Chitosan-Coated Nanostructured Lipid Carriers (NLCs) Incorporating Esters of Ferulic Acid with Photoprotective Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
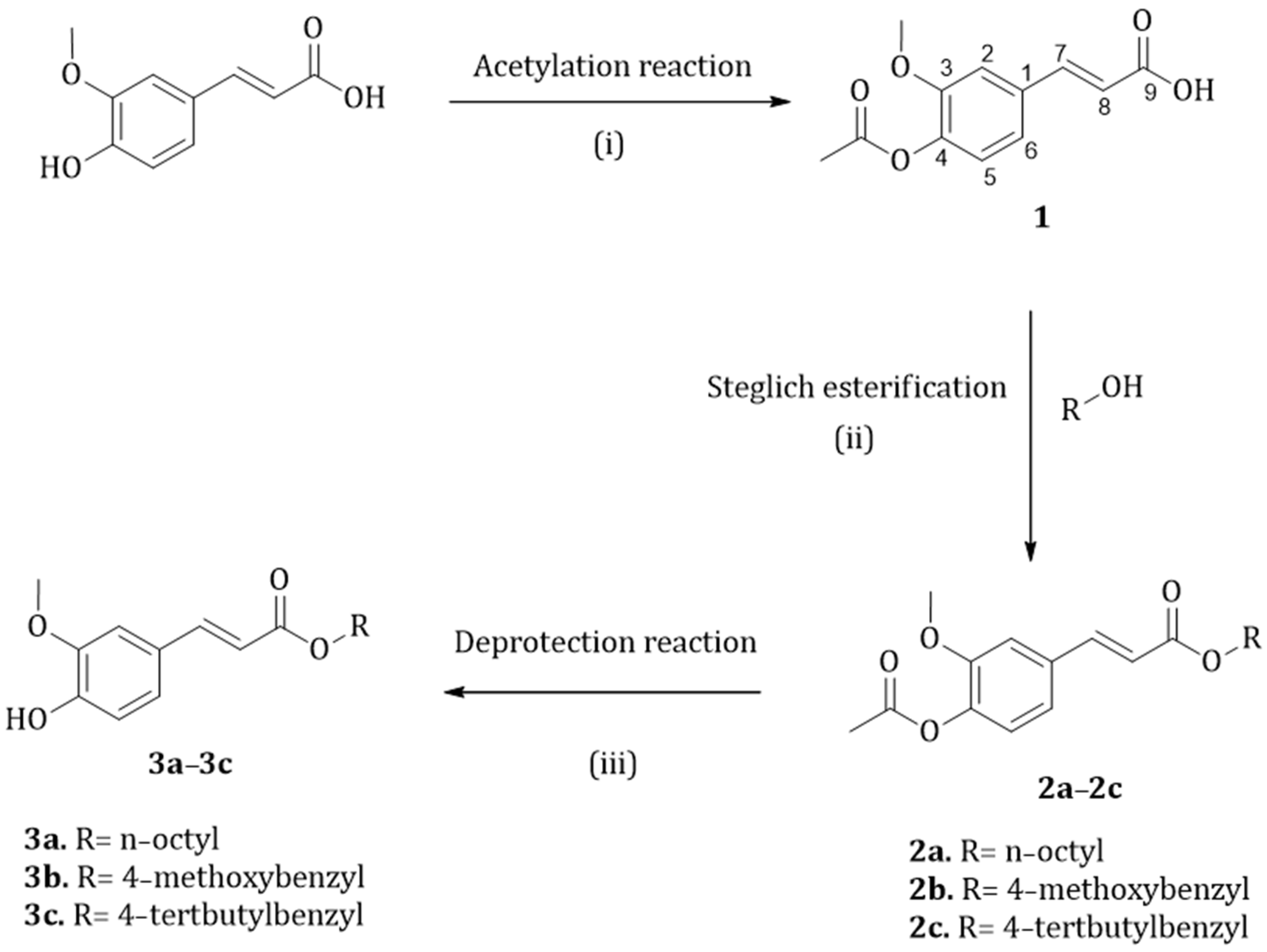
2.2. Synthesis of the Esters
2.3. Synthesis of NADES
2.4. Isolation of Trimyristin from Nutmeg
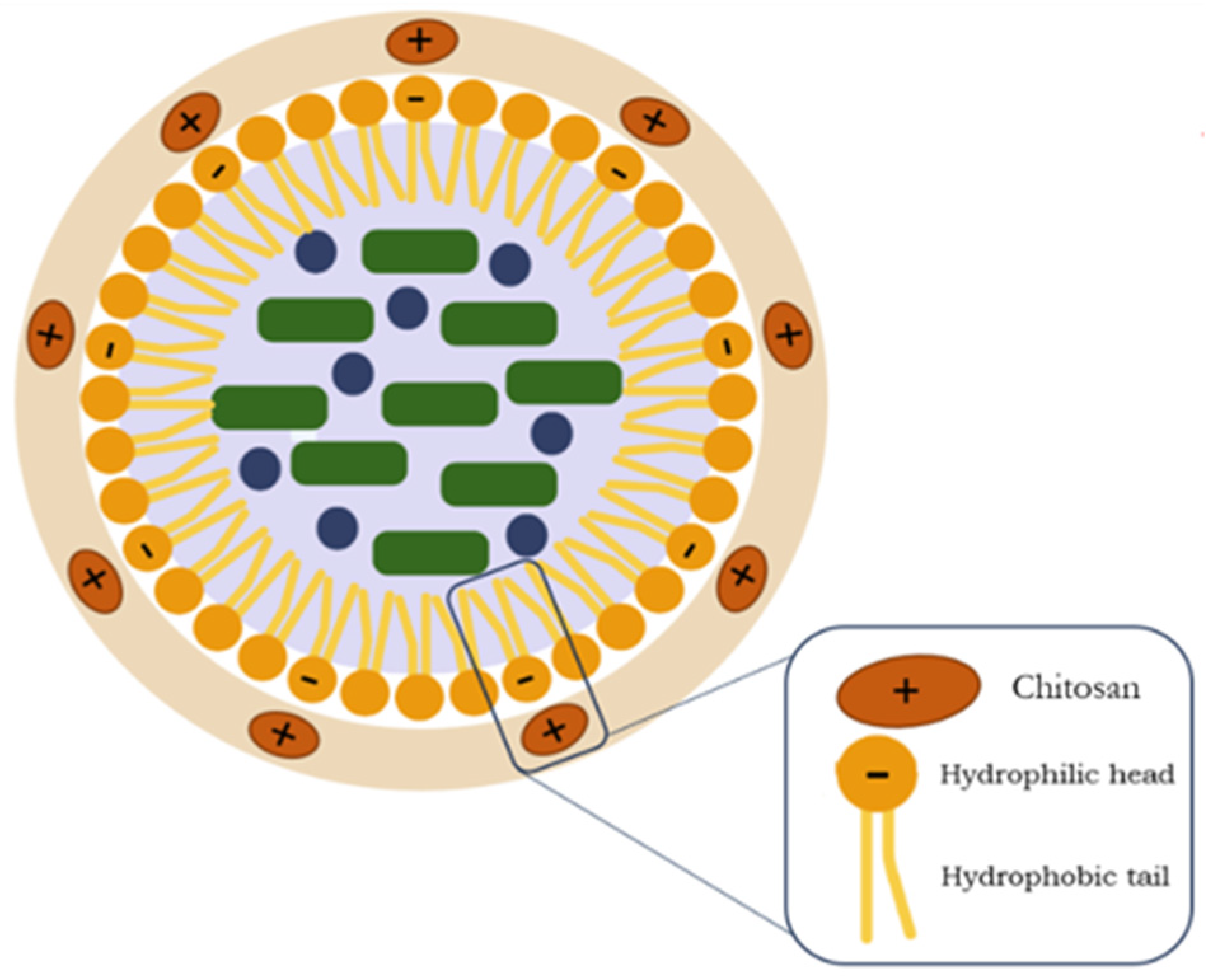
2.5. Preparation of NLCs
2.6. NLC Coating Process
2.7. Characterization of the NLCs
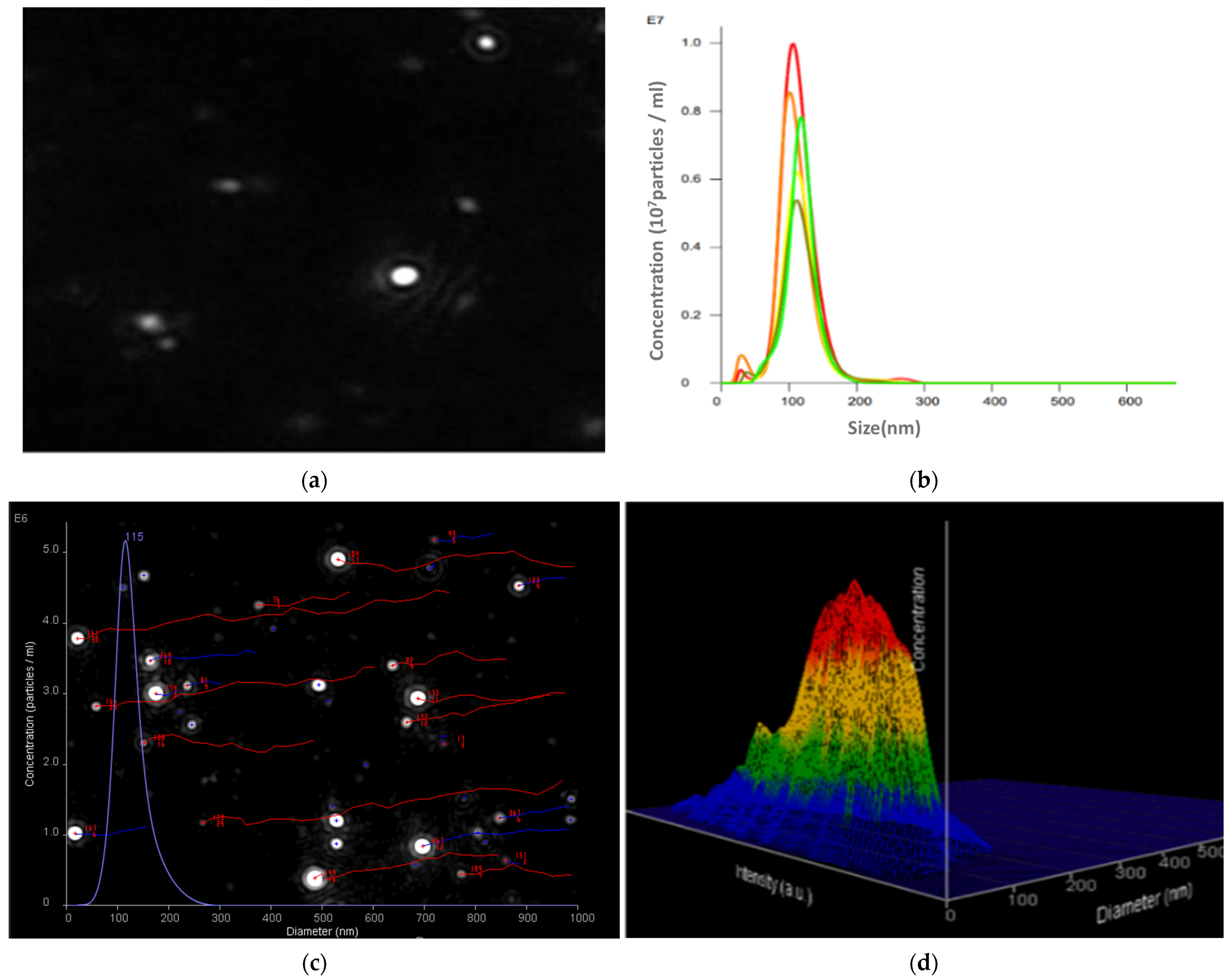
2.7.1. Nanoparticle Tracking Analysis
2.7.2. Hydrodynamic Diameter, Polydispersity Index (PDI), and ζ-Potential
2.7.3. Calculation of the Encapsulation Efficiency
2.7.4. Fourier-Transform Infrared Spectroscopy (FTIR)
2.7.5. Thermogravimetric Analysis (TGA)
2.7.6. Transmission Electron Microscopy (TEM)
2.8. Experimental Design for the Optimization of the Encapsulation-Coating Process
2.9. Assessment of Inhibition to Lipid Peroxidation (AAPH)
2.10. SPF Measurement
3. Results
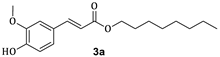
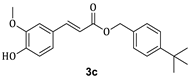
3.1. Synthesis of Esters 3a–3c
3.2. Preliminary Experiments for the Encapsulation Process
3.2.1. Study of ζ-Potential Before and After the Coating of the NLCs
3.2.2. Selection of Solid and Liquid Lipid
3.3. Box–Behnken Experimental Design
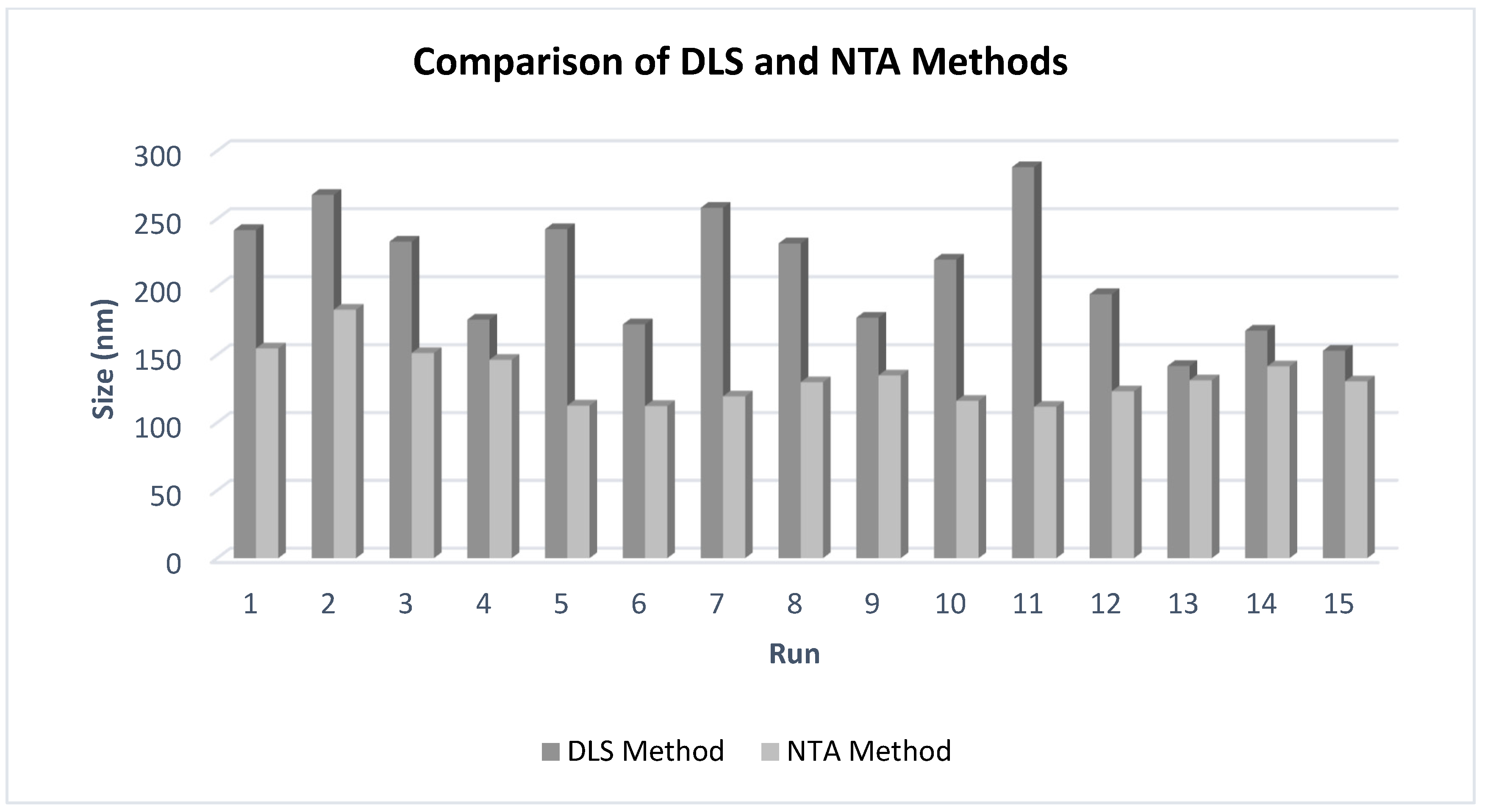
3.3.1. Results of DLS Method for Size and Size Distribution of [NLC-3a] and Comparison with the NΤA Method
3.3.2. Size of NLCs
3.3.3. Encapsulation Efficiency of Octyl Ferulate (3a) in CS-NLCs
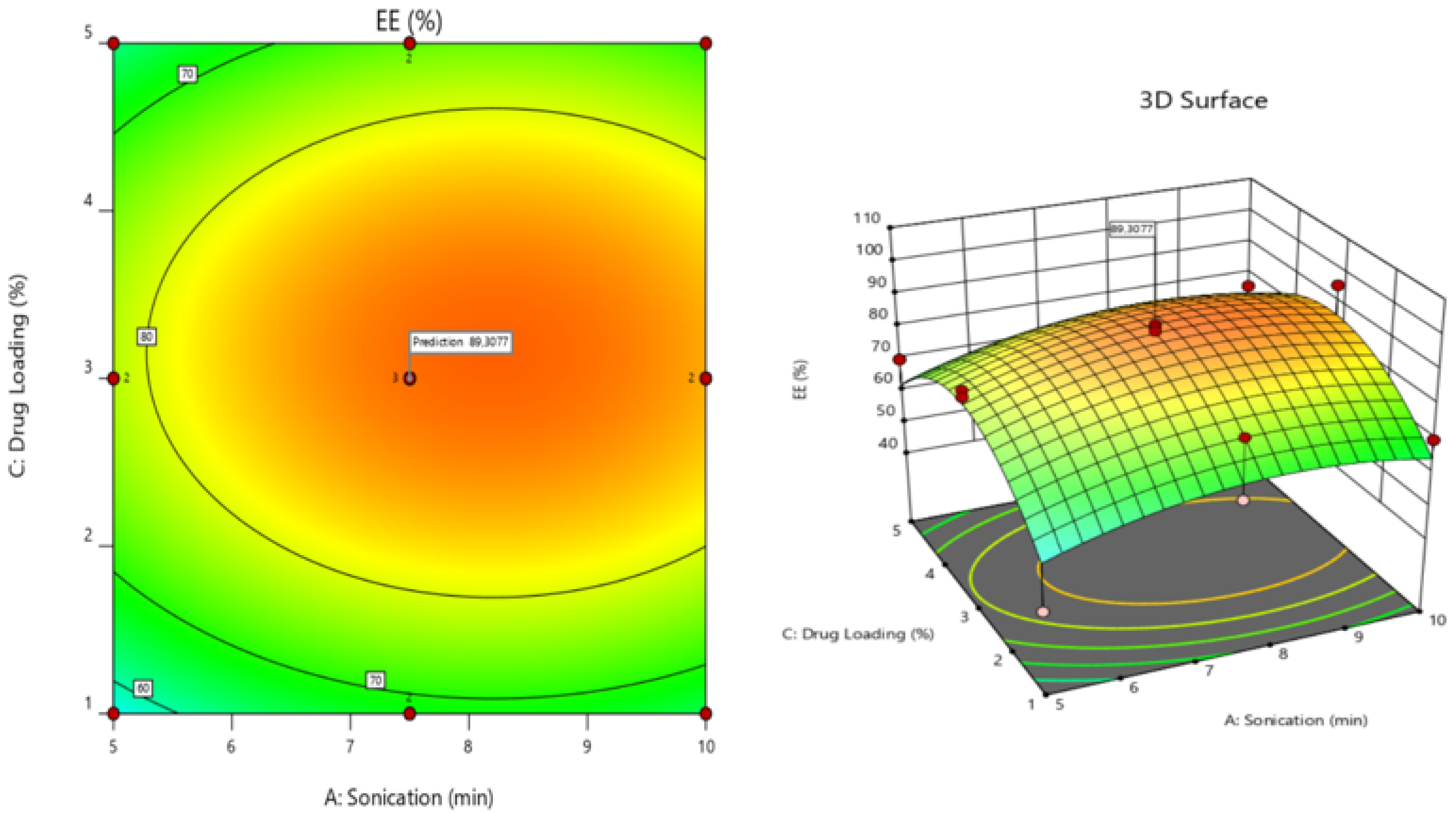
3.3.4. Validation of the Model—Optimization of the Encapsulation Process
3.3.5. Testing the Model with Esters 3b and 3c
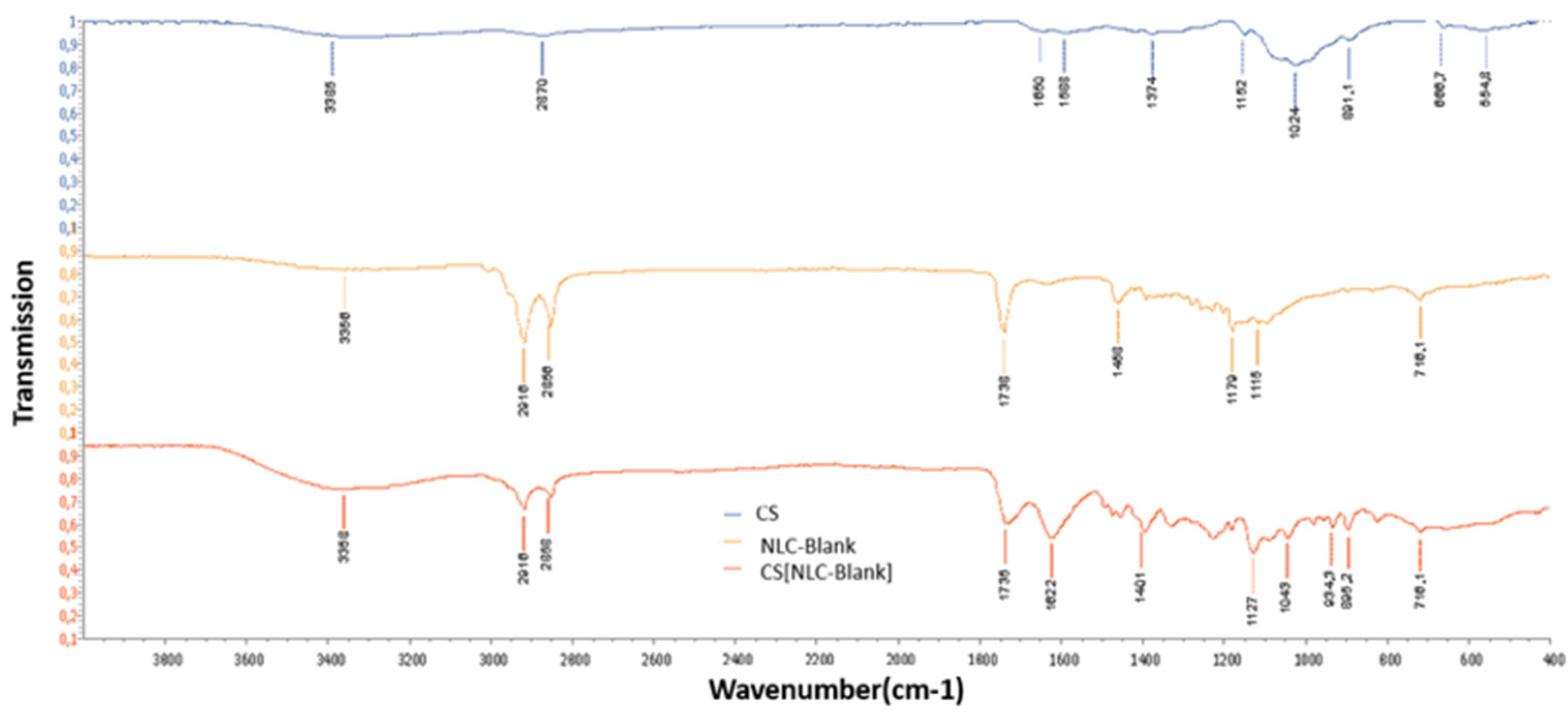
3.4. FTIR Analysis of NLC-Blank and CS[NLC-Blank] Nanosystems
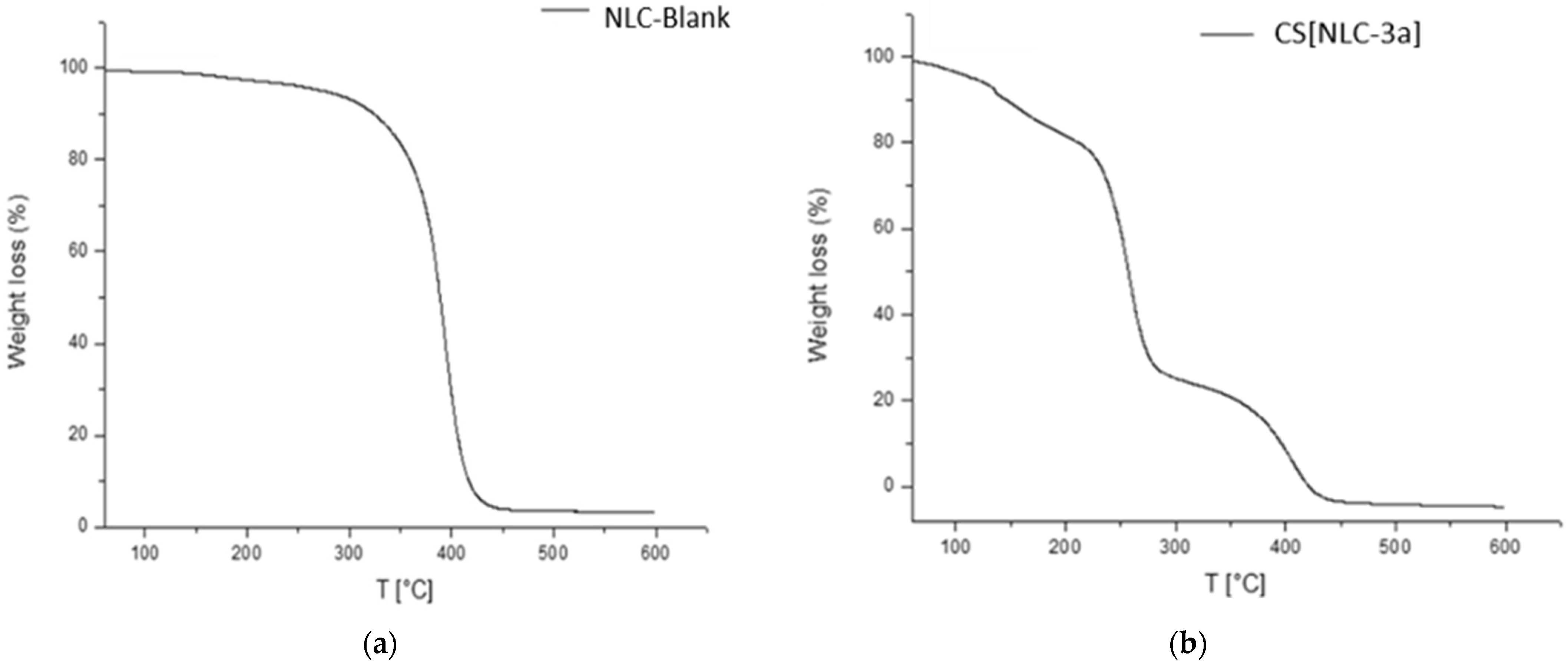
3.5. Thermogravimetric Analysis (TGA)
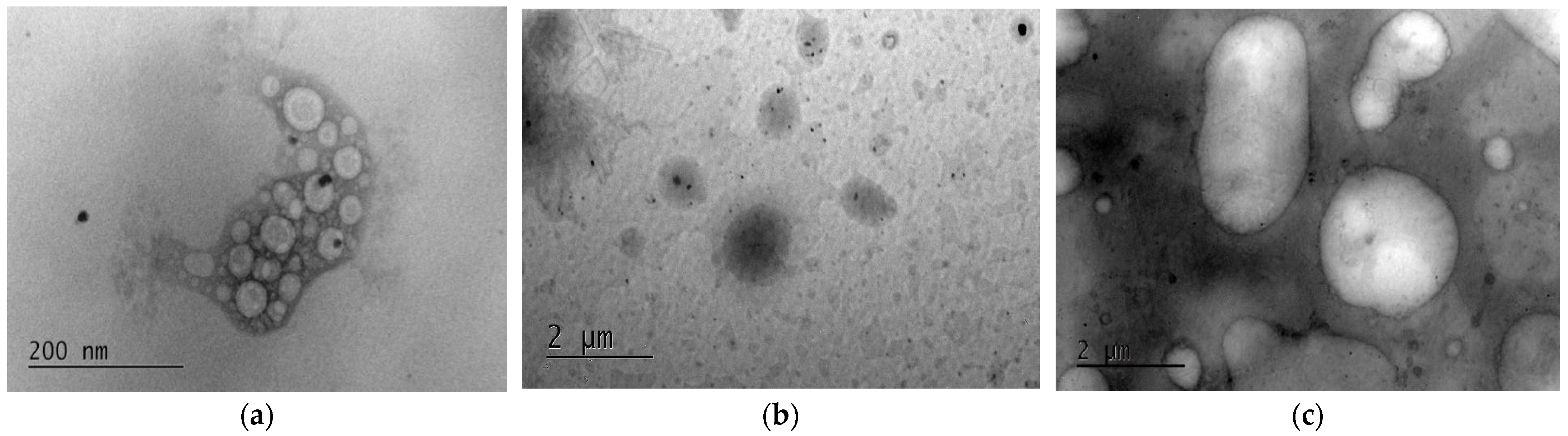
3.6. Morphology—Transmission Electron Microscopy (TEM)
3.7. Antioxidant Activity
3.8. Evaluation of Photoprotective Activity
3.9. Evaluation of the Effect of the Coating on the Stability over Time
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yaar, M.; Gilchrest, B.A. Photoageing: Mechanism, Prevention and Therapy. Br. J. Dermatol. 2007, 157, 874–887. [Google Scholar] [CrossRef]
- Ma, Y.; Yoo, J. History of Sunscreen: An Updated View. J. Cosmet. Dermatol. 2021, 20, 1044–1049. [Google Scholar] [CrossRef]
- Tanner, P.R. Sunscreen Product Formulation. Dermatol. Clin. 2006, 24, 53–62. [Google Scholar] [CrossRef]
- Schalka, S.; Ravelli, N.; Perim, N.; Vasconcelos, R. Chemical and Physical Sunscreens. In Daily Routine in Cosmetic Dermatology; Clinical Approaches and Procedures in Cosmetic Dermatology; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- López-García, R.; Ganem-Rondero, A. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC): Occlusive Effect and Penetration Enhancement Ability. J. Cosmet. Dermatol. Sci. Appl. 2015, 5, 62–72. [Google Scholar] [CrossRef]
