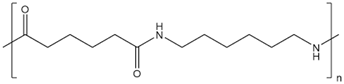
Abstract
The escalating production of virgin plastics has resulted in an unprecedented generation of microplastics (MPs), posing significant environmental and health risks. Biodegradable plastics have emerged as an alternative, but their degradation also releases microplastic-sized particles, referred to as biodegradable microplastics (BMPs). This review evaluates the current understanding of BMPs, focusing on their environmental fate, degradation kinetics, and comparative persistence relative to conventional MPs. The degradation process of biodegradable plastics involves sequential abiotic and biotic mechanisms, with factors such as polymer chemistry, geometry, and environmental conditions influencing BMPs’ formation and mineralization. Studies highlight the temporal advantage of BMPs, which exhibit significantly shorter lifetimes than traditional MPs; however, their environmental impact remains context-dependent, particularly in soil and aquatic systems. Despite promising results under controlled conditions, challenges in standardizing biodegradation assessments and discrepancies between laboratory and real-world scenarios complicate evaluations of the temporal fate and the effects of BMPs. This work underscores the need for long-term studies and improved modeling approaches to accurately predict BMP behavior and mitigate their ecological impact. Poly(hydroxyalkanoates) are a class of fully biodegradable polymers that do not leave behind persistent microplastics. Biodegradable plastics should be prioritized over non-degradable, traditional polymers, as they can replace them in a large fraction of applications, yet with a significantly reduced footprint and without leaving behind persistent micro- and nanoplastics. They can also be recycled.
1. Introduction
The production of virgin plastic materials from fossil sources increases every year [1], creating a growing burden for waste management systems. Every year, more than 400 million tons of plastics articles are produced. Unfortunately, these objects are usually short-lived and often mismanaged. Global waste management systems are simply incapable of absorbing such staggering volumes [2]. End-of-life options are mostly limited to linear “solutions”. There is a rapidly increasing body of knowledge on the formation of micro- and nanoplastics (MPs, NPs), which are generated throughout the life cycle of plastic products and thereby in a multitude of environments across the globe, representing a tremendous threat to the environment and human health [3,4,5]. MPs are insoluble polymeric particles of a size smaller than 5 mm. MP occurrence is either due to deliberate manufacturing purposes (primary MPs, e.g., microbeads in body peeling products or spilled and leaked virgin plastic pellets) or to the uncontrolled environmental fragmentation of larger polymeric items (secondary MPs, stemming, e.g., from littered packaging items, such as PE- and PP-based containers floating on water bodies). Major contributors to the global MP and NP freight are tire attrition and fibers emitted from textiles. Also, paints and coatings are a large source. While the previous belief was that only MPs and NPs from the environment, e.g., attrition from tires, paints, and coatings, containing additives and adsorbed toxins and pathogens, would pose a health threat to humans, mainly through their function as “vectors” for toxins and pathogens, it is now clear that MPs and NPs from “food-grade” packaging can also enter and accumulate in the human body and cause serious harm. Prominent examples are kitchen cutting boards made from polyolefins, or MPs emitted by PET drinking bottles. Given the extreme persistence of MPs derived from traditional plastic materials in the environment [6], the interest in biodegradable alternatives has soared in recent decades [7]. Biobased materials have the advantage of a reduced carbon footprint, and another benefit is that biodegradable materials will not accumulate in the environment. Through MPs and NPs, “properly managed” plastic articles will also leak into the environment. While the main bulky item might be collected and recycled, the “shredded” MPs and NPs can cause harm. More recently, an animated debate arose around the adoption of biodegradable plastics. It has been argued that fossil plastics can be recycled, while biodegradable materials would promote single-use items. That framing is not correct, as biodegradable materials can in principle be collected and recycled just as non-degradable materials, and after decades, recycling rates of fossil plastics are well below 10%. This failure of circularity is due to many aspects, amongst them the low value of classic plastic materials, the externalization of costs, and unsustainable product design. Composite materials can be tailored to specific needs; however, they make recycling difficult to impossible. Plastic articles have been found to create MPs and NPs throughout their intended and unintended uses, and biodegradable materials are no exception here. In fact, it has been observed how biodegradable polymers like PLA (Polylactic Acid), PHA (Polyhydroxyalkanoates), PBAT (Polybutylene Adipate Terephthalate), PBS (Polybutylene Succinate), and PCL (Polycaprolactone) generate MPs during their degradation, for instance [8,9,10,11], before complete mineralization has been achieved. A new expression has been coined to describe MPs derived from biodegradable plastics: biodegradable microplastics (BMPs) [12]. Hence, researchers started asking themselves whether biodegradable plastics are truly safe for the environment [13]. Nevertheless, considering the polymeric nature of biodegradable plastics, it is not surprising that smaller particles are temporarily released during the degradation process. Indeed, that temporal factor is of the utmost importance if one wants to address the environmental impact of a polymeric material. In this paper, the most recent works studying BMPs are reviewed, dedicating great attention to the lifespan of BMPs in comparison to traditional MPs and their long environmental persistence.
2. Biodegradation: Phases and Conditions
To discuss BMPs, it is first imperative to describe biodegradation in broad terms. The degradation of biodegradable plastics follows a surface erosion process and consists of three phases [14]. Initially, abiotic factors like temperature, UV rays, and chemical and mechanical stresses are mainly responsible for breaking down bulky plastic items into smaller fragments and polymer chains. As a consequence, the material loses its mechanical properties and is fragmented into particles and further on into oligomers. At the same time, extracellular bacterial and fungal enzymes also contribute to oligomer release during this initial phase of biodeterioration. Afterwards, during a phase named biofragmentation, enzymes such as lipases, cutinases, and proteases hydrolyze the oligomers further into smaller molecules. Eventually, under a process called mineralization, plastic degradation products such as monomers and oligomers are internalized by hydrolytic bacteria and fungi and metabolized into biomass, CO2, and water under aerobic conditions and into CH4 under anaerobic conditions (biodegradable materials can yield disadvantageous life cycle assessment results through a strong climate impact when this process occurs in the open environment). Analytically, different techniques can be adopted to monitor biodegradation rates. The choice of the methodology is based both on the stage of biodegradation and the size of the particles at that specific moment (Figure 1). Overall, morphological properties like larger surface-to-volume ratios and porosity, higher wettability, or reduced molecular weight and crystallinity facilitate access and action by hydrolytic enzymes, hence accelerating biodegradation kinetics [15].
