbers. AIDS Treat. News 1995, 237, 1–2. [Google Scholar]
- Pereira, M.; Vale, N. Saquinavir: From HIV to COVID-19 and cancer treatment. Biomolecules 2022, 12, 944. [Google Scholar] [CrossRef]
- Lam, A.M.; Frick, D.N. Hepatitis C virus subgenomic replicon requires an active NS3 RNA helicase. J. Virol. 2006, 80, 404–411. [Google Scholar] [CrossRef]
- Beran, R.K.F.; Pyle, A.M. Hepatitis C viral NS3-4A protease activity is enhanced by the NS3 helicase. J. Biol. Chem. 2008, 283, 29929–29937. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Ishii, K.; Aizaki, H.; Wakita, T. Hepatitis C viral life cycle. Adv. Drug Deliv. Rev. 2007, 59, 1200–1212. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.H.; Nie, Q.H.; Zhao, X.T. Drug-drug interactions of newly approved direct-acting antiviral agents in patients with hepatitis C. Int. J. Gen. Med. 2021, 14, 289–301. [Google Scholar] [CrossRef]
- de Leuw, P.; Stephan, C. Protease inhibitors for the treatment of hepatitis C virus infection. GMS Infect. Dis. 2017, 5, Doc08. [Google Scholar] [CrossRef]
- Yu, W.; Krishnan, M.K.; Weekly, M.; Shanker, R.M.; Doshi, P.; Ragan, J.A.; Greene, R.A.; Gampper, B.; Caron, S.; McKillop, A.; et al. The unprecedented Paxlovid journey from milligrams to millions of patient doses during the COVID-19 pandemic. Commun. Med. 2025, 5, 80. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Q.; Guo, D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J. Med. Virol. 2020, 92, 418–423. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, H.; Yuan, Z.; Yang, H. Structural biology of SARS-CoV-2 M(pro) and drug discovery. Curr. Opin. Struct. Biol. 2023, 82, 102667. [Google Scholar] [CrossRef]
- Morse, J.S.; Lalonde, T.; Xu, S.; Liu, W.R. Learning from the past: Possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019-nCoV. Chembiochem 2020, 21, 730–738. [Google Scholar] [CrossRef]
- Zvornicanin, S.N.; Shaqra, A.M.; Flynn, J.; Martinez, H.C.; Jia, W.; Moquin, S.; Dovala, D.; Bolon, D.N.; Yilmaz, N.K.; Schiffer, C.A. Molecular mechanisms of drug resistance and compensation in SARS-CoV-2 main protease: The interplay between E166 and L50. mBio 2025, 16, e04068-24. [Google Scholar] [CrossRef] [PubMed]
- Tingle, B.I.; Tang, K.G.; Castanon, M.; Gutierrez, J.J.; Khurelbaatar, M.; Dandarchuluun, C.; Moroz, Y.S.; Irwin, J.J. ZINC-22–A free multi-billion-scale database of tangible compounds for ligand discovery. J. Chem. Inf. Model. 2023, 63, 1166–1176. