Development of a Drug Delivery System with Bacterial Cellulose and Gelatin: Physicochemical and Microbiological Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Production of the Release System
2.2. Determination of the Minimum Inhibitory Concentration
2.3. Diffusion Assay
2.4. Antioxidant Activity
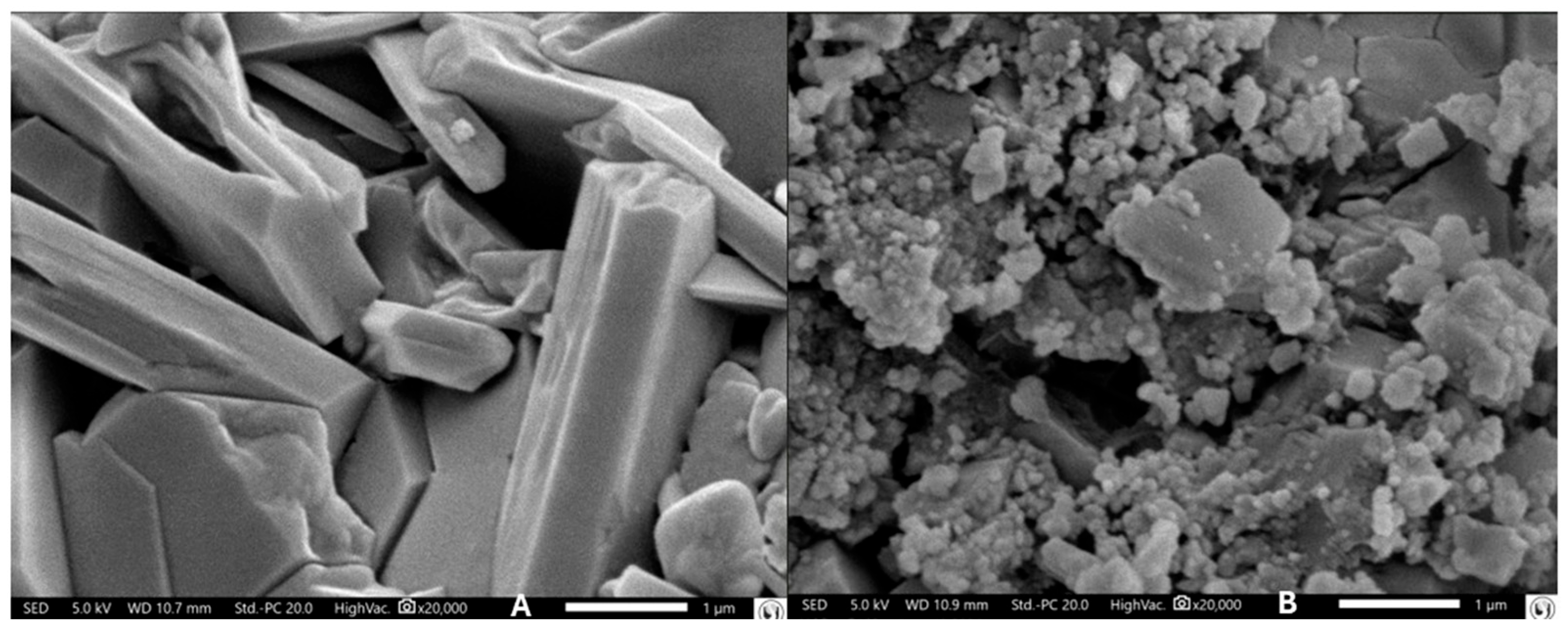
2.5. Scanning Electron Microscopy
3. Results
3.1. Analysis of the Release System
3.2. Results from Antimicrobial Assays: Minimum Inhibitory Concentration and Agar Diffusion
3.3. Matrix Antioxidant Capacity
3.4. Microscopic Observations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Antimicrobial Resistance. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 28 April 2025).