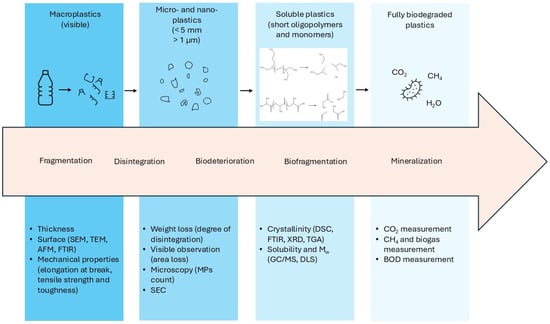
Figure 1.
Phases of biodegradation. As the size of the plastic material reduces, different analytical techniques can be employed to monitor macro- and microscopic changes. SEC = size exclusion chromatography; DLS = dynamic light scattering.
However, to obtain full biodegradation, some environmental conditions need to be met, depending on the chemistry and geometry of the biodegradable material [16]. Firstly, optimal pH and temperature higher than the glass transition temperature support the induction of polymer structural changes and hydrolytic enzyme access. Additionally, the degree of humidity must enable the proliferation of biodegrading microorganisms and the activity of their hydrolytic enzymes. As already mentioned, abiotic factors like UV exposure and mechanical stress have a significant impact on biodeterioration and the generation of BMPs. In this regard, Tong and colleagues [17] tested pellets and films of PBAT, PBS, and PLA. They observed by fluorescence microscopy that after three weeks, because of photooxidation, PBS and PLA released 10 and 3 times more BMPs compared to MPs from PE. This is not surprising, since degradation (to which biodegradable materials are more prone than non-degradable ones) results in material comminution. The authors highlighted how the large majority of BMPs had a size of 1–100 µm. Differently, the action of UV rays induced the release of a similar number of particles from PE and PBAT that instead were more affected by mechanical stress, especially when in film form or blended with PBS [17]. Conversely, the extrinsic properties of plastic materials, such as shape and thickness, play an equally important role. For example, it was theoretically calculated that an HDPE film can degrade more than 250 times faster than a fiber and more than 1000 times faster than a bead of the same volume and chemical composition [18]. In a similar fashion, yet at a highly different magnitude, for PHA (poly(hydroxyalkanoates)), biodegradation rates are strictly bound to geometry. In the marine environment, it is estimated that the complete biodegradation of PHA-based materials spans from 0.1 to 0.2 years in the case of bags, and 2.3 to 5.7 years for cutleries (Figure 2) [19].
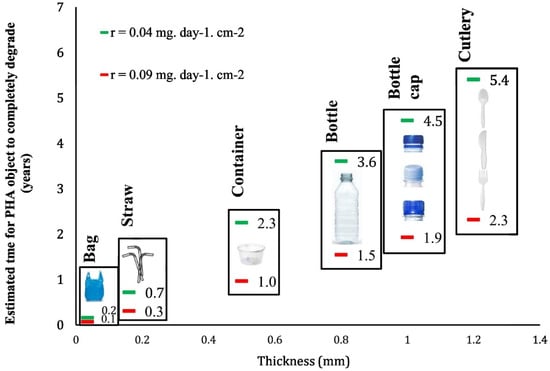
Figure 2.
PHAs are marine-degradable materials. Source: [19]. How long will it take a PHA item to degrade? Lifetime values estimated using the 95% confidence interval for the mean of the rate of biodegradation of PHA in the marine environment. Reproduced with permission.
PHAs are known to biodegrade in virtually all environments. The rate of biodegradation is between 20 µm per day in hot, humid compost and 1 µm per day in cold sea water at optimum conditions. Cellulose is another example of a widely biodegradable biopolymer, like silk or proteins. PLA, on the other hand, is an example of a less biodegradable material. Through copolymerization and the blending of biodegradable materials, the lifetime in a specific target environment can be controlled, e.g., for mulching film or protective sleeves for young trees.
Plastic pellets (nurdles, resin) are a relevant emission source of microplastics too. Thus, the polymer industry has started a program (Operation Clean Sweep (OCS), also known as OCS Europe and OCS Blue) to increase awareness and reduce the amount of pellet spills and leakages [20]. For instance, it is estimated that between 52,140 and 184,290 tons of pellets were lost to the environment in the EU alone in 2019 [20]. Given the fact that less than 10% of all primary plastic production takes place in the EU, the global emission of pellets from spills and subsequent leakages will be at least an order of magnitude higher. Like many forms of littering, such pellet leakage—unthinkable in other industries—is avoidable and more efforts are needed.
Additionally, when it comes to a biodegradable polymer, one must consider the formation of biofilms on the plastic surface during biodegradation. This constitutes a further layer of complexity that does not have to be factored in when one attempts to model biodegradation behaviors of traditional plastic like HDPE, as detailed in [18]. Biofilms are complex systems that also influence the aggregation of different BMPs and their sedimentation if found, for instance, in the sea [21]. Overall, given the complexity of environments and plastic materials, it is impossible to create theoretical models that can simulate the biodegradation of a material using only linear equations, neither will it be feasible to derive universal correlations.
3. Limitations of Current Biodegradation Studies
Likewise for traditional plastics, considering different blends and geometries, there is a great variety of biodegradable polymers, and copolymers and blends derived thereof. These are characterized by very different behaviors, especially if contextualized in dissimilar environments [22]. Therefore, it is challenging to set common biodegradation standards. Current tests in laboratories replicate the conditions described in official standards of biodegradation like those by ISO and ASTM. However, in the real world and in dedicated facilities for organic recycling (industrial composting and anaerobic digestion for biogas production), the same conditions are often not met. Additionally, it is well known that a large fraction of plastic waste is not disposed of properly or leaked from various sources, such as waste management systems. When this happens, the waste materials’ fate becomes unpredictable, and it is almost impossible to assume that the conditions described in biodegradation standards will be met [23]. Also, many standards only demand 90% biodegradation and not complete mineralization. Similarly, during LCA (life cycle assessment) studies analyzing the environmental impact of materials after their use phase, hence including the material behavior once it is disposed of, there is no consideration of MPs and BMPs and their persistence in the environment. As a result, the final score is not influenced by the completely different impact that MPs and BMPs can have [24]. The costs of MPs and NPs today are fully externalized and not considered at all. No current LCA standard protocol for non-degradable plastics, applied to tires, textiles, mulching film, or packaging items made from fossil fuels, for instance, evaluates the impacts of persistent MPs and NPs on ecosystems or humans.
