Abstract
Biopolymers are revolutionizing the materials landscape, driven by a growing demand for sustainable alternatives to traditional petroleum-based materials. Sourced from biological origins, these polymers are not only environment friendly but also present exciting solutions in healthcare, packaging, biosensors, high performance, and durable materials as alternatives to crude oil-based products. Recently, biopolymers derived from plants, such as lignin and cellulose, alongside those produced by bacteria, like polyhydroxyalkanoates (PHAs), have captured the spotlight, drawing significant interest for their industrial and eco-friendly applications. The growing interest in biopolymers stems from their potential as sustainable, renewable materials across diverse applications. This review provides an in-depth analysis of the current advancements in plant-based and bacterial biopolymers, covering aspects of bioproduction, downstream processing, and their integration into high-performance next-generation materials. Additionally, we delve into the technical challenges of cost-effectiveness, processing, and scalability, which are critical barriers to widespread adoption. By highlighting these issues, this review aims to equip researchers in the bio-based domain with a comprehensive understanding of how plant-based and bacterial biopolymers can serve as viable alternatives to petroleum-derived materials. Ultimately, we envision a transformative shift from a linear, fossil fuel-based economy to a circular, bio-based economy, fostering more sustainable and environmentally conscious material solutions using novel biopolymers aligning with the framework of the United Nations Sustainable Development Goals (SDGs), including clean water and sanitation (SDG 6), industry, innovation, and infrastructure (SDG 9), affordable and clean energy (SDG 7), sustainable cities and communities (SDG 11), responsible production and consumption (SDG 12), and climate action (SDG 13).
1. Introduction
The extensive reliance on non-renewable sources and raw materials, combined with the overwhelming production of recalcitrant waste in most industrial processes, significantly affects environmental sustainability [1,2]. This trend poses challenges to maintaining ecological balance and underscores the need for more sustainable practices in industry [3]. Biomaterials hold significant promise for enhancing environmental sustainability through the creation of biodegradable materials, thereby mitigating global waste generation [4,5,6,7]. These biomaterials are engineered to mimic biological systems, facilitating interactions that can lead to improved ecological outcomes. The integration of biomaterials into industrial processes is gaining traction as it effectively diminishes the environmental footprint of products, contributing to a more sustainable manufacturing paradigm with reduced ecological impact.
Biopolymers are essential in various applications due to their structural and functional roles in living organisms [8,9]. Composed of natural polymers like polysaccharides, polynucleotides, and polypeptides, biopolymers consist of long chains formed from monomeric subunits through covalent bonding. Their key advantages include high biodegradability and biocompatibility. In the food industry, they are used for emulsions, edible films, and packaging. In medicine, biopolymers function as drug delivery systems, medical implants, synthetic organs, and wound healing materials. They also play a role in creating tissue scaffolds and smart textiles and have applications in aerospace research and aviation equipment. Biopolymers play a crucial role in enhancing technology and environmental sustainability. They serve as effective nanocarriers for targeted cancer therapeutics and have applications in drug and gene delivery and tissue engineering [10,11,12]. Recent research highlights various biopolymers, including cellulose, lignin, PHAs, gelatin, chitosan, agarose, pectin, and alginate, which can be sourced from agricultural resources (Figure 1). Their versatility spans industries such as cosmetics, food, pharmaceuticals, and biomedicine. Notably, chitin and chitosan come from fungal sources and crustacean exoskeletons [13,14]. Alginates, extracted from marine algae, are among the most prevalent polysaccharides, alongside cellulose. Various polysaccharides are noted for their remarkable biocompatibility, biodegradability, stability, and abundant natural availability [15,16,17,18,19]. Enhancing their properties involves adding functionalities like conductivity, magnetic properties, bioactivity, and flame retardance. Additionally, solvent-free electro (spinning) of nanocelluloses allows for the integration of additives while avoiding harmful solvents [20].