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Saranraj, K.; Kiran, P.U. Drug repurposing: Clinical practices and regulatory pathways. Perspect. Clin. Res. 2025, 16, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Oprea, T.I.; Mestres, J. Drug repurposing: Far beyond new targets for old drugs. AAPS J. 2012, 14, 759–763. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug. Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Shivanika, C.; Deepak Kumar, S.; Venkataraghavan, R.I.; Pawan, T.; Sumitha, A.; Brindha, D.P. Molecular docking, validation, dynamics simulations, and pharmacokinetic prediction of natural compounds against the SARS-CoV-2 main-protease. J. Biomol. Struct. Dyn. 2022, 40, 585–611. [Google Scholar] [CrossRef]
- Luttens, A.; Gullberg, H.; Abdurakhmanov, E.; Vo, D.D.; Akaberi, D.; Talibov, V.O.; Nekhotiaeva, N.; Vangeel, L.; De Jonghe, S.; Jochmans, D.; et al. Ultralarge virtual screening identifies SARS-CoV-2 main protease inhibitors with broad-spectrum activity against coronaviruses. J. Am. Chem. Soc. 2022, 144, 2905–2920. [Google Scholar] [CrossRef]
- Gevorgyan, S.; Khachatryan, H.; Shavina, A.; Gharaghani, S.; Zakaryan, H. Targeting SARS-CoV-2 main protease: A comprehensive approach using advanced virtual screening, molecular dynamics, and in vitro validation. Virol. J. 2024, 21, 330. [Google Scholar] [CrossRef]
- Fu, L.; Ye, F.; Feng, Y.; Yu, F.; Wang, Q.; Wu, Y.; Zhao, C.; Sun, H.; Huang, B.; Niu, P.; et al. Both Boceprevir and GC376 efficaciously inhibit SARS-CoV-2 by targeting its main protease. Nat. Commun. 2020, 11, 4417. [Google Scholar] [CrossRef] [PubMed]
- Owen, D.R.; Allerton, C.M.N.; Anderson, A.S.; Aschenbrenner, L.; Avery, M.; Berritt, S.; Boras, B.; Cardin, R.D.; Carlo, A.; Coffman, K.J.; et al. An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science 2021, 374, 1586–1593. [Google Scholar] [CrossRef]
- Jiang, X.; Su, H.; Shang, W.; Zhou, F.; Zhang, Y.; Zhao, W.; Zhang, Q.; Xie, H.; Jiang, L.; Nie, T.; et al. Structure-based development and preclinical evaluation of the SARS-CoV-2 3C-like protease inhibitor simnotrelvir. Nat. Commun. 2023, 14, 6463. [Google Scholar] [CrossRef] [PubMed]
- Allerton, C.M.N.; Arcari, J.T.; Aschenbrenner, L.M.; Avery, M.; Bechle, B.M.; Behzadi, M.A.; Boras, B.; Buzon, L.M.; Cardin, R.D.; Catlin, N.R.; et al. A second-generation oral SARS-CoV-2 main protease inhibitor clinical candidate for the treatment of COVID-19. J. Med. Chem. 2024, 67, 13550–13571. [Google Scholar] [CrossRef] [PubMed]
- Unoh, Y.; Uehara, S.; Nakahara, K.; Nobori, H.; Yamatsu, Y.; Yamamoto, S.; Maruyama, Y.; Taoda, Y.; Kasamatsu, K.; Suto, T.; et al. Discovery of S-217622, a noncovalent oral SARS-CoV-2 3CL protease inhibitor clinical candidate for treating COVID-19. J. Med. Chem. 2022, 65, 6499–6512. [Google Scholar] [CrossRef]