- Akova, M. Epidemiology of antimicrobial resistance in bloodstream infections. Virulence 2016, 7, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz Abdelmoneim, S.; Mohamed Ghazy, R.; Anwar Sultan, E.; Hassaan, M.A.; Anwar Mahgoub, M. Antimicrobial resistance burden pre and post-COVID-19 pandemic with mapping the multidrug resistance in Egypt: A comparative cross-sectional study. Sci. Rep. 2024, 14, 7176. [Google Scholar] [CrossRef]
- World Health Organization. WHO Reports Widespread Overuse of Antibiotics in Patients Hospitalized with COVID-19. 2024. Available online: https://www.who.int/news/item/26-04-2024-who-reports-widespread-overuse-of-antibiotics-in-patients--hospitalized-with-covid-19 (accessed on 28 April 2025).
- Langford, B.J.; So, M.; Simeonova, M.; Leung, V.; Lo, J.; Kan, T.; Raybardhan, S.; Sapin, M.E.; Mponponsuo, K.; Farrell, A.; et al. Antimicrobial resistance in patients with COVID-19: A systematic review and meta-analysis. Lancet Microbe 2023, 4, e179–e191. [Google Scholar] [CrossRef]
- Petrakis, V.; Panopoulou, M.; Rafailidis, P.; Lemonakis, N.; Lazaridis, G.; Terzi, I.; Papazoglou, D.; Panagopoulos, P. The Impact of the COVID-19 Pandemic on Antimicrobial Resistance and Management of Bloodstream Infections. Pathogens 2023, 12, 780. [Google Scholar] [CrossRef]
- Botelho, J.; Grosso, F.; Peixe, L. Antibiotic resistance in Pseudomonas aeruginosa—Mechanisms, epidemiology and evolution. Drug Resist. Updates 2019, 44, 100640. [Google Scholar] [CrossRef]
- MacKinnon, M.C.; McEwen, S.A.; Pearl, D.L.; Lyytikäinen, O.; Jacobsson, G.; Collignon, P.; Gregson, D.B.; Valiquette, L.; Laupland, K.B. Increasing incidence and antimicrobial resistance in Escherichia coli bloodstream infections: A multinational population-based cohort study. Antimicrob. Resist. Infect. Control 2021, 10, 131. [Google Scholar] [CrossRef]
- van Duin, D.; Paterson, D.L. Multidrug-Resistant Bacteria in the Community. Infect. Dis. Clin. N. Am. 2020, 34, 709–722. [Google Scholar] [CrossRef]
- Leite Mdos, S.; Gusmão Ado, C.; Gontijo Bde, A.V.; Garcia, P.G. Antimicrobial resistance profile of Escherichia coli isolated from urine specimens of an Intensive Care Unit patients. Rev. Bras. Anál. Clín. 2020, 52, 243–247. [Google Scholar] [CrossRef]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- Tizhe, D.T.; Ogra, I.O.; Apollos, S.D.; Enesi, K.O.; Yohanna, A.; Aminu, R.; Kwaga, J.K.P. Antimicrobial resistance as a global public health threat: The way forward. EUREKA Life Sci. 2024, 52–57. [Google Scholar] [CrossRef]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef]
