Upcycling Microalgal Residues: Physicochemical Insights and Biocomposite Enhancement
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
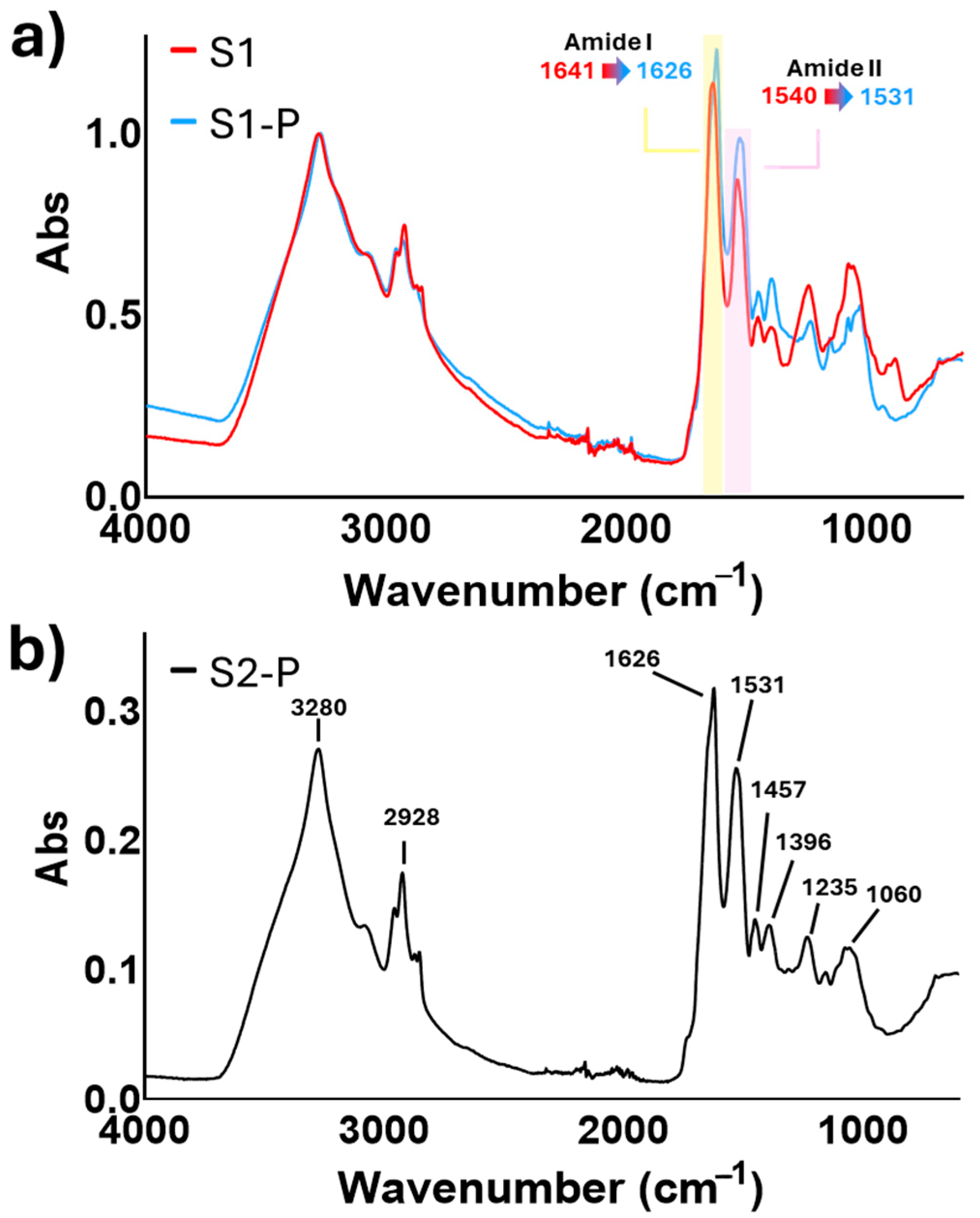
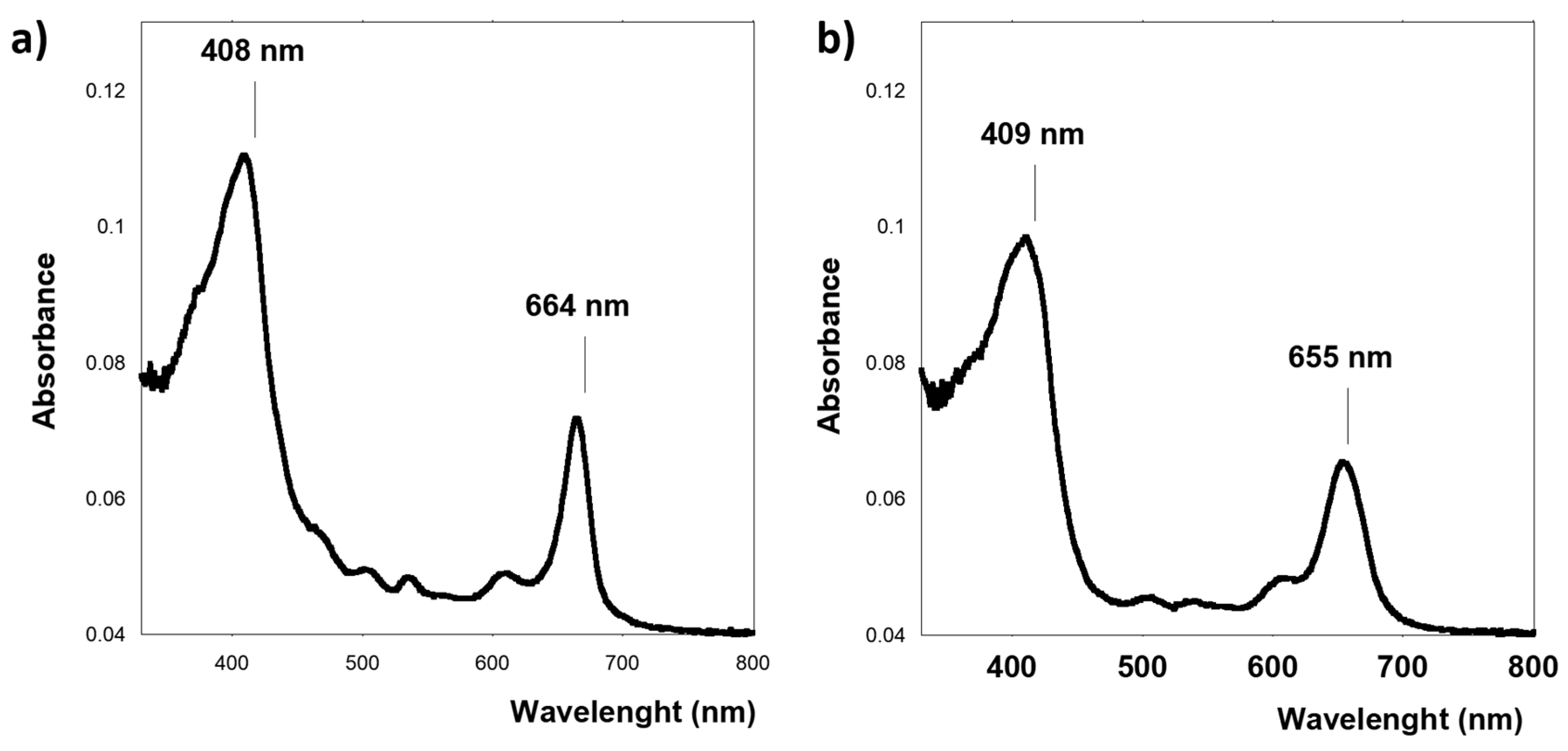
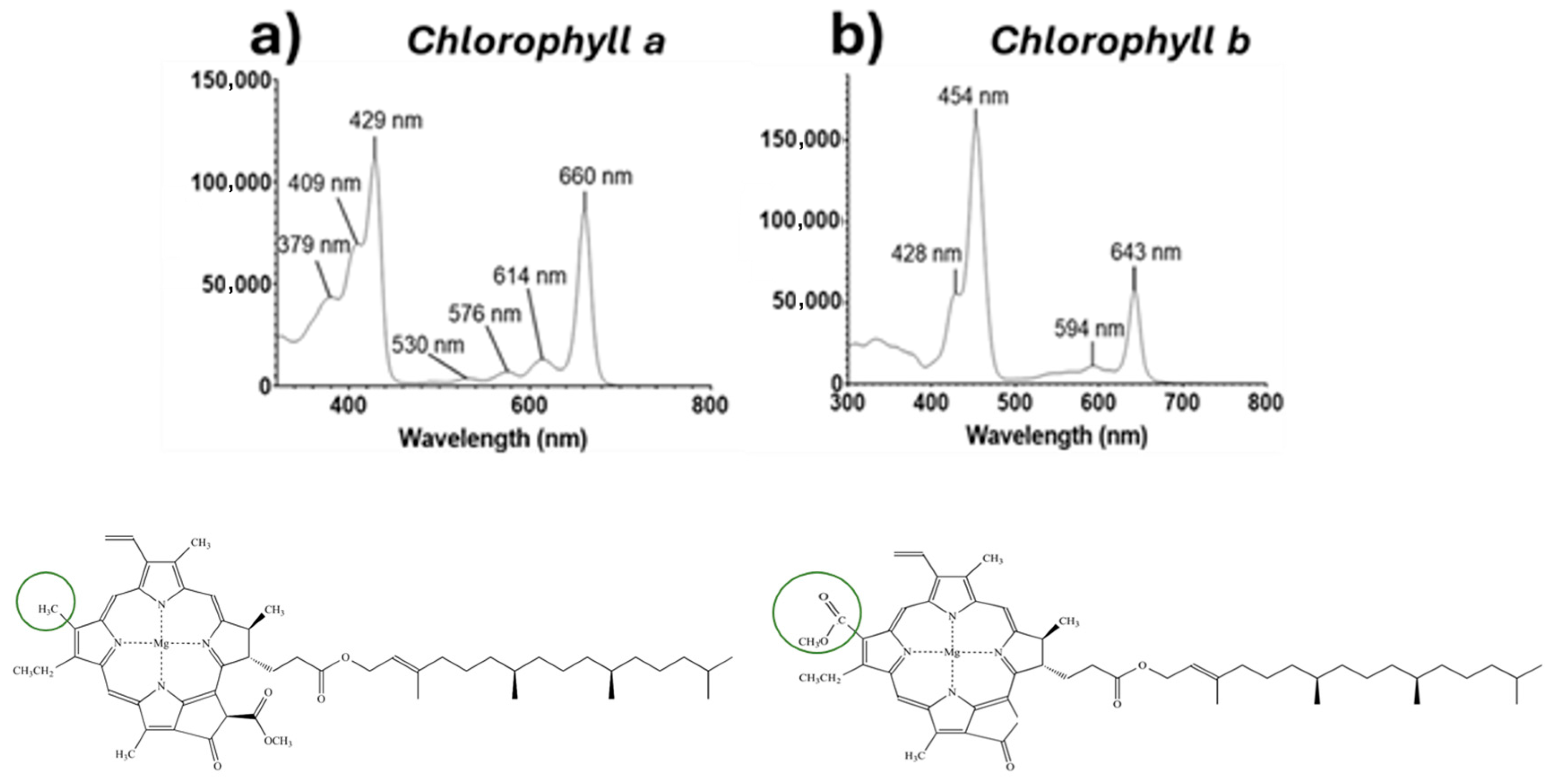
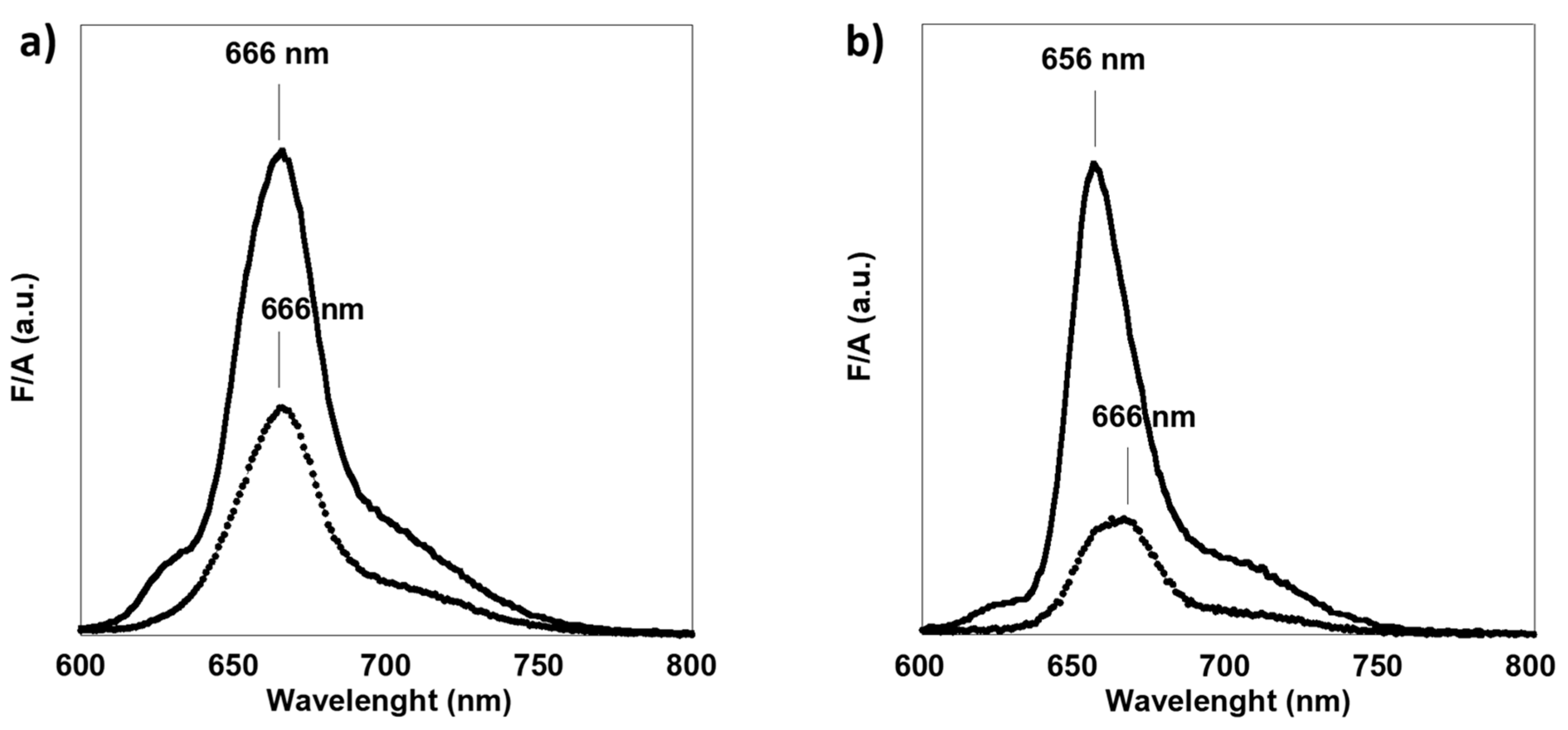
3.1. Spectroscopical Analysis of Microalgae
3.2. Utilization of Microalgae as Additives for Biobased Materials
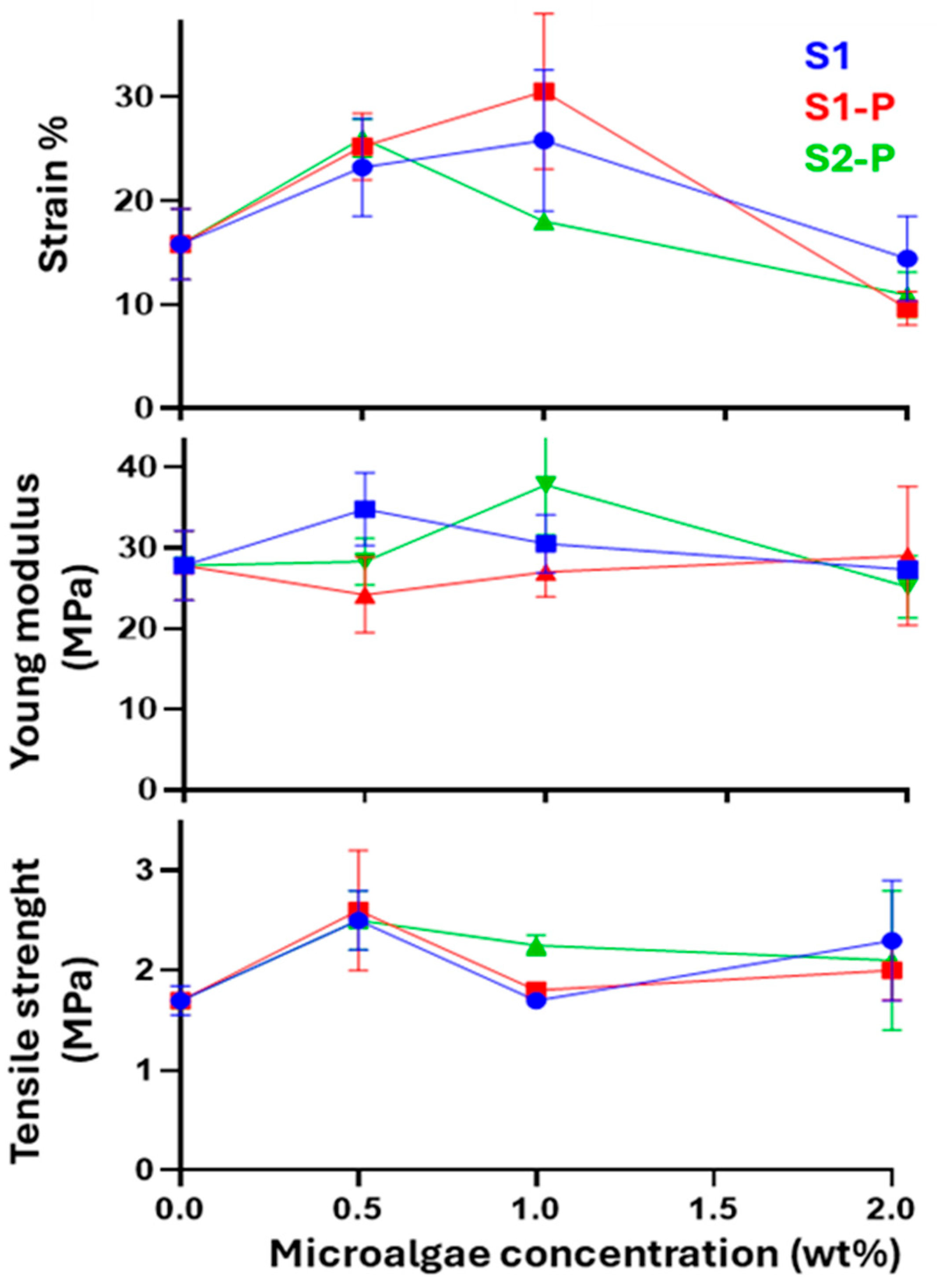
3.2.1. Mechanical Properties
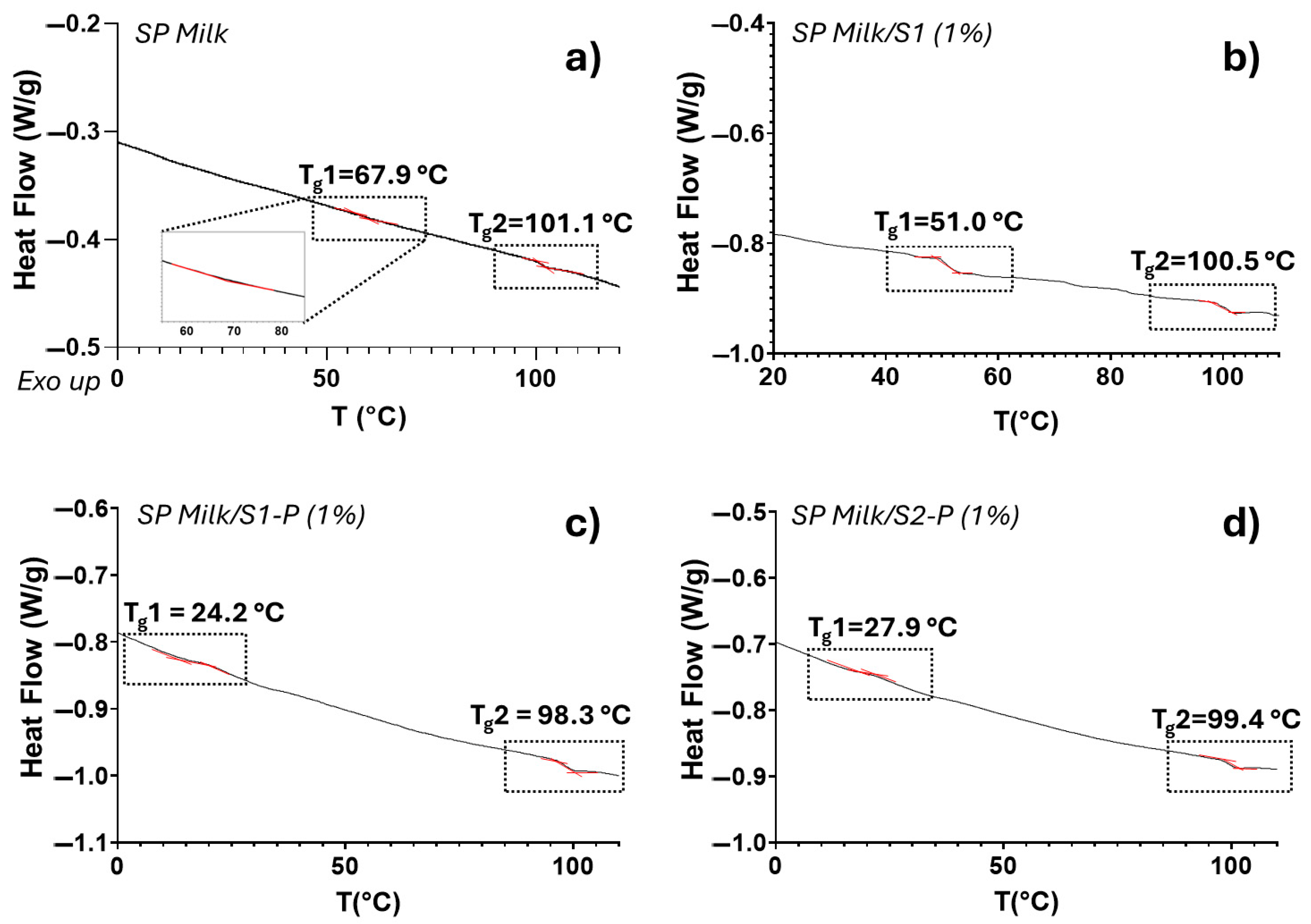
3.2.2. Thermal Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lettieri, R.; Fazio, V.; Abruzzese, D.; di Bartolomeo, E.; D’Ottavi, C.; Micheletti, A.; Tiero, A.; Duranti, L.; Armuzza, V.; Licoccia, S.; et al. Influence of natural