Smart Theranostic Platforms Based on Carbohydrate Hydrogels
Abstract
1. Introduction
| [Natural Carbohydrate Sources] | |
| (e.g., chitosan, alginate, and dextran) | |
| [Synthesis of Hydrogels] | |
| - 3D polymeric network; | |
| - Biocompatible, biodegradable; | |
| - Modifiable functional groups. | |
| [Hydrogel Properties] | |
| - Tunable porosity; | |
| - High water content; | |
| - Stimuli-responsiveness (pH, T°, and enzymes). | |
| [Integrated Theranostic Functions] | |
| BIOSENSING | DRUG DELIVERY |
| - Glucose monitoring; | - Targeted release; |
| - Cancer biomarkers; | - Site-specific action; |
| - Respiratory markers. | - pH-/T°-/enzyme-triggered. |
| [Clinical Applications] | |
| - Cardiac health monitoring; | |
| - Respiratory diagnostics; | |
| - Controlled drug administration; | |
| - Oncology and postoperative care; | |
| - Gene therapy and regenerative medicine. | |
2. Carbohydrate-Based Hydrogels
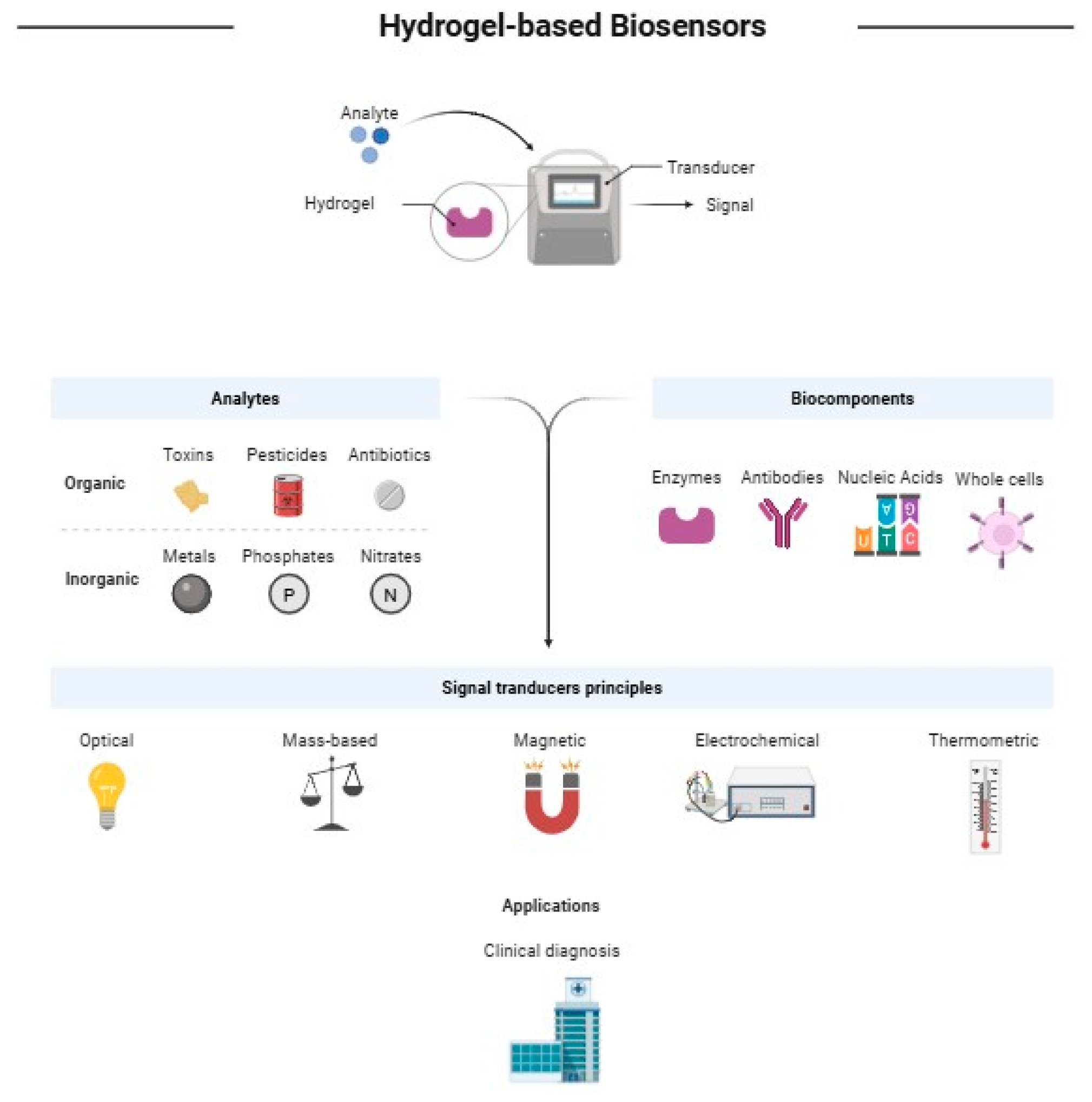
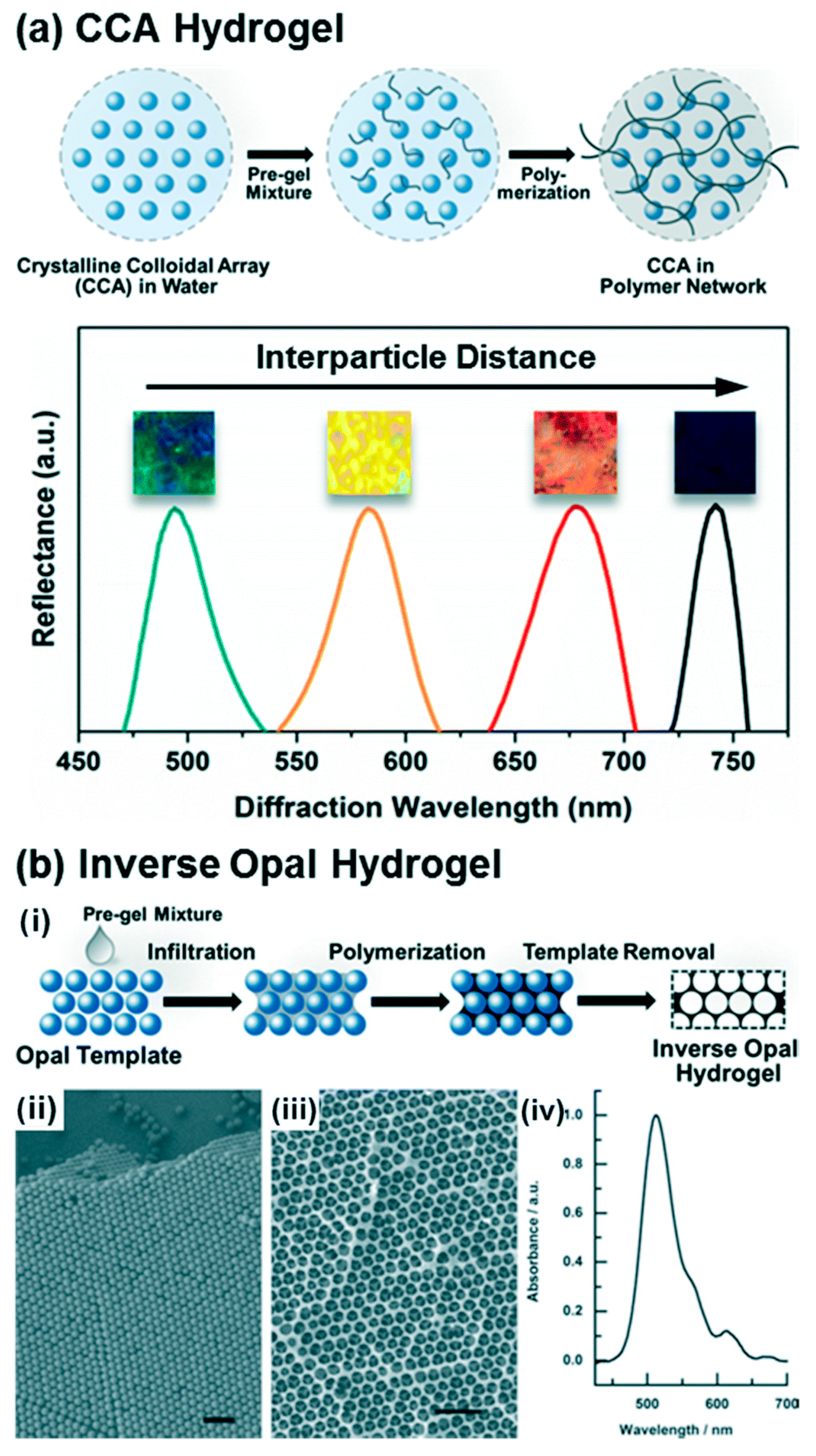
3. Core Concepts in Biosensing and Drug Delivery Carbohydrate-Based Hydrogels
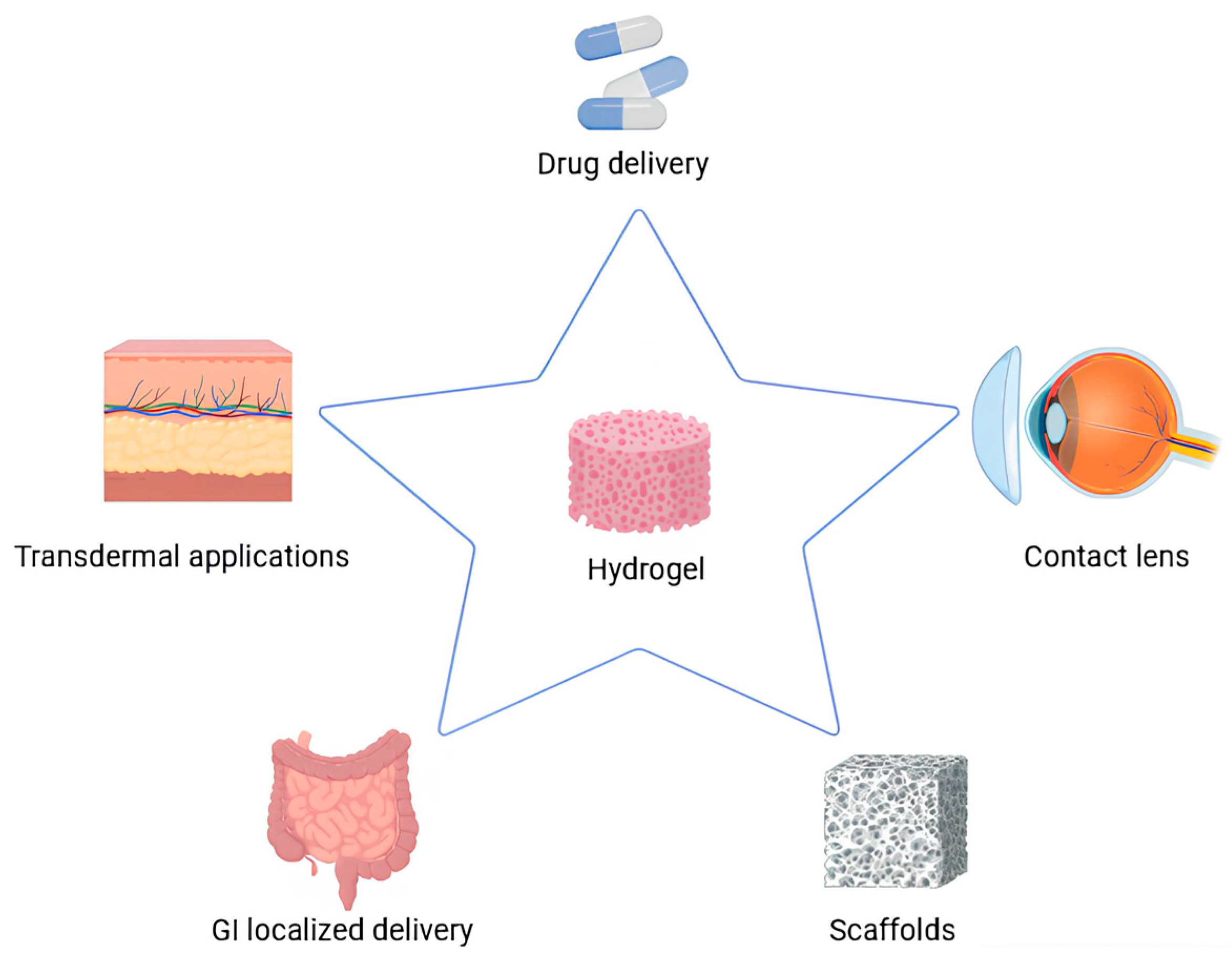
4. Application of Carbohydrate-Based Hydrogel as Theranostic Devices
4.1. Monitoring Cardiac Wellness
4.2. Respiratory Monitoring
4.3. Drug Delivery Devices
5. Conclusions and Future Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hemdan, M.; Ali, M.A.; Doghish, A.S.; Mageed, S.S.A.; Elazab, I.M.; Khalil, M.M.; Mabrouk, M.; Das, D.B.; Amin, A.S. Innovations in Biosensor Technologies for Healthcare Diagnostics and Therapeutic Drug Monitoring: Applications, Recent Progress, and Future Research Challenges. Sensors 2024, 24, 5143. [Google Scholar] [CrossRef]
- Lino, C.; Barrias, S.; Chaves, R.; Adega, F.; Martins-Lopes, P.; Fernandes, J.R. Biosensors as diagnostic tools in clinical applications. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188726. [Google Scholar] [CrossRef]
- Ho, T.-C.; Chang, C.-C.; Chan, H.-P.; Chung, T.-W.; Shu, C.-W.; Chuang, K.-P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef]
- Vicentini, F.C.; Silva, L.R.G.; Stefano, J.S.; Lima, A.R.; Prakash, J.; Bonacin, J.A.; Janegitz, B.C. Starch-Based Electrochemical Sensors and Biosensors: A Review. Biomed. Mater. Devices 2023, 1, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Di, X.; Liang, X.; Shen, C.; Pei, Y.; Wu, B.; He, Z. Carbohydrates Used in Polymeric Systems for Drug Delivery: From Structures to Applications. Pharmaceutics 2022, 14, 739. [Google Scholar] [CrossRef]
- Valentino, A.; Yazdanpanah, S.; Conte, R.; Calarco, A.; Peluso, G. Smart Nanocomposite Hydrogels as Next-Generation Therapeutic and Diagnostic Solutions. Gels 2024, 10, 689. [Google Scholar] [CrossRef] [PubMed]
- Conte, R.; Valentino, A.; Romano, S.; Margarucci, S.; Petillo, O.; Calarco, A. Stimuli-Responsive Nanocomposite Hydrogels for Oral Diseases. Gels 2024, 10, 478. [Google Scholar] [CrossRef]
- Sun, S.; Chen, J. Recent Advances in Hydrogel-Based Biosensors for Cancer Detection. ACS Appl. Mater. Interfaces 2024, 16, 46988–47002. [Google Scholar] [CrossRef] [PubMed]
- Conte, R.; Foggia, R.; Valentino, A.; Di Salle, A.; Kandsi, F.; Calarco, A. Nanotechnology advancements transforming molecular diagnostics: Applications in precision healthcare. Int. J. Nano Dimens. 2024, 15, 1–8. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, Y.; Yang, Q.; Yuan, N.; Zhang, W. Review of Electrochemical DNA Biosensors for Detecting Food Borne Pathogens. Sensors 2019, 19, 4916. [Google Scholar] [CrossRef]