- Halawa, E.M.; Fadel, M.; Al-Rabia, M.W.; Behairy, A.; Nouh, N.A.; Abdo, M.; Olga, R.; Fericean, L.; Atwa, A.M.; El-Nablaway, M.; et al. Antibiotic action and resistance: Updated review of mechanisms, spread, influencing factors, and alternative approaches for combating resistance. Front. Pharmacol. 2023, 14, 1305294. [Google Scholar] [CrossRef]
- Ferraboschi, P.; Ciceri, S.; Grisenti, P. Applications of Lysozyme, an Innate Immune Defense Factor, as an Alternative Antibiotic. Antibiotics 2021, 10, 1534. [Google Scholar] [CrossRef]
- Singha, B.; Singh, V.; Soni, V. Alternative therapeutics to control antimicrobial resistance: A general perspective. Front. Drug Discov. 2024, 4, 1385460. [Google Scholar] [CrossRef]
- Prima, M.J.; Hassan, M.; Sharma, J. Novel Approaches for Combating Antibiotic Resistance in Pathogenic Bacteria. Microb. Bioact. 2023, 6, 1–8. [Google Scholar] [CrossRef]
- Pünnel, L.C.; Lunter, D.J. Film-Forming Systems for Dermal Drug Delivery. Pharmaceutics 2021, 13, 932. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. The Antibiotic Resistance Crisis: Part 1: Causes and Threats. P T 2015, 40, 277–283. [Google Scholar] [PubMed]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An economic evaluation of the impact, cost, and Medicare policy implications of chronic nonhealing wounds. Value Health 2018, 21, 27–32. [Google Scholar] [CrossRef]
- Williamson, D.A.; Carter, G.P.; Howden, B.P. Current and emerging topical antibacterials and antiseptics: Agents, action, and resistance patterns. Clin. Microbiol. Rev. 2017, 30, 827–860. [Google Scholar] [CrossRef] [PubMed]
- Ataíde, J.A.; de Carvalho, N.M.; Rebelo, M.A.; Chaud, M.V.; Grotto, D.; Gerenutti, M.; Rai, M.; Mazzola, P.G.; Jozala, A.F. Bacterial nanocellulose loaded with bromelain: Assessment of antimicrobial, antioxidant, and physical-chemical properties. Sci. Rep. 2017, 7, 2–10. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, C.A.; dos Santos, G.R.; Soeiro, V.S.; dos Santos, J.R.; de Araujo Rebelo, M.; Chaud, M.V.; Gerenutti, M.; Grotto, D.; Pandit, R.; Rai, M.; et al. Bacterial nanocellulose membranes combined with nisin: A strategy to prevent microbial growth. Cellulose 2018, 25, 6681–6689. [Google Scholar] [CrossRef]
- Kupnik, K.; Primožič, M.; Kokol, V.; Leitgeb, M. Nanocellulose in Drug Delivery and Antimicrobially Active Materials. Polymers 2020, 12, 2825. [Google Scholar] [CrossRef]
- Liang, S. Advances in drug delivery applications of modified bacterial cellulose-based materials. Front. Bioeng. Biotechnol. 2023, 11, 1252706. [Google Scholar] [CrossRef]
- Pandey, A.; Singh, M.K.; Singh, A. Bacterial cellulose: A smart biomaterial for biomedical applications. J. Mater. Res. 2024, 39, 2–18. [Google Scholar] [CrossRef]
- Lamboni, L.; Gauthier, M.; Yang, G.; Wang, Q. Silk sericin: A versatile material for tissue engineering and drug delivery. Biotechnol. Adv. 2015, 33, 1855–1867. [Google Scholar] [CrossRef]