additives on the properties of a milk-based compostable bioplastic. RSC Adv. 2024, 14, 3737–3755. [Google Scholar] [CrossRef] [PubMed]
- Boey, J.Y.; Lee, C.K.; Tay, G.S. Factors Affecting Mechanical Properties of Reinforced Bioplastics: A Review. Polymers 2022, 14, 3737. [Google Scholar] [CrossRef] [PubMed]
- Brennan, L.; Owende, P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Wijffels, R.H.; Kruse, O.; Hellingwerf, K.J. Potential of industrial biotechnology with cyanobacteria and eukaryotic microalgae. Curr. Opin. Biotechnol. 2013, 24, 405–413. [Google Scholar] [CrossRef]
- Trivedi, J.; Aila, M.; Bangwal, D.; Kaul, S.; Garg, M.O. Algae based biorefinery—How to make sense? Renew. Sustain. Energy Rev. 2015, 47, 295–307. [Google Scholar] [CrossRef]
- Cheah, W.Y.; Er, A.C.; Aiyub, K.; Mohd Yasin, N.H.; Ngan, S.L.; Chew, K.W.; Khoo, K.S.; Ling, T.C.; Juan, J.C.; Ma, Z.; et al. Current status and perspectives of algae-based bioplastics: A reviewed potential for sustainability. Algal Res. 2023, 71, 103078. [Google Scholar] [CrossRef]
- Ciapponi, R.; Turri, S.; Levi, M. Mechanical Reinforcement by Microalgal Biofiller in Novel Thermoplastic Biocompounds from Plasticized Gluten. Materials 2019, 12, 1476. [Google Scholar] [CrossRef]
- Merchant, S.S.; Prochnik, S.E.; Vallon, O.; Harris, E.H.; Karpowicz, S.J.; Witman, G.B.; Terry, A.; Salamov, A.; Fritz-Laylin, L.K.; Maréchal-Drouard, L.; et al. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science 2007, 318, 245–250. [Google Scholar] [CrossRef]