- Polat, E.O.; Cetin, M.M.; Tabak, A.F.; Bilget Güven, E.; Uysal, B.Ö.; Arsan, T.; Kabbani, A.; Hamed, H.; Gül, S.B. Transducer Technologies for Biosensors and Their Wearable Applications. Biosensors 2022, 12, 385. [Google Scholar] [CrossRef]
- Pandey, R.R.; Chusuei, C.C. Carbon Nanotubes, Graphene, and Carbon Dots as Electrochemical Biosensing Composites. Molecules 2021, 26, 6674. [Google Scholar] [CrossRef]
- Chu, S.S.; Nguyen, H.A.; Zhang, J.; Tabassum, S.; Cao, H. Towards Multiplexed and Multimodal Biosensor Platforms in Real-Time Monitoring of Metabolic Disorders. Sensors 2022, 22, 5200. [Google Scholar] [CrossRef]
- Johnston, L.; Wang, G.; Hu, K.; Qian, C.; Liu, G. Advances in Biosensors for Continuous Glucose Monitoring Towards Wearables. Front. Bioeng. Biotechnol. 2021, 9, 733810. [Google Scholar] [CrossRef] [PubMed]
- Psoma, S.D.; Kanthou, C. Wearable Insulin Biosensors for Diabetes Management: Advances and Challenges. Biosensors 2023, 13, 719. [Google Scholar] [CrossRef]
- Akbari Nakhjavani, S.; Mirzajani, H.; Carrara, S.; Onbaşlı, M.C. Advances in biosensor technologies for infectious diseases detection. TrAC Trends Anal. Chem. 2024, 180, 117979. [Google Scholar] [CrossRef]
- Hara, T.O.; Singh, B. Electrochemical Biosensors for Detection of Pesticides and Heavy Metal Toxicants in Water: Recent Trends and Progress. ACS EST Water 2021, 1, 462–478. [Google Scholar] [CrossRef]
- Palladino, F.; Marcelino, P.R.F.; Schlogl, A.E.; José, Á.H.M.; Rodrigues, R.d.C.L.B.; Fabrino, D.L.; Santos, I.J.B.; Rosa, C.A. Bioreactors: Applications and Innovations for a Sustainable and Healthy Future—A Critical Review. Appl. Sci. 2024, 14, 9346. [Google Scholar] [CrossRef]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Nair, A.B.; Shah, J.; Sreeharsha, N.; Gupta, S.; Shinu, P. Emerging Role of Hydrogels in Drug Delivery Systems, Tissue Engineering and Wound Management. Pharmaceutics 2021, 13, 357. [Google Scholar] [CrossRef]
- Parhi, R. Cross-Linked Hydrogel for Pharmaceutical Applications: A Review. Adv. Pharm. Bull. 2017, 7, 515–530. [Google Scholar] [CrossRef]
- Cicha, I.; Priefer, R.; Severino, P.; Souto, E.B.; Jain, S. Biosensor-Integrated Drug Delivery Systems as New Materials for Biomedical Applications. Biomolecules 2022, 12, 1198. [Google Scholar] [CrossRef]
- Wu, J.; Xue, W.; Yun, Z.; Liu, Q.; Sun, X. Biomedical applications of stimuli-responsive “smart” interpenetrating polymer network hydrogels. Mater. Today Bio 2024, 25, 100998. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Palomino, K.; Magaña, H.; Bucio, E. Hydrogels Classification According to the Physical or Chemical Interactions and as Stimuli-Sensitive Materials. Gels 2021, 7, 182. [Google Scholar] [CrossRef] [PubMed]
- Basu, P.; Banerjee, A.; Okoro, P.D.; Masoumi, A.; Kanjilal, B.; Akbari, M.; Martins-Green, M.; Armstrong, D.G.; Noshadi, I. Integration of Functional Polymers and Biosensors to Enhance Wound Healing. Adv. Healthc. Mater. 2024, 13, e2401461. [Google Scholar] [CrossRef]
- Valentino, A.; Conte, R.; De Luca, I.; Di Cristo, F.; Peluso, G.; Bosetti, M.; Calarco, A. Thermo-Responsive Gel Containing Hydroxytyrosol-Chitosan Nanoparticles (Hyt@tgel) Counteracts the Increase of Osteoarthritis Biomarkers in Human Chondrocytes. Antioxidants 2022, 11, 1210. [Google Scholar] [CrossRef]