- Han, S.H.; Goins, C.M.; Arya, T.; Shin, W.-J.; Maw, J.; Hooper, A.; Sonawane, D.P.; Porter, M.R.; Bannister, B.E.; Crouch, R.D.; et al. Structure-based optimization of ML300-derived, noncovalent inhibitors targeting the severe acute respiratory syndrome coronavirus 3CL protease (SARS-CoV-2 3CLpro). J. Med. Chem. 2022, 65, 2880–2904. [Google Scholar] [CrossRef]
- Jin, Z.; Zhao, Y.; Sun, Y.; Zhang, B.; Wang, H.; Wu, Y.; Zhu, Y.; Zhu, C.; Hu, T.; Du, X.; et al. Structural basis for the inhibition of SARS-CoV-2 main protease by antineoplastic drug carmofur. Nat. Struct. Mol. Biol. 2020, 27, 529–532. [Google Scholar] [CrossRef]
- Boby, M.L.; Fearon, D.; Ferla, M.; Filep, M.; Koekemoer, L.; Robinson, M.C.; The COVID Moonshot Consortium; Chodera, J.D.; Lee, A.A.; London, N.; et al. Open science discovery of potent noncovalent SARS-CoV-2 main protease inhibitors. Science 2023, 382, eabo7201. [Google Scholar] [CrossRef]
- Sun, Q.; Ye, F.; Liang, H.; Liu, H.; Li, C.; Lu, R.; Huang, B.; Zhao, L.; Tan, W.; Lai, L. Bardoxolone and bardoxolone methyl, two Nrf2 activators in clinical trials, inhibit SARS-CoV-2 replication and its 3C-like protease. Signal Transduct. Target Ther. 2021, 6, 212. [Google Scholar] [CrossRef]
- Tong, X.; Keung, W.; Arnold, L.D.; Stevens, L.J.; Pruijssers, A.J.; Kook, S.; Lopatin, U.; Denison, M.; Kwong, A.D. Evaluation of in vitro antiviral activity of SARS-CoV-2 M(pro) inhibitor pomotrelvir and cross-resistance to nirmatrelvir resistance substitutions. Antimicrob. Agents Chemother. 2023, 67, e0084023. [Google Scholar] [CrossRef]
- Allais, C.; Bernhardson, D.; Brown, A.R.; Chinigo, G.M.; Desrosiers, J.-N.; DiRico, K.J.; Hotham, I.; Jones, B.P.; Kulkarni, S.A.; Lewis, C.A.; et al. Early clinical development of lufotrelvir as a potential therapy for COVID-19. Org. Process Res. Dev. 2023, 27, 2223–2239. [Google Scholar] [CrossRef]
- Chen, W.; Shao, Y.; Peng, X.; Liang, B.; Xu, J.; Xing, D. Review of preclinical data of PF-07304814 and its active metabolite derivatives against SARS-CoV-2 infection. Front. Pharmacol. 2022, 13, 1035969. [Google Scholar] [CrossRef]
- Amporndanai, K.; Meng, X.; Shang, W.; Jin, Z.; Rogers, M.; Zhao, Y.; Rao, Z.; Liu, Z.-J.; Yang, H.; Zhang, L.; et al. Inhibition mechanism of SARS-CoV-2 main protease by ebselen and its derivatives. Nat. Commun. 2021, 12, 3061. [Google Scholar] [CrossRef] [PubMed]