- Oresajo, C.; Stephens, T.; Hino, P.D.; Law, R.M.; Yatskayer, M.; Foltis, P.; Pillai, S.; Pinnell, S.R. Protective Effects of a Topical Antioxidant Mixture Containing Vitamin C, Ferulic Acid, and Phloretin against Ultraviolet-Induced Photodamage in Human Skin. J. Cosmet. Dermatol. 2008, 7, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Saija, A.; Tomaino, A.; Trombetta, D.; De Pasquale, A.; Uccella, N.; Barbuzzi, T.; Paolino, D.; Bonina, F. In Vitro and in Vivo Evaluation of Caffeic and Ferulic Acids as Topical Photoprotective Agents. Int. J. Pharm. 2000, 199, 39–47. [Google Scholar] [CrossRef]
- Gasparro, F.P.; Mitchnick, M.; Nash, J.F. A Review of Sunscreen Safety and Efficacy. Photochem. Photobiol. 1998, 68, 243–256. [Google Scholar] [CrossRef] [PubMed]
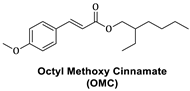
- Xu, C.; Zeng, X.; Yang, Z.; Ji, H. Sunscreen Enhancement of Octyl Methoxycinnamate Microcapsules by Using Two Biopolymers as Wall Materials. Polymers 2021, 13, 866. [Google Scholar] [CrossRef]
- Cahova, J.; Blahova, J.; Marsalek, P.; Doubkova, V.; Franc, A.; Garajová, M.; Tichy, F.; Mares, J.; Svobodova, Z. The Biological Activity of the Organic UV Filter Ethylhexyl Methoxycinnamate in Rainbow Trout (Oncorhynchus mykiss). Sci. Total Environ. 2021, 774, 145570. [Google Scholar] [CrossRef]
- Yamada, M.; Mohammed, Y.; Prow, T.W. Advances and Controversies in Studying Sunscreen Delivery and Toxicity. Adv. Drug Deliv. Rev. 2020, 153, 72–86. [Google Scholar] [CrossRef]
- Mall, J.; Naseem, N.; Haider, M.F.; Rahman, M.A.; Khan, S.; Siddiqui, S.N. Nanostructured Lipid Carriers as a Drug Delivery System: A Comprehensive Review with Therapeutic Applications. Intell. Pharm. 2025, 3, 243–255. [Google Scholar] [CrossRef]
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Structure, Preparation and Application. Adv. Pharm. Bull. 2015, 5, 305. [Google Scholar] [CrossRef] [PubMed]
- Beloqui, A.; Solinís, M.Á.; Rodríguez-Gascón, A.; Almeida, A.J.; Préat, V. Nanostructured Lipid Carriers: Promising Drug Delivery Systems for Future Clinics. Nanomedicine 2016, 12, 143–161. [Google Scholar] [CrossRef]
- Garcês, A.; Amaral, M.H.; Sousa Lobo, J.M.; Silva, A.C. Formulations Based on Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) for Cutaneous Use: A Review. Eur. J. Pharm. Sci. 2018, 112, 159–167. [Google Scholar] [CrossRef]
- Chauhan, I.; Yasir, M.; Verma, M.; Singh, A.P. Nanostructured Lipid Carriers: A Groundbreaking Approach for Transdermal Drug Delivery. Adv. Pharm. Bull. 2020, 10, 150–165. [Google Scholar] [CrossRef]
- Elmowafy, M.; Al-Sanea, M.M. Nanostructured Lipid Carriers (NLCs) as Drug Delivery Platform: Advances in Formulation and Delivery Strategies. Saudi Pharm. J. 2021, 29, 999–1012. [Google Scholar] [CrossRef]