- D’souza, A.A.; Shegokar, R. Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef]
- Strickley, R.G. Solubilizing excipients in oral and injectable formulations. Pharm. Res. 2004, 21, 201–230. [Google Scholar] [CrossRef]
- Mohamed, M.F.; Abdelkhalek, A.; Seleem, M.N. Evaluation of short synthetic antimicrobial peptides for treatment of drug-resistant and intracellular Staphylococcus aureus. Sci. Rep. 2016, 6, 29707. [Google Scholar] [CrossRef] [PubMed]
- Hren, M.; Trček, J.; Šakanović, A.; Obradović, H.; Kreft, M.E.; Hribernik, S.; Gorgieva, S. Nisin-loaded gelatin microparticles for the enhanced bioactivity of bacterial nanocellulose. Int. J. Biol. Macromol. 2025, 305, 141203. [Google Scholar] [CrossRef] [PubMed]
- Jozala, A.F.; Pértile, R.A.N.; dos Santos, C.A.; de Carvalho Santos-Ebinuma, V.; Seckler, M.M.; Gama, F.M.; Pessoa, A., Jr. Bacterial cellulose production by Gluconacetobacter xylinus by employing alternative culture media. Appl. Microbiol. Biotechnol. 2014, 99, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Soeiro, V.S.; Silva-Carvalho, R.; Martins, D.; Parpot, P.; Grotto, D.; Chaud, M.V.; da Gama, F.M.P.; Jozala, A.F. Alginate-amphotericin B nanocomplexes covered by nanocrystals from bacterial cellulose: Physico-chemical characterization and in vitro toxicity. Sci. Rep. 2021, 11, 23944. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48 (Suppl. 1), 5–16, Erratum in J. Antimicrob. Chemother. 2002, 49, 1049. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, P.G.; Jozala, A.F.; Novaes LCde, L.; Moriel, P.; Penna, T.C.V. Minimal inhibitory concentration (MIC) determination of disinfectant and/or sterilizing agents. Braz. J. Pharm. Sci. 2009, 45, 241–248. [Google Scholar] [CrossRef]
- dos Santos, G.R.; Soeiro, V.S.; Talarico, C.F.; Ataide, J.A.; Lopes, A.M.; Mazzola, P.G.; Oliveira, T.J.; Oliveira Junior, J.M.; Grotto, D.; Jozala, A.F. Bacterial Cellulose Membranes as Carriers for Nisin: Incorporation, Antimicrobial Activity, Cytotoxicity and Morphology. Polymers 2022, 14, 3497. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Goldstein, J.I.; Newbury, D.E.; Michael, J.R.; Ritchie, N.W.M.; Scott, J.H.J.; Joy, D.C. Scanning Electron Microscopy and X-Ray Microanalysis, 4th ed.; Springer: New York, NY, USA, 2018. [Google Scholar]
- Egerton, R.F. Physical Principles of Electron Microscopy: An Introduction to TEM, SEM, and AEM, 2nd ed.; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Burel, C.; Kala, A.; Purevdorj-Gage, L. Impact of pH on citric acid antimicrobial activity against Gram-negative bacteria. Lett. Appl. Microbiol. 2020, 72, 332–340. [Google Scholar] [CrossRef]