- Sun, X.; Agate, S.; Salem, K.S.; Lucia, L.; Pal, L. Hydrogel-Based Sensor Networks: Compositions, Properties, and Applications—A Review. ACS Appl. Bio Mater. 2021, 4, 140–162. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, X.; Tao, S.; Wang, Q.; Ma, P.Q.; Li, Z.B.; Wu, Y.L.; Li, D.W. Research advances in smart responsive-hydrogel dressings with potential clinical diabetic wound healing properties. Mil. Med. Res. 2023, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Omidian, H.; Chowdhury, S.D. High-Performing Conductive Hydrogels for Wearable Applications. Gels 2023, 9, 549. [Google Scholar] [CrossRef]
- Fedi, A.; Vitale, C.; Giannoni, P.; Caluori, G.; Marrella, A. Biosensors to Monitor Cell Activity in 3D Hydrogel-Based Tissue Models. Sensors 2022, 22, 1517. [Google Scholar] [CrossRef]
- Song, J.; Zhang, Y.; Chan, S.Y.; Du, Z.; Yan, Y.; Wang, T.; Li, P.; Huang, W. Hydrogel-based flexible materials for diabetes diagnosis, treatment, and management. npj Flex. Electron. 2021, 5, 26. [Google Scholar] [CrossRef]
- Ahmed, A.; Aziz, S.; Abd-Alrazaq, A.; Farooq, F.; Househ, M.; Sheikh, J. The Effectiveness of Wearable Devices Using Artificial Intelligence for Blood Glucose Level Forecasting or Prediction: Systematic Review. J. Med. Internet Res. 2023, 25, e40259. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Ershad, F.; Zhao, M.; Isseroff, R.R.; Duan, B.; Zhou, Y.; Wang, Y.; Yu, C. Wearable electronics for skin wound monitoring and healing. Soft Sci. 2022, 2, 9. [Google Scholar] [CrossRef]
- Van Hoovels, K.; Xuan, X.; Cuartero, M.; Gijssel, M.; Swarén, M.; Crespo, G.A. Can Wearable Sweat Lactate Sensors Contribute to Sports Physiology? ACS Sens. 2021, 6, 3496–3508. [Google Scholar] [CrossRef]
- Kumi, M.; Ejeromedoghene, O.; Sudane, W.D.; Zhang, Z. Unlocking the biological response of smart Stimuli-Responsive hydrogels and their application in biological systems. Eur. Polym. J. 2024, 209, 112906. [Google Scholar] [CrossRef]
- Gerlach, G.; Guenther, M.; Sorber, J.; Suchaneck, G.; Arndt, K.-F.; Richter, A. Chemical and pH sensors based on the swelling behavior of hydrogels. Sens. Actuators B Chem. 2005, 111–112, 555–561. [Google Scholar] [CrossRef]
- Lee, C.H.; Bae, Y.C. Effect of Salt on Swelling Behaviors of Thermosensitive Hydrogels: Applicability of the Nonrandom Contact Model. Macromolecules 2015, 48, 4063–4072. [Google Scholar] [CrossRef]
- Morariu, S. Advances in the Design of Phenylboronic Acid-Based Glucose-Sensitive Hydrogels. Polymers 2023, 15, 582. [Google Scholar] [CrossRef]
- Elsherif, M.; Moreddu, R.; Alam, F.; Salih, A.E.; Ahmed, I.; Butt, H. Wearable Smart Contact Lenses for Continual Glucose Monitoring: A Review. Front. Med. 2022, 9, 858784. [Google Scholar] [CrossRef]
- Thirumalai, D.; Santhamoorthy, M.; Kim, S.-C.; Lim, H.-R. Conductive Polymer-Based Hydrogels for Wearable Electrochemical Biosensors. Gels 2024, 10, 459. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Kaar, J.L.; Stoykovich, M.P. Design and functionalization of responsive hydrogels for photonic crystal biosensors. Mol. Syst. Des. Eng. 2016, 1, 225–241. [Google Scholar] [CrossRef]
- Xue, H.; Wang, D.; Jin, M.; Gao, H.; Wang, X.; Xia, L.; Li, D.a.; Sun, K.; Wang, H.; Dong, X.; et al. Hydrogel electrodes with conductive and substrate-adhesive layers for noninvasive long-term EEG acquisition. Microsyst. Nanoeng. 2023, 9, 79. [Google Scholar] [CrossRef]
- Xu, S.; Li, T.; Ren, H.; Mao, X.; Ye, X.; Liang, B. PEDOT: PSS Hydrogel based Flexible Electrodes for Wearable ECG Monitoring. In Proceedings of the 2020 IEEE SENSORS, Rotterdam, The Netherlands, 25–28 October 2020; pp. 1–4. [Google Scholar]