- Jaiswal, P.; Gidwani, B.; Vyas, A. Nanostructured Lipid Carriers and Their Current Application in Targeted Drug Delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 27–40. [Google Scholar] [CrossRef]
- Li, Q.; Cai, T.; Huang, Y.; Xia, X.; Cole, S.P.C.; Cai, Y. A Review of the Structure, Preparation, and Application of NLCs, PNPs, and PLNs. Nanomaterials 2017, 7, 122. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, S.; Keck, C.M.; Anselmi, C.; Müller, R.H. Skin Photoprotection Improvement: Synergistic Interaction between Lipid Nanoparticles and Organic UV Filters. Int. J. Pharm. 2011, 414, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Niculae, G.; Lacatusu, I.; Bors, A.; Stan, R. Photostability Enhancement by Encapsulation of α-Tocopherol into Lipid-Based Nanoparticles Loaded with a UV Filter. Comptes Rendus Chim. 2014, 17, 1028–1033. [Google Scholar] [CrossRef]
- Prado, A.H.D.; Araújo, V.H.S.; Eloy, J.O.; Fonseca-Santos, B.; Pereira-Da-Silva, M.A.; Peccinini, R.G.; Chorilli, M. Synthesis and Characterization of Nanostructured Lipid Nanocarriers for Enhanced Sun Protection Factor of Octyl P-Methoxycinnamate. AAPS PharmSciTech 2020, 21, 125. [Google Scholar] [CrossRef]
- Damiani, E.; Puglia, C. Nanocarriers and Microcarriers for Enhancing the UV Protection of Sunscreens: An Overview. J. Pharm. Sci. 2019, 108, 3769–3780. [Google Scholar] [CrossRef]
- Santos, A.C.; Marto, J.; Chá-Chá, R.; Martins, A.M.; Pereira-Silva, M.; Ribeiro, H.M.; Veiga, F. Nanotechnology-Based Sunscreens—A Review. Mater. Today Chem. 2022, 23, 100709. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Q.J.; Miao, Y.L.; Luo, S.Q.; Wang, H.C.; Zhang, W.P. Effect of Solid Lipid’s Structure on Nanostructured Lipid Carriers Encapsulated with Sun Filter: Characterisation, Photo-Stability and in Vitro Release. J. Microencapsul. 2017, 34, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.C.; Tan, C.P.; Nyam, K.L. Development of Nanostructured Lipid Carriers (NLCs) Using Pumpkin and Kenaf Seed Oils with Potential Photoprotective and Antioxidative Properties. Eur. J. Lipid Sci. Technol. 2019, 121, 1900082. [Google Scholar] [CrossRef]
- Badea, G.; Lăcătuşu, I.; Badea, N.; Ott, C.; Meghea, A. Use of Various Vegetable Oils in Designing Photoprotective Nanostructured Formulations for UV Protection and Antioxidant Activity. Ind. Crops Prod. 2015, 67, 18–24. [Google Scholar] [CrossRef]
- Mazzotta, S.; Baron, G.; Fumagalli, L. Stable Isotopic Labelling of β-Sitosteryl Ferulate for Use as Analytical Tool. Food Chem. X 2022, 13, 100227. [Google Scholar] [CrossRef]
- Gilles, V.; Vieira, M.A.; Lacerda, V.; Castro, E.V.R.; Santos, R.B.; Orestes, E.; Carneiro, J.W.M.; Greco, S.J. A New, Simple and Efficient Method of Steglich Esterification of Juglone with Long-Chain Fatty Acids: Synthesis of a New Class of Non-Polymeric Wax Deposition Inhibitors for Crude Oil. J. Braz. Chem. Soc. 2015, 26, 74–83. [Google Scholar] [CrossRef]