- Rhodin, M.H.J.; Reyes, A.C.; Balakrishnan, A.; Bisht, N.; Kelly, N.M.; Gibbons, J.S.; Lloyd, J.; Vaine, M.; Cressey, T.; Crepeau, M.; et al. The small molecule inhibitor of SARS-CoV-2 3CLpro EDP-235 prevents viral replication and transmission in vivo. Nat. Commun. 2024, 15, 6503. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, R.L.; Kania, R.S.; Brothers, M.A.; Davies, J.F.; Ferre, R.A.; Gajiwala, K.S.; He, M.; Hogan, R.J.; Kozminski, K.; Li, L.Y.; et al. Discovery of ketone-based covalent inhibitors of coronavirus 3CL proteases for the potential therapeutic treatment of COVID-19. J. Med. Chem. 2020, 63, 12725–12747. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lin, D.; Sun, X.; Curth, U.; Drosten, C.; Sauerhering, L.; Becker, S.; Rox, K.; Hilgenfeld, R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science 2020, 368, 409–412. [Google Scholar] [CrossRef]
- Cooper, M.S.; Zhang, L.; Ibrahim, M.; Zhang, K.; Sun, X.; Röske, J.; Göhl, M.; Brönstrup, M.; Cowell, J.K.; Sauerhering, L.; et al. Diastereomeric resolution yields highly potent inhibitor of SARS-CoV-2 main potease. J. Med. Chem. 2022, 65, 13328–13342. [Google Scholar] [CrossRef]
- Kitamura, N.; Sacco, M.D.; Ma, C.; Hu, Y.; Townsend, J.A.; Meng, X.; Zhang, F.; Zhang, X.; Ba, M.; Szeto, T.; et al. Expedited approach toward the rational design of noncovalent SARS-CoV-2 main protease inhibitors. J. Med. Chem. 2022, 65, 2848–2865. [Google Scholar] [CrossRef]
- Alvarez, N.; Adam, G.C.; Howe, J.A.; Sharma, V.; Zimmerman, M.D.; Dolgov, E.; Rasheed, R.; Nizar, F.; Sahay, K.; Nelson, A.M.; et al. Novel pan-coronavirus 3CL protease inhibitor MK-7845: Biological and pharmacological profiling. Viruses 2024, 16, 1158. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, D.; Kusov, Y.; Nian, Y.; Ma, Q.; Wang, J.; von Brunn, A.; Leyssen, P.; Lanko, K.; Neyts, J.; et al. α-Ketoamides as broad-spectrum inhibitors of coronavirus and enterovirus replication: Structure-based design, synthesis, and activity assessment. J. Med. Chem. 2020, 63, 4562–4578. [Google Scholar] [CrossRef] [PubMed]
- Syed, Y.Y. Ensitrelvir fumaric acid: First approval. Drugs 2024, 84, 721–728. [Google Scholar] [CrossRef]
- Narayanan, A.; Narwal, M.; Majowicz, S.A.; Varricchio, C.; Toner, S.A.; Ballatore, C.; Brancale, A.; Murakami, K.S.; Jose, J. Identification of SARS-CoV-2 inhibitors targeting Mpro and PLpro using in-cell-protease assay. Commun. Biol. 2022, 5, 169. [Google Scholar] [CrossRef]
- Atatreh, N.; Mahgoub, R.E.; Ghattas, M.A. Evaluating the potential of PLpro as a drug target in SARS-CoV-2: MD simulations and druggability analysis. Results Chem. 2025, 17, 102565. [Google Scholar] [CrossRef]