Studying the different persistence of BMPs and MPs is nevertheless complex. During their existence, all micro- and nanoplastics can do harm, e.g., by acting as vectors for toxins and pathogens into the human body. This can also be the case for BMPs. More quantitative data is still needed to confirm and quantify that the effects of BMPs are less long-lived and less detrimental compared to the ones caused by MPs. To study the difference between BMPs and MPs, first, one could observe the presence of these particles in specific environments. During this kind of environmental assessment, it should not be ignored that the plastic market is still dominated by non-biodegradable products at approx. 98–99% (and even more in coatings), thereby representing most plastic waste and hence microscopic particle sources. Shi and colleagues collected several studies on the occurrence of BMPs in aquatic environments, emphasizing how BMPs can also be found in wastewater influent, sewage, and marine sediment, but at a much lower concentration than MPs [25,26]. More importantly, while MPs were detected in drinking water, no trace of BMPs was found [25]. On the other hand, to scientifically understand the behavior and persistence of BMPs in the environment, few-days-long observation is insufficient [27], while several months- or years-long experiments should be conceived. As brilliantly theorized by Colwell et al. [28], while every plastic material goes through the same phases of degradation, generating smaller and smaller fragments in the order of macroplastics → microplastics → nanoplastics → soluble plastics oligomers → monomers, the overall dynamics may be extremely different [28]. When the authors compared PE, PLA, and PHA, extrapolating data from the literature, they highlighted how important it is to consider the temporal factor. As such, biodegradable plastics are characterized by an initial larger release of BMPs compared to non-biodegradable plastics, for which the release of MPs is steady and spread across a much wider time window. Overall, MPs linger for an extremely longer period in the environment compared to BMPs, as summarized in Figure 3. Although it is practically complicated to design experiments lasting for decades, and current studies assessing BMP degradation are performed with much higher particle concentrations compared to real-world conditions, mathematical models addressing the degree of degradation have become extremely helpful. In the wake of these analyses, one can draw the hypothesis that the long persistence of MPs and NPs in the environment causes highly severe damage compared to BMPs.
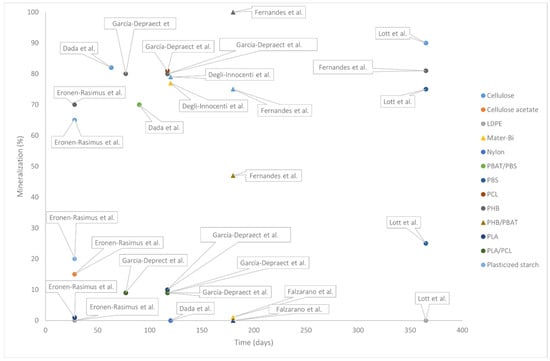
Figure 3.
Mineralization levels of different biodegradable and non-biodegradable plastics. Data derived from works reported in the following paragraphs [22,29,30,31,32,33,34].
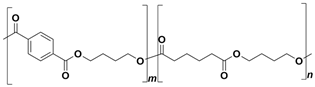
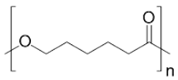
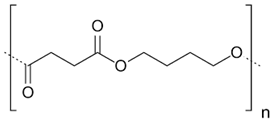
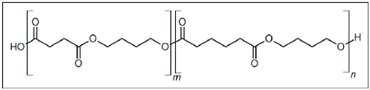
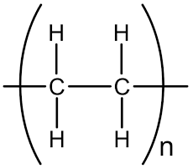
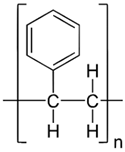
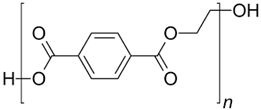
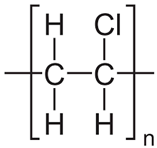
In the following sections, we summarize the most recent works on the persistence of BMPs derived from several types of biodegradable plastics in different environments, in comparison with MPs stemming from traditional plastics. Briefly, the complete list of the polymers’ names and chemical structures is reported in Table 1.

Table 1.
List of degradable and non-degradable polymers (or class of polymers) described in this paper.
Although the maximum duration of these experiments is about 1–2 years, which is not always enough to reach the full mineralization of some BMPs, this review frames the current state of the art about biodegradation studies on BMPs. In this sense, a key parameter to discriminate against the behavior of different BMPs is the environmental one. For this reason, the reported papers have been clustered in two sets depending on the context: fates of BMPs in soil on the one hand, and in marine and freshwater environments on the other hand.
4. Biodegradable Microplastics in Soil
When soil biodegradation tests are performed in a laboratory, a sample of environmental soil is incubated together with the tested plastic materials under well-defined conditions, e.g., in bioreactors. Parameters such as humidity, temperature, and aeration are maintained under controlled levels to replicate real-world scenarios. Amid the main analyses to assess biodegradation, mineralization tests are the most informative to describe the fate of BMPs. Under aerobic conditions, by measuring CO2 levels and knowing the plastic sample’s maximum theoretical CO2 production, one can estimate the degree of mineralization, and thereby the degradation rate of BMPs. On the other hand, the degree of fragmentation can be assessed by visual analysis or mass loss measurements. Despite the ease of conducting this type of analysis, the results are descriptive only at a macroscopical level and cannot provide any information regarding the end of life of BMPs. Table 2 summarizes the most recent works on BMP degradation in soil. In a study carried out by Novamont, one of the largest biodegradable plastic resin manufacturers, films of their flagship biodegradable polymer Mater-Bi™, grade EF04P, were tested in the laboratory for 120 days to observe mineralization and fragmentation in soil [29]. The results were compared to cellulose, which is typically adopted as a reference biodegradable material. For Mater-Bi™ EF04P, which consists of TPS, biodegradable polyesters, and plasticizers, the degree of mineralization and disintegration reached 77% to 80% during the time of the experiment, marking basically the same performance as cellulose (79% to 84% for the same tests, respectively). The only difference consisted in the speed at which BMPs were generated and degraded: when comparing the persistency curves of BMPs, in the case of cellulose, a higher but narrower peak is observable, reflected in a steeper mineralization curve. Additionally, the authors attempted to estimate, based on the fragmentation and mineralization rate, the time to achieve 100% biodegradation for both biopolymers, in comparison to PE, both at 14 and 28 °C. Remarkably, for cellulose and Mater-Bi, 0.3 and 0.8 years, respectively, were calculated at the two studied temperatures. On the other hand, to fully degrade MPs derived from PE, 548 and 1175 years were projected. A longer persistence in the environment was reflected by considerably higher MP emission potential (MPEP). An index was created by the authors which takes into account the total number of MPs derived from a plastic sample and their persistence time. The higher the MPEP, the higher the ecological pressure. While the theoretical number of total MPs released across the entire degradation process is not supposed to differ between biodegradable and non-biodegradable materials (all other conditions such as temperature, stiffness, product, etc.), fragmentation and mineralization rates plummeted for non-biodegradable plastics like PE. As a result, the MPEP for PE was estimated to be almost 2000 times higher than for cellulose and Mater-Bi™.