- Bashiri, S.; Ghanbarzadeh, B.; Ayaseh, A.; Dehghannya, J.; Ehsani, A. Preparation and Characterization of Chitosan-Coated Nanostructured Lipid Carriers (CH-NLC) Containing Cinnamon Essential Oil for Enriching Milk and Anti-Oxidant Activity. LWT 2020, 119, 108836. [Google Scholar] [CrossRef]
- Tzani, A.; Kalafateli, S.; Tatsis, G.; Bairaktari, M.; Kostopoulou, I.; Pontillo, A.R.N.; Detsi, A. Natural Deep Eutectic Solvents (NaDESs) as Alternative Green Extraction Media for Ginger (Zingiber officinale Roscoe). Sustain. Chem. 2021, 2, 576–598. [Google Scholar] [CrossRef]
- Katopodi, A.; Safari, K.; Kalospyros, A.; Politopoulos, K.; Alexandratou, E.; Detsi, A. Preparation and Characterization of Solid Lipid Nanoparticles Incorporating Bioactive Coumarin Analogues as Photosensitizing Agents. Colloids Surf. B Biointerfaces 2023, 229, 113439. [Google Scholar] [CrossRef]
- Tzani, A.; Pitterou, I.; Divani, F.; Tsiaka, T.; Sotiroudis, G.; Zoumpoulakis, P.; Detsi, A. Green Extraction of Greek Propolis Using Natural Deep Eutectic Solvents (NADES) and Incorporation of the NADES-Extracts in Cosmetic Formulation. Sustain. Chem. 2022, 4, 8–25. [Google Scholar] [CrossRef]
- Qosa, H.; Mohamed, L.A.; Batarseh, Y.S.; Alqahtani, S.; Ibrahim, B.; LeVine, H.; Keller, J.N.; Kaddoumi, A. Extra-Virgin Olive Oil Attenuates Amyloid-β and Tau Pathologies in the Brains of TgSwDI Mice. J. Nutr. Biochem. 2015, 26, 1479–1490. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Kendall, C.W.C.; Marchie, A.; Parker, T.L.; Connelly, P.W.; Qian, W.; Haight, J.S.; Faulkner, D.; Vidgen, E.; Lapsley, K.G.; et al. Dose Response of Almonds on Coronary Heart Disease Risk Factors: Blood Lipids, Oxidized Low-Density Lipoproteins, Lipoprotein(a), Homocysteine, and Pulmonary Nitric Oxide: A Randomized, Controlled, Crossover Trial. Circulation 2002, 106, 1327–1332. [Google Scholar] [CrossRef]
- Zafar, A.; Imam, S.S.; Yasir, M.; Alruwaili, N.K.; Alsaidan, O.A.; Warsi, M.H.; Ullah, S.N.M.N.; Alshehri, S.; Ghoneim, M.M. Preparation of NLCs-Based Topical Erythromycin Gel: In Vitro Characterization and Antibacterial Assessment. Gels 2022, 8, 116. [Google Scholar] [CrossRef]
- Pitterou, I.; Kalogeropoulou, F.; Tzani, A.; Tsiantas, K.; Gatou, M.A.; Pavlatou, E.; Batrinou, A.; Fountzoula, C.; Kriebardis, A.; Zoumpoulakis, P.; et al. Development of Alginate Hydrogels Incorporating Essential Oils Loaded in Chitosan Nanoparticles for Biomedical Applications. Molecules 2024, 29, 5318. [Google Scholar] [CrossRef] [PubMed]