- Luo, Q.; Hossen, M.A.; Zeng, Y.; Dai, J.; Li, S.; Qin, W.; Liu, Y. Gelatin-based composite films and their application in food packaging: A review. J. Food Eng. 2022, 313, 110762. [Google Scholar] [CrossRef]
- Tamo, A.K. Nanocellulose-based hydrogels as versatile materials with interesting functional properties for tissue engineering applications. J. Mater. Chem. B 2024, 12, 7692–7759. [Google Scholar] [CrossRef]
- Soeiro, V.S.; Tundisi, L.L.; Amaral, V.A.; Batain, F.; Mazzola, P.G.; Tambourgi, E.B.; de Oliveira Júnior, J.M.; Chaud, M.V.; Grotto, D.; Aranha, N.; et al. Bacterial nanocellulose and fibroin: Natural products to produce a structure membranes. Matéria 2021, 26, e13086. [Google Scholar] [CrossRef]
- Roy, S.; Biswas, D.; Rhim, J.W. Gelatin/Cellulose Nanofiber-Based Functional Nanocomposite Film Incorporated with Zinc Oxide Nanoparticles. J. Compos. Sci. 2022, 6, 223. [Google Scholar] [CrossRef]
- Bessalah, S.; Faraz, A.; Dbara, M.; Khorcheni, T.; Hammadi, M.; Ajose, D.J.; Saeed, S.I. Antibacterial, anti-biofilm, and anti-inflammatory properties of gelatin-chitosan-moringa-biopolymer-based wound dressings toward Staphylococcus aureus and Escherichia coli. Pharmaceuticals 2024, 17, 545. [Google Scholar] [CrossRef]
- Aad, R.; Dragojlov, I.; Vesentini, S. Sericin Protein: Structure, Properties, and Applications. J. Funct. Biomater. 2024, 15, 322. [Google Scholar] [CrossRef]
- Liu, J.; Shi, L.; Deng, Y.; Zou, M.; Cai, B.; Song, Y.; Wang, Z.; Wang, L. Silk sericin-based materials for biomedical applications. Biomaterials 2022, 287, 121638. [Google Scholar] [CrossRef]
- Domján, A.; Bajdik, J.; Pintye-Hódi, K. Understanding of the Plasticizing Effects of Glycerol and PEG 400 on Chitosan Films Using Solid-State NMR Spectroscopy. Macromolecules 2009, 42, 4667–4673. [Google Scholar] [CrossRef]
- Zhang, Y.; Lane, M.E.; Moore, D.J. An Investigation of the Influence of PEG 400 and PEG-6-Caprylic/Capric Glycerides on Dermal Delivery of Niacinamide. Polymers 2020, 12, 2907. [Google Scholar] [CrossRef]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew. Chem. Int. Ed. Engl. 2010, 49, 6288–6308. [Google Scholar] [CrossRef] [PubMed]
- Vessoni, C.; Jozala, A.F.; Gentille, T.R.; Pessoa, A.; Cholewa, O. Detection of Nisin Expression by Lactococcus lactis Using Two Susceptible Bacteria to Associate the Effects of Nisin with EDTA. Appl. Biochem. Biotechnol. 2006, 129, 334–346. [Google Scholar] [CrossRef]
- Yang, F.C.; Yan, J.J.; Hung, K.H.; Wu, J.J. Characterization of Ertapenem-Resistant Enterobacter cloacae in a Taiwanese University Hospital. J. Clin. Microbiol. 2012, 50, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Tavares-Carreon, F.; De Anda-Mora, K.; Rojas-Barrera, I.C.; Andrade, A. Serratia marcescens antibiotic resistance mechanisms of an opportunistic pathogen: A literature review. PeerJ 2023, 11, e14399. [Google Scholar] [CrossRef] [PubMed]