In another study, when Mater-Bi™ cups were tested, as well as PLA cups, sensibly different results were obtained [22]. The experiment was conducted in the laboratory for 6 months, and at the end there was no visible sign of mineralization. Some degree of mineralization was detected only when PLA and Mater-Bi™ digestate were tested; however, their mineralization levels recorded were 26% and 20%, respectively, while 62% of cellulose was fully biodegraded. This observation reinforces the crucial role that geometry plays during degradation. Thicker items such as cups require longer degradation times or more favorable conditions. In this sense, the same PLA and Mater-Bi™ cups were also tested under composting conditions. Within 45 days, the samples underwent full mass loss, and within 130 and 180 days, PLA and Mater-Bi™ cups were fully mineralized, behaving similarly to cellulose (120 days). Natural materials such as leaves and wood can also take a very long time to biodegrade under cold, anoxic, and microorganism-devoid conditions.
When mineralization is not fully achieved, important information regarding the biodegradation of a polymer can still be inferred. This is especially relevant in the case of composite materials or blends like Mater-Bi™. In fact, two or more copolymers or blends constituting the same material could have significantly different biodegradation profiles. When a PHB/PBAT 55/45 wt % bilayer in the form of film was incubated for 6 months in soil under controlled conditions according to standard ASTM D 5988 (2018), the degree of mineralization stopped at 47% (cellulose, the positive control, recorded 75%) [30]. To better understand the degradation behavior of the blend, i.e., of both PHB and PBAT individually, further analysis was conducted. Crystallinity and surface properties were investigated using DSC, FTIR, and TGA. The results pinpointed how no PHB was detected at the end of the experiment, whereas PBAT crystallinity levels increased. Therefore, it was speculated that PHB was likely fully mineralized whereas PBAT was only partially degraded in the more amorphous regions. It is important to note that different biodegradation tests can generate very different results, even when similar samples are the subject of the study. When PHBV sheets were buried in soil for 112 days, following ASTM G160-12 guidelines (Standard Practice for Evaluating Microbial Susceptibility of Nonmetallic Materials By Laboratory Soil Burial, ASTM, 2019) although a drop in the mechanical properties of the material was demonstrated, only a very small decrease in the total film area was observed [35]. This is likely because, under ASTM G160-12 guidelines, conditions are not controlled like in ASTM D 5988 (Standard Test Method for Determining Aerobic Biodegradation of Plastic Materials in Soil, ASTM, 2018), where instead parameters like temperature and humidity are optimized. In the same paper, the authors elucidated how blending PHBV with biodegradable copolymers such as PBSA and PCL decreased the crystallinity of the material, inducing an increased overall visual degradation. Additionally, the authors demonstrated that PBSA can biodegrade more efficiently than PBS in soil and confirmed that PLA does not biodegrade under environmental conditions, but can instead be fully mineralized after 200 days under “home composting” (and obviously also under the hotter “industrial composting”) conditions. These results for PLA are in accordance with the majority of the literature: especially under thermophilic conditions, both during anaerobic digestion and industrial composting, PLA reaches a total or a quasi-total degree of biodegradation. On the other hand, when tested in soil ecosystems, the degree of PLA degradation spans between 10 and 60% [35]. PLA is an example of an “industrially compostable” but not “home-compostable” material. This means that it will meet the conditions of the “industrially compostable” standard, but not those stipulated under the “home-compostable” standard, yet the PLA will still biodegrade over time—not as fast as, e.g., PHA, but still much faster than, e.g., PE. The common biodegradability standards are not to be seen as absolute for a certain polymer, but need to be applied to a specific product, which, depending, e.g., on its thickness, might or might not pass the criteria.
Demonstrating soil biodegradability and BMP persistence in soil is not only related to end-of-life scenarios or leakages from waste management streams. Indeed, some plastic commodities find their applications and use phase in soil environments, including slow-release fertilizers [36], seed coatings [37], tree guards [38], and mulch films [39]. One prominent example is the case of plastic mulch films used in agriculture. Likewise with any other plastic object, the concern around MPs released from mulch films created a rising interest in biodegradable alternatives. Nevertheless, the detection of BMPs in fields alarmed researchers, questioning the effective sustainability of biodegradable mulch films. One of the longest experimental plans tracked macro- and microplastic dynamics released from thin PLA/PBAT mulch films for 3.5 years [40]. After 2.5 years, FTIR analysis confirmed the total elimination of macroplastics, whereas at that time, microplastics recorded their peak concentration, which eventually plummeted to 50 to 125 particles/kg of soil. Several correlations were found between BMPs deriving from PHA and PLA mulch films, and it was shown that they altered soil nutrient cycling and microbial diversity [41]. However, these findings were not linked to any proven negative effect on soil and microbial health. It has even been hypothesized how the typical large release of BMPs in a narrow window of time could be beneficial by inducing accelerated microbial turnover and favoring soil carbon storage [42]. Additionally, when the impacts of MPs from traditional mulch films and BMPs from biodegradable ones were compared, contrasting results were highlighted [43]. In fact, elucidating BMPs’ toll in this type of environment and on crop yields is highly complex. Experimental times are extended and several variables, usually difficult to control and interdependent, contribute to the influence of the results. For this reason, a clever strategy is to look at the overall plastic mass flow. In this sense, de Sadeleer and Woodhouse observed that if the application time of biodegradable mulch films is long enough, the concentration of BMPs reaches a plateau [44]. This is possible considering the biodegradable nature of the film; in their study, they adopted PBAT/starch-based polymers. Increasing the rotation time to 3 years allowed the mulch enough time to biodegrade and leave a reduced concentration of BMPs that were fully mineralized during the following cycle. On the other hand, using traditional mulch films like LDPE causes a smaller amount of generated MPs within one cycle; however, these persist in the field for many more rotations. Finally, after their use, biodegradable mulch films may offer the option to be efficiently disposed of in composting facilities, which typically are present nearby in agricultural fields [45].

Table 2.
Degradation studies of biodegradable plastics in soil.
Table 2.