- Fu, Z.; Huang, B.; Tang, J.; Liu, S.; Liu, M.; Ye, Y.; Liu, Z.; Xiong, Y.; Zhu, W.; Cao, D.; et al. The complex structure of GRL0617 and SARS-CoV-2 PLpro reveals a hot spot for antiviral drug discovery. Nat. Commun. 2021, 12, 488. [Google Scholar] [CrossRef]
- Ma, C.; Sacco, M.D.; Xia, Z.; Lambrinidis, G.; Townsend, J.A.; Hu, Y.; Meng, X.; Szeto, T.; Ba, M.; Zhang, X.; et al. Discovery of SARS-CoV-2 papain-like protease inhibitors through a combination of high-throughput screening and a FlipGFP-based reporter assay. ACS Cent. Sci. 2021, 7, 1245–1260. [Google Scholar] [CrossRef]
- Weglarz-Tomczak, E.; Tomczak, J.M.; Talma, M.; Burda-Grabowska, M.; Giurg, M.; Brul, S. Identification of ebselen and its analogues as potent covalent inhibitors of papain-like protease from SARS-CoV-2. Sci. Rep. 2021, 11, 3640. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, P.; Liang, X.; Ansari, A.; Tan, B.; Tan, H.; Li, K.; Chi, X.; Ford, A.; Ruiz, F.X.; Arnold, E.; et al. Design of quinoline SARS-CoV-2 papain-like protease inhibitors as oral antiviral drug candidates. Nat. Commun. 2025, 16, 1604. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.; Liu, J.; Shen, J.; Dai, J.; Xu, G.; Lu, K.; Han, C.; Wang, Y.; Xu, X.; Tong, Y.; et al. Development of potent and selective inhibitors targeting the papain-like protease of SARS-CoV-2. Cell. Chem. Biol. 2021, 28, 855–865.e9. [Google Scholar] [CrossRef] [PubMed]
- Bader, S.; Calleja, D.J.; Devine, S.M.; Kuchel, N.W.; Lu, B.G.C.; Wu, X.; Birkinshaw, R.W.; Bhandari, R.; Loi, K.; Volpe, R.; et al. A novel PLpro inhibitor improves outcomes in a pre-clinical model of long COVID. Nat. Commun. 2025, 16, 2900. [Google Scholar] [CrossRef]
- Tan, H.; Hu, Y.; Jadhav, P.; Tan, B.; Wang, J. Progress and challenges in targeting the SARS-CoV-2 papain-like protease. J. Med. Chem. 2022, 65, 7561–7580. [Google Scholar] [CrossRef]
- Jiang, H.; Yang, P.; Zhang, J. Potential inhibitors targeting papainl-like protease of SARS-CoV-2: Two birds with one stone. Front. Chem. 2022, 10, 822785. [Google Scholar] [CrossRef]
- Calleja, D.J.; Lessene, G.; Komander, D. Inhibitors of SARS-CoV-2 PLpro. Front. Chem. 2022, 10, 876212. [Google Scholar] [CrossRef]
- Yue, Q.; Hua-Juan, J.; Yu-Shun, Y.; Xiao-Qin, H.; Xue-Wen, Z. Review of the crystallized structures of the SARS-CoV-2 papain-like protease. J. Mol. Struct. 2025, 1333, 141730. [Google Scholar] [CrossRef]
- Fais, A.; Era, B. Phytochemical composition and biological activity. Plants 2024, 13, 331. [Google Scholar] [CrossRef]