- Lee, Y.; Yim, S.-G.; Lee, G.W.; Kim, S.; Kim, H.S.; Hwang, D.Y.; An, B.-S.; Lee, J.H.; Seo, S.; Yang, S.Y. Self-Adherent Biodegradable Gelatin-Based Hydrogel Electrodes for Electrocardiography Monitoring. Sensors 2020, 20, 5737. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Liu, T.; Wu, Q.; Ding, Y.; Ou, R.; Guo, C.; Liu, Z.; Wang, Q. Multimodal Hydrogel-Based Respiratory Monitoring System for Diagnosing Obstructive Sleep Apnea Syndrome. Adv. Funct. Mater. 2022, 32, 2204686. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, W.; Li, J.; Li, C.; Xu, S.; Sun, Y.; Ma, Z.; Zhao, H.; Ren, L. Multimodal and flexible hydrogel-based sensors for respiratory monitoring and posture recognition. Biosens. Bioelectron. 2024, 243, 115773. [Google Scholar] [CrossRef]
- Hong, X.; Wu, Z.; Chen, L.; Wu, F.; Wei, L.; Yuan, W. Hydrogel Microneedle Arrays for Transdermal Drug Delivery. Nano-Micro Lett. 2014, 6, 191–199. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, N.; Chauhan, N. Synthesis, characterization and swelling studies of pH responsive psyllium and methacrylamide based hydrogels for the use in colon specific drug delivery. Carbohydr. Polym. 2007, 69, 631–643. [Google Scholar] [CrossRef]
- Das, A.; Wadhwa, S.; Srivastava, A.K. Cross-linked guar gum hydrogel discs for colon-specific delivery of ibuprofen: Formulation and in vitro evaluation. Drug Deliv. 2006, 13, 139–142. [Google Scholar] [CrossRef]
- Miyazaki, S.; Suzuki, S.; Kawasaki, N.; Endo, K.; Takahashi, A.; Attwood, D. In situ gelling xyloglucan formulations for sustained release ocular delivery of pilocarpine hydrochloride. Int. J. Pharm. 2001, 229, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Burgalassi, S.; Chetoni, P.; Panichi, L.; Boldrini, E.; Saettone, M.F. Xyloglucan as a novel vehicle for timolol: Pharmacokinetics and pressure lowering activity in rabbits. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2000, 16, 497–509. [Google Scholar] [CrossRef]
- Cooper, R.C.; Yang, H. Hydrogel-based ocular drug delivery systems: Emerging fabrication strategies, applications, and bench-to-bedside manufacturing considerations. J. Control. Release Off. J. Control. Release Soc. 2019, 306, 29–39. [Google Scholar] [CrossRef]
- Torres-Luna, C.; Fan, X.; Domszy, R.; Hu, N.; Wang, N.S.; Yang, A. Hydrogel-based ocular drug delivery systems for hydrophobic drugs. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2020, 154, 105503. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.R.; Kondiah, P.P.D.; Choonara, Y.E.; du Toit, L.C.; Ally, N.; Pillay, V. Hydrogel Biomaterials for Application in Ocular Drug Delivery. Front. Bioeng. Biotechnol. 2020, 8, 228. [Google Scholar] [CrossRef]
- Xinming, L.; Yingde, C.; Lloyd, A.W.; Mikhalovsky, S.V.; Sandeman, S.R.; Howel, C.A.; Liewen, L. Polymeric hydrogels for novel contact lens-based ophthalmic drug delivery systems: A review. Contact Lens Anterior Eye J. Br. Contact Lens Assoc. 2008, 31, 57–64. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, H.; Wang, J.; Ge, L.; Zhu, J. Development of a thermally responsive nanogel based on chitosan-poly(N-isopropylacrylamide-co-acrylamide) for paclitaxel delivery. J. Pharm. Sci. 2014, 103, 2012–2021. [Google Scholar] [CrossRef]
- Mennini, N.; Casella, G.; Cirri, M.; Maestrelli, F.; Mura, P. Development of cyclodextrin hydrogels for vaginal delivery of dehydroepiandrosterone. J. Pharm. Pharmacol. 2016, 68, 762–771. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Wu, W.; Li, H. Vaginal delivery of carboplatin-loaded thermosensitive hydrogel to prevent local cervical cancer recurrence in mice. Drug Deliv. 2016, 23, 3544–3551. [Google Scholar] [CrossRef] [PubMed]