- McComiskey, K.P.M.; Tajber, L. Comparison of Particle Size Methodology and Assessment of Nanoparticle Tracking Analysis (NTA) as a Tool for Live Monitoring of Crystallisation Pathways. Eur. J. Pharm. Biopharm. 2018, 130, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Matarazzo, A.P.; Rios, C.A.; Gerônimo, G.; Ondei, R.; de Paula, E.; Breitkreitz, M.C. Development of a Versatile Nanostructured Lipid Carrier (NLC) Using Design of Experiments (DoE)—Part II: Incorporation and Stability of Butamben with Different Surfactants. Pharmaceutics 2024, 16, 863. [Google Scholar] [CrossRef] [PubMed]
- Golalipour, A.; Mohammadi, A.; Hosseinzadeh, S.; Soltani, A.; Erfani-Moghadam, V. Synergistic Cytotoxicity of Olive Leaf Extract-Loaded Lipid Nanocarriers Combined with Newcastle Disease Virus against Cervical Cancer Cells. PLoS ONE 2024, 19, e0308599. [Google Scholar] [CrossRef]
- Taha, E.; Nour, S.A.; Mamdouh, W.; Selim, A.A.; Swidan, M.M.; Ibrahim, A.B.; Naguib, M.J. Cod Liver Oil Nano-Structured Lipid Carriers (Cod-NLCs) as a Promising Platform for Nose to Brain Delivery: Preparation, in Vitro Optimization, Ex Vivo Cytotoxicity & in Vivo Biodistribution Utilizing Radioiodinated Zopiclone. Int. J. Pharm. X 2023, 5, 100160. [Google Scholar] [CrossRef]
- Zewail, M.; Nafee, N.; Helmy, M.W.; Boraie, N. Coated Nanostructured Lipid Carriers Targeting the Joints—An Effective and Safe Approach for the Oral Management of Rheumatoid Arthritis. Int. J. Pharm. 2019, 567, 118447. [Google Scholar] [CrossRef]
- Lee, S.A.; Joung, H.J.; Park, H.J.; Shin, G.H. Preparation of Chitosan-Coated Nanostructured Lipid Carriers (CH-NLCs) to Control Iron Delivery and Their Potential Application to Food Beverage System. J. Food Sci. 2017, 82, 904–912. [Google Scholar] [CrossRef]
- Fang, X.; Shima, M.; Kadota, M.; Tsuno, T.; Adachi, S. Suppressive Effect of Alkyl Ferulate on the Oxidation of Linoleic Acid. Biosci. Biotechnol. Biochem. 2006, 70, 457–461. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, L.; Zhao, P.; Ma, G.; Li, Q.; Yu, X. Synthesized Alkyl Ferulates with Different Chain Lengths Inhibited the Formation of Lipid Oxidation Products in Soybean Oil during Deep Frying. Food Chem. 2023, 410, 135458. [Google Scholar] [CrossRef] [PubMed]
- Cantele, C.; Martina, K.; Potenziani, G.; Rossi, A.M.; Cardenia, V.; Bertolino, M. Antioxidant Properties of Ferulic Acid-Based Lipophenols in Oil-in-Water (O/W) Emulsions. LWT 2023, 189, 115505. [Google Scholar] [CrossRef]
- Garoli, D.; Pelizzo, M.G.; Bernardini, B.; Nicolosi, P.; Alaibac, M. Sunscreen Tests: Correspondence between in Vitro Data and Values Reported by the Manufacturers. J. Dermatol. Sci. 2008, 52, 193–204. [Google Scholar] [CrossRef]
- Battistin, M.; Bonetto, A.; Nicoli, F.; Torreggiani, E.; Brunetta, A.; Cesa, E.; Manfredini, S.; Baldisserotto, A.; Vertuani, S. Synthesis and Evaluation of a ZnO-Chitosan Adduct for Safe and Sustainable Enhanced Ultra-Violet (UV) Sunscreens Protection. Molecules 2024, 29, 5204. [Google Scholar] [CrossRef] [PubMed]