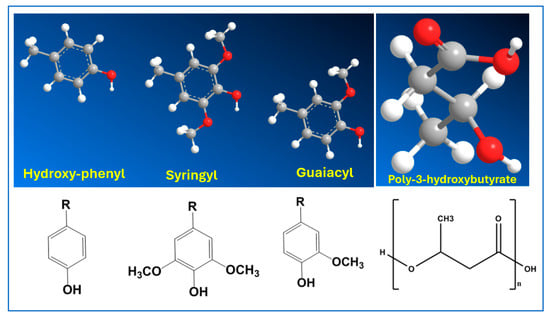
Figure 1.
Structures of monomeric units of lignin and poly-3-hydroxybutyrate (PHB).
This review aims to explore a range of plant- and bacterial-derived biopolymers, examining their emerging trends and applications in next-generation materials. We explore advancements aimed at improving the structural integrity and functional properties of these biopolymers, providing a comprehensive analysis of their potential and the current state of the art in biopolymer research. The discussion will focus on their applications within the biomaterials domain, highlighting the innovative uses and future directions for these materials in various industries.
2. Bacterial Biopolymers
2.1. Polyhydroxyalkanoates
Polyhydroxyalkanoates are natural polymers produced by bacteria and have important applications in biotechnology, medicine, and sustainable materials. These biopolymers have become popular substitutes for synthetic polymers because they are renewable, biodegradable, and have unique physicochemical characteristics. Maurice Lemoigne originally described polyhydroxyalkanoates (PHAs) as a class of biodegradable polyesters, in 1926. These biopolymers are found naturally and are stored by a variety of species, primarily prokaryotes, in excess of carbon source, as reserve material in the form of intracellular granules, frequently in contrast to stressful situations like high temperatures or surroundings with increased salinity or oxygenation [21]. Bacteria produce polyhydroxybutyrate (PHB), a kind of polyhydroxyalkanoate (PHA), as an internal polymer for storing carbon and energy. They can store up to 90% of their dry weight in PHAs [22]. Therefore, biological processes are the primary, but not the only, means of producing them industrially [23]. A wide variety of microorganisms are used in batch, fed-batch, or continuous fermentation, including Ralstonia insidiosa [6], the halophilic bacterium Halomonas bluephagenesis [24], modified Escherichia coli [25], Pseudomonas strains [26], and the Gram-negative Cupriavidus necator [4]. However, due to their high production costs, the PHA market is still fairly small today. In particular, the high cost of feedstocks necessitates the switch to second-generation biorefineries, where waste is used to create new, inexpensive substrates [27]. Furthermore, strain performance needs to be improved by genetic engineering [28].
Hydroxyl acid (HA) monomers joined by ester linkages generate PHAs, which are linear polyesters [29]. HAs are fatty acids with lengths ranging from three to more than fourteen carbon atoms [30]. While medium-chain-length (MCL) monomers have between six and fourteen carbon atoms, short-chain-length (SCL) monomers have between three and five. For units longer than 14 carbon atoms, the class of long-chain-length (LCL) monomers has recently been defined [31]. Despite being biodegradable and biocompatible, PHB has drawbacks such as low elongation at break, poor thermal stability, and brittleness. Other hydroxyalkanoate (HA) monomers have been added to PHB copolymers in order to avoid these problems (Table 1) [32]. These copolymers are known for their unique thermal and mechanical properties (Table 2).

Table 1.
Types of PHA copolymers.

Table 2.
Thermal and mechanical properties of PHA copolymers.