Degradation studies of biodegradable plastics in soil.
| Biodegradable Polymer | Geometry | Testing Environment | Test Duration | Methodologies | Results | Reference |
|---|---|---|---|---|---|---|
| Mater-Bi EF04P | Film | Laboratory | 120 days |
|
| [29] |
| Mater-Bi PLA | Cups | Laboratory | 6 months |
|
| [22] |
| PHB/PBAT | Film | Laboratory | 6 months |
|
| [30] |
| PHBV PBS PCL PBS PLA | Sheets | Laboratory and in field | 112/120 days |
|
| [35] |
| PLA/PBAT | Mulch films | Field | 3.5 years |
| No BMPs after 3.5 years | [41] |
| PBAT/starch | Mulch films | Field | 2 years | Material Flow Analysis | Increasing rotation of mulch film to 3 years decreases the overall BMP release | [44] |
5. Biodegradable Microplastics in Marine and Fresh Water
In general terms, the behaviors of biodegradable plastics in water environments are not dissimilar to what has been described for soil. Unfilled PE and PE float on fresh and sea water, whereas biodegradable plastics typically have higher densities.
Table 3 summarizes the most recent works on BMP degradation in soil. There is great variability depending both on polymer chemistry and geometry, but at the same time the role played by environmental factors is no less impactful. Therefore, although it is difficult to draw broad conclusions regarding mineralization levels for specific biodegradable polymers, it is important to highlight how the majority of studies are conducted on sea water (76.6%), with a strong focus on the biodegradability of polymers from the PHA and PLA families [46]. As previously mentioned, drivers of the process of biodegradability include both abiotic factors and microorganisms present in the environment. While abiotic factors have an impact on breaking the polymer into smaller residues and oligomers, the action of hydrolytic bacteria and fungi mediates the digestion and mineralization of BMP. When Mater-Bi™, PLA, and rPET (recycled PET) microplastic granules smaller than 500 µm were incubated for 4 weeks in sterilized sea water, only UV light, mechanical forces, and temperature acted as degrading agents [47]. Thanks to FTIR and GC/MS analysis, it was clear that monomers and several oligomers were released from Mater-Bi™ and PLA granules, yet not in the case of rPET. Non-degradable plastics typically only disintegrate into smaller fragments, MPs and NPs, comparable to the end point of biodegradable materials, which, too, only fragment into non-visible particles but do not truly (bio)degrade.

Table 3.
Degradation studies of biodegradable plastic in marine and fresh water.
During biodegradation tests in water environments, PHA-based materials tend to perform best when compared to other biodegradable plastics. However, PHA disruption is highly dependent on the type of water environment. In a 1-year long experiment in the field, PHBV sheets were tested in river benthic, marina surface, marina benthic, mesocosm benthic, and sea benthic environments and compared to LDPE [48]. Within 350 days, PHBV sheets lost a lot of weight, with the only exception being samples incubated in marina surface, the full biodegradation of which was calculated to be achieved within 500 days. These results are in line with another work measuring the visual disintegration of PHB in seafloor sand, which was found to be complete after 1 year [49]. Differently, no degree of degradation was observed for LDPE [48]. Before the full disruption of the samples, the material properties were monitored: consistently in the different areas, PHBV was characterized by a loss of mechanical properties and thickness, whereas it maintained its molecular weight and crystallinity level. The same rationale was adopted by Lott and colleagues. In their work, 85 µm thick PHB films were tested in benthic, eulittoral, and pelagic habitats, both in the laboratory and in three differently warm seas (Mediterranean and Southeast Asia) [31]. The variability in terms of results encountered was very high, and it was predicted by statistical models that the half-lives of PHB could span from 54 to 1247 days. Additionally, to better understand the fate of BMPs, the authors conducted CO2 evolution tests for one year, witnessing 81% and almost 75% degrees of mineralization for PHB in benthic and eulittoral habitats, respectively, while this value was 0% for LDPE in both environments. Even more interestingly, PHB/PHV films also demonstrated excellent biodegradability in cold water. Experimentally, PHB/PHV was studied in Baltic Sea water, both in the laboratory to study mineralization across 4 weeks (in darkness at 15 °C) and in situ for 1 year to investigate weight loss [32]. By measuring CO2 levels, it was calculated that 70% of PHB/PHV was mineralized, while after 6 months the films completely lost their weight in Finnish sea water. Conversely, PLA films were only fragmented, their weight loss was neglectable, and ultimately no mineralization was observed in the laboratory. Similarly, the ability of PHB and PLA to biodegrade in water was compared, adopting the ISO 14852 (Determination of the ultimate aerobic biodegradability of plastic materials in an aqueous medium—Method by analysis of evolved carbon dioxide, 2021, ISO) and 14853 (Plastics—Determination of the ultimate anaerobic biodegradation of plastic materials in an aqueous system—Method by measurement of biogas production, 2016, ISO) standards, in aerobic and anaerobic conditions for 117 and 77 days, respectively [33]. In that work, PBS, PCL, and PLA/PCL granules of a maximum of 1 mm were tested as well. Given the initial size of the samples, the results accurately frame the behavior of BMPs from different materials under typical end-of-life scenarios. PHB stood out and reached an 80% degree of mineralization (more than 90% if compared to cellulose) for both tests, while PCL recorded the same degree of mineralization only in the presence of oxygen. On the other hand, CO2 measurements did not exceed 9% of the maximum theoretical levels for all other samples. Overall, it was underlined how smaller particles had faster biodegradation kinetics, which can be attributed to a larger surface/volume ratio.
As previously discussed, Mater-Bi™ shows great levels of biodegradation in soil environments. Likewise, this bioplastic material behaves effectively in water habitats. When tested on the seafloor, both in beach sand and mud, for 12 and 10 months, respectively, the disintegration of Mater-Bi™ films was found to be total [49]. In a similar way, Mater-Bi™ was seen to have a great degree of fragmentation and weight loss in marsh. This was deducted after an in-field, 32-week-long test conducted on both Mater-Bi™ and PLA films [50]. Despite lacking information on BMP mineralization levels, the released particle count provided important insights into macroscopic levels of degradation. Differently from Mater-Bi™, PLA films showed a degree of fragmentation comparable to PET and PS, and no evident weight loss. Indeed, PLA requires specific conditions to efficiently biodegrade, e.g., elevated temperatures. Alternatively, blending PLA with other biodegradable polymers like PBAT may decrease the crystallinity and facilitate hydrolysis. This was demonstrated on PLA/PBAT granules smaller than 200 µm incubated for a short time, with a lipase that gave a hydrolysis rate of 30 nmol·min−1 as result [51]. Although PBAT is commonly considered not to biodegrade as efficiently in the marine environment or in fresh water as it does in soil, it was observed by microscopy, DSC (differential scanning calorimetry), and SEC (size exclusion chromatography) that a great release of BMPs after 10 weeks occurred in sea water [52]. In comparison, LDPE films during the same period of time generated much smaller amounts of MPs, reinforcing the notion that BMPs are typically quickly released in high amounts to then be biodegraded in a relatively short time, while MPs’ release and persistence are spread across extremely long periods. In fact, when films of PBAT, blended with PBS, were submerged in sea or fresh water for 4 months and CO2 evolution was monitored, an almost complete mineralization of the material was already observed [34].