- Ntohogian, S.; Gavriliadou, V.; Christodoulou, E.; Nanaki, S.; Lykidou, S.; Naidis, P.; Mischopoulou, L.; Barmpalexis, P.; Nikolaidis, N.; Bikiaris, D.N. Chitosan Nanoparticles with Encapsulated Natural and Uf-Purified Annatto and Saffron for the Preparation of UV Protective Cosmetic Emulsions. Molecules 2018, 23, 2107. [Google Scholar] [CrossRef]
- Rabelo, R.S.; Oliveira, I.F.; da Silva, V.M.; Prata, A.S.; Hubinger, M.D. Chitosan Coated Nanostructured Lipid Carriers (NLCs) for Loading Vitamin D: A Physical Stability Study. Int. J. Biol. Macromol. 2018, 119, 902–912. [Google Scholar] [CrossRef]



| Factors | Responses |
|---|---|
| Sonication time (ST, min) | Size of NLCs (size, nm) |
| NADES (% v/v) | Encapsulation efficiency (%EE) |
| Drug loading (DL % w/w) |
| Independent Variables | ||||||
|---|---|---|---|---|---|---|
| Run | Sonication Time (ST) (min) | NADES (% v/v) | DL (% w/w) | |||
| 1 | 7.5 | 0 | 4 | 0 | 3 | 0 |
| 2 | 7.5 | 0 | 6 | +1 | 1 | −1 |
| 3 | 7.5 | 0 | 4 | 0 | 3 | 0 |
| 4 | 7.5 | 0 | 4 | 0 | 3 | 0 |
| 5 | 5 | −1 | 6 | +1 | 3 | 0 |
| 6 | 10 | +1 | 2 | –1 | 3 | 0 |
| 7 | 7.5 | 0 | 2 | −1 | 5 | +1 |
| 8 | 7.5 | 0 | 2 | –1 | 1 | −1 |
| 9 | 5 | −1 | 2 | –1 | 3 | 0 |
| 10 | 10 | +1 | 4 | 0 | 5 | +1 |
| 11 | 5 | –1 | 4 | 0 | 5 | +1 |
| 12 | 7.5 | 0 | 6 | +1 | 5 | +1 |
| 13 | 10 | +1 | 6 | +1 | 3 | 0 |
| 14 | 10 | +1 | 4 | 0 | 1 | −1 |
| 15 | 5 | −1 | 4 | 0 | 1 | −1 |
| Compound | Structure | Yield (%) | Compound | Structure | Yield (%) |
|---|---|---|---|---|---|
| 2a | 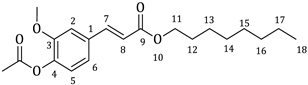 | 65 | 3a | 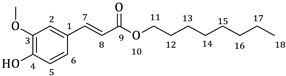 | 54 |
| 2b | 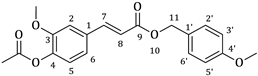 | 57 | 3b | 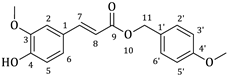 | 38 |
| 2c |  | 40 | 3c | 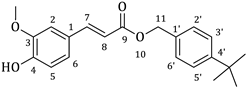 | 45 |
| Conditions | Results | |||||||
|---|---|---|---|---|---|---|---|---|
| Almond Oil | Olive Oil | |||||||
| Sonication Time (min) | ΝADES (% v/v) | DL (% w/w) | Hydrodynamic Diameter (nm) | ζ-Potential (mV) | EE (%) | Hydrodynamic Diameter (nm) | ζ-Potential (mV) | EE (%) |
| 7.5 | 4 | 0 | 126.4 ± 1.7 | 21.9 ± 1.2 | - | 109.9 ± 0.6 | 21.6 ± 1.5 | - |
| 7.5 | 6 | 1 | 155.0 ± 9.5 | 30.7 ± 0.5 | 98 | 183.2 ± 17.1 | 24.3 ± 8.8 | 96 |
| 7.5 | 4 | 3 | 188.7 ± 19.4 | 20.1 ± 3.9 | 90 | 154.7 ± 28.8 | 24.5 ± 5.7 | 96 |
| 7.5 | 6 | 3 | 205.5 ± 3.7 | 36.3 ± 4.6 | 91 | 112.5 ± 4.4 | 21.1 ± 1.9 | 97 |
| Conditions | Results | |||||||
|---|---|---|---|---|---|---|---|---|
| Run | Sonication Time (ST) (min) | NADES (% v/v) | DL (% w/w) | Hydrodynamic Diameter NTA (nm) | ΕΕ (%) | |||
| 1 | 7.5 | 0 | 4 | 0 | 3 | 0 | 154.7 ± 28.8 | 90 |
| 2 | 7.5 | 0 | 6 | +1 | 1 | −1 | 183.2 ± 17.1 | 81 |
| 3 | 7.5 | 0 | 4 | 0 | 3 | 0 | 151.3 ± 4.5 | 91 |
| 4 | 7.5 | 0 | 4 | 0 | 3 | 0 | 146.3 ± 5.0 | 92 |
| 5 | 5 | −1 | 6 | +1 | 3 | 0 | 112.5 ± 4.4 | 80 |
| 6 | 10 | +1 | 2 | –1 | 3 | 0 | 112.2 ± 5.3 | 95 |
| 7 | 7.5 | 0 | 2 | −1 | 5 | +1 | 119.3 ± 0.8 | 67 |