- Luo, Z.; Yin, F.; Wang, X.; Kong, L. Progress in approved drugs from natural product resources. Chin. J. Nat. Med. 2024, 22, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Modes of action of herbal medicines and plant secondary metabolites. Medicines 2015, 2, 251–286. [Google Scholar] [CrossRef]
- Sagandira, C.R.; Mathe, F.M.; Guyo, U.; Watts, P. The evolution of Tamiflu synthesis, 20 years on: Advent of enabling technologies the last piece of the puzzle? Tetrahedron 2020, 76, 131440. [Google Scholar] [CrossRef] [PubMed]
- Tsilimingkra, N.T.; Papaneophytou, C. Phytochemicals: Promising inhibitors of human rhinovirus type 14 3C protease as a strategy to fight the common cold. Curr. Top. Med. Chem. 2024, 24, 1343–1358. [Google Scholar] [CrossRef] [PubMed]
- Balsera-Manzanero, M.; Ghirga, F.; Ruiz-Molina, A.; Mori, M.; Pachón, J.; Botta, B.; Cordero, E.; Quaglio, D.; Sánchez-Céspedes, J. Inhibition of adenovirus transport from the endosome to the cell nucleus by rotenone. Front. Pharmacol. 2023, 14, 1293296. [Google Scholar] [CrossRef] [PubMed]
- Musarra-Pizzo, M.; Pennisi, R.; Ben-Amor, I.; Mandalari, G.; Sciortino, M.T. Antiviral activity exerted by natural products against human viruses. Viruses 2021, 13, 828. [Google Scholar] [CrossRef]
- Antoniou, G.; Papakyriacou, I.; Papaneophytou, C. Optimization of soluble expression and purification of recombinant human rhinovirus type-14 3C protease using statistically designed experiments: Isolation and characterization of the enzyme. Mol. Biotechnol. 2017, 59, 407–424. [Google Scholar] [CrossRef]
- Onyeogaziri, F.C.; Papaneophytou, C. A General guide for the optimization of enzyme assay conditions using the design of experiments approach. SLAS Discov. 2019, 24, 587–596. [Google Scholar] [CrossRef]
- Papaneophytou, C.; Zervou, M.-E.; Theofanous, A. Optimization of a colorimetric assay to determine lactate dehydrogenase B activity using design of experiments. SLAS Discov. 2021, 26, 383–399. [Google Scholar] [CrossRef]
- Papaneophytou, C. Design of experiments as a tool for optimization in recombinant protein biotechnology: From constructs to crystals. Mol. Biotechnol. 2019, 61, 873–891. [Google Scholar] [CrossRef]
- Papaneophytou, C.P.; Kontopidis, G.A. Optimization of TNF-α overexpression in Escherichia coli using response surface methodology: Purification of the protein and oligomerization studies. Protein Expr. Purif. 2012, 86, 35–44. [Google Scholar] [CrossRef]
- Papaneophytou, C.P.; Rinotas, V.; Douni, E.; Kontopidis, G. A statistical approach for optimization of RANKL overexpression in Escherichia coli: Purification and characterization of the protein. Protein Expr. Purif. 2013, 90, 9–19. [Google Scholar] [CrossRef]