- Yunus, I.S.; Wichmann, J.; Wördenweber, R.; Lauersen, K.J.; Kruse, O.; Jones, P.R. Synthetic metabolic pathways for photobiological conversion of CO2 into hydrocarbon fuel. Metab. Eng. 2018, 49, 201–211. [Google Scholar] [CrossRef]
- Poulhazan, A.; Arnold, A.A.; Mentink-Vigier, F.; Muszyński, A.; Azadi, P.; Halim, A.; Vakhrushev, S.Y.; Joshi, H.J.; Wang, T.; Warschawski, D.E.; et al. Molecular-level architecture of Chlamydomonas reinhardtii’s glycoprotein-rich cell wall. Nat. Commun. 2024, 15, 986. [Google Scholar] [CrossRef]
- Milledge, J.J.; Heaven, S. A review of the harvesting of micro-algae for biofuel production. Rev. Environ. Sci. Biotechnol. 2013, 12, 165–178. [Google Scholar] [CrossRef]
- Safi, C.; Zebib, B.; Merah, O.; Pontalier, P.-Y.; Vaca-Garcia, C. Morphology, composition, production, processing and applications of Chlorella vulgaris: A review. Renew. Sustain. Energy Rev. 2014, 35, 265–278. [Google Scholar] [CrossRef]
- Suvorov, N.; Pogorilyy, V.; Diachkova, E.; Vasil’ev, Y.; Mironov, A.; Grin, M. Derivatives of Natural Chlorophylls as Agents for Antimicrobial Photodynamic Therapy. Int. J. Mol. Sci. 2021, 22, 6392. [Google Scholar] [CrossRef] [PubMed]
- Caravella, A.; Lettieri, R.; Vezza, R.; Gatto, E. Aerobic biodegradation at a seawater-sediment interface of a new bioplastic 100% based on natural polymers. ACS Sustain. Chem. Eng. 2024, 1, 1099–1111. [Google Scholar] [CrossRef]
- Masi, A.; Leonelli, F.; Di Luca, D.; Celio, L.; Gasperuzzo, G.; Lunardi, S.; Rigon, C.; Pitocco, M.; Ioele, M.; Storace, M.S.; et al. Extraction and characterization of polysaccharide mixtures from Chlamydomonas reinhardtii: A case study as a consolidant for paper-based artworks. Carbohydr. Polym. Technol. Appl. 2025, 9, 100713. [Google Scholar] [CrossRef]
- Farobie, O.; Anis, L.A.; Nurcahyani, P.R.; Hartulistiyoso, E.; Rahman, D.Y.; Fatriasari, W.; Nafisyah, A.L.; Amrullah, A.; Aziz, M. Extraction of Bio-pigments from the Green Microalgae Chlorella pyrenoidosa Under Different Solvent Ratios. IOP Conf. Ser. Earth Environ. Sci. 2023, 1187, 012009. [Google Scholar] [CrossRef]
- International Standard ISO 527-1; Plastics—Determination of tensile properties. International Organization for Standardization (ISO): Geneva, Switzerland, 2019.