| 8 | 7.5 | 0 | 2 | –1 | 1 | −1 | 129.8 ± 1.3 | 63 |
| 9 | 5 | −1 | 2 | –1 | 3 | 0 | 134.8 ± 0.7 | 82 |
| 10 | 10 | +1 | 4 | 0 | 5 | +1 | 116.0 ± 2.1 | 76 |
| 11 | 5 | –1 | 4 | 0 | 5 | +1 | 111.6 ± 2.0 | 70 |
| 12 | 7.5 | 0 | 6 | +1 | 5 | +1 | 123.1 ± 1.3 | 66 |
| 13 | 10 | +1 | 6 | +1 | 3 | 0 | 131.2 ± 2.4 | 66 |
| 14 | 10 | +1 | 4 | 0 | 1 | −1 | 141.5 ± 4.5 | 70 |
| 15 | 5 | −1 | 4 | 0 | 1 | −1 | 130.4 ± 1.8 | 42 |
| Conditions | Results | |||||||
|---|---|---|---|---|---|---|---|---|
| Run | Sonication Time (ST) (min) | NADES (% v/v) | DL (% w/w) | Hydrodynamic Diameter DLS (nm) | PDI | |||
| 1 | 7.5 | 0 | 4 | 0 | 3 | 0 | 241.7 ± 7.3 | 0.557 ± 0.103 |
| 2 | 7.5 | 0 | 6 | +1 | 1 | −1 | 267.6 ± 3.0 | 0.343 ± 0.031 |
| 3 | 7.5 | 0 | 4 | 0 | 3 | 0 | 233.2 ± 2.4 | 0.298 ± 0.009 |
| 4 | 7.5 | 0 | 4 | 0 | 3 | 0 | 175.8 ± 7.1 | 0.317 ± 0.015 |
| 5 | 5 | −1 | 6 | +1 | 3 | 0 | 242.4 ± 2.9 | 0.342 ± 0.026 |
| 6 | 10 | +1 | 2 | –1 | 3 | 0 | 172.4 ± 57.2 | 0.426 ± 0.269 |
| 7 | 7.5 | 0 | 2 | −1 | 5 | +1 | 258.1 ± 6.1 | 0.315 ± 0.015 |
| 8 | 7.5 | 0 | 2 | –1 | 1 | −1 | 231.9 ± 8.6 | 0.298 ± 0.002 |
| 9 | 5 | −1 | 2 | –1 | 3 | 0 | 177.3 ± 1.2 | 0.304 ± 0.016 |
| 10 | 10 | +1 | 4 | 0 | 5 | +1 | 220.0 ± 10.3 | 0.313 ± 0.025 |
| 11 | 5 | –1 | 4 | 0 | 5 | +1 | 288.0 ± 6.8 | 0.149 ± 0.009 |
| 12 | 7.5 | 0 | 6 | +1 | 5 | +1 | 194.6 ± 8.8 | 0.284 ± 0.068 |
| 13 | 10 | +1 | 6 | +1 | 3 | 0 | 141.7 ± 9.2 | 0.474 ± 0.056 |
| 14 | 10 | +1 | 4 | 0 | 1 | −1 | 167.7 ± 2.1 | 0.337 ± 0.033 |
| 15 | 5 | −1 | 4 | 0 | 1 | −1 | 153.0 ± 2.3 | 0.395 ± 0.005 |
| Model | Lack of Fit | A | B | C | AB | BC | A2 | B2 | C2 | |
|---|---|---|---|---|---|---|---|---|---|---|
| p-value | 0.0254 | 0.7563 | 0.6241 | 0.3014 | 0.0085 | 0.0407 | 0.2324 | 0.0087 | 0.0735 | 0.1534 |
| F-value | 5.56 | 0.4874 | 0.2666 | 1.28 | 14.79 | 6.76 | 1.76 | 14.62 | 4.69 | 2.67 |
| (3) |
| Model | Lack of Fit | A | C | A2 | C2 | |
|---|---|---|---|---|---|---|
| p-value | 0.045 | 0,3337 | 0.3006 | 0.4643 | 0.2181 | 0.0069 |
| F-value | 3.60 | 1.42 | 1.19 | 0.5789 | 1.73 | 11.48 |
| Lower Limit | Upper Limit | Criteria | |
|---|---|---|---|
| A: ST (min) | 5 | 10 | - |
| B: NADES (% v/v) | 2 | 6 | - |
| C: DL (% w/w) | 1 | 5 | - |
| Size (nm) | 111.6 | 154.7 | Minimize |
| EE (%) | 42 | 95 | Maximize |
| Hydrodynamic Diameter (nm) | ΕΕ (%) | |
|---|---|---|
| Mean | 116.7 ± 5.0 | 78.7 ± 23.0 |
| Predicted (%) | 118.40 ± 11.37 | 77.50 ± 10.69 |
| 95% PI low | 99.22 | 61.93 |
| 95% PI high | 139.58 | 99.86 |
| Compound | Hydrodynamic Diameter (nm) | EE (%) |
|---|---|---|
| CS[NLC-3b] | 105.3 ± 13.4 | 75.5 ± 9.1 |
| CS[NLC-3c] | 138.7 ± 9.3 | 38.0 ± 5.6 |
| Predicted (%) | 118.40 ± 11.37 | 77.50 ± 10.69 |
| Sample | (%) Inhibition of Lipid Peroxidation (100 μM) |
|---|---|
 | 83.7 ± 3.6 |
| CS[NLC-3a] | 81.6 ± 11.8 |