- Moradali, M.F.; Ghods, S.; Rehm, B.H. Pseudomonas aeruginosa lifestyle: A paradigm for adaptation, survival, and persistence. Front. Cell Infect. Microbiol. 2017, 7, 39. [Google Scholar] [CrossRef]
- Rozwadowski, M.; Gawel, D. Molecular factors and mechanisms driving multidrug resistance in uropathogenic Escherichia coli—An update. Genes 2022, 13, 1397. [Google Scholar] [CrossRef]
- Shao, W.; Liu, H.; Wang, S.; Wu, J.; Huang, M.; Min, H.; Liu, X. Controlled release and antibacterial activity of tetracycline hydrochloride-loaded bacterial cellulose composite membranes. Carbohydr. Polym. 2016, 145, 114–120. [Google Scholar] [CrossRef]
- Araujo, L.; Nunes, K.; Mello, J.; Nakamura, C.; Gomes, R.; Bergamasco, R. Evaluation of the Antioxidant, Photoprotective and Wound Healing Capacity of Guazuma ulmifolia lam. Extracts in L-929 Cells Exposed to UV-A and UV-B Irradiation. J. Braz. Chem. Soc. 2024, 35, e-20230149. [Google Scholar] [CrossRef]
- Takechi, T.; Wada, R.; Fukuda, T.; Harada, K.; Takamura, H. Antioxidant activities of two sericin proteins extracted from cocoon of silkworm (Bombyx mori) measured by DPPH, chemiluminescence, ORAC and ESR methods. Biomed. Rep. 2014, 2, 364–369. [Google Scholar] [CrossRef]
- Singh, S.K.; Kaldate, R.; Bisht, A. Chapter 4.5—Citric acid, antioxidant effects in health. In Antioxidants Effects in Health; Nabavi, S.M., Sanches Silva, A., Eds.; Elsevier eBooks: Amsterdam, The Netherlands, 2022; pp. 309–322. [Google Scholar] [CrossRef]
- Luo, L.; Wu, Y.; Li, C.; Zou, Y.; Huang, L.; Liang, Y.; Ren, J.; Liu, Y.; Lin, Q. Elaboration and characterization of curcumin-loaded soy soluble polysaccharide (SSPS)-based nanocarriers mediated by antimicrobial peptide nisin. Food Chem. 2021, 336, 127669. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.S.; Rodrigues, P.; Pintado, M.; Tavaria, F.K. A systematic review of natural products for skin applications: Targeting inflammation, wound healing, and photo-aging. Phytomedicine 2023, 115, 154824. [Google Scholar] [CrossRef]
- Jangra, N.; Singla, A.; Puri, V.; Dheer, D.; Chopra, H.; Malik, T.; Sharma, A. Herbal bioactive-loaded biopolymeric formulations for wound healing applications. RSC Adv. 2025, 15, 12402–12442. [Google Scholar] [CrossRef]
- Bai, Q.; Hu, F.; Gou, S.; Gao, Q.; Wang, S.; Zhang, W.; Zhang, Y.; Lu, T. Curcumin-loaded chitosan-based hydrogels accelerating S. aureus-infected wound healing. Int. J. Biol. Macromol. 2024, 259 Pt 1, 129111. [Google Scholar] [CrossRef]