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef]
- Jackson, M.; Mantsch, H.H. The use and misuse of FTIR spectroscopy in the determination of protein structure. Crit. Rev. Biochem. Mol. Biol. 1995, 30, 95–120. [Google Scholar] [CrossRef]
- Kong, J.; Yu, S. Fourier Transform Infrared Spectroscopic Analysis of Protein Secondary Structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef]
- Guillén, M.D.; Cabo, N. Characterization of edible oils and lard by Fourier transform infrared spectroscopy. Relationships between composition and frequency of concrete bands in the fingerprint region. J. Am. Oil Chem. Soc. 1997, 74, 1281–1286. [Google Scholar] [CrossRef]
- Bobroff, V.; Rubio, C.; Vigier, V.; Petibois, C. FTIR spectroscopy characterization of fatty-acyl-chain conjugates. Anal. Bioanal. Chem. 2016, 408, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Kareem, J. Effect of Chlorophyll and Anthocyanin on the Secondary Bonds of Poly Methyl Methacrylate (PMMA). Int. J. Tech. Res. Appl. 2014, 2, 73–80. [Google Scholar]
- Tyubaeva, P.M.; Gasparyan, K.G.; Romanov, R.R.; Kolesnikov, E.A.; Martirosyan, L.Y.; Larkina, E.A.; Tyubaev, M.A. Biomimetic materials based on poly-3-hydroxybutyrate and chlorophyll derivatives. Polymers 2024, 16, 101. [Google Scholar] [CrossRef] [PubMed]
- Hotos, G.N.; Bekiari, V. Absorption Spectra as Predictors of Algal Biomass and Pigment Content of the Cultured Microalgae Amphidinium carterae, Isochrysis galbana, Nephroselmis sp., and Anabaena sp. Int. J. Plant Biol. 2023, 14, 879–895. [Google Scholar] [CrossRef]
- Esteves, A.F.; Pardilhó, S.; Gonçalves, A.L.; Vilar, V.J.P.; Pires, J.C.M. Unravelling the impact of light spectra on microalgal growth and biochemical composition using principal component analysis and artificial neural network models. Algal Res. 2025, 85, 103820. [Google Scholar] [CrossRef]
- Eregie, S.B.; Sanusi, I.A.; Kana, G.E.B.; Olaniran, A.O. Effect of ultra-violet light radiation on Scenedesmus vacuolatus growth kinetics, metabolic performance, and preliminary biodegradation study. Biodegradation 2024, 35, 71–86. [Google Scholar] [CrossRef]
- Na Ayudhya, T.I.; Posey, F.T.; Tyus, J.C.; Dingra, N.N. Using a microscale approach to rapidly separate and characterize three photosynthetic pigment species from fern. J. Chem. Educ. 2015, 92, 920–923. [Google Scholar] [CrossRef]
- Lípová, L.; Krchňák, P.; Komenda, J.; Ilík, P. Heat-induced disassembly and degradation of chlorophyll-containing protein complexes in vivo. Biochim. Biophys. Acta 2010, 1797, 63–70. [Google Scholar] [CrossRef]
- Östbring, K.; Rayner, M.; Sjöholm, I.; Otterström, J.; Albertsson, P.-Å.; Emek, S.C.; Erlanson-Albertsson, C. The effect of heat treatment of thylakoids on their ability to inhibit in vitro lipase/co-lipase activity. Food Funct. 2014, 5, 2157–2165. [Google Scholar] [CrossRef]
- Zhang, Y.; Nian, Y.; Shi, Q.; Hu, B. Protein fibrillation and hybridization with polysaccharides enhance strength, toughness, and gas selectivity of bioplastic packaging. J. Mater. Chem. A 2023, 11, 9884–9901. [Google Scholar] [CrossRef]
- Lanfer-Marquez, U.M.; Barros, R.M.; Sinnecker, P. Antioxidant activity of chlorophylls and their derivatives. Food Res. Int. 2005, 38, 885–891. [Google Scholar] [CrossRef]
- Pérez-Gálvez, A.; Viera, I.; Roca, M. Carotenoids and chlorophylls as antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef] [PubMed]