6. Conclusions
Biodegradable plastics were introduced as a sustainable alternative to traditional plastics, with the aim of reducing environmental persistence and pollution. However, their degradation results in the formation of biodegradable microplastics (BMPs), which have raised new concerns regarding their ecological impact. This review highlights that BMPs, while shorter-lived than conventional microplastics (MPs), still pose potential risks depending on environmental conditions and polymer composition, yet for a considerably shorter time.
The degradation of biodegradable plastics involves complex abiotic and biotic processes influenced by factors such as polymer chemistry, geometry, and external conditions like temperature, pH, and humidity, as well as the presence and abundance of microorganisms. While BMPs are typically generated in higher quantities during the initial degradation phases, their mineralization occurs significantly faster than that of MPs, reducing their long-term environmental persistence. Soil and aquatic environments exhibit diverse degradation behaviors, with biodegradable polymers such as PHA demonstrating higher biodegradation efficiency compared to PLA, PBAT, or blends. The PHA family stands out amongst bioplastics as being fully biodegradable, comparable to cellulose and silk, which are also naturally occurring polyesters [53,54].
Despite advancements in understanding BMP behavior, limitations in current studies, such as the reliance on laboratory conditions and short experimental durations, hinder the ability to fully assess their environmental impact. Real-world scenarios often deviate significantly from controlled settings, emphasizing the need for long-term studies and improved biodegradation models to evaluate BMP persistence and interactions in natural ecosystems.
Ultimately, while biodegradable plastics represent a step toward sustainability, their use must be carefully assessed within the context of their entire life cycle, including BMP formation and degradation. Further research is essential to develop standards and strategies that minimize BMP generation, ensuring biodegradable plastics fulfill their promise of environmental safety. The plastics industry needs a full transformation—not only does the feedstock have to become renewable (biobased) and the materials be intrinsically biodegradable to prevent persistent MPs and NPs, as well as “white pollution” from leakages, more sustainable product design and use profiles need to be developed based on the classic waste hierarchy to first reduce, then reuse, and finally recycle materials to draw multiple benefits from cascaded use and to achieve full circularity.
Author Contributions
Conceptualization, P.C. and M.L.; writing—original draft preparation, P.C.; writing—review and editing, M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| MP | Microplastic |
| NP | Nanoplastic |
| BMP | Biodegradable microplastic |
| PLA | Poly(lactic acid) |
| PHA | Poly(hydroxyalkanoates) |
| PHB | Poly(hydroxybutyrate) |
| PHBV | Poly(hydroxybutyrate-co-valerate) |
| PBAT | Poly(butylene adipate terephthalate) |
| PBS | Poly(butylene succinate) |
| PBSA | Poly(butylene succinate-co-adipate) |
| PCL | Poly(caprolactone) |
| PE | Poly(ethylene) |
| LDPE | Low-Density Poly(ethylene) |
| HDPE | High-Density Poly(ethylene) |
| PS | Poly(styrene) |
| PET | Poly(ethylene terephthalate) |
| PVC | Poly(vinyl chloride) |
References
- OECD. Global Plastics Outlook; Organisation for Economic Co-Operation and Development (OECD): Paris, France, 2022. [Google Scholar] [CrossRef]
- Berger, R. The Plastic Waste Management Framework. Alliance to End Plastic Waste. 2023. Available online: https://endplasticwaste.org/en/our-stories/plastic-waste-management-framework (accessed on 19 May 2025).
- Wang, B.; Yuan, H.; Yang, Y.; Jiang, Z.; Xi, D. Toxicological effects and molecular metabolic of polystyrene nanoplastics on soybean (Glycine max L.): Strengthening defense ability by enhancing secondary metabolisms. Environ. Pollut. 2025, 366, 125522. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Li, J.; Xiong, R.G.; Zhou, D.D.; Huang, S.Y.; Wu, S.X.; Shang, A.; Tang, G.Y.; Li, H.B.; Gan, R.Y. Potentially harmful effects of micro-/nanoplastics on humans as well as protective actions of dietary natural products. Trends Food Sci. Technol. 2025, 156, 104841. [Google Scholar] [CrossRef]
- Perkins, D.M.; Müller, H.L.; Grünewald, S.; Reiss, J.; Restrepo-Sulez, K.; Robertson, A.; Perna, A. Microplastic ingestion by an aquatic ciliate: Functional response, modulation, and reduced population growth. Sci. Total Environ. 2025, 963, 178272. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; You, F. Microplastic Human Dietary Uptake from 1990 to 2018 Grew across 109 Major Developing and Industrialized Countries but Can Be Halved by Plastic Debris Removal. Environ. Sci. Technol. 2024, 58, 8709–8723. [Google Scholar] [CrossRef]
- European Bioplastics, Institute for Bioplastics and Biocomposites. Bioplastics Market Development Update 2024. 2024. Available online: https://www.european-bioplastics.org/bioplastics-market-development-update-2024/ (accessed on 19 May 2025).