- Vanić, Ž.; Hurler, J.; Ferderber, K.; Golja Gašparović, P.; Škalko-Basnet, N.; Filipović-Grčić, J. Novel vaginal drug delivery system: Deformable propylene glycol liposomes-in-hydrogel. J. Liposome Res. 2014, 24, 27–36. [Google Scholar] [CrossRef]
- Chang, J.Y.; Oh, Y.K.; Kong, H.S.; Kim, E.J.; Jang, D.D.; Nam, K.T.; Kim, C.K. Prolonged antifungal effects of clotrimazole-containing mucoadhesive thermosensitive gels on vaginitis. J. Control. Release Off. J. Control. Release Soc. 2002, 82, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Brunner, C.A.; Gröner, R.W. Carboxy-methyl-cellulose hydrogel-filled breast implants—An ideal alternative? A report of five years’ experience with this device. Can. J. Plast. Surg. J. Can. De Chir. Plast. 2006, 14, 151–154. [Google Scholar] [CrossRef]
- Ajji, Z.; Othman, I.; Rosiak, J. Production of hydrogel wound dressing using gamma radiation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2005, 229, 375–380. [Google Scholar] [CrossRef]
- Gupta, A.; Kowalczuk, M.; Heaselgrave, W.; Britland, S.T.; Martin, C.; Radecka, I. The production and application of hydrogels for wound management: A review. Eur. Polym. J. 2019, 111, 134–151. [Google Scholar] [CrossRef]
- García-Astrain, C.; Avérous, L. Synthesis and evaluation of functional alginate hydrogels based on click chemistry for drug delivery applications. Carbohydr. Polym. 2018, 90, 271–280. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, Q.; Tao, Y.; Wang, X. Rheological and pH dependent properties of injectable and controlled release hydro-gels based on mushroom hyperbranched polysaccharide and xan-than gum. Carbohydr. Polym. 2021, 2, 100063. [Google Scholar]
- Huang, J.; Deng, Y.; Ren, J.; Chen, G.; Wang, G.; Wang, F.; Wu, X. Novel in situ forming hydrogel based on xanthan and chitosan re-gelifying in liquids for local drug delivery. Carbohydr. Polym. 2018, 186, 54–63. [Google Scholar] [CrossRef]
- Huh, H.W.; Zhao, L.; Kim, S.Y. Biomineralized biomimetic organic/inorganic hybrid hydrogels based on hyaluronic acid and poloxamer. Carbohydr. Polym. 2015, 126, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.-S.; Park, W.; Park, H.; Lee, D.-K.; Na, K. Thermo-sensitive injectable hydrogel based on the physical mixing of hyaluronic acid and Pluronic F-127 for sustained NSAID delivery. Carbohydr. Polym. 2017, 156, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, B.; Li, X.; Zhang, L.; Xu, Y.; Liu, Z.; Cheng, Z.; Zhu, X. Self-assembly of BODIPY based pH-sensitive near-infrared polymeric micelles for drug controlled delivery and fluorescence imaging applications. Nanoscale 2015, 7, 16399–16416. [Google Scholar] [CrossRef]
- Yang, J.; Chen, J.; Pan, D.; Wan, Y.; Wang, Z. pH-sensitive interpenetrating network hydrogels based on chitosan derivatives and alginate for oral drug delivery. Carbohydr. Polym. 2013, 92, 719–725. [Google Scholar] [CrossRef]
- Emman, H.E.; Shaheen, T.Y. Design of a dual pH and temperature responsive hydrogel based on esterified cellulose nanocrystals for potential drug release. Carbohydr. Polym. 2022, 278, 118925. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Chen, S.; Cheong, K.-L.; Teng, B. Carboxymethyl β-cyclodextrin grafted carboxymethyl chitosan hydrogel-based microparticles for oral insulin delivery. Carbohydr. Polym. 2020, 246, 116617. [Google Scholar] [CrossRef] [PubMed]