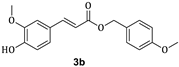 | 73.6 ± 10.4 |
| CS[NLC-3b] | 88.7 ± 7.6 |
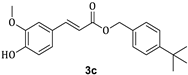 | 73.6 ± 10.5 |
| CS[NLC-3c] | 90.7± 5.5 |
 Trolox | 81.0 ± 0.1 |
| Sunscreen Filters | Content (% w/w) |
|---|---|
| Diethylamino hydroxybenzoyl hexyl benzoate | 4.0 |
| Ethylhexyl salicylate | 5.0 |
| Ethylhexyl triazone | 1.5 |
| Octocrylene | 3.0 |
| Tris-biphenyl triazine | 2.0 |
| Sample | SPF | UVA/UVB | λc | Ester Concentration in DBA (% w/w) |
|---|---|---|---|---|
 | 4.45 ± 0.88 | 0.405 ± 0.01 | 349.8 ± 0.41 | 2.5 |
 | 9.22 ± 1.98 | 0.381 ± 0.01 | 343.9 ± 0.22 | 2.5 |
 | 5.05 ± 0.54 | 0.375 ± 0.01 | 342.4 ± 0.01 | 2.5 |
 | 8.19 ± 0.86 | 0.153 ± 0.01 | 337.7 ± 0.19 | 2.5 |
| Sample | Ester Content in the Formulation (%w/w) | SPF | SPF Boost | UVA/UVB | λc |
|---|---|---|---|---|---|
| Reference sunscreen formulation | 0 | 15.12 ± 5.43 | - | 0.694 ± 0.01 | 375.3 ± 0.54 |
| NLC-Blank | 0 | 15.35 ± 3.96 | 0.23 | 0.700 ± 0.01 | 375.6 ± 0.70 |
| CS[NLC-Blank] | 0 | 15.47 ± 2.20 | 0.35 | 0.684 ± 0.01 | 374.9 ± 0.25 |
| NLC-3b | 0.007 | 15.66 ± 3.07 | 0.54 | 0.696 ± 0.01 | 375.2 ± 0.38 |
| CS[NLC-3b] | 0.007 | 22.47± 6.20 | 7.35 | 0.701 ± 0.01 | 375.7 ± 0.31 |
| Size (nm) | ||
|---|---|---|
| 1 Day | 60 Days | |
| NLC-Blank | 131.3 ± 2.2 | 162.4 ± 4.8 |
| CS[NLC-Blank] | 116.0 ± 2.1 | 132.3 ± 1.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pitterou, I.; Kostopoulou, I.; Karadendrou, M.-A.; Mitsioni, M.F.; Fountzoula, C.; Kriebardis, A.; Miliaronikolaki, M.; Paraskevopoulos, N.; Tzani, A.; Detsi, A. Chitosan-Coated Nanostructured Lipid Carriers (NLCs) Incorporating Esters of Ferulic Acid with Photoprotective Activity. Macromol 2025, 5, 50. https://doi.org/10.3390/macromol5040050
Pitterou I, Kostopoulou I, Karadendrou M-A, Mitsioni MF, Fountzoula C, Kriebardis A, Miliaronikolaki M, Paraskevopoulos N, Tzani A, Detsi A. Chitosan-Coated Nanostructured Lipid Carriers (NLCs) Incorporating Esters of Ferulic Acid with Photoprotective Activity. Macromol. 2025; 5(4):50. https://doi.org/10.3390/macromol5040050
Chicago/Turabian StylePitterou, Ioanna, Ioanna Kostopoulou, Maria-Anna Karadendrou, Marianna Fanouria Mitsioni, Christina Fountzoula, Anastasios Kriebardis, Marianthi Miliaronikolaki, Nikolaos Paraskevopoulos, Andromachi Tzani, and Anastasia Detsi. 2025. "Chitosan-Coated Nanostructured Lipid Carriers (NLCs) Incorporating Esters of Ferulic Acid with Photoprotective Activity" Macromol 5, no. 4: 50. https://doi.org/10.3390/macromol5040050
APA StylePitterou, I., Kostopoulou, I., Karadendrou, M.-A., Mitsioni, M. F., Fountzoula, C., Kriebardis, A., Miliaronikolaki, M., Paraskevopoulos, N., Tzani, A., & Detsi, A. (2025). Chitosan-Coated Nanostructured Lipid Carriers (NLCs) Incorporating Esters of Ferulic Acid with Photoprotective Activity. Macromol, 5(4), 50. https://doi.org/10.3390/macromol5040050