- De Felice, B.; Gazzotti, S.; Ortenzi, M.A.; Parolini, M. Multi-level toxicity assessment of polylactic acid (PLA) microplastics on the cladoceran Daphnia magna. Aquat. Toxicol. 2024, 272, 106966. [Google Scholar] [CrossRef]
- Lv, H.; Park, J.; Lim, H.K.; Abraham, I.J.; Yin, X.; Gao, Y.; Hur, J. Impacts of polyhydroxybutyrate (PHB) microplastic exposure on physiology and metabolic profiles of Litopenaeus vannamei. Sci. Total Environ. 2024, 951, 175588. [Google Scholar] [CrossRef]
- Xie, M.; Cai, K.; Zhang, J.; Tu, S.; Feng, J. Preparation of PBAT microplastics and their potential toxicity to zebrafish embryos and juveniles. Aquat. Toxicol. 2024, 275, 107065. [Google Scholar] [CrossRef]
- Tang, L.; Su, C.; Chen, Y.; Xian, Y.; Hui, X.; Ye, Z.; Chen, M.; Zhu, F.; Zhong, H. Influence of biodegradable polybutylene succinate and non-biodegradable polyvinyl chloride microplastics on anammox sludge: Performance evaluation, suppression effect and metagenomic analysis. J. Hazard. Mater. 2021, 401, 123337. [Google Scholar] [CrossRef]
- Allemann, M.N.; Tessman, M.; Reindel, J.; Scofield, G.B.; Evans, P.; Pomeroy, R.S.; Burkart, M.D.; Mayfield, S.P.; Simkovsky, R. Rapid biodegradation of microplastics generated from bio-based thermoplastic polyurethane. Sci. Rep. 2024, 14, 6036. [Google Scholar] [CrossRef]
- Mut, N.N.N.; Na, J.; Jung, J. A review on fate and ecotoxicity of biodegradable microplastics in aquatic system: Are biodegradable plastics truly safe for the environment? Environ. Pollut. 2024, 344, 123399. [Google Scholar] [CrossRef]
- García-Depraect, O.; Bordel, S.; Lebrero, R.; Santos-Beneit, F.; Börner, R.A.; Börner, T.; Muñoz, R. Inspired by nature: Microbial production, degradation and valorization of biodegradable bioplastics for life-cycle-engineered products. Biotechnol. Adv. 2021, 53, 107772. [Google Scholar] [CrossRef] [PubMed]
- Laycock, B.; Nikolić, M.; Colwell, J.M.; Gauthier, E.; Halley, P.; Bottle, S.; George, G. Lifetime prediction of biodegradable polymers. Prog. Polym. Sci. 2017, 71, 144–189. [Google Scholar] [CrossRef]
- Miri, S.; Saini, R.; Davoodi, S.M.; Pulicharla, R.; Brar, S.K.; Magdouli, S. Biodegradation of microplastics: Better late than never. Chemosphere 2022, 286, 131670. [Google Scholar] [CrossRef]
- Tong, H.; Zhong, X.; Duan, Z.; Yi, X.; Cheng, F.; Xu, W.; Yang, X. Micro- and nanoplastics released from biodegradable and conventional plastics during degradation: Formation, aging factors, and toxicity. Sci. Total Environ. 2022, 833, 155275. [Google Scholar] [CrossRef]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef]
- Dilkes-Hoffman, L.S.; Lant, P.A.; Laycock, B.; Pratt, S. The rate of biodegradation of PHA bioplastics in the marine environment: A meta-study. Mar. Pollut. Bull. 2019, 142, 15–24. [Google Scholar] [CrossRef]
- Operation Clean Sweep. Available online: https://www.opcleansweep.eu/ (accessed on 19 May 2025).
- Wang, C.; Yu, J.; Lu, Y.; Hua, D.; Wang, X.; Zou, X. Biodegradable microplastics (BMPs): A new cause for concern? Environ. Sci. Pollut. Res. 2021, 28, 66511–66518. [Google Scholar] [CrossRef]
- Falzarano, M.; Marìn, A.; Cabedo, L.; Polettini, A.; Pomi, R.; Rossi, A.; Zonfa, T. Alternative end-of-life options for disposable bioplastic products: Degradation and ecotoxicity assessment in compost and soil. Chemosphere 2024, 362, 142648. [Google Scholar] [CrossRef]
- Folino, A.; Pangallo, D.; Calabrò, P.S. Assessing bioplastics biodegradability by standard and research methods: Current trends and open issues. J. Environ. Chem. Eng. 2023, 11, 109424. [Google Scholar] [CrossRef]
- Keyes, A.; Saffron, C.M.; Manjure, S.; Narayan, R. Biobased Compostable Plastics End-of-Life: Environmental Assessment Including Carbon Footprint and Microplastic Impacts. Polymers 2024, 16, 3073. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, Y.; Shao, Y.; Ray, S.S.; Wang, B.; Zhao, Z.; Yu, B.; Zhang, X.; Li, W.; Ding, J.; et al. A review on the occurrence, detection methods, and ecotoxicity of biodegradable microplastics in the aquatic environment: New cause for concern. TrAC Trends Anal. Chem. 2024, 178, 117832. [Google Scholar] [CrossRef]
- Lau, P.; Stein, J.; Reinhold, L.; Barjenbruch, M.; Fuhrmann, T.; Urban, I.; Bauerfeld, K.; Holte, A. Reduction in the Input of Microplastics into the Aquatic Environment via Wastewater Treatment Plants in Germany. Microplastics 2024, 3, 276–292. [Google Scholar] [CrossRef]
- Wei, X.F.; Capezza, A.J.; Cui, Y.; Li, L.; Hakonen, A.; Liu, B.; Hedenqvist, M.S. Millions of microplastics released from a biodegradable polymer during biodegradation/enzymatic hydrolysis. Water Res. 2022, 211, 118068. [Google Scholar] [CrossRef]
- Colwell, J.; Pratt, S.; Lant, P.; Laycock, B. Hazardous state lifetimes of biodegradable plastics in natural environments. Sci. Total Environ. 2023, 894, 165025. [Google Scholar] [CrossRef] [PubMed]
- Degli-Innocenti, F.; Barbale, M.; Chinaglia, S.; Esposito, E.; Pecchiari, M.; Razza, F.; Tosin, M. Analysis of the microplastic emission potential of a starch-based biodegradable plastic material. Polym. Degrad. Stab. 2022, 199, 109934. [Google Scholar] [CrossRef]
- Fernandes, M.; Salvador, A.F.; Vicente, A.A. Biodegradation of PHB/PBAT films and isolation of novel PBAT biodegraders from soil microbiomes. Chemosphere 2024, 362, 142696. [Google Scholar] [CrossRef]
- Lott, C.; Eich, A.; Makarow, D.; Unger, B.; van Eekert, M.; Schuman, E.; Reinach, M.S.; Lasut, M.T.; Weber, M. Half-Life of Biodegradable Plastics in the Marine Environment Depends on Material, Habitat, and Climate Zone. Front. Mar. Sci. 2021, 8, 662074. [Google Scholar] [CrossRef]