- Anugrah, D.S.B.; Ramesh, K.; Kim, M.; Hyun, K.; Lim, K.T. Near-infrared light-responsive alginate hydrogels based on dis-elenide-containing cross-linkage for on demand degradation and drug release. Carbohydr. Polym. 2019, 223, 115070. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, H.; Ding, H.; Fan, Z.; Pi, P.; Cheng, J.; Wen, X. Allylated chitosan-poly(N-isopropylacrylamide) hydrogel based on a functionalized double network for controlled drug release. Carbohydr. Polym. 2019, 214, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Maiz-Fernández, S.; Guaresti, O.; Pérez-Álvarez, L.; Ruiz-Rubio, L.; Gabilondo, N.; Vilas-Vilela, J.L.; Lanceros-Mendes, S. β-Glycerol phosphate/genipin chitosan hydrogels: A comparative study of their properties and diclofenac delivery. Carbohydr. Polym. 2020, 248, 116811. [Google Scholar] [CrossRef]
- Yuan, X.; Praphakar, R.A.; Munusamy, M.A.; Alarfaj, A.A.; Kumar, S.S.; Rajan, M. Mucoadhesive guargum hydrogel inter-connected chitosan-g-polycaprolactone micelles for rifampicin delivery. Carbohydr. Polym. 2019, 206, 1–10. [Google Scholar] [CrossRef]
- Gupta, E.; Saxena, J.; Kumar, S.; Sharma, U.; Rastogi, S.; Srivastava, V.K.; Kaushik, S.; Jyoti, A. Fast Track Diagnostic Tools for Clinical Management of Sepsis: Paradigm Shift from Conventional to Advanced Methods. Diagnostics 2023, 13, 277. [Google Scholar] [CrossRef]
- Son, Y.S.; Kwon, K.H. Utilization of smart devices and the evolution of customized healthcare services focusing on big data: A systematic review. mHealth 2024, 10, 7. [Google Scholar] [CrossRef]
- Alshangiti, D.M.; El-damhougy, T.K.; Zaher, A.; Madani, M.; Mohamady ghobashy, M. Revolutionizing biomedicine: Advancements, applications, and prospects of nanocomposite macromolecular carbohydrate-based hydrogel biomaterials: A review. RSC Adv. 2023, 13, 35251–35291. [Google Scholar] [CrossRef]
- Gideon, O.; Samuel, H.S.; Okino, I.A. Biocompatible materials for next-generation biosensors. Discov. Chem. 2024, 1, 34. [Google Scholar] [CrossRef]
- Nayak, S.; Blumenfeld, N.R.; Laksanasopin, T.; Sia, S.K. Point-of-Care Diagnostics: Recent Developments in a Connected Age. Anal. Chem. 2017, 89, 102–123. [Google Scholar] [CrossRef] [PubMed]
- Lazo-Porras, M.; Penniecook, T. Health equity: Access to quality services and caring for underserved populations. Health Policy Plan. 2023, 38, ii1–ii2. [Google Scholar] [CrossRef]
- Olatunji, A.O.; Maha, C.; Kolawole; Abdul, P. Revolutionizing infectious disease management in low-resource settings: The impact of rapid diagnostic technologies and portable devices. Int. J. Appl. Res. Soc. Sci. 2024, 6, 1417–1432. [Google Scholar] [CrossRef]
- Hosseini, E.S.; Dervin, S.; Ganguly, P.; Dahiya, R. Biodegradable Materials for Sustainable Health Monitoring Devices. ACS Appl. Bio Mater. 2020, 4, 163–194. [Google Scholar] [CrossRef]
- Zhu, J.; Tao, J.; Yan, W.; Song, W. Pathways toward wearable and high-performance sensors based on hydrogels: Toughening networks and conductive networks. Natl. Sci. Rev. 2023, 10, nwad180. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh Fakhr, M.; Lopez Carrasco, I.; Belyaev, D.; Kang, J.; Shin, Y.; Yeo, J.-S.; Koh, W.-G.; Ham, J.; Michaelis, A.; Opitz, J.; et al. Recent advances in wearable electrochemical biosensors towards technological and material aspects. Biosens. Bioelectron. X 2024, 19, 100503. [Google Scholar] [CrossRef]

| Material | Applications | Characteristics | Reference |
|---|---|---|---|
| Self-healing alginate hydrogel electrode with anti-freezing and moisturizing properties | Real-time ECG monitoring | Excellent electrical conductivity, soft and flexible, no adverse skin reactions, high reproducibility, suitable for long-term health monitoring. | [43] |