- Papaneophytou, C.; Kontopidis, G. A comparison of statistical approaches used for the optimization of soluble protein expression in Escherichia coli. Protein Expr. Purif. 2016, 120, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Ruzindana-Umunyana, A.; Imbeault, L.; Weber, J.M. Substrate specificity of adenovirus protease. Virus Res. 2002, 89, 41–52. [Google Scholar] [CrossRef] [PubMed]



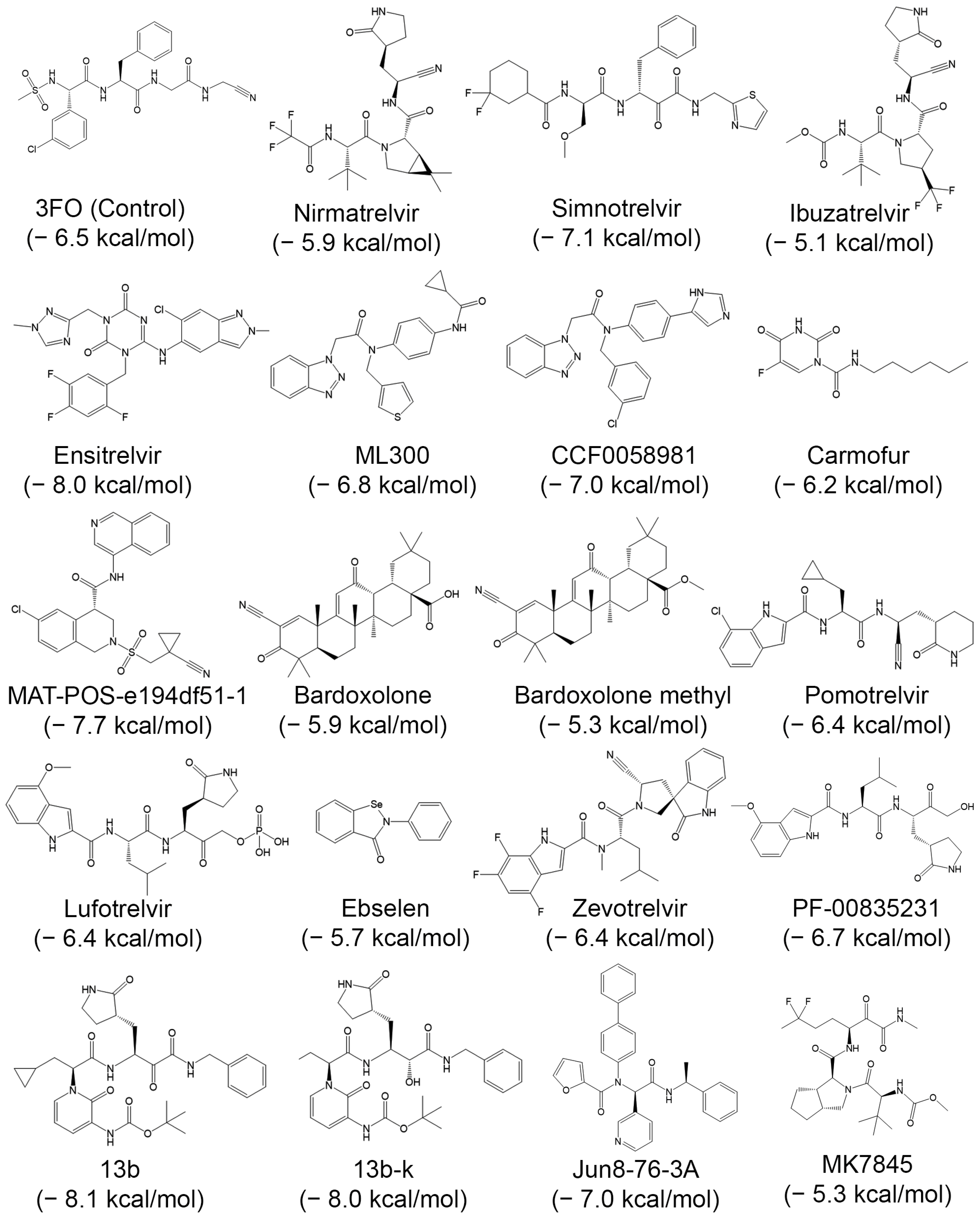
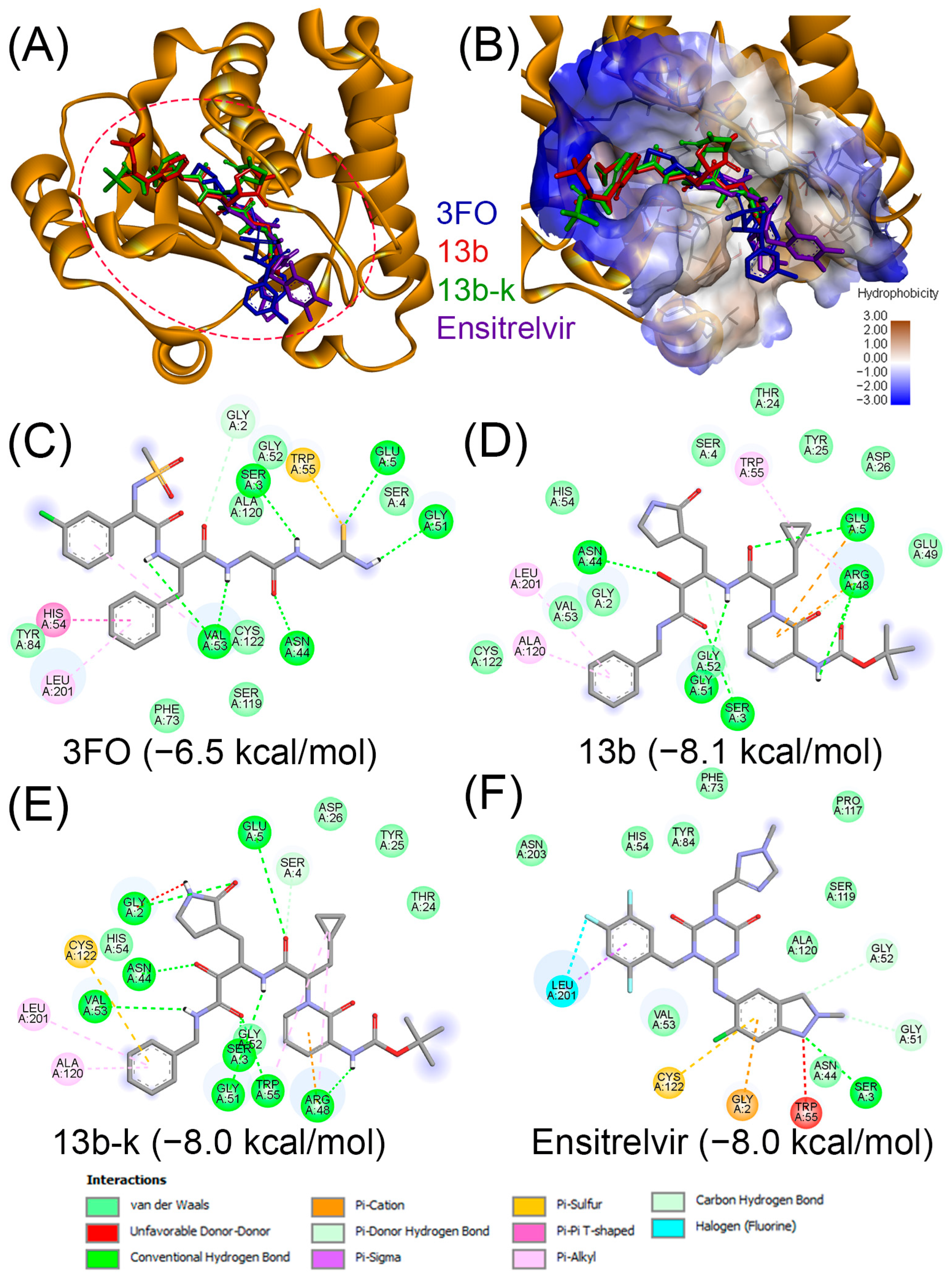
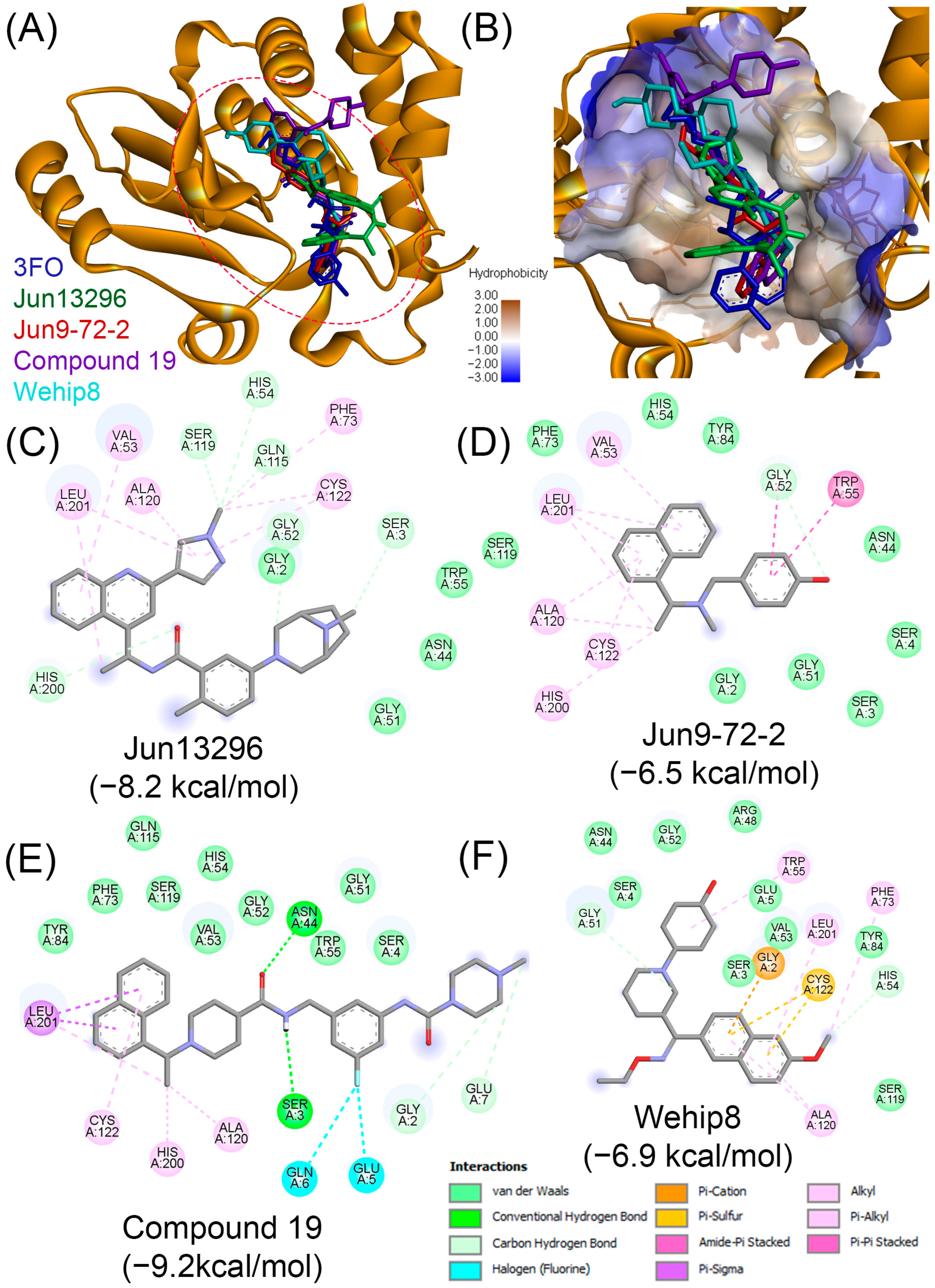
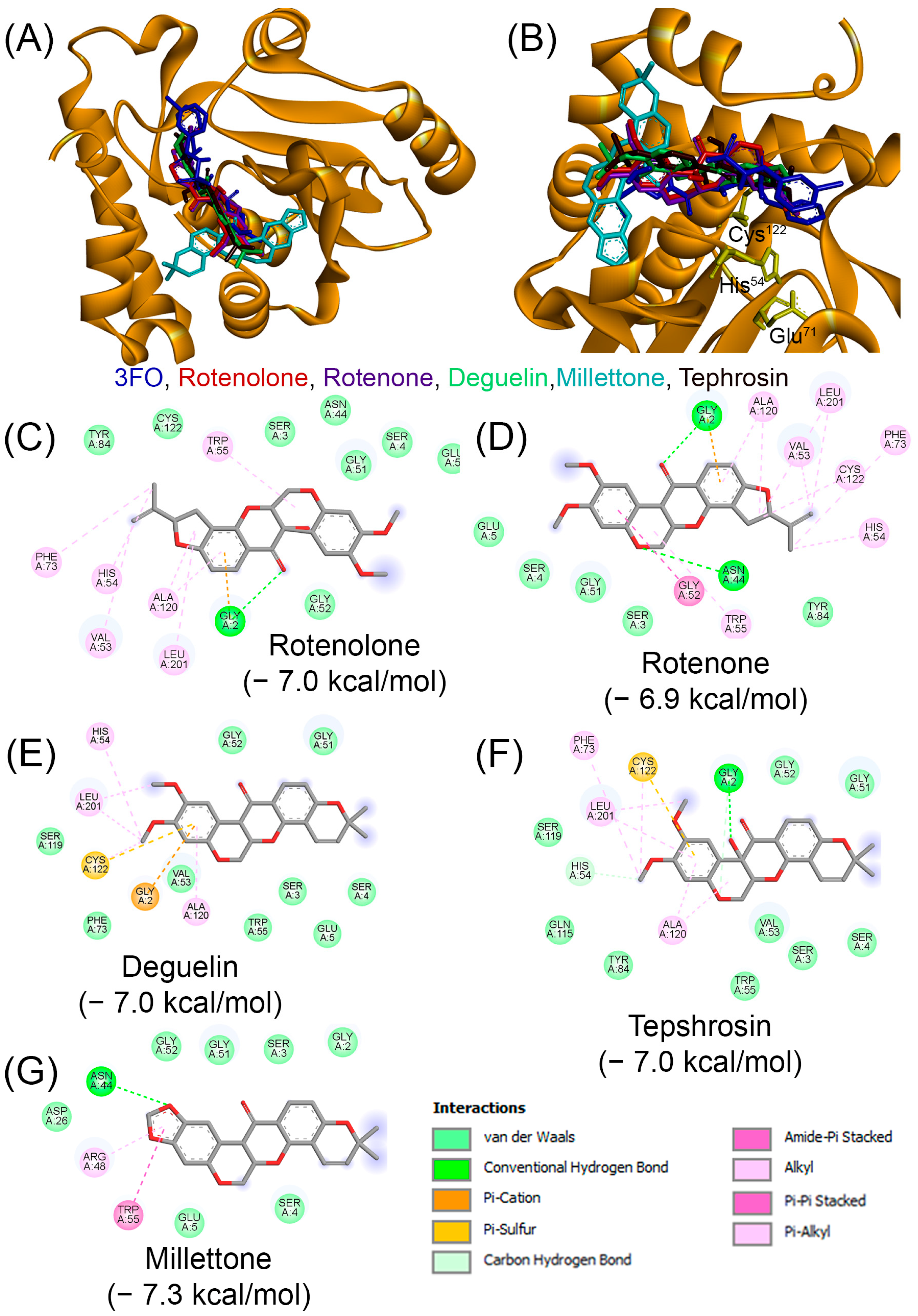

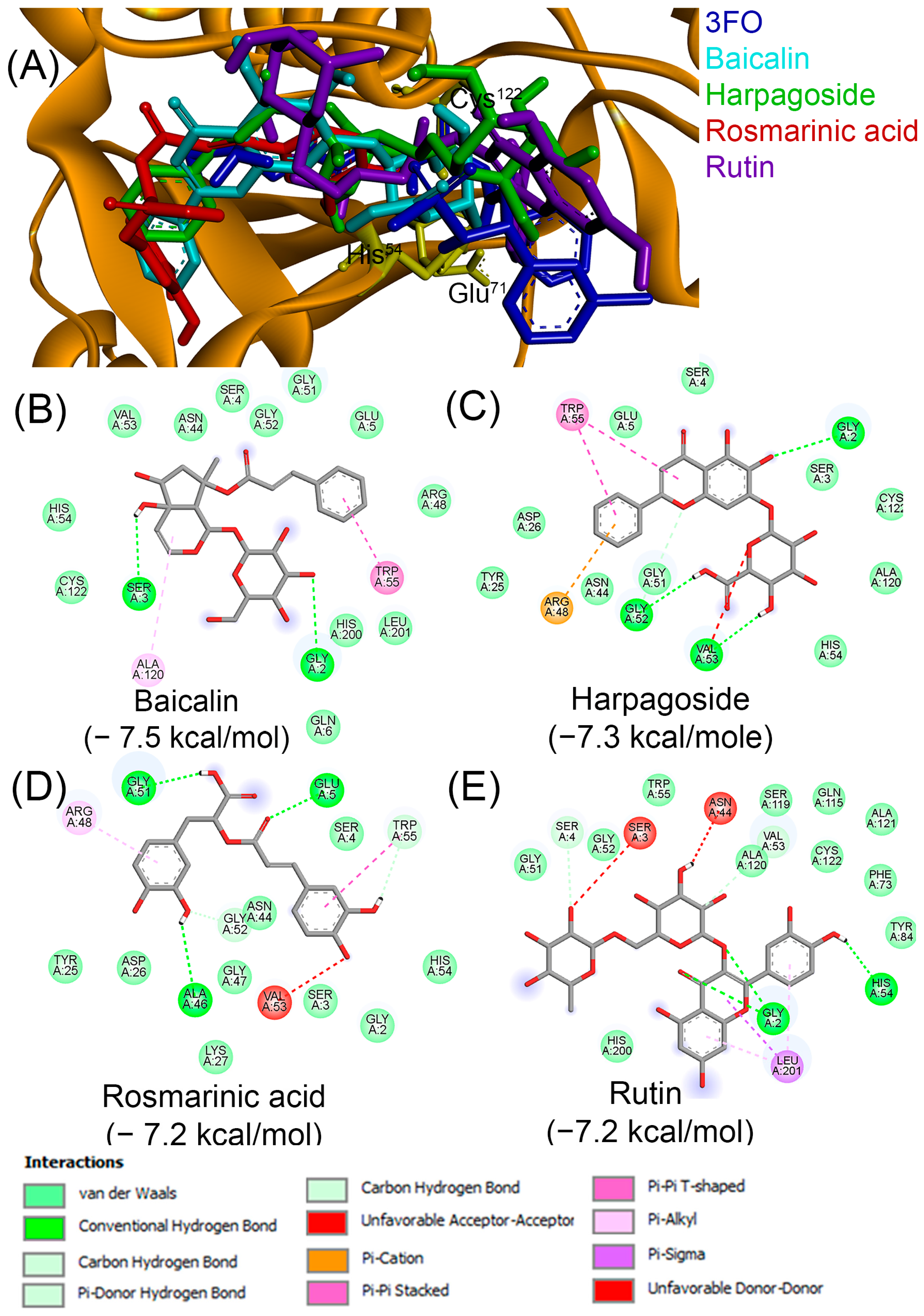

| Compound | PubChem | Remarks | Refs. |
|---|---|---|---|
| Nirmatrelvir (PF-07321332) | 155903259 | Good selectivity and safety profiles. Part of a nirmatrelvir/ritonavir (Paxlovid) combination used to treat COVID-19 | [88] |
| Simnotrelvir | 167312484 | Identified after structure-based optimization of boceprevir (HCV/NS3 protease inhibitor) | [89] |
| Ibuzatrelvir (PF-07817883) | 163362000 | A 2nd-generation, inhibitor with improved metabolic stability compared to nirmatrelvir | [90] |
| Ensitrelvir (S-217622) | 162533924 | Nonpeptidic, noncovalent inhibitor approved in Japan | [91] |
| ML300 | 46861530 | Noncovalent (small molecule) inhibitor | [92] |
| CCF0058981 | 156027237 | An optimized ML300 derivative with nanomolar IC50 and sub-100 nM cellular antiviral potency. | [92] |
| Carmofur | 2577 | An antineoplastic drug that covalently binds to catalytic Cys145. It inhibits viral replication in cells (EC50 = 24.3 µM) | [93] |
| MAT-POS-e194df51-1 | 156906151 | A noncovalent, nonpeptidic inhibitor with nanomolar potency and robust cellular efficacy | [94] |
| Bardoxolone | 400010 | Nrf2-activating clinical candidates They reversibly covalently inhibit Mpro (EC50 ~0.3–0.4 µM), and block viral replication | [95] |
| Bardoxolone methyl | 400769 | ||
| Pomotrelvir (PBI-0451) | 162396309 | A selective, competitive, orally active covalent inhibitor, with an IC50 of 24 nM. | [96] |
| Lufotrelvir (PF-07304814) | 154699467 | A first in class inhibitor with good tolerability, pharmacology, pharmacodynamics, pharmacokinetics, and safety in preclinical trials. | [97,98] |
| Ebselen | 3194 | An organoselenium molecule exhibiting potent Mpro inhibition and antiviral activity. | [99] |
| Zevotrelvir (EDP-235) | 163373364 | Exhibits potent nanomolar activity against all SARS-CoV-2 variants | [100] |
| PF-00835231 | 11561899 | A potent covalent ketone-based with favorable solubility and stability | [101] |