2.2. Bacterial Nanocellulose
Bacterial nanocellulose (BNC) is recognized as the purest form of cellulose [33]. It is produced through a bottom-up process from sugars, making it particularly valuable in pharmaceutical technology. Though BNC has a lower degree of crystallinity and polymerization compared to plant-derived cellulose, it has a higher content of the Iα allomorph [33]. This unique composition endows nanocellulose with remarkable properties, enabling its application in a variety of fields, including wound dressings, textiles, food packaging, cosmetics, regenerative medicine, tissue engineering, energy solutions, optoelectronics, bioprinting, and environmental remediation. Chemical modifications of nanocellulose can impart enhanced properties such as improved adhesion, preservation, and antimicrobial activity. This is particularly relevant in the context of the increasing demand for textile products. The production of raw materials—especially crops like cotton—requires substantial quantities of water and chemicals, including fertilizers and pesticides, throughout the processes of cultivation, spinning, weaving, and dyeing. Furthermore, the environmental impact continues downstream, as consumers contribute significantly to resource depletion through water and energy consumption during laundering, drying, and ironing. To gain a competitive edge in the market, lignocellulosic materials must exhibit attributes that are comparable to, or exceed, those presented by conventional fossil fuel-based alternatives. Bacterial nanocellulose (BNC) presents an intriguing alternative to traditional wood and plant cellulose. BNC is a homo-polysaccharide produced by specific Gram-negative bacterial species from the genera Komagataeibacter, Acetobacter, Rhizobium, Agrobacterium, Pseudomonas, Alcaligenes, and Sarcina, all of which are known for their unique cellulose synthesis capabilities [34]. The morphology, structural characteristics, mechanical properties, and cellulose yields vary notably among these bacterial species, highlighting the diverse potential applications of BNC in various fields [35]. Komagataeibacter xylinus, often regarded as one of the most prolific cellulose-producing bacteria, is particularly favored for obtaining high yields of BNC [36]. The historical context of BNC production traces back to the discovery of kombucha, a popular fermented beverage. The symbiotic culture of bacteria and yeast within kombucha generates a distinctive cellulose film that forms at the air interface of the culture media [37,38]. This remarkable phenomenon was first documented by Brown in 1886, who noted a film possessing structural and chemical properties akin to those of plant cellulose [39]. BNC is characterized by its unique structure, consisting of ribbon-shaped fibrils measuring less than 100 nanometers in width (Figure 2). These nanofibrils, typically about 7 to 8 nanometers wide, aggregate randomly into bundles, creating a highly intricate network. Notably, BNC is devoid of lignin and hemicellulose, which are commonly found in plant-based cellulose sources, thereby enhancing its purity and suitability for various applications [40,41,42,43]. This distinct composition and structure lend BNC unique properties, making it an exciting material for research and industry, particularly in areas such as biomedicine, food technology, and environmental sustainability [44,45,46,47,48].
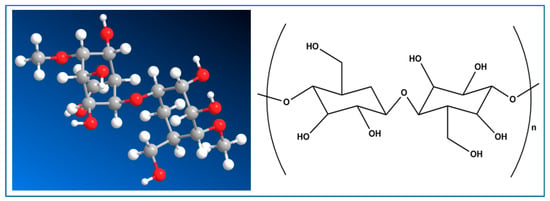
Figure 2.
Structure of bacterial nanocellulose (BNC).
The chemical structure of bacterial nanocellulose (BNC) exhibits similarities to that of plant cellulose, characterized as a linear homopolymer of glucose monomers interconnected by β-(1,4) glycosidic linkages, represented by the chemical formula (C6H10O5)n. However, BNC differs in its macromolecular architecture and corresponding properties [49,50,51,52]. During the processes of pulping and purification, the degree of polymerization for BNC decreases, ranging between 2000 and 6000, in contrast to plant cellulose, which demonstrates a degree of polymerization between 13,000 and 14,000 [53,54,55]. The unbranched cellulose chains in BNC are stabilized by robust intra- and intermolecular hydrogen bonding, facilitating the formation of its supramolecular structure and elementary fibers [56]. Due to these attributes, BNC has found extensive application within the biomedical sector, notably in wound dressings, tissue engineering, drug delivery systems, and biosensors, as well as for cancer diagnostics (Table 3). Its utility extends into the paper and textile industries for fiber composites and coatings, and within the food and cosmetