Degradation Mechanisms of Cellulose-Based Transformer Insulation: The Role of Dissolved Gases and Macromolecular Characterisation
Abstract
1. Introduction
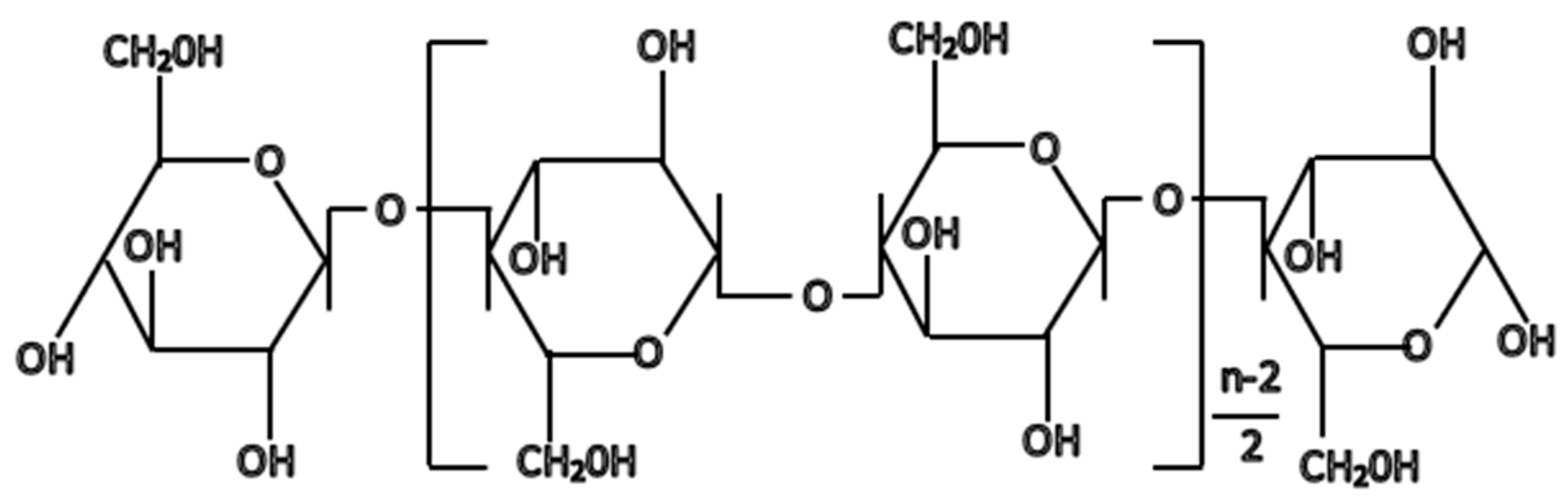
2. Cellulose Pulp: Composition and Properties
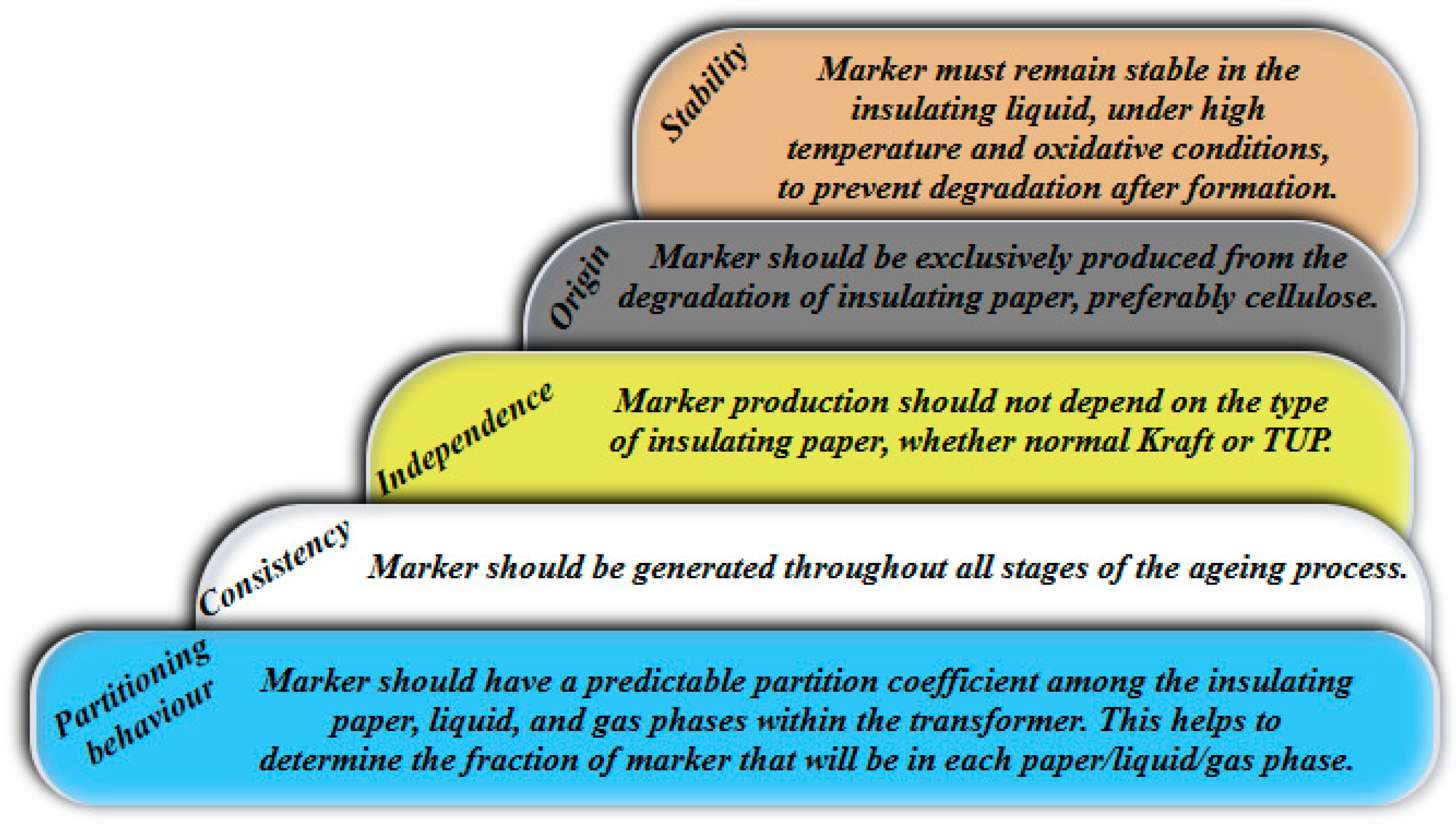
3. Marker to Capture DP
4. Characterisation of Paper Ageing
4.1. Molecular Dynamics Simulations
4.2. Glass Transition Temperature
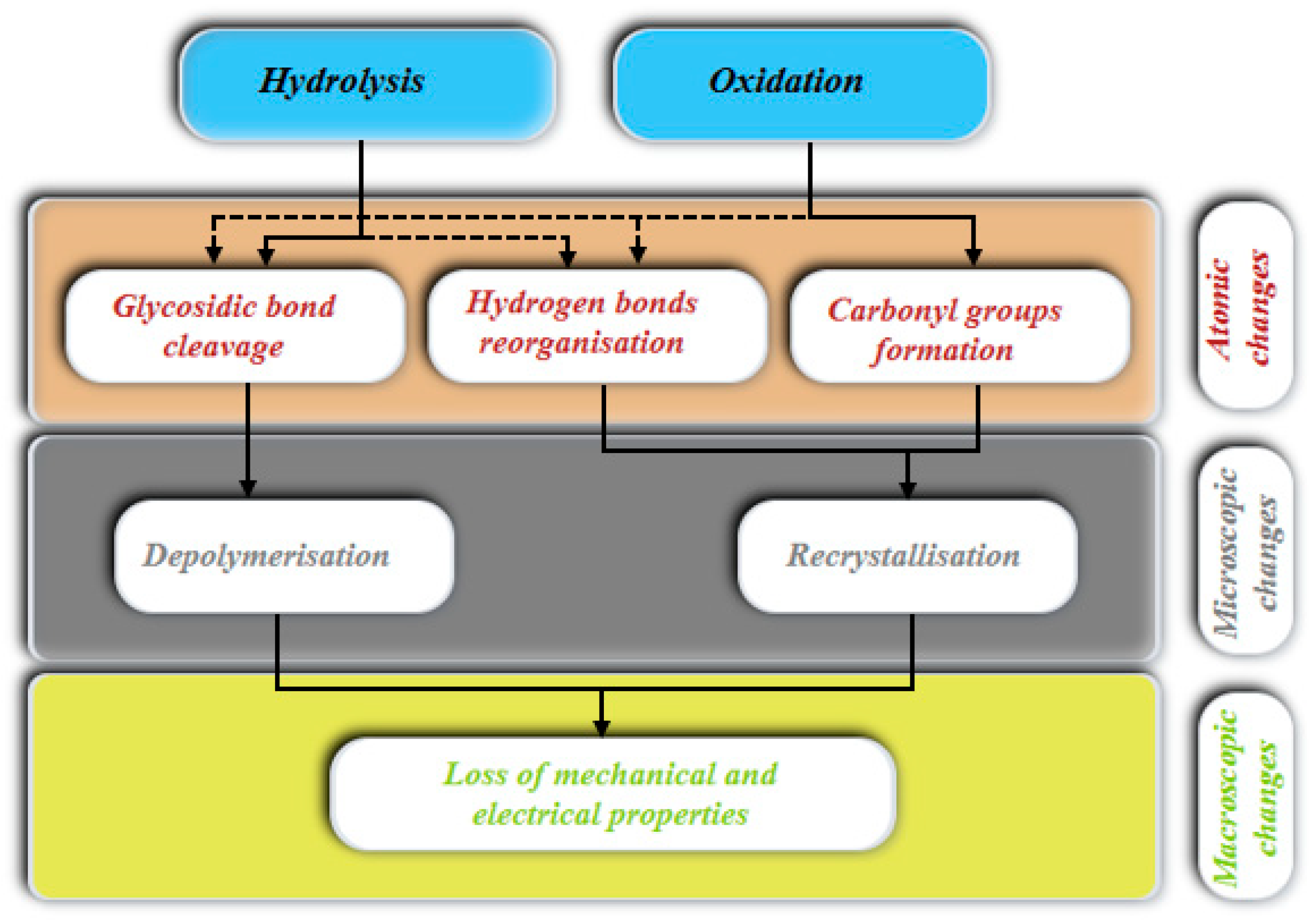
5. Cellulose Degradation
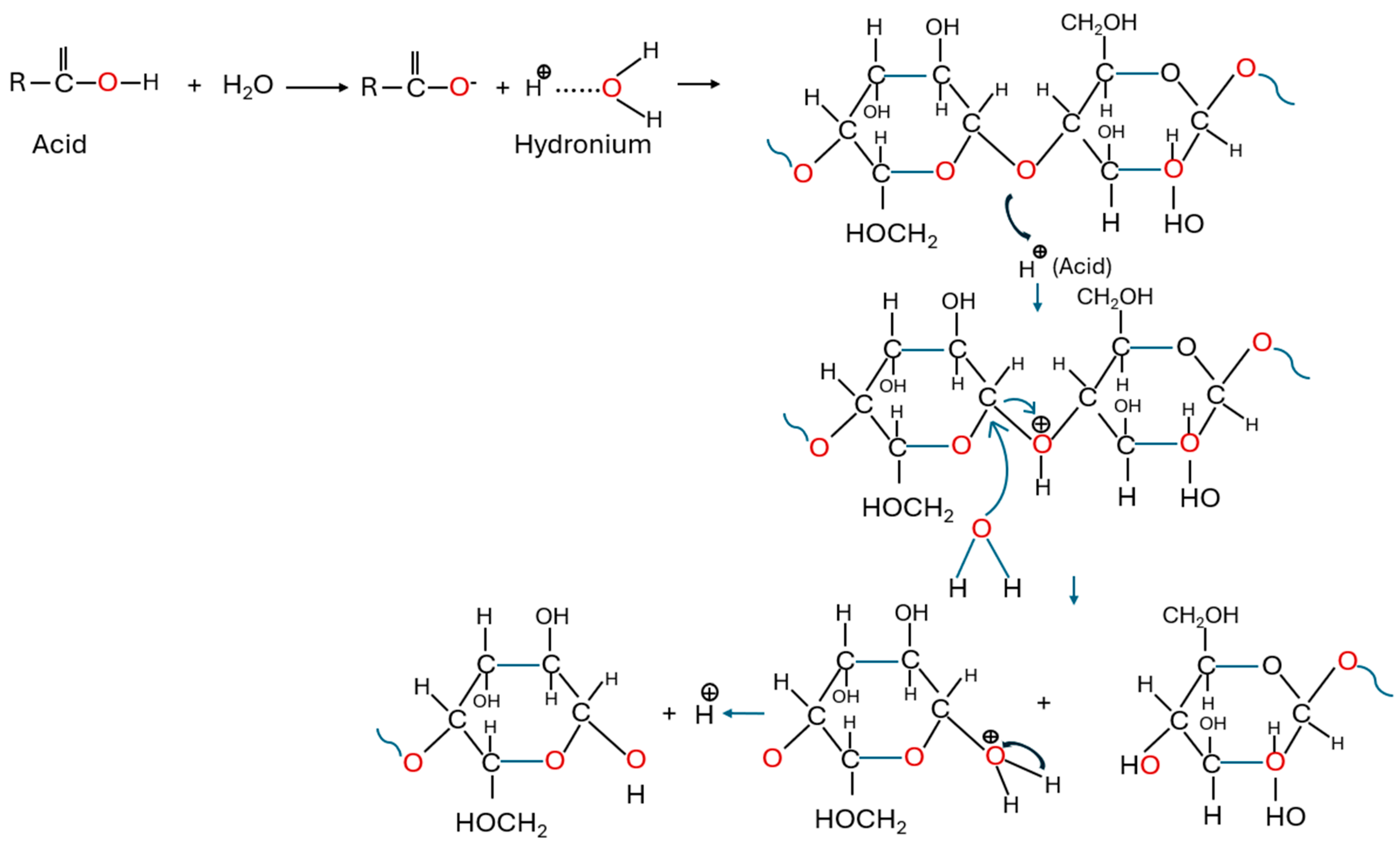
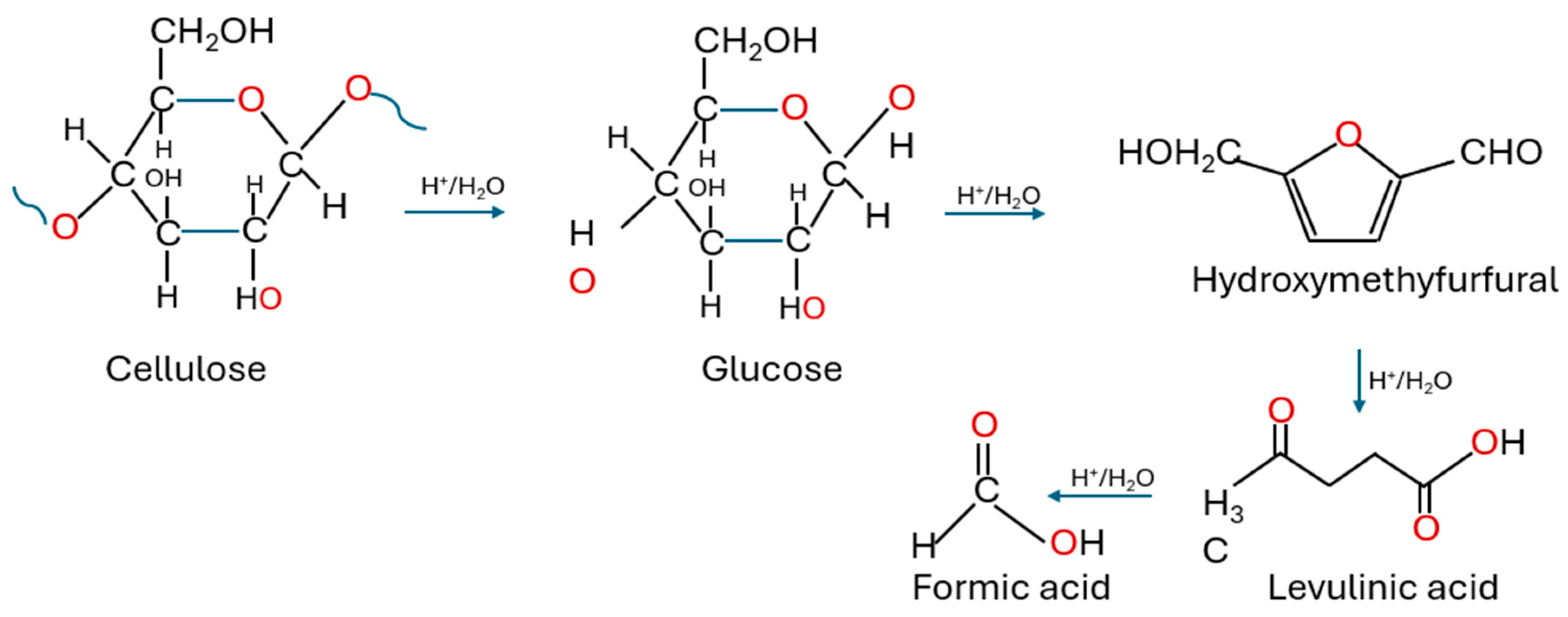
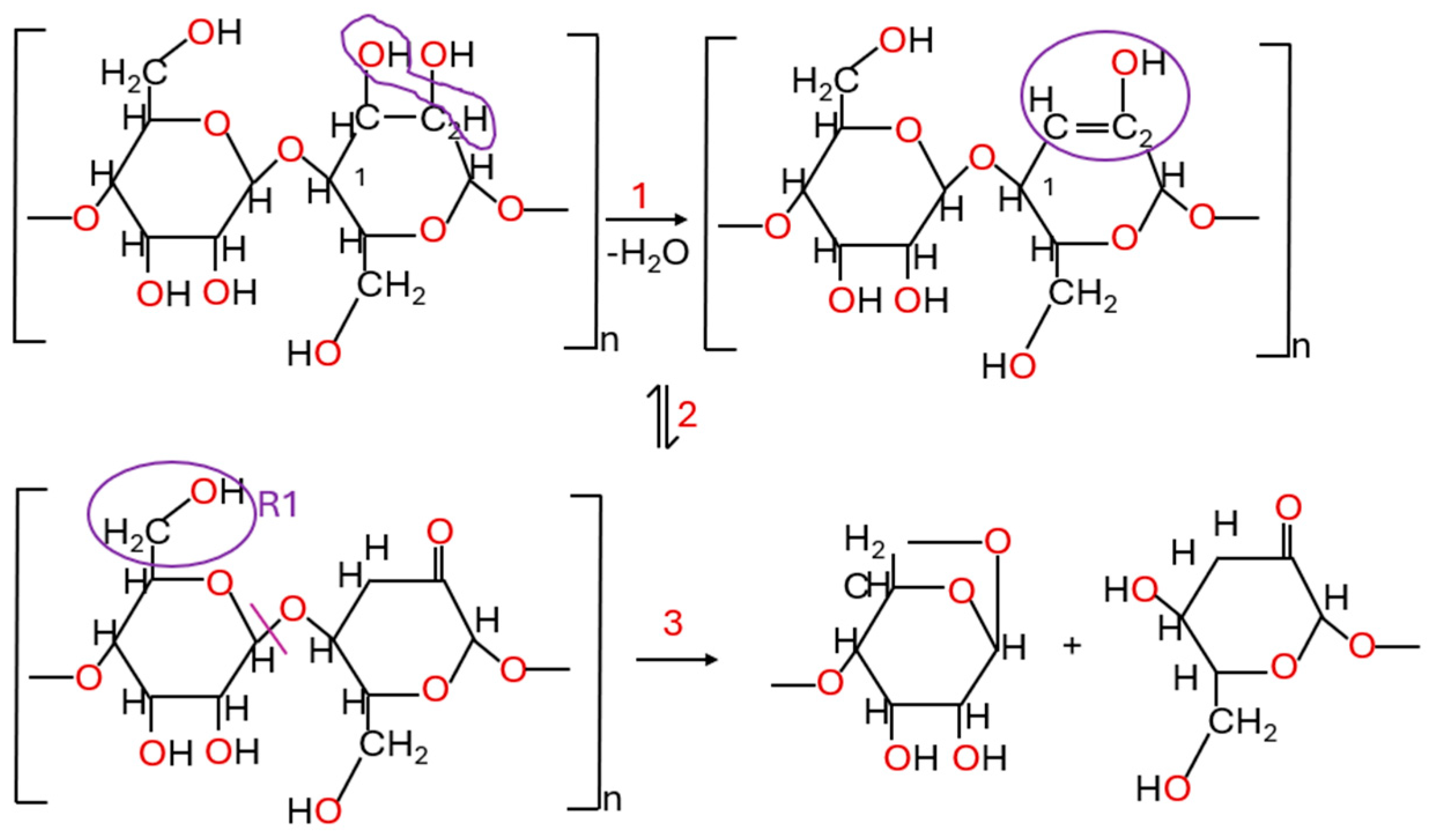
5.1. Acid Hydrolysis
Acid Detection
5.2. Number of Ruptured Bonds Within Cellulose
5.3. Yield Behaviour of Cellulose Paper
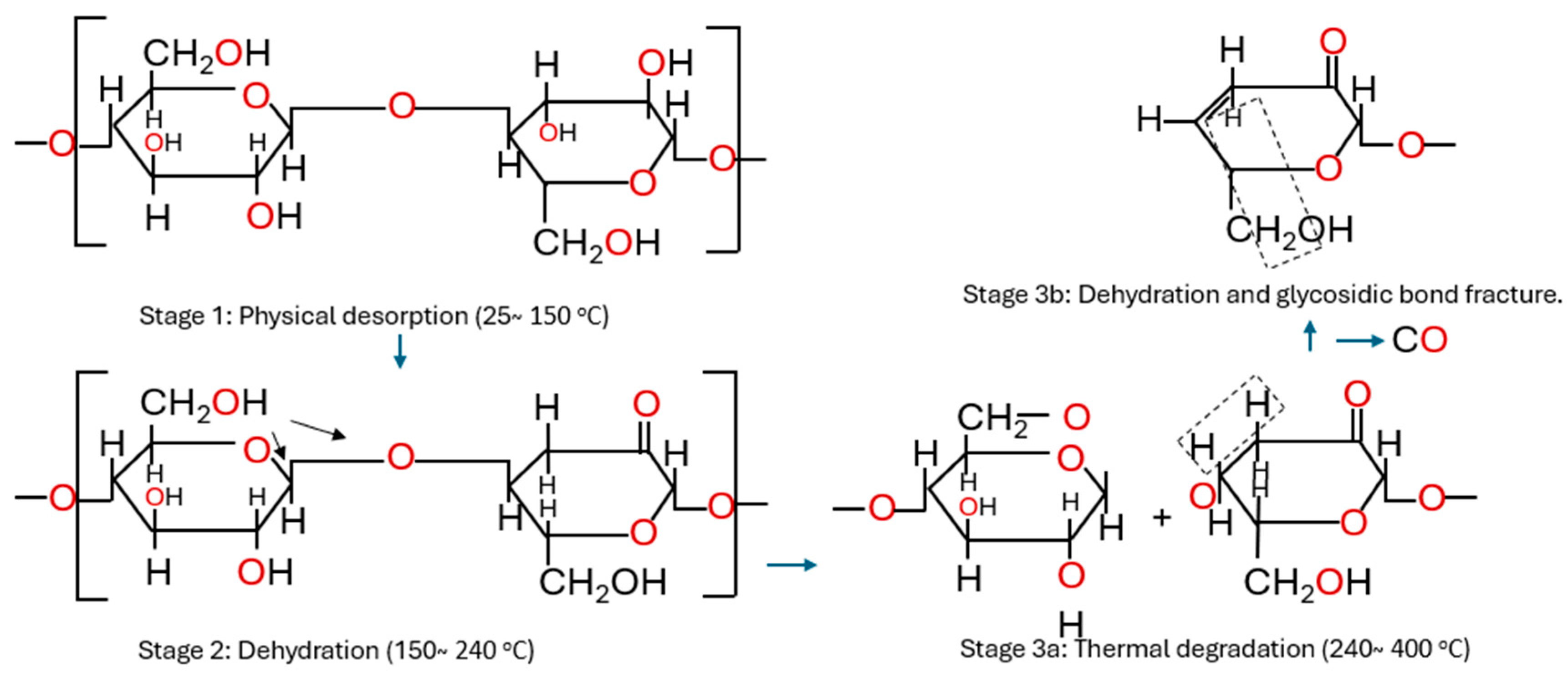
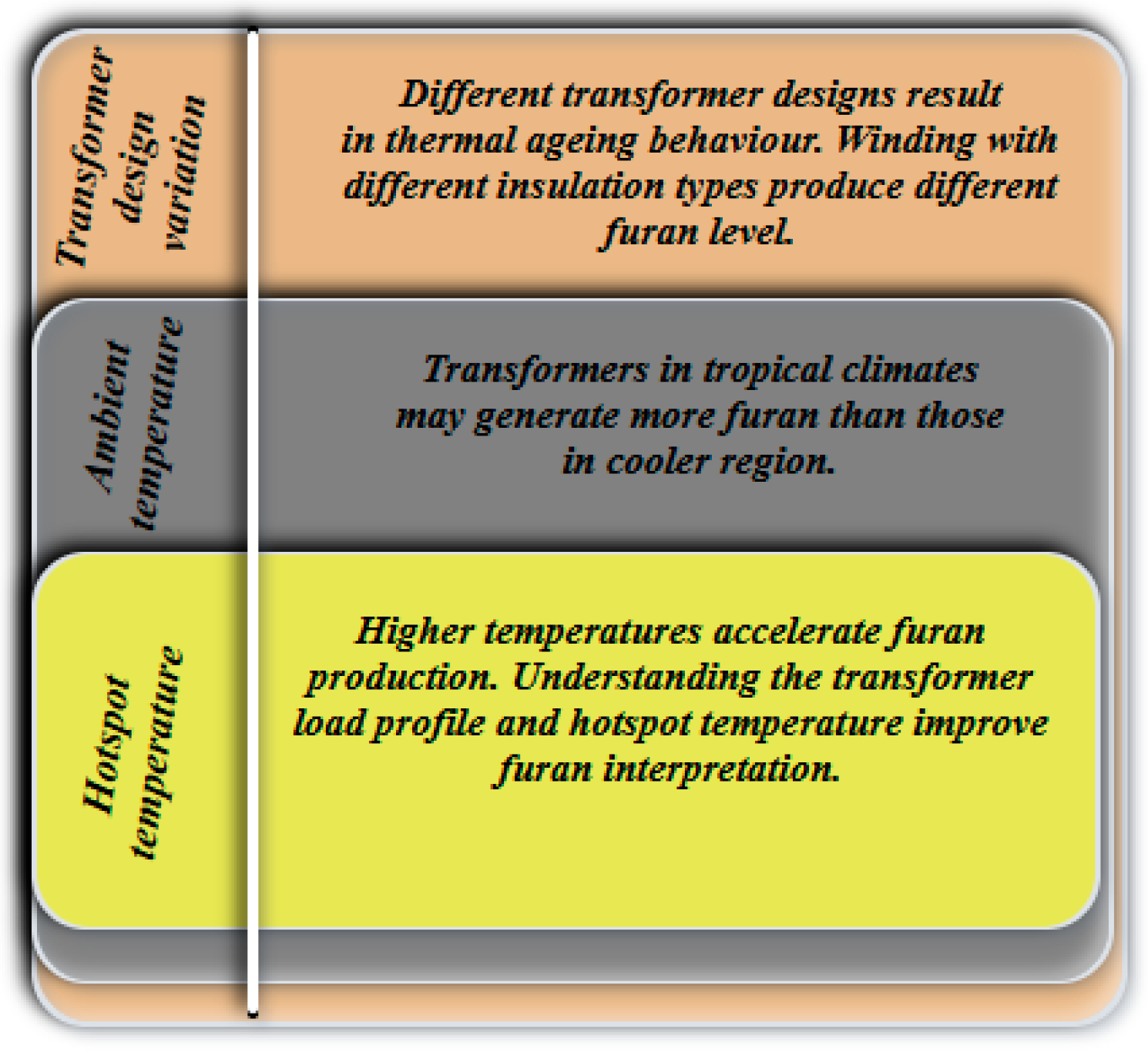
5.4. Temperature Condition
5.5. Effect of Hydrogen Sulphide
5.6. Cellulose Degradation in Insulating Liquid
6. Key Ageing Characteristics: Natural Ester vs. Mineral Oil-Impregnated Paper
6.1. Insulating Liquid–Paper Moisture Equilibrium
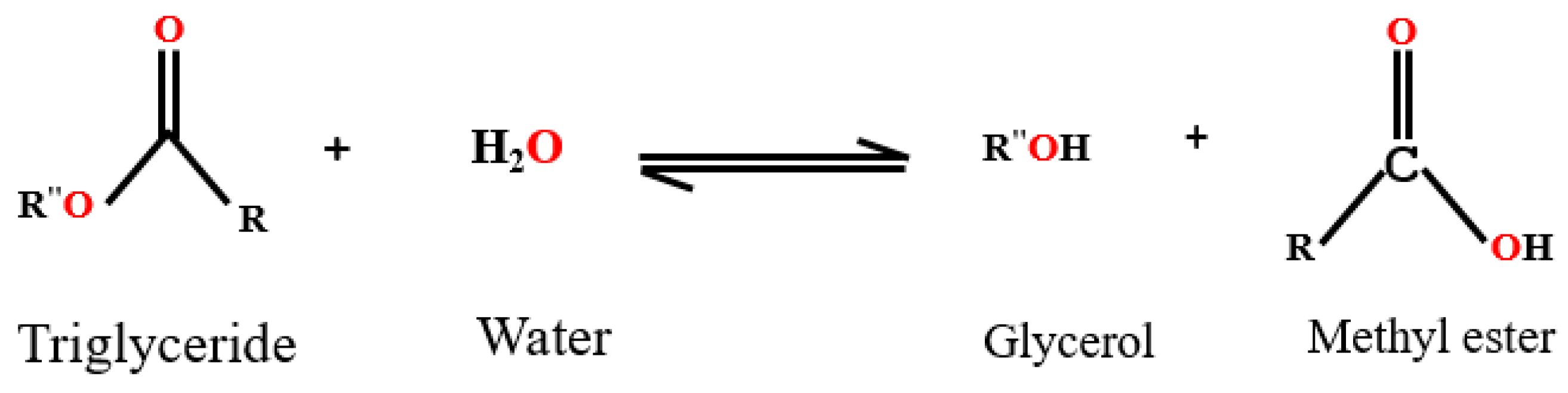
6.2. Hydrolysis of Esters
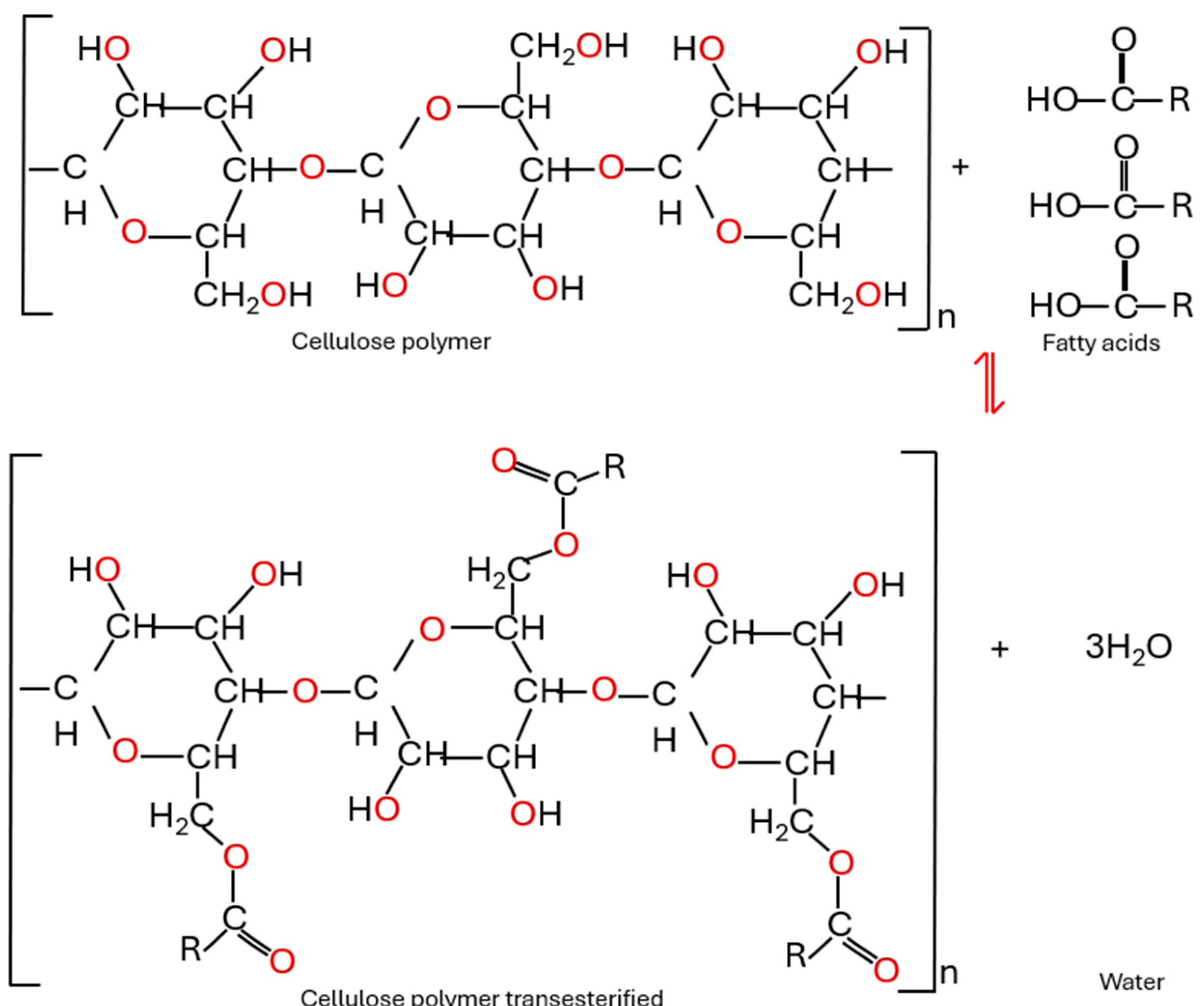
6.3. Transesterification Reaction
7. Models for DP Prediction
- The complexity of degradation and furan generation mechanisms and variability in stress factors (thermal and electrical).
- Differences in the analytical methods (sampling, detection, and calibration standards) that might vary between laboratories or studies.
- Variability in the transformer design, materials, and operational conditions, which complicates the generalisation of 2FAL as a reliable indicator across different studies.
- Disagreements on the reliability and interpretation of the relationship between 2FAL levels and transformer degradation (linear vs. non-linear models depending on factors such as the severity of stress, temperature, and operational conditions).
- The simplification of models that may not account for the full range of factors influencing transformer ageing. Over time, the characteristics of the transformer oil change due to oxidation, moisture absorption, and other chemical processes. Aged oils may have different chemical properties that affect 2FAL concentration, making it harder to draw direct correlations with the transformer’s degradation state. In addition, the presence of additives or impurities in the transformer oil, such as antioxidants or other chemical stabilisers, may interfere with the formation of 2FAL or change its concentration, leading to variation across studies using different types of oil formulations.
7.1. Limitations and Factors to Consider for 2FAL
7.2. Limitations and Factors to Consider for Methanol
8. Perspectives
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fernández-Diego, C.; Carrascal, I.A.; Ortiz, A.; Fernández, I.; Ferreño, D.; Diego, S.; Casado, A. Fracture toughness as an alternative approach to quantify the ageing of insulation paper in oil. Cellulose 2021, 28, 11533–11550. [Google Scholar] [CrossRef]
- Hassan, R. Thermal degradation of paper: The structural changes of fibres. Egypt. J. Archaeol. Restor. Stud. 2016, 6, 71. [Google Scholar]
- Saavedra, E.F.Q. High Performance Cellulosic Materials to Increase the Life and Reliability of Power Transformers. Ph.D. Thesis, Université Grenoble Alpes, Saint-Martin-d’Hères, France, 2023. [Google Scholar]
- Wang, Y.; Fei, R.; Gao, M.; Shang, J. Analysis of SEM characteristics of transformer oil-immersed insulation pressboard before and after thermal aging. In Proceedings of the 2020 IEEE 9th Joint International Information Technology and Artificial Intelligence Conference (ITAIC), Chongqing, China, 11–13 December 2020; IEEE: Chongqing, China, 2020; Volume 9, pp. 1248–1251. [Google Scholar]
- Mariano, M.; El Kissi, N.; Dufresne, A. Cellulose nanocrystals and related nanocomposites: Review of some properties and challenges. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 791–806. [Google Scholar] [CrossRef]
- Li, Y. Studies on Cellulose Hydrolysis and Hemicellulose Monosaccharide Degradation in Concentrated Hydrochloric Acid. Ph.D. Thesis, Université d’Ottawa/University of Ottawa, Ottawa, ON, Canada, 2014. [Google Scholar]
- Tazhibayev, A.; Amitov, Y.; Arynov, N.; Shingissov, N.; Kural, A. Experimental investigation and evaluation of drying methods for solid insulation in transformers: A comparative analysis. Results Eng. 2024, 23, 102470. [Google Scholar] [CrossRef]
- Boiron, L.; Ramousse, A. Effect of a cold delignification prior to determination of the IEC 60450 viscometric degree of polymerization of cellulosic insulation materials. IEEE Trans. Dielectr. Electr. Insul. 2020, 27, 1619–1626. [Google Scholar] [CrossRef]
- Emadifar, R.; Kalantari, N.T.; Behjat, V.; Najjar, R. Monitoring and condition assessment of insulation paper of distribution transformers with novel oil spectroscopy method. IEEE Trans. Dielectr. Electr. Insul. 2022, 29, 1904–1912. [Google Scholar] [CrossRef]
- Emadifar, R.; Taghizadegan, N.; Kalantari Behjat, V.; Najjar, R. Evaluation of Paper Insulation Condition of Distribution Transformers Based on the Concentration of 2-FAL and Methanol. TABRIZ J. Electr. Eng. 2022, 52, 43–49. [Google Scholar]
- Betie, A.; Meghnefi, F.; Fofana, I.; Yeo, Z. Modeling the insulation paper drying process from thermogravimetric analyses. Energies 2018, 11, 517. [Google Scholar] [CrossRef]
- Frimpong, G.K.; Melzer, L. Evaluation of mechanical condition of transformer paper insulation after factory drying. IEEE Electr. Insul. Mag. 2019, 35, 23–32. [Google Scholar] [CrossRef]
- Adekunle, A.A.; Oparanti, S.O.; Fofana, I. Performance assessment of cellulose paper impregnated in nanofluid for power transformer insulation application: A review. Energies 2023, 16, 2002. [Google Scholar] [CrossRef]
- Gao, T. 2-FAL; Francis Academic Press: London, UK, 2018. [Google Scholar]
- Adekunle, A.; Oparanti, S. A review on physicochemical and electrical performance of vegetable oil-based nanofluids for high voltage equipment. Electr. Power Syst. Res. 2023, 214, 108873. [Google Scholar] [CrossRef]
- He, Y.; Yang, L.; Cheng, L.; Chen, Q.; Yu, H.; Hou, W. Cellulose hydrogen bond detection using terahertz time-domain spectroscopy to indicate deterioration of oil–paper insulation. Cellulose 2023, 30, 727–740. [Google Scholar] [CrossRef]
- Adekunle, A.A.; Fofana, I.; Picher Rodriguez-Celis, E.M.; Arroyo-Fernandez, O.H. Analyzing transformer insulation paper prognostics and health management: A modeling framework perspective. IEEE Access 2024, 12, 58349–58377. [Google Scholar] [CrossRef]
- Kaliappan, G.; Rengaraj, M. Aging assessment of transformer solid insulation: A review. Mater. Today: Proc. 2021, 47, 272–277. [Google Scholar] [CrossRef]
- Zhang, E.; Liu, J.; Zhang, C.; Zheng Nakanishi, Y.; Wu, T. State-of-art review on chemical indicators for monitoring the aging status of oil-immersed transformer paper insulation. Energies 2023, 16, 1396. [Google Scholar] [CrossRef]
- Thiviyanathan, V.A.; Ker, J.; Leong, Y.S.; Abdullah, F.; Ismail, A.; Jamaludin, M.Z. Power transformer insulation system: A review on the reactions, fault detection, challenges and future prospects. Alex. Eng. J. 2022, 61, 7697–7713. [Google Scholar] [CrossRef]
- Cong, H.; Hu, X.; Liu, Z.; Wang, Y.; Li, Q. Micromechanism study on deterioration effect of vegetable oil and mineral oil on insulating paper by molecular dynamics. IEEE Trans. Dielectr. Electr. Insul. 2023, 30, 1460–1469. [Google Scholar] [CrossRef]
- Bagniuk, J.; Pawcenis, D.; Conte, A.M.; Pulci, O.; Aksamit-Koperska, M.; Missori, M.; Łojewska, J. How to estimate cellulose condition in insulation transformers papers? Combined chromatographic and spectroscopic study. Polym. Degrad. Stab. 2019, 168, 108951. [Google Scholar] [CrossRef]
- George, M.; Montemagno, C. Cellulose based materials: In-depth property survey and assessment. Cellulose 2017, 6, 55–76. [Google Scholar]
- Mokhena, T.C.; Sadiku, E.R.; Mochane, M.J.; Ray, S.S.; John, M.J.; Mtibe, A. Mechanical properties of cellulose nanofibril papers and their bionanocomposites: A review. Carbohydr. Polym. 2021, 273, 118507. [Google Scholar] [CrossRef]
- Naidu, D.S.; Hlangothi, S.; John, M.J. Bio-based products from xylan: A review. Carbohydr. Polym. 2018, 179, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Xu, D.; Luo, L.; Zhou, Y.; Yan, W.; Leng, X.; Fan, M. Overview of nanocellulose as additives in paper processing and paper products. Nanotechnol. Rev. 2021, 10, 264–281. [Google Scholar] [CrossRef]
- Gigante, V.; Aliotta, L.; Coltelli, M.B.; Lazzeri, A. Mechanical Response of Reactive Extruded Biocomposites Based on Recycled Poly (lactic Acid)(R-PLA)/Recycled Polycarbonate (R-PC) and Cellulosic Fibers with Different Aspect Ratios. Macromol 2022, 2, 509–521. [Google Scholar] [CrossRef]
- Hollertz, R.; Wågberg, L.; Pitois, C. Effect of composition and Morphology on the dielectric response of cellulose-based electrical insulation. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 2339–2348. [Google Scholar] [CrossRef]
- Wilhelm, H.; Fernandes, P.O.; Dill, L.P.; Moscon, K.G.; Marin, M.A.; Moraes, G.F.; Bender, V.C. Evaluation of solid insulating paper properties aged in natural ester and mineral insulating oil. In Proceedings of the 2022 IEEE Conference on Electrical Insulation and Dielectric Phenomena (CEIDP), Denver, CO, USA, 30 October–2 November 2022; IEEE: Denver, CO, USA, 2022; pp. 422–426. [Google Scholar]
- Adekunle, A.; Oparanti, S.; Hamzat, A.; Abdelmalik, A. Dielectric response of vegetable oil-based nanofluid and impregnated Kraft paper for high voltage transformer insulation. J. Mol. Liq. 2023, 391, 123391. [Google Scholar] [CrossRef]
- Geissler, D.; Leibfried, T. Mechanical breakdown of aged insulating paper around continuously transposed conductors for power transformers under the influence of short-circuit forces-Analysis by numerical simulations. In Proceedings of the 2015 IEEE Electrical Insulation Conference (EIC), Seattle, WA, USA, 7–10 June 2015; IEEE: Seattle, WA, USA, 2015; pp. 401–406. [Google Scholar]
- Fernández-Diego, C.; Ortiz, A.; Fernández, I.; Renedo, C.J.; Delgado, F. Ageing of crepe paper in mineral oil and natural ester. In Proceedings of the 2020 International Symposium on Electrical Insulating Materials (ISEIM), Tokyo, Japan, 13–17 September 2020; IEEE: Tokyo, Japan, 2020; pp. 217–220. [Google Scholar]
- Lyutikova, M.N.; Korobeynikov, S.M.; Ridel, A.V. Evaluation of the Effect of Essential Oil Blends on the Condition of Paper Insulation. In Proceedings of the 2023 IEEE 24th International Conference of Young Professionals in Electron Devices and Materials (EDM), Novosibirsk, Russia, 29 June–3 July 2023; IEEE: Novosibirsk, Russia, 2023; pp. 940–944. [Google Scholar]
- Rodriguez, E.; Jalbert, J.; Duchesne, S.; Arroyo, O.H.; Rodriguez, L.; Cross, J.; Lewand, L. On the experimental determination of the nitrogen content of thermally upgraded electrical papers. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 3092–3098. [Google Scholar] [CrossRef]
- Arroyo, O.H.; Jalbert, J.; Fofana, I.; Ryadi, M. Temperature dependence of methanol and the tensile strength of insulation paper: Kinetics of the changes of mechanical properties during ageing. Cellulose 2017, 24, 1031–1039. [Google Scholar] [CrossRef]
- Wilhelm, H.; Fernandes, P.; Moscon, K.; Steffens, C.; Peres, S.; Ziliotto, M.; Marek, R.P. New composite paper: Determination of degree of polymerization (DP) and end-of-life criteria. In Proceedings of the 2020 IEEE 3rd International Conference on Dielectrics (ICD), Valencia, Spain, 5–31 July 2020; IEEE: Valencia, Spain, 2020; pp. 681–684. [Google Scholar]
- Liu, B.; Lv, F.; Fan, X.; Sui, Y.; Wang, J.; Yin, S. [Retracted] Preparation and Performance Analysis of Transformer Aramid Nanopaper-Based Insulating Material Based on Deep Learning. Comput. Intell. Neurosci. 2022, 2022, 2282870. [Google Scholar]
- Wilhelm, H.M.; Fernandes, P.; Fernandes, K.M.P.; Fornari, M.; Peres, S.; Bender, V.; Marin, M. Comparative effect of hybrid and thermally upgraded kraft insulating papers on degradation of mineral and natural ester insulating oils by accelerated ageing tests. In Proceedings of the 2023 IEEE Electrical Insulation Conference (EIC), Quebec City, QC, Canada, 18–21 June 2023; IEEE: Quebec City, QC, Canada, 2023; pp. 1–4. [Google Scholar]
- Matharage, S.; Liu, Q.; Wang, Z. Aging assessment of kraft paper insulation through methanol in oil measurement. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 1589–1596. [Google Scholar] [CrossRef]
- Arroyo, O.H.; Fofana, I.; Jalbert, J.; Ryadi, M. Relationships between methanol marker and mechanical performance of electrical insulation papers for power transformers under accelerated thermal aging. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 3625–3632. [Google Scholar] [CrossRef]
- Tokunaga, J.; Koide, H.; Mogami, K.; Hikosaka, T. Gas generation of cellulose insulation in palm fatty acid ester and mineral oil for life prediction marker in nitrogen-sealed transformers. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 420–427. [Google Scholar] [CrossRef]
- Teymouri, A.; Vahidi, B. CO2/CO concentration ratio: A complementary method for determining the degree of polymerization of power transformer paper insulation. IEEE Electr. Insul. Mag. 2017, 33, 24–30. [Google Scholar] [CrossRef]
- Zheng, H.; Yang, E.; Wu, S.; Lv, W.; Yang, H.; Li, X.; Hu, W. Investigation on formation mechanisms of carbon oxides during thermal aging of cellulosic insulating paper. IEEE Trans. Dielectr. Electr. Insul. 2022, 29, 1226–1233. [Google Scholar] [CrossRef]
- Fernández, O.H.A.; Fofana, I.; Jalbert, J.; Gagnon, S.; Rodriguez-Celis, E.; Duchesne, S.; Ryadi, M. Aging characterization of electrical insulation papers impregnated with synthetic ester and mineral oil: Correlations between mechanical properties, depolymerization and some chemical markers. IEEE Trans. Dielectr. Electr. Insul. 2018, 25, 217–227. [Google Scholar] [CrossRef]
- Aciu, A.M.; Nițu, M.C.; Nicola, M.; Nicola, C.I. Determination of the condition of solid insulation in high-power transformers based on 2-furfuraldehyde and methanol markers using neural networks. In Proceedings of the 2021 International Conference on Electromechanical and Energy Systems (SIELMEN), Iasi, Romania, 6–8 October 2021; IEEE: Iasi, Romania, 2021; pp. 175–180. [Google Scholar]
- Duval, M.; De Pablo, A.; Atanasova-Hoehlein, I.; Grisaru, M. Significance and detection of very low degree of polymerization of paper in transformers. IEEE Electr. Insul. Mag. 2017, 33, 31–38. [Google Scholar] [CrossRef]
- Liu, T.; Li, J.; Huang, Z.; Hou, S.; Duan, Y.; Li, C. Ageing Characterization of Transformer Paper Insulation Based on Dispersion Staining Colors of Cellulose Fibers in Oil. In Proceedings of the 2020 IEEE International Conference on High Voltage Engineering and Application (ICHVE), Beijing, China, 6–10 September 2020; IEEE: Beijing, China, 2020; pp. 1–4. [Google Scholar]
- Chen, C.; Li, S.; He, T.; Liu, Y. Concentration of total sugar produced by insulating paper in oil-paper insulation system. IEEE Trans. Dielectr. Electr. Insul. 2019, 26, 2036–2040. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, Z.; Li, H.; Tang, C. Properties of ladder-like polysilsesquioxane-modified insulation paper cellulose with different substituents. J. Ind. Eng. Chem. 2024, 132, 437–447. [Google Scholar] [CrossRef]
- Mazeau, K.; Vergelati, C. Atomistic modeling of the adsorption of benzophenone onto cellulosic surfaces. Langmuir 2002, 18, 1919–1927. [Google Scholar] [CrossRef]
- Du, D.; Tang, C.; Zhang, J.; Hu, D. Effects of hydrogen sulfide on the mechanical and thermal properties of cellulose insulation paper: A molecular dynamics simulation. Mater. Chem. Phys. 2020, 240, 122153. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, S.; Xie, J.; Lv, C. Molecular simulation and experimental analysis of Al2O3-nanoparticle-modified insulation paper cellulose. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 1018–1026. [Google Scholar] [CrossRef]
- Wang, W.; He, D.; Yang, K.; Liu, S.; Song, S.; Yi, D. Research of the thermal aging mechanism of polycarbonate and polyester film. e-Polymers 2017, 17, 45–56. [Google Scholar] [CrossRef]
- Lelekakis, N.; Wijaya, J.; Martin, D.; Susa, D. The effect of acid accumulation in power-transformer oil on the aging rate of paper insulation. IEEE Electr. Insul. Mag. 2014, 30, 19–26. [Google Scholar] [CrossRef]
- Kouassi, K.D.; Fofana, I.; Cissé, L.; Hadjadj, Y.; Yapi, K.M.L.; Diby, K.A. Impact of low molecular weight acids on oil impregnated paper insulation degradation. Energies 2018, 11, 111465. [Google Scholar] [CrossRef]
- Wan, F.; Du, L.; Chen, W.; Wang, P.; Wang, J.; Shi, H. A novel method to directly analyze dissolved acetic acid in transformer oil without extraction using Raman spectroscopy. Energies 2017, 10, 967. [Google Scholar] [CrossRef]
- Li, N.; Li, J.; Yang, L.; Liao, R. Effect of acidic substances on thermal life of vegetable oil-paper insulation. In Proceedings of the 2014 ICHVE International Conference on High Voltage Engineering and Application, Poznan, Poland, 8–11 September 2014; IEEE: Poznan, Poland, 2014; pp. 1–4. [Google Scholar]
- Lan, S.; Huang, M.; Zhang, Y.; Yuan, Y. Experimental study of oil-paper insulation under combined thermal stress and corona discharge. IEEE Trans. Dielectr. Electr. Insul. 2019, 26, 1001–1008. [Google Scholar] [CrossRef]
- Yan, K.; Wang, G.; Li, Z.; Cong, H. Study on Electrothermal Aging of Insulation Pressboard Based on Molecular Simulation. In Proceedings of the 2023 2nd Asia Power and Electrical Technology Conference (APET), Shanghai, China, 28–30 December 2023; IEEE: Shanghai, China, 2023; pp. 1–5. [Google Scholar]
- Fan Wang, Y.; Tian, M.; Wu, J. Molecular dynamics simulation on the impact of electric field on the yield behavior of insulation paper. In Proceedings of the 2014 International Symposium on Electrical Insulating Materials, Niigata, Japan, 1–5 June 2014; IEEE: Niigata, Japan, 2014; pp. 457–460. [Google Scholar]
- Wang, Y.Y.; Yang, T.; Tian, M.; Liao, R.J. The relationship between DP, fracture degree and mechanical strength of cellulose Iβ in insulation paper by molecular dynamic simulations. Int. J. Mod. Phys. B 2013, 27, 1350183. [Google Scholar] [CrossRef]
- Han, S.; Li, Q.; Li, C.; Yan, J. Electrical and mechanical properties of the oil-paper insulation under stress of the hot spot temperature. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 179–185. [Google Scholar] [CrossRef]
- Zhang, M.; Cong, H.; Shu, X.; Li, Q. Study on microscopic mechanism of the transformer insulating paper’s degradation under synergistic effect of DBDS and Hexadecanethiol. Proc. CSEE 2018, 38, 7156–7165, 7442. [Google Scholar]
- Mc Shane, C.; Rapp, K.J.; Corkran, J.L.; Gauger, G.A.; Luksich, J. Aging of paper insulation in natural ester dielectric fluid. In Proceedings of the 2001 IEEE/PES Transmission and Distribution Conference and Exposition. Developing New Perspectives (Cat. No. 01CH37294), Atlanta, GA, USA, 2 November 2001; IEEE: Atlanta, GA, USA, 2001; Volume 2, pp. 675–679. [Google Scholar]
- Liao, R.; Liang, S.; Sun, C.; Yang, L.; Sun, H. A comparative study of thermal aging of transformer insulation paper impregnated in natural ester and in mineral oil. Eur. Trans. Electr. Power 2010, 20, 518–533. [Google Scholar] [CrossRef]
- Martins, M.A.G. Vegetable oils, an alternative to mineral oil for power transformers-experimental study of paper aging in vegetable oil versus mineral oil. IEEE Electr. Insul. Mag. 2010, 26, 7–13. [Google Scholar] [CrossRef]
- Tokunaga, J.; Koide, H.; Mogami, K.; Hikosaka, T. Comparative studies on the aging of thermally upgraded paper insulation in palm fatty acid ester, mineral oil, and natural ester. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 258–265. [Google Scholar] [CrossRef]
- Tokunaga, J.; Koide, H.; Mogami, K.; Hikosaka, T. Comparative study on the aging of kraft paper insulation in palm fatty acid ester and mineral oil in air sealed tank. IEEJ Trans. Fundam. Mater. 2016, 136, 541–546. [Google Scholar] [CrossRef]
- Garcia, B.; Garcia, T.; Primo, V.; Burgos, J.C.; Urquiza, D. Studying the loss of life of natural-ester-filled transformer insulation: Impact of moisture on the aging rate of paper. IEEE Electr. Insul. Mag. 2017, 33, 15–23. [Google Scholar] [CrossRef]
- Dombek, G.; Nadolny, Z. Influence of paper type and liquid insulation on heat transfer in transformers. IEEE Trans. Dielectr. Electr. Insul. 2018, 25, 1863–1870. [Google Scholar] [CrossRef]
- Sayed, N.; Jacob, J.; Sindhu, T.; Preetha, P. Compatibility analysis of paper insulation with natural ester. In Proceedings of the 2019 IEEE 4th International Conference on Condition Assessment Techniques in Electrical Systems (CATCON), Chennai, India, 21–23 November 2019; IEEE: Chennai, India, 2019; pp. 1–5. [Google Scholar]
- Gao, C.; Hao, J.; Chen, X.; Jian, Z.; Hao, W.; Zhu, T. Microcosmic Mechanism Analysis of Insulation Paper Thermally Aged in Mineral Oil and Ester Oils. In Proceedings of the 2020 IEEE International Conference on High Voltage Engineering and Application (ICHVE), Beijing, China, 6–10 September 2020; IEEE: Beijing, China, 2020; pp. 1–4. [Google Scholar]
- Wang, Y.; Chen, G.; Chen, X.; Liu, T.; Feng, D. Production pattern of furfural in esters-immersed paper and aging evaluation methods. IEEE Trans. Dielectr. Electr. Insul. 2024, 31, 1. [Google Scholar] [CrossRef]
- Oria, C.; Méndez, C.; Carrascal, I.; Ortiz, A.; Ferreño, D. Impact of the use of vegetable oil on the mechanical failure of the cellulosic insulation of continuously transposed conductors in power transformers. IEEE Trans. Dielectr. Electr. Insul. 2022, 29, 607–613. [Google Scholar] [CrossRef]
- Gutiérrez, C.M.; Salmas, C.O.; Estébanez, C.J.R.; Kozako, M.; Hikita, M.; Fernández, A.O. Study of the Thermal Degradation of Different Insulating Papers Impregnated with a Natural Ester. In Proceedings of the 2022 9th International Conference on Condition Monitoring and Diagnosis (CMD), Kitakyushu, Japan, 13–18 November 2022; IEEE: Kitakyushu, Japan, 2022; pp. 167–171. [Google Scholar]
- Mihajlovic, D.; Vasovic, V.; Lukic, J. Development of New Accelerated Aging Test for Comparison of the Quality of Different Insulating Papers Based on Cellulose. Polymers 2023, 15, 2556. [Google Scholar] [CrossRef]
- Wilhelm, H.M.; Fernandes, P.O.; Marek, R.; Marin, M.; Moraes, G.F.; Marchesan, T.; Bender, V. Mechanical Tensile Properties of Insulating Papers, Hybrid and Thermally Upgraded Kraft Paper, Aged in Natural Ester and Mineral Insulating Oils. In Proceedings of the 2023 IEEE Conference on Electrical Insulation and Dielectric Phenomena (CEIDP), East Rutherford, NJ, USA, 15–19 October 2023; IEEE: East Rutherford, NJ, USA, 2023; pp. 1–4. [Google Scholar]
- Ye, W.; Hao, J.; Gao, C.; Zhang, J.; Yang, L.; Liao, R. Natural ester replacement effect on thermal degradation of cellulose insulation from macroscopic behavior to atomic-scale mechanism. IEEE Trans. Dielectr. Electr. Insul. 2023, 30, 1582–1589. [Google Scholar] [CrossRef]
- Gmati, G.; Fofana, I.; Picher, P.; Meghnefi, F.; Arroyo-Fernàndez, O.; Rebaine, D.; Kouba, Y.M.L. Bubbling Inception Temperature in Power Transformers–Part 1: Comparative Study of Kraft Paper, Thermally Upgraded Kraft Paper, and Aramid Paper with Mineral Oil. IEEE Access 2025, 13, 51287–51299. [Google Scholar] [CrossRef]
- Jeong, H.; Lee, S.; Park, J.Y.; Ryu, J.; Bae, C.Y. A Study on the Insulation breakdown voltage according to degradation condition of natural ester oil. In Proceedings of the 2024 IEEE Electrical Insulation Conference (EIC), Minneapolis, MN, USA, 2–5 June 2024; IEEE: Minneapolis, MN, USA, 2024; pp. 300–303. [Google Scholar]
- Wang, B.; Li, J. Thermal aging results between oil-paper insulation impregnated by mineral and vegetable insulating oil. In Proceedings of the 2014 ICHVE International Conference on High Voltage Engineering and Application, Poznan, Poland, 8–11 September 2014; IEEE: Poznan, Poland, 2014; pp. 1–4. [Google Scholar]
- Mtetwa, N.S. Accuracy of Furan Analysis in Estimating the Degree of Polymerization in Power Transformers. Master’s Thesis, University of the Witwatersrand, Johannesburg, South Africa, 2011. [Google Scholar]
- Cheim, L.; Platts, D.; Prevost, T.; Xu, S. Furan analysis for liquid power transformers. IEEE Electr. Insul. Mag. 2012, 28, 8–21. [Google Scholar] [CrossRef]
- Talib, M.A.; Aziz, M.A.A.; Balasubramaniam, Y.; Ghazali, Y.Z.Y.; Abidin, M.R.Z.; Yousof, M.F.M. Transformer ageing investigation and correlation of furanic compound and degree of polymerization for power transformer life assessment. In Proceedings of the 2020 IEEE International Conference on Power and Energy (PECon), Penang, Malaysia, 7–8 December 2020; IEEE: Penang, Malaysia, 2020; pp. 240–244. [Google Scholar]
- Ortiz Fernández, F.; Fernández, C.; Diego Santisteban, A.; Díaz Delgado, F.; San Román, S.; Ortiz Fernández, A. Estimating the age of power transformers using the concentration of furans in dielectric oil. In Proceedings of the International Conference on Renewable Energies and Power Quality (ICREPQ’16), Madrid, Spain, 4–6 May 2016. [Google Scholar]
- CIGRÉ. Technical Brochure 494: Furanic Compounds for Diagnosis. Final Report of Working Group D1.03; CIGRÉ: Paris, France, 2012; pp. 1–71. [Google Scholar]
- Lin, C.; Zhang, B.; Yuan, Y. The aging diagnosis of solid insulation for oil-immersed power transformers and its remaining life prediction. In Proceedings of the 2010 Asia-Pacific Power and Energy Engineering Conference, Chengdu, China, 28–31 March 2010; IEEE: Chengdu, China, 2010; pp. 1–3. [Google Scholar]
- García, B.; Urquiza, D.; Burgos, J. Investigating the influence of moisture on the 2FAL generation rate of transformers: A new model to estimate the DP of cellulosic insulation. Electr. Power Syst. Res. 2016, 140, 87–94. [Google Scholar] [CrossRef]
- Leibfried, T.; Jaya, M.; Majer, N.; Schafer, M.; Stach, M.; Voss, S. Postmortem investigation of power transformers—Profile of degree of polymerization and correlation with furan concentration in the oil. IEEE Trans. Power Deliv. 2013, 28, 886–893. [Google Scholar] [CrossRef]
- Jalbert, J.; Hernandez, L.; Coulibaly, M.L.; Lessard, L.; Lesaint, C.; Lukic, J.; Martins, M.A. Field Experience with Transformer Solid Insulation Ageing Markers, Cigré Technical Brochure; CIGRE: Paris, France, 2019; Volume 779. [Google Scholar]
- Ghoneim, S.S. The degree of polymerization in a prediction model of insulating paper and the remaining life of power transformers. Energies 2021, 14, 670. [Google Scholar] [CrossRef]
- Lütke, H.; Höhlein, I.; Kachler, A. Transformer Aging Research on Furanic Compounds Dissolved in Insulating Oil. In Proceedings of the Large High Voltage Electrical Systems Conference (CIGRÉ), Paris, France, August 2002; pp. 25–30. [Google Scholar]
- Jalbert, J.; Gilbert, R.; Tétreault Morin, B.; Lessard-Déziel, D. Identification of a chemical indicator of the rupture of 1, 4-β-glycosidic bonds of cellulose in an oil-impregnated insulating paper system. Cellulose 2007, 14, 295–309. [Google Scholar] [CrossRef]
- Jalbert, J.; Rodriguez-Celis, E.M.; Arroyo-Fernández, O.H.; Duchesne, S.; Morin, B. Methanol marker for the detection of insulating paper degradation in transformer insulating oil. Energies 2019, 12, 3969. [Google Scholar] [CrossRef]
- ASTM D8086-20; Standard Test Method for Determination of Methanol and Ethanol in Electrical Insulating Liquids of Petroleum Origin by Headspace (HS)-Gas Chromatography (GC) Using Mass Spectrometry (MS) or Flame Ionization Detection (FID). ASTM International: West Conshohocken, PA, USA, 2020.
- Adewunmi, A.; Adekunle Brahami, Y.; Coulibaly, M.L.; Courtellemont, R.; Meghnefi, F.; Fofana, I. Bio-based hydrocarbon and Gas-to-Liquid (GTL) insulating liquids for power transformers: A comprehensive review. Transform. Mag. 2025, 12, 104–117. [Google Scholar]
- Xu, T.; Wang, Q.; Song, S.; Zhang, M.; Peng, Z.; Liu, P.; Wang, R. A Potential Substitute for Traditional Insulating Paper: Low-Ash Paper Processed from Natural Bamboo. IEEE Trans. Dielectr. Electr. Insul. 2023, 31, 713–721. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, R.; Xu, T.; Tian, S.; Wei, D.; Peng, Z.; Zhou, X. Thermal Aging Characteristics of New Insulating Oil-Bamboo Paper Composite Material. IEEE Trans. Dielectr. Electr. Insul. 2024, 32, 779–788. [Google Scholar] [CrossRef]
- Beheshti Asl, M.; Fofana, I.; Meghnefi, F. Review of various sensor technologies in monitoring the condition of power transformers. Energies 2024, 17, 3533. [Google Scholar] [CrossRef]
- Münster, T.; Werle, P.; Peter, I.; Hämel, K.; Barden, R.; Preusel, J. Optical sensor for determining the degree of polymerization of the insulation paper inside transformers. Transform. Mag. 2021, 8, 106–117. [Google Scholar]
- Seifaddini, N.; Fofana, I.; Rajesh, N.; Kandala, V.S.; Lim, K.-S.; Ooi, C.W.; Udos, W.; Sekongo, B.; Chehri, A.; Ouhrouche, M.; et al. Aging characterization of thermally aged transformer paper based on its reflectance. Results Opt. 2024, 16, 100716. [Google Scholar] [CrossRef]




| Techniques | Explanation | Strength | Drawback |
|---|---|---|---|
| Indirect drying | - Uses absorbent materials to remove water from transformer liquid, which indirectly dries the solid insulation | - Continuous moisture control - Cost-effective preventive maintenance | - Primarily affects liquid rather than solid insulation |
| Vapour phase drying | - Uses solvent vapour for heating and condensation, with vacuum pumps enhancing the moisture level | - Efficient moisture removal - Suitable for large transformers | - Complex process as it requires controlled conditions |
| Hot air drying | - Applies hot air at 100 °C to 110 °C, which is typically used in convection ovens for transformer drying | - Simple and widely employed - Effective for small transformers | - Uneven heat distribution - Limited to small units |
| Heat and vacuum treatment | - Uses heat and vacuum to lower the boiling point of water, allowing for moisture removal at safe temperatures | - Highly efficient - Prevents insulation degradation and is suitable for all transformers | - Requires specialised chambers and vacuum pumps |
| Insulating Paper | Manufacturing Process | Composition | Strength | Limitation | Thermal Class | Ref. |
|---|---|---|---|---|---|---|
| Kraft paper | Kraft process/sulphate process | Cellulose (primary component), hemicellulose, lignin (partially removed), and residual sulphates from Kraft process | - Cost-effective and widely available - Slightly alkaline, aiding insulation longevity | - More vulnerable to thermal, mechanical, and electrical stress | Class A (105 °C) | [3,13,29,30] |
| Crepe paper | Kraft process with an extra creeping step | Cellulose, hemicellulose, and modified with crimping (mechanical process) to enhance extensibility | - Higher stretch (elongation) capacity to wrap around copper wiring without tearing - Used to insulate irregular shapes and connections - Provides reliable pairing of insulation layers during winding | - More expensive than standard Kraft paper - Less durable under prolonged mechanical stress | Class B (130 °C) | [3,29,31,32,33] |
| Thermally upgraded paper | Kraft process with additional nitrous compound | Cellulose, hemicellulose (partially replaced), and stabilising nitrogen compounds (dicyandiamide, urea, melanine) | - Better thermal performance than standard Kraft paper - Slower ageing rate (up to 2.5 times slower than Kraft paper) - Higher resistance to hydrolysis and oxidation - Partially neutralise the attack of the acids against the cellulose and reacts in contact with water | - More expensive than standard Kraft paper - Reduced mechanical strength due to hydroxyl group substitution - Some chemical processes s(cyanoethylation modification) involve toxic nitrile organic compounds | Class A (105 °C) | [3,29,34,35,36] |
| Diamond-dotted paper | Kraft process with an epoxy layer | Kraft paper-based, thermosetting resin (diamond-pattern coating) | - The epoxy layer provides internal strengthening of the coil for better adhesion to the conductor - High mechanical strength due to large bonding surface | - More expensive than standard Kraft paper - Increased manufacturing complexity | Class A (105 °C) | [3,31] |
| Synthetic paper (Aramid/Nomex) | A blend of wood pulp, synthetic fibres, and a binder | Aramid fibres (polymeric aromatic polyamides), aramid pulp, and no cellulose content | - Mostly polyaramid-based papers with better thermal and mechanical performance for traction transformers - Higher thermal rating than thermally upgraded Kraft (TUK) - Resistance to moisture, acids, and abrasion - Higher bubble initiation temperature than cellulose-based papers | - Low moisture adsorption but easy to hydrolyse at high temperatures - More expensive than other alternatives - Less flexible compared to crepe paper - The aramid portion of the paper cannot be dissolved by most solvents | Nomex 910 Class B (130 °C) Nomex 410 Class C (220 °C) | [3,29,31,36,37,38] |
| Markers | Strength | Limitation | Ref. |
|---|---|---|---|
| Carbon oxides | - Widely used in transformer diagnostics - Excellent indicator for overheating fault as it is being generated through the thermal decomposition of insulating paper - A standardised online technique with dissolved gas analysis (DGA) - C-O bond within the glucose ring undergoes cleavage to form a carbonyl group and release CO2 molecules - Dehydrogenation of the secondary alcohol hydroxyl group on the glucose ring forms a carbonyl group and then close bonds weaken to release CO molecules | - Not specific to cellulose degradation as they can also originate from insulating liquid oxidation - Low sensitivity to early stages of paper degradation - Different rates of diffusion of CO from paper to liquid at higher temperature - Concentration influenced by leakage from the atmosphere in a free-breathing transformer - Can be generated by partial discharges in the transformer | [19,40,41,42,43] |
| 2-furfural (2FAL) | - Specific to cellulose degradation - Concentration is sensitive to cellulose depolymerisation in mineral and ester liquid - Popularly used in the industry | - Unreliable for TUK due to the influence of alkaline inhibitors, which interfere with the acid hydrolysis reaction responsible for 2FAL formation - Can originate from any pyranose-based compound as its production may result from hemicellulose degradation and not just cellulose breakdown - Only accurate in the later stages of degradation, when acid hydrolysis has reduced the cellulose DP to approximately 400 or lower - Not stable above 110 °C - Concentration affected by the presence of water, acids, and oxygen - There is an effect of humidity on the rate of production | [3,40,42,44,45] |
| Ethanol (EtOH or CH3OH) | - Can indicate localised faults such as hotspots for abnormal cellulose degradation - Serves as a hot-spot chemical marker for cellulose insulation - Has a higher concentration than MeOH at a higher temperature - Generation not influenced by environmental parameters - Helps to differentiate between abnormal and normal ageing | - Not a general indicator of paper ageing - Generated more from insulating liquid ageing than paper - Related to abnormal paper ageing that occurs at very high temperatures since it is generated from levoglucosan, which is the byproduct of pyrolysis - Particularity of the EtOH behaviour appears at temperatures of over 250 °C | [39,40,45] |
| Methanol (MeOH or CH3CH2OH) | - Effective for both Kraft and TUK paper - Capable to detect early paper ageing - More stable among paper degradation byproducts compared to acetone, butanol, and ethanol - Linked to the rupture of 1,4-β-glycosidic bonds of cellulose - The time required to recover MeOH after processing the liquid of a specific transformer is half the duration needed for the recovery of 2FAL | - High volatility may lead to loss over time - May undergo esterification with LMA from paper and liquid, which will affect its accuracy - Requires multiple experimental validations, including stability, partitioning, and origin studies - Requires extensive validation with DP and tensile strength - The rate of methanol formation stabilises and no longer increases significantly when the DP is below 400 - Shows a partial sensitivity to the cellulose ageing in ester liquid | [3,40,44,45,46] |
| Dispersion staining colour | - Identifies ageing stages through colour transition | - Depends on a different colour ratio ageing marker - Subject to variations in lighting and imaging conditions - May not have a universal calibration for all insulation types | [47] |
| Refractive index | - Correlates with the degradation of cellulose fibres - Can be studied using molecular simulation software | - Can be influenced by bulk density and polarisation changes - Requires specialised equipment and simulation tools - May not be directly measurable in in-service transformers | [47] |
| Total sugar | - Sugar is one of the principal decomposition products of cellulose - Can serve as an early ageing marker than 2FAL | - Different types of sugar are dissolved in the insulating liquid, so further investigation is required to determine the total sugar concentration - Unreliable to judge the paper ageing by the concentration of one type of sugar - May not be detected and measurable in real transformers, since sugar decomposes rapidly and may not persist long | [48] |
| Acid | Properties | Type | Structure | Ref. |
|---|---|---|---|---|
| LMAs | - Produce by insulating liquid oxidation and are highly soluble in insulating liquid - Are hydrophilic and react chemically to give a proton (H+) - Significant influence on the paper degradation rate - Readily interact with cellulose and water due to their ability to form bonds with the hydroxyl (OH) group - Readily dissolve in cellulose paper and, at the same time, damage the crystalline and amorphous parts of the paper - Have a high diffusion rate - Influence water distribution between paper and insulating liquid - Contain a strong polar carboxyl group and a non-polar alkyl group - Are also known as short-chain organic acids | Formic acid | 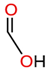 | [19,54,55,56,57] |
| Levulinic acid | 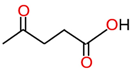 | |||
| Acetic acid | 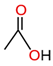 | |||
| HMAs | - Hardly soluble in insulating liquid - Not aggressive and are hydrophobic causing them to have a lower affinity for cellulose - Little participation in insulating paper degradation - Can deposit on radiator pipes, reducing heat transfer efficiency - Have little or no influence on water distribution between paper and insulating liquid - Have a low diffusion rate - Contain a strong polar carboxyl group and a non-polar alkyl group - Are also known as long-chain organic acids | Stearic acid (CH3(CH2)16COOH) | 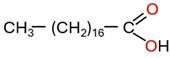 | [19,55,56,57] |
| - Naphthenic acid (R(C5H8)(CH2)nCOOH | 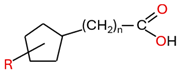 |
| Ref | Insulating Paper | Insulating Liquid | Ageing Temperature | Test | Finding | Explanation |
|---|---|---|---|---|---|---|
| [29] | - Hybrid paper (aramid/cellulose) - TUK | - Corn-based natural ester - Mineral oil | 165 °C and 185 °C | - Tensile strength - DP | - Hybrid paper has better performance than TUK in both liquids - Slower degradation observed in paper in a natural ester | - No transesterification reaction in both papers impregnated in insulating liquid |
| [38] | - Hybrid paper (cellulose/Aramid) - TUK | - Soya bean-based natural ester - Mineral oil | 165 °C, 175 °C, 185 °C, and 195 °C | - Tensile strength - DP - Acidity - Moisture content - Viscosity - Power loss | - Final ageing duration of TUK in ester liquid is 5.9 times longer than that of TUK in mineral oil and 4.6 longer than hybrid paper in mineral oil; this indicates that TUK in ester liquid exhibited significantly greater resistance to ageing compared to the other two combinations | - Hybrid paper, which has the least effect on insulating liquid degradation, shows that the degradation of insulating liquid depends on the type of insulating paper |
| [74] | - Crepe paper | - Natural ester - Naphthenic mineral oil | 150 °C for seven months | - Tensile strength - DP - Viscosity - Breakdown voltage | - Rapid deterioration was observed during early ageing but more pronounced in mineral oil (53%) than natural ester (27%) - Decrease in DP is less pronounced when using natural than mineral oil | - There is greater protection of cellulose when in natural ester as it consumes moisture from the cellulose due to hydrolysis |
| [75] | - TUK - Diamond-dotted paper - Mixture of wood pulp and cotton paper (Grade 3) | - Sunflower liquid (Natural ester) | 150 °C for 5232 h | - Dielectric loss Permittivity - Resistivity - Moisture content - DP | - TUK and diamond-dotted paper exhibited similar levels of degradation - Grade 3 paper experienced the most severe deterioration - Insulating liquid showed greater degradation when aged with Grade 3 paper compared to the other two papers | - Grade 3 paper performs worst because of the high acidity detected in its samples despite the fact that natural ester generates high molecular acid, which has no significant influence on cellulose degradation |
| [76] | - Kraft paper - TUK | - Natural ester - Mineral oil | 150, °C, 160, °C, 170, °C, and 180 °C | - DP - Tensile strength - Furan and MEOH - Acidity - Interfacial tension - Dielectric loss | - Furan concentration was observed to be 10 times lower in ester liquid than in mineral oil - Concentration of methanol was reported to be much lower in ester liquid. - Reported that natural ester insulating liquid extends the lifespan of Kraft paper and TUK as compared to mineral oil | - More furans in ester liquid may be due to their consumption in the reaction with nitrogen compounds in the TUK - There is a stronger bonding of ester groups to cellulose OH groups, which limits the dissolution of furans in ester liquid - More MeOH produced may be a result of the reaction of methanol with acids |
| [33] | - Crepe paper | - Aromatic mineral oil - Synthetic ester (Midel 7131) | 90 °C, 110 °C, and 130 °C for 2000 h | - DP | - Report reveals that, within the temperature of 90 °C to 110 °C, the depolymerisation of cellulose occurred most extensively in the mineral oil - Incorporating synthetic ester into mineral oil at a concentration of 10% and 30% by volume enhances the thermal stability of both the insulating liquid and the paper impregnated in the blends | - Result obtained may be due to hydrolysis reactions in synthetic esters, which lead to the formation of long-chain fatty acids that do not have a negative influence on insulating paper - Ester groups block the cellulose OH sites, creating a protective barrier against the negative effect of polar water molecules |
| [77] | - Hybrid paper (aramid/cellulose) - TUK | - Mineral oil - Natural ester | 165 °C, 175 °C, and 185 °C | - Tensile strength - Young’s modulus - Elongation | - Natural ester enhances the mechanical characteristics of the TUK and hybrid paper at all aged temperatures - TUK reaches the 25% retained tensile strength target faster than hybrid paper in both mineral oil and natural ester; this suggests that hybrid paper may have a longer lifespan than TUK | - Report may be due to natural ester capacity to offer a protective role for cellulose, which preserves the elasticity of the interlamellar amorphous phase by controlling van der Waals interactions among cellulose chains and promoting selective water absorption |
| [21] | Cellulose Kraft paper | - Mineral oil - Vegetable insulating liquid | Not stated | - Number of hydrogen, water, and formic acid molecules | - The number of H2, H2O, CO, and formic acid molecules in the insulating paper is diminished when vegetable insulating liquid is present, with a notable decrease compared to when mineral oil is used | - The higher number of hydrogen bonds, binding energy, and electrostatic energy between vegetable insulating liquid and paper, compared to mineral oil, is the reason why there is a stronger interaction between vegetable insulating liquid and paper |
| [78] | - Kraft paper - TUK | - Mineral oil - FR3 ester liquid | 120 °C | - DP - Furan content | - Report indicates that Kraft paper and TUK impregnated in FR3 exhibited higher DP values and slower ageing rate - Substitution of mineral oil with FR3 leads to a notable reduction in the chain scission of both papers - FR3 demonstrates a more significant ageing-retarding effect on TUK than on Kraft paper | - The observation was analysed based on atomic-scale mechanisms, which reveal that FR3 ester liquid effectively restricts the diffusion of water and acid within the insulating liquid–paper insulation system; this inhibition plays a crucial role in slowing down the acid hydrolysis of cellulose |
| [79] | - Kraft paper - TUK - Aramid paper | - Mineral oil | Between 100 °C and 180 °C | - Bubbling inception temperature | - TUK insulating paper exhibits the highest bubbling inception temperature, followed by Kraft and aramid paper; this observation shows that TUK has the highest resistance to the formation of paper in the insulating liquid compared to the other two insulating papers | - It was reported that the bubbling inception temperature is highly dependent on the water content in the insulating paper and pressure, as these factors determine the threshold at which trapped water within the fibres vapourises and generates bubbles |
| [80] | - Kraft paper | - Mineral oil - Natural ester | 130 °C and 150 °C | - DP - Tensile strength - Water content - AC breakdown voltage - Dielectric loss | - Report shows that natural ester exhibits a slower degradation rate, with only a 27% reduction after 8000 h, in contrast to mineral oil, which experiences a 58% decrease after 3500 h - Natural ester displays superior performance in dielectric loss, AC dielectric strength, and moisture content | - The observation shows that the high water saturation level of natural ester has a drying impact on the insulating paper, which helps to slow its degradation rate as compared to mineral oil with low-water-level saturation |
| Mechanisms | Summary | Ref. |
|---|---|---|
| Water absorption | Natural ester absorbs water from the transformer paper due to its high water solubility. This reduces the moisture in the insulating paper, slowing down its ageing process. The water saturation level of the natural ester is significantly higher than that of mineral oil, helping to maintain moisture balance. | [65,66,67,69] |
| Promotion of hydrogen bond | Natural ester enhances hydrogen bonding between cellulose fibres, strengthening the structure of the insulation paper. Also, it reduces the water content in the paper, preventing water molecules from disrupting the hydrogen bonds and thereby stabilising cellulose by strengthening the C-O and C-C bonds in the cellulose. | [65,66,67] |
| Reduction in hydrolysis | Cellulose degradation involves hydrolysis, where water competes with hydroxyl groups on the cellulose sugar ring for protons. Since natural ester absorbs more water from the paper, it reduces this competition, thereby slowing down hydrolysis. | [65,66,67,69] |
| Restriction in pore swelling | Natural ester helps to maintain the ordered structure of cellulose molecules, preventing excessive swelling of fibril pores. This helps preserve the mechanical integrity of the paper insulation as it causes the neighbouring open pores in the cellulose to swell. | [65,66] |
| Water consumption through hydrolysis | Natural ester undergoes hydrolysis, consuming available water in the insulation system. This reduces the damaging effects of water on cellulose, unlike mineral oil, which does not react with water and allows for moisture accumulation. | [65,66,67] |
| Esterification of insulating paper | During ageing, long-chain fatty acids produced from natural ester hydrolysis can esterify the cellulose paper, chemically modifying its structure. This reaction enhances the paper stability and provides additional protection against degradation. | [65,66,67,69] |
| Models | Formula | Strength | Limitation | Ref. |
|---|---|---|---|---|
| Cheim-Dupont | λ—shortening expression d—parameter representing the type of paper and on the winding longitudinal temperature gradient | - Considers both hotspot temperature and paper type - Incorporates the effect of temperature gradients inside the transformer | - Does not account for localised degradation - Insulating liquid regeneration or replacement alters 2FAL concentration - Does not directly consider the effect of oxygen and moisture on paper ageing - Typically applicable to mineral oil - Depends on transformer design | [82,83] |
| Chendong | 2FAL is in ppm | - Developed from real transformer data, making it useful for practical diagnostics - More reliable than using only hotspot gradient since furans are direct byproducts of paper ageing - Account for DP between 150 and 1000 | - Limited to transformers with normal Kraft paper - Limited to transformers with free-breathing conservators - If insulating liquid has been replaced or regenerated, furan concentration changes, leading to inaccurate DP estimation - Typically applicable to mineral oil | [82,84] |
| Stebbins | 2FAL is in ppb | - Modified Chendong model by using TUK - More reliable than using only hotspot gradient since furans are direct byproducts of paper ageing | - Only applicable to certain transformers depending on the type of insulation paper - Assumes that furans fully represent paper degradation, ignoring other influencing factors - Typically applicable to mineral oil | [82] |
| De Pablo 1 | 2FAL is in ppm | - Accounts for the fact that paper rarely decomposes evenly - Considers the effect of hotspot and thermal gradient - Polymer degradation main chain theory is utilised | - Accounts for DP between 150 and 600 - Typically applicable to mineral oil | [85] |
| De Pablo 2 | 2FAL is in ppm | - It is a simple linear equation - Utilises the theory of cellulose chain scissions - Accounts for the fact that paper rarely decomposes evenly | - Paper is assumed to degrade uniformly - The initial DP of the paper was assumed to be 800, which is lower than the typical value in a real transformer - Typically applicable to mineral oil - Assumes molecular weight of 2FAL is 96, three cellulose chain scission give one 2FAL molecule, and the ratio of insulating liquid to paper is 25 | [82,83,84,86] |
| Pahlavanpour | 2FAL is in ppm | - Accounts for non-uniform paper ageing within transformer winding - Modifies De Pablo model | - Assumes a fixed 20% of inner paper layers degrade twice as fast as the remaining 80% of the paper, which may not reflect real-world variations - Typically applicable to mineral oil - Furan concentration is affected by insulating liquid replacement or reconditioning process | [82,83] |
| Vaurchex | 2FAL is in ppm | - Model was developed based on experimental results - Accurate results for transformers less than 8 years in operation | - Does not utilise data from a real-world transformer - Typically applicable to mineral oil - The experiment was conducted exclusively on Kraft paper | [83,84,85] |
| Burton | 2FAL is in ppm | - Model was developed based on experimental results | - The experiment was conducted exclusively on Kraft paper - Typically applicable to mineral oil - Does not utilise data from a real-world transformer | [83,84] |
| Chaouhui | ) 2FAL is in ppm | - Based on real-world transformer data - Suitable for various transformer conditions | - Limited dataset used for model development - Typically applicable to mineral oil- Does not consider external influencing factors, like moisture | [87] |
| Myers et al. | ) 2FAL is in ppb | - For non-TUK paper - Accurate results for transformers less than 8 years in operation | - The presence of other furan compounds aside from 2FAL indicates unusual paper degradation - Typically applicable to mineral oil | [84,85] |
| Myers et al. | ) 2FAL is in ppb | - For TUK paper | - 2FAL has low stability in paper treated with dicyandiamide - Typically applicable to mineral oil | [85] |
| Serena | 2FAL is in ppm | - Validates De Pablo model by suggesting that one 2FAL molecule is generated for every three broken cellulose chains | - Assumes a fixed relationship between furan generation and paper breakdown, which may not always be accurate - Typically applicable to mineral oil | [87] |
| Li Song | 2FAL is in ppm | - Accurate result for older transformers around 30 years in operation | - Assumes a specific degradation pattern for aged transformers, which may not generalise well - Typically applicable to mineral oil | [85] |
| Heisler and Banzer | - Account for DP between 100 and 900 | - Developed from a limited dataset, restricting broader applicability - Typically applicable to mineral oil | [19] | |
| Dong et al. | 2FAL is in ppm | - Accurate result for older transformers around 30 years in operation | - Model may not be suitable for newer transformers - Typically applicable to mineral oil | [85] |
| Shkolnik et al. | - Model exclusively for TUK | - Model is exclusively for TUK - Typically applicable to mineral oil | [86] | |
| Garcia et al. | 2FAL is in ppm MP—moisture content of the paper in % | - Considers the impact of moisture content in the evolution of 2FAL - Model developed from real transformer data, making it real for practical diagnostics - Accelerated thermal ageing was performed to validate the model | - Not widely used by utility - The type of insulating paper used was not mentioned - Validation was performed using Kraft paper - Typically applicable to mineral oil | [88] |
| Thomas Leibfried et al. | 2FAL is in ppm | - Gives accurate results between DP of 200 and 800 - Model developed with real transformer data through post-mortem techniques | - The number of investigated transformers is comparably low for a statistical data evaluation - Typically applicable to mineral oil - More accurate for a grid transformer than a generator set-up transformer | [89] |
| Jalbert el al | M—methanol in ppm | - Model built on a real operating transformer | - Only 6 transformers were considered - Typically applicable to mineral oil | [10,90] |
| Ghoneim | 2FAL is in ppb | - Provides estimates for DP in aged transformers - Developed from empirical data | - Assumes a fixed relationship between 2FAL and DP, which may not account for real-world variations - Typically applicable to mineral oil | [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adekunle, A.A.; Oparanti, S.O.; Fofana, I.; Picher, P.; Rodriguez-Celis, E.M.; Arroyo-Fernandez, O.H.; Meghnefi, F. Degradation Mechanisms of Cellulose-Based Transformer Insulation: The Role of Dissolved Gases and Macromolecular Characterisation. Macromol 2025, 5, 20. https://doi.org/10.3390/macromol5020020
Adekunle AA, Oparanti SO, Fofana I, Picher P, Rodriguez-Celis EM, Arroyo-Fernandez OH, Meghnefi F. Degradation Mechanisms of Cellulose-Based Transformer Insulation: The Role of Dissolved Gases and Macromolecular Characterisation. Macromol. 2025; 5(2):20. https://doi.org/10.3390/macromol5020020
Chicago/Turabian StyleAdekunle, Andrew Adewunmi, Samson Okikiola Oparanti, Issouf Fofana, Patrick Picher, Esperanza Mariela Rodriguez-Celis, Oscar Henry Arroyo-Fernandez, and Fethi Meghnefi. 2025. "Degradation Mechanisms of Cellulose-Based Transformer Insulation: The Role of Dissolved Gases and Macromolecular Characterisation" Macromol 5, no. 2: 20. https://doi.org/10.3390/macromol5020020
APA StyleAdekunle, A. A., Oparanti, S. O., Fofana, I., Picher, P., Rodriguez-Celis, E. M., Arroyo-Fernandez, O. H., & Meghnefi, F. (2025). Degradation Mechanisms of Cellulose-Based Transformer Insulation: The Role of Dissolved Gases and Macromolecular Characterisation. Macromol, 5(2), 20. https://doi.org/10.3390/macromol5020020