| Self-adherent, biocompatible hydrogel electrodes composed of biodegradable gelatin, geniposide and poly(3,4-ethylenedioxythiophene)/poly(4-styrenesulfonate) | Real-time ECG monitoring | Device designed to form a patch and used as a wearable device to detect the ECG signals of volunteer from static to dynamic conditions. | [44] |
| Self-adherent, biocompatible hydrogel electrodes composed of crosslinked gelatin, geniposide and poly(3,4-ethylenedioxythiophene)/poly(4-styrenesulfonate) | Real-time ECG monitoring | Improved mechanical properties and electrical conductivity. Comparable performance in 12-lead human ECG measurement with commercial ECG clinical electrodes (3M Red Dot). | [45] |
| Material | Applications | Characteristics | Reference |
|---|---|---|---|
| Cellulose-based hydrogel | Real-time respiratory monitoring, OSAS diagnosis | Outstanding tensile strength, extreme resistance to temperature fluctuations, multimodal sensing (mechanical and thermal changes), high robustness and reliability. | [46] |
| Hydrogel electrolyte (polyvinyl alcohol, aluminum hydroxide, and starch) | Respiration monitoring, posture recognition | Superior flexibility and adaptability, multimodal functionality (mechanical and thermal signals), integrated with machine learning (99.259% recognition accuracy). | [47] |
| Material | Applications | Characteristics | Reference |
|---|---|---|---|
| Collagen-based hydrogel masks containing sodium hyaluronate | Maintain skin elasticity, hydration, and a healthy glow | Ability to provide essential nutrients to skin | [48] |
| pH-responsive psyllium and methacrylamide-based hydrogels | Colon specific drug delivery | These hydrogels leverage the high concentration of polysaccharide enzymes in the colon and pH responsivity to enable site-specific drug release | [49] |
| Crosslinked guar gum hydrogel disks | Colon-specific delivery of ibuprofen | Altering enzymatic activity or utilizing pH-sensitive mechanisms, this hydrogel can deliver ibuprofen at a controlled rate | [50] |
| Gel-forming xyloglucan | Sustained delivery of pilocarpine | The 3D network enables responsiveness to stimuli like pH and temperature, making this hydrogel suitable for drug-eluting soft contact lenses and intraocular lenses | [51] |
| Gel-forming xyloglucan | Sustained delivery of timolol | The 3D network enables responsiveness to stimuli like pH and temperature, making this hydrogel suitable for drug-eluting soft contact lenses and intraocular lenses | [52] |
| Thermoresponsive hydrogel based on chitosan–poly(N-isopropyl acrylamide-co-acrylamide) | Drug delivery in tumor environments | These hydrogels exploit the increased temperature of cancerous tissues to release drugs in a temperature-dependent manner, enhancing treatment efficacy | [57] |
| Cyclodextrin hydrogels and deformable propylene glycol liposomes-in-hydrogel | Vaginal delivery of dehydroepiandrosterone, carboplatin, and clotrimazole | Enhance drug retention and controlled release within the vaginal environment | [58,59,60,61] |
| Breast implants filled with hydroxyl propyl cellulose gel | Delivery of antioxidant bioactives | Biodegradable and radiolucent gel with reduced capsular contraction particularly suitable for breast cancer patients | [62] |
| Polyvinyl pyrrolidine, agar, and PEG-based hydrogels | Dressing for wound management | Ability to maintain a moist environment and to provide a barrier against microbes, offering flexibility, softness, and non-thrombogenic properties | [63,64] |