| 13b | 146026181 | A potent α-ketoamide inhibitor optimized with a P2 cyclopropyl group for enhanced antiviral activity against SARS-CoV-2 and SARS-CoV | [102] |
| 13b-K (S,S,S)-13b | 146018708 | S,S,S diastereomer of 13b; IC50: 120 nM; EC50: 0.8–3.4 µM; favorable oral/inhalation Pharmacokinetics. | [103] |
| Jun8-76-3A | 155289416 | High selectivity. Binds to a novel binding pocket between the S2 and S4 subsites | [104] |
| MK7845 | 168976112 | Pan-Coronavirus 3CL Protease Inhibitor | [105] |
| Molecule | Molar Mass (g/mol) | GI 1 Absorption | Lipinski Violations | PAINS 2 Alerts | Lead- Likeness | BA 3 Score | SA 4 Score |
|---|---|---|---|---|---|---|---|
| Baicalin | 446.36 | Low | 2 | 1 (catechol_A) | No (MW > 350) | 0.11 | 5.09 |
| Harpagoside | 494.49 | Low | 2 | 0 | No (MW > 350) | 0.17 | 6.13 |
| Rosmarinic acid | 494.49 | Low | 2 | 0 | No (MW > 350) | 0.17 | 6.13 |
| Rutin | 610.52 | Low | 3 | 1 (catechol_A) | No (MW > 350) | 0.17 | 6.52 |
| Apigenin | 270.24 | High | 0 | 0 | Yes | 0.55 | 2.96 |
| Camptothecin | 348.35 | High | 0 | 0 | Yes | 0.55 | 3.84 |
| Kaempferol | 286.24 | High | 0 | 0 | Yes | 0.55 | 3.14 |
| Piperine | 285.34 | High | 0 | 0 | Yes | 0.55 | 2.92 |
| Endpoint | Apigenin | Camptothecin | Kaempferol | Piperine |
|---|---|---|---|---|
| Acute inhalation toxicity | Non-Toxic (73%) | Non-Toxic (74%) | Non-Toxic (68%) | Non-Toxic (63%) |
| Acute oral toxicity | Non-Toxic (58%) | Toxic (90%) | Non-Toxic (70%) | Toxic (90%) |
| Acute Dermal Toxicity | Toxic (54%) | Non-Toxic (68%) | Toxic (65%) | Non-Toxic (72%) |
| Eye irritation and corrosion | Toxic (71%) | Toxic (71%) | Non-Toxic (50%) | Toxic (52%) |
| Skin sensitization | Sensitizer (60%) | Non-Sensitizer (70%) | Sensitizer (70%) | Sensitizer (60%) |
| Skin irritation and corrosion | Negative (70%) | Negative (90%) | Negative (80%) | Negative (70%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belova, P.; Papaneophytou, C. Adenovirus Protease: An Overlooked but Druggable Antiviral Target. Macromol 2025, 5, 52. https://doi.org/10.3390/macromol5040052
Belova P, Papaneophytou C. Adenovirus Protease: An Overlooked but Druggable Antiviral Target. Macromol. 2025; 5(4):52. https://doi.org/10.3390/macromol5040052
Chicago/Turabian StyleBelova, Polina, and Christos Papaneophytou. 2025. "Adenovirus Protease: An Overlooked but Druggable Antiviral Target" Macromol 5, no. 4: 52. https://doi.org/10.3390/macromol5040052
APA StyleBelova, P., & Papaneophytou, C. (2025). Adenovirus Protease: An Overlooked but Druggable Antiviral Target. Macromol, 5(4), 52. https://doi.org/10.3390/macromol5040052