- Nanni, A.; Parisi, M.; Colonna, M. Wine by-products as raw materials for the production of biopolymers and natural reinforcing fillers: A critical review. Polymers 2021, 13, 381. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, A.M.; Di Caprio, F.; Piozzi, A.; Pagnanelli, F.; Francolini, I. Sustainable Bioactive Packaging Based on Thermoplastic Starch and Microalgae. Int. J. Mol. Sci. 2021, 23, 178. [Google Scholar] [CrossRef]
- Alee, M.; Duan, Q.; Chen, Y.; Liu, H.; Ali, A.; Zhu, J.; Jiang, T.; Rahaman, A.; Chen, L.; Yu, L. Plasticization Efficiency and Characteristics of Monosaccharides, Disaccharides, and Low-Molecular-Weight Polysaccharides for Starch-Based Materials. ACS Sustain. Chem. Eng. 2021, 9, 11960. [Google Scholar] [CrossRef]
- Li-Beisson, Y.; Beisson, F.; Riekhof, W. Metabolism of acyl-lipids in Chlamydomonas reinhardtii. Plant J. 2015, 82, 504–522. [Google Scholar] [CrossRef]
- Masi, A.; Leonelli, F.; Scognamiglio, V.; Gasperuzzo, G.; Antonacci, A.; Terzidis, M.A. Chlamydomonas reinhardtii: A factory of nutraceutical and food supplements for human health. Molecules 2023, 28, 1185. [Google Scholar] [CrossRef]
- Dahash, F.K.; Aobaid, A.K.; Al Maamori, M.H. The effect of chlorophyll on the mechanical properties of natural rubber (NR) and the penetration of X-ray. In Recent Trends and Advances in Artificial Intelligence; Garcia, F.P., Jamil, A., Hameed, A.A., Ortis, A., Ramirez, I.S., Eds.; Springer: Cham, Switzerland, 2024; Volume 1138, pp. 1099–1111. [Google Scholar] [CrossRef]
- Aversa, C.; Barletta, M.; Cappiello, G.; Gisario, A. Compatibilization strategies and analysis of morphological features of poly(butylene adipate-co-terephthalate) (PBAT)/poly(lactic acid) PLA blends: A state-of-art review. Eur. Polym. J. 2022, 173, 111304. [Google Scholar] [CrossRef]


| Acronym | Strain | Treatment |
|---|---|---|
| S1 | CC125 | None |
| S1-P | CC125 | Polysaccharides extracted |
| S2-P | SAG 11-32b | Polysaccharides extracted |
| Sample Name | % w/w Microalgae |
|---|---|
| SP-Milk | 0 |
| SP-Milk/S1 (0.5%) | 0.5 |
| SP-Milk/S1 (1.0%) | 1.0 |
| SP-Milk/S1 (2.0%) | 2.0 |
| SP-Milk/S1-P (0.5%) | 0.5 |
| SP-Milk/S1-P (1.0%) | 1.0 |
| SP-Milk/S1-P (2.0%) | 2.0 |
| SP-Milk/S2-P (0.5%) | 0.5 |
| SP-Milk/S2-P (1.0%) | 1.0 |
| SP-Milk/S2-P (2.0%) | 2.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuboni, V.; Lettieri, R.; Caravella, A.; Corvino, M.; Scognamiglio, V.; Antonacci, A.; Gatto, E. Upcycling Microalgal Residues: Physicochemical Insights and Biocomposite Enhancement. Macromol 2025, 5, 32. https://doi.org/10.3390/macromol5030032
Cuboni V, Lettieri R, Caravella A, Corvino M, Scognamiglio V, Antonacci A, Gatto E. Upcycling Microalgal Residues: Physicochemical Insights and Biocomposite Enhancement. Macromol. 2025; 5(3):32. https://doi.org/10.3390/macromol5030032
Chicago/Turabian StyleCuboni, Valerio, Raffaella Lettieri, Alice Caravella, Martina Corvino, Viviana Scognamiglio, Amina Antonacci, and Emanuela Gatto. 2025. "Upcycling Microalgal Residues: Physicochemical Insights and Biocomposite Enhancement" Macromol 5, no. 3: 32. https://doi.org/10.3390/macromol5030032
APA StyleCuboni, V., Lettieri, R., Caravella, A., Corvino, M., Scognamiglio, V., Antonacci, A., & Gatto, E. (2025). Upcycling Microalgal Residues: Physicochemical Insights and Biocomposite Enhancement. Macromol, 5(3), 32. https://doi.org/10.3390/macromol5030032