- Eronen-Rasimus, E.L.; Näkki, P.P.; Kaartokallio, H.P. Degradation Rates and Bacterial Community Compositions Vary among Commonly Used Bioplastic Materials in a Brackish Marine Environment. Environ. Sci. Technol. 2022, 56, 15760–15769. [Google Scholar] [CrossRef]
- García-Depraect, O.; Lebrero, R.; Rodriguez-Vega, S.; Bordel, S.; Santos-Beneit, F.; Martínez-Mendoza, L.J.; Börner, R.A.; Börner, T.; Muñoz, R. Biodegradation of bioplastics under aerobic and anaerobic aqueous conditions: Kinetics, carbon fate and particle size effect. Bioresour. Technol. 2022, 344, 126265. [Google Scholar] [CrossRef]
- Dada, O.; Bada, A.; Okorodo, E. Natural Biodegradation Rates of Single-Use Blended Bioplastic Packaging Nylon Entrenched In Freshwater and Marine Water Environments of the Tropics. Pollution 2023, 9, 1428–1438. [Google Scholar] [CrossRef]
- van der Zee, M.; Zijlstra, M.; Kuijpers, L.J.; Hilhorst, M.; Molenveld, K.; Post, W. The effect of biodegradable polymer blending on the disintegration rate of PHBV, PBS and PLA in soil. Polym. Test. 2024, 140, 108601. [Google Scholar] [CrossRef]
- Priya, E.; Sarkar, S.; Maji, P.K. A review on slow-release fertilizer: Nutrient release mechanism and agricultural sustainability. J. Environ. Chem. Eng. 2024, 12, 113211. [Google Scholar] [CrossRef]
- Javed, T.; Afzal, I.; Shabbir, R.; Ikram, K.; Zaheer, M.S.; Faheem, M.; Ali, H.H.; Iqbal, J. Seed coating technology: An innovative and sustainable approach for improving seed quality and crop performance. J. Saudi Soc. Agric. Sci. 2022, 21, 536–545. [Google Scholar] [CrossRef]
- Briassoulis, D. Agricultural plastics as a potential threat to food security, health, and environment through soil pollution by microplastics: Problem definition. Sci. Total Environ. 2023, 892, 164533. [Google Scholar] [CrossRef]
- Khalid, N.; Aqeel, M.; Noman, A.; Rizvi, Z.F. Impact of plastic mulching as a major source of microplastics in agroecosystems. J. Hazard. Mater. 2023, 445, 130455. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ding, F.; Flury, M.; Wang, J. Dynamics of macroplastics and microplastics formed by biodegradable mulch film in an agricultural field. Sci. Total Environ. 2023, 894, 164674. [Google Scholar] [CrossRef]
- Song, D.; Jin, G.; Su, Z.; Ge, C.; Fan, H.; Yao, H. Influence of biodegradable microplastics on soil carbon cycling: Insights from soil respiration, enzyme activity, carbon use efficiency and microbial community. Environ. Res. 2025, 266, 120558. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Jia, R.; Brown, R.W.; Yang, Y.; Zeng, Z.; Jones, D.L.; Zang, H. The long-term uncertainty of biodegradable mulch film residues and associated microplastics pollution on plant-soil health. J. Hazard. Mater. 2023, 442, 130055. [Google Scholar] [CrossRef]
- Liu, S.; Jin, R.; Li, T.; Yang, S.; Shen, M. Are biodegradable plastic mulch films an effective way to solve residual mulch film pollution in farmland? Plant Soil 2024, 494, 85–94. [Google Scholar] [CrossRef]
- de Sadeleer, I.; Woodhouse, A. Environmental impact of biodegradable and non-biodegradable agricultural mulch film: A case study for Nordic conditions. Int. J. Life Cycle Assess. 2024, 29, 275–290. [Google Scholar] [CrossRef]
- Yu, Y.; Griffin-LaHue, D.E.; Miles, C.A.; Hayes, D.G.; Flury, M. Are micro- and nanoplastics from soil-biodegradable plastic mulches an environmental concern? J. Hazard. Mater. Adv. 2021, 4, 100024. [Google Scholar] [CrossRef]
- Lavagnolo, M.C.; Poli, V.; Zampini, A.M.; Grossule, V. Biodegradability of bioplastics in different aquatic environments: A systematic review. J. Environ. Sci. 2024, 142, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Barlucchi, L.; Biale, G.; La Nasa, J.; Mattonai, M.; Pezzini, S.; Corti, A.; Castelvetro, V.; Modugno, F. Abiotic degradation and accelerated ageing of microplastics from biodegradable and recycled materials in artificial seawater. Sci. Total Environ. 2024, 954, 176832. [Google Scholar] [CrossRef] [PubMed]
- Read, T.; Chaléat, C.; Laycock, B.; Pratt, S.; Lant, P.; Chan, C.M. Lifetimes and mechanisms of biodegradation of polyhydroxyalkanoate (PHA) in estuarine and marine field environments. Mar. Pollut. Bull. 2024, 209, 117114. [Google Scholar] [CrossRef]
- Eich, A.; Weber, M.; Lott, C. Disintegration half-life of biodegradable plastic films on different marine beach sediments. PeerJ 2021, 9, e11981. [Google Scholar] [CrossRef]
- Weinstein, J.E.; Dekle, J.L.; Leads, R.R.; Hunter, R.A. Degradation of bio-based and biodegradable plastics in a salt marsh habitat: Another potential source of microplastics in coastal waters. Mar. Pollut. Bull. 2020, 160, 111518. [Google Scholar] [CrossRef]
- Miksch, L.; Köck, M.; Gutow, L.; Saborowski, R. Bioplastics in the Sea: Rapid In-Vitro Evaluation of Degradability and Persistence at Natural Temperatures. Front. Mar. Sci. 2022, 9, 920293. [Google Scholar] [CrossRef]
- Wei, X.F.; Bohlén, M.; Lindblad, C.; Hedenqvist, M.; Hakonen, A. Microplastics generated from a biodegradable plastic in freshwater and seawater. Water Res. 2021, 198, 117123. [Google Scholar] [CrossRef]
- Lackner, M.; Costa, P.; Koller, M.; Zinn, M. More than PHB—The PHAmily of Copolymers: Justifications for Broader Use and Summary of Biodegradation Facts on Polyhydroxyalkanoates (PHA)—A Review. Chem. Biochem. Eng. Q. 2024, 38, 265–291. [Google Scholar] [CrossRef]
- Lackner, M.; Mukherjee, A.; Koller, M. What Are “Bioplastics”? Defining Renewability, Biosynthesis, Biodegradability, and Biocompatibility. Polymers 2023, 15, 4695. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).