| pH-sensitive hydrogels using modified alginate | Drug delivery device | Pronounced dependence of the swelling behavior on the surrounding pH, ranging from acidic to basic environments | [65] |
| Injectable hydrogels using hyperbranched mushroom polysaccharides in combination with xanthan gum | System for ciprofloxacin delivery | Optimal release properties | [66] |
| Injectable hydrogels by crosslinking aldehyde-functionalized xanthan gum with carboxymethyl chitosan | Drug delivery device | Excellent rheological recovery and enhanced resistance to enzymatic degradation over 72 h | [67] |
| Organic–inorganic hydrogel matrix based on hyaluronic acid and poloxamer | Bone regeneration delivery device | Potential for biomineralization via urea-induced mineral deposition, particularly useful in bone regeneration | [68] |
| Hyaluronic acid with Pluronic F-127 hydrogels | Hydrogels for NSAID delivery | Drug release beyond 50 h | [69] |
| β-cyclodextrin, epichlorohydrin, and succinic anhydride hydrogels delivering indomethacin | Anti-inflammatory DDS | Effective prevention of inflammation during bone biomineralization processes | [70] |
| Hydrogels of methoxy polyethylene glycol, alginate, and carboxymethyl chitosan | Oral drug delivery in dental applications | Alginate ratio played a key role in enhancing delivery performance | [71] |
| Smart hydrogels of esterified cellulose | Responsive drug delivery system | Improved hydrophobicity and dual responsiveness to pH and temperature, making the system suitable for complex physiological environments | [72] |
| β-cyclodextrin-loaded microparticles into carboxymethyl chitosan hydrogel | Oral delivery system for insulin | Sustained insulin release over 12 h. Enhanced drug retention and bioavailability | [73] |
| Alginate-based hydrogels containing doxorubicin | Drug delivery system | NIR exposure promoted additional crosslinking within the hydrogel matrix, enabling precise control over drug release kinetics. | [74] |
| Thermoresponsive hydrogels using alkylated chitosan and poly(N-isopropylacrylamide) for diclofenac release | Anti-inflammatory drug delivery device | Prolonged diclofenac release, with 25% of the drug released over 300 min | [75] |
| Hydrogels from β-glycerol phosphate and genipin-crosslinked chitosan | System for diclofenac delivery | Mechanical and rheological properties suitable for biomedical use | [76] |
| Hybrid hydrogel system consisting of chitosan and polycaprolactone | System for rifampicin delivery | This formulation exhibited potent antibacterial activity against Klebsiella pneumoniae and Staphylococcus aureus | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romano, S.; Yazdanpanah, S.; Conte, R.; De Rosa, A.; Fico, A.; Peluso, G.; Pedram, P.; Moeini, A. Smart Theranostic Platforms Based on Carbohydrate Hydrogels. Macromol 2025, 5, 37. https://doi.org/10.3390/macromol5030037
Romano S, Yazdanpanah S, Conte R, De Rosa A, Fico A, Peluso G, Pedram P, Moeini A. Smart Theranostic Platforms Based on Carbohydrate Hydrogels. Macromol. 2025; 5(3):37. https://doi.org/10.3390/macromol5030037
Chicago/Turabian StyleRomano, Silvia, Sorur Yazdanpanah, Raffaele Conte, Agnello De Rosa, Antonio Fico, Gianfranco Peluso, Parisa Pedram, and Arash Moeini. 2025. "Smart Theranostic Platforms Based on Carbohydrate Hydrogels" Macromol 5, no. 3: 37. https://doi.org/10.3390/macromol5030037
APA StyleRomano, S., Yazdanpanah, S., Conte, R., De Rosa, A., Fico, A., Peluso, G., Pedram, P., & Moeini, A. (2025). Smart Theranostic Platforms Based on Carbohydrate Hydrogels. Macromol, 5(3), 37. https://doi.org/10.3390/macromol5030037