- Falbo, F.; Spizzirri, U.G.; Restuccia, D.; Aiello, F. Natural compounds and biopolymers-based hydrogels join forces to promote wound healing. Pharmaceutics 2023, 15, 271. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, D.; Ning, X.; Jin, L.; Lin, Y.; Liang, C.; Wen, X.; Huang, T.; Zhou, J.; Zhang, Y. An antibacterial, antioxidant and hemostatic hydrogel accelerates infectious wound healing. J. Nanobiotechnol. 2025, 23, 49. [Google Scholar] [CrossRef] [PubMed]
- Haririan, Y.; Asefnejad, A. Biopolymer hydrogels and synergistic blends for tailored wound healing. Int. J. Biol. Macromol. 2024, 279 Pt 4, 135519. [Google Scholar] [CrossRef] [PubMed]
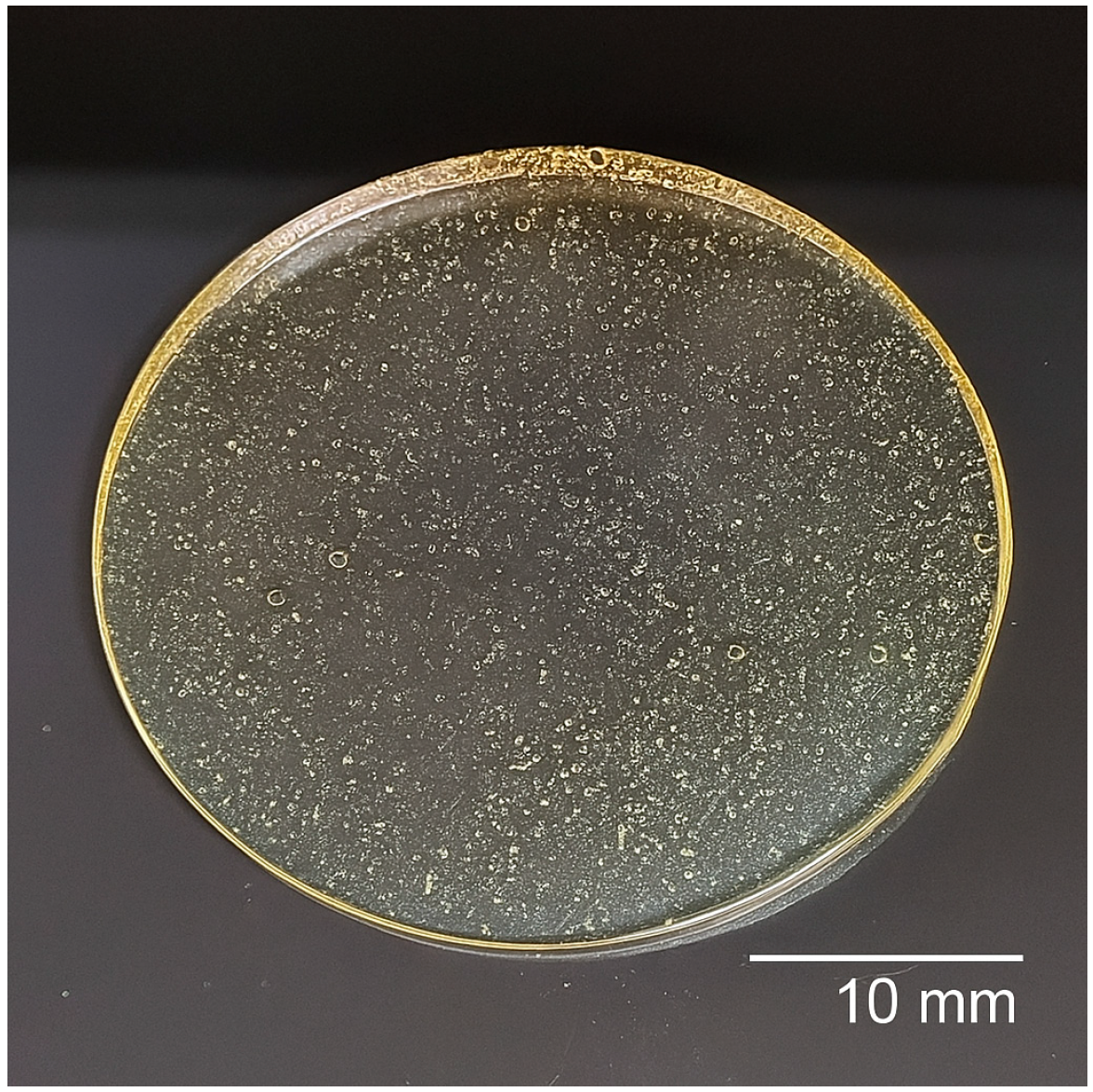

| Component | Amount (per 10 mL Solution) | Function |
|---|---|---|
| Gelatin | 1.5 g | Matrix/base |
| BC * | 2 g | Structural agent |
| PEG 400 | 0.2 g | Plasticizer |
| Citric acid | 0.45 g | Crosslinker |
| Sericin ** | 1.5 g | Functional agent |
| Nisin ** | 0.1 g | Antimicrobial |
| Microorganisms | E5 Halo (mm) | SD | BE Halo (mm) | SD |
|---|---|---|---|---|
| S. aureus (ATCC 10390) | 18.96 | ±0.15 | 0 | 0 |
| E. coli (ATCC 25922) | 21.68 | ±0.59 | 8.95 | ±0.06 |
| P. aeruginosa (ATCC 9721) | 20.35 | ±0.33 | 0 | 0 |
| E. cloacae R | 18.34 | ±0.13 | 0 | 0 |
| S. marcescens R | 13.34 | ±0.16 | 0 | 0 |
| P. aeruginosa R | 18.54 | ±0.24 | 0 | 0 |
| E. coli R | 17.40 | ±0.11 | 5.18 | ±0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, G.P.; Ibanez, N.L.A.; Alves, P.L.M.; Chacon, A.C.; Simões, L.; Schultz, V.; Oliveira, S.; Grotto, D.; Jozala, A.F. Development of a Drug Delivery System with Bacterial Cellulose and Gelatin: Physicochemical and Microbiological Evaluation. Macromol 2025, 5, 39. https://doi.org/10.3390/macromol5030039
Machado GP, Ibanez NLA, Alves PLM, Chacon AC, Simões L, Schultz V, Oliveira S, Grotto D, Jozala AF. Development of a Drug Delivery System with Bacterial Cellulose and Gelatin: Physicochemical and Microbiological Evaluation. Macromol. 2025; 5(3):39. https://doi.org/10.3390/macromol5030039
Chicago/Turabian StyleMachado, Gabriel P., Natasha L. A. Ibanez, Patricia L. M. Alves, Ana C. Chacon, Larissa Simões, Victoria Schultz, Samanta Oliveira, Denise Grotto, and Angela F. Jozala. 2025. "Development of a Drug Delivery System with Bacterial Cellulose and Gelatin: Physicochemical and Microbiological Evaluation" Macromol 5, no. 3: 39. https://doi.org/10.3390/macromol5030039
APA StyleMachado, G. P., Ibanez, N. L. A., Alves, P. L. M., Chacon, A. C., Simões, L., Schultz, V., Oliveira, S., Grotto, D., & Jozala, A. F. (2025). Development of a Drug Delivery System with Bacterial Cellulose and Gelatin: Physicochemical and Microbiological Evaluation. Macromol, 5(3), 39. https://doi.org/10.3390/macromol5030039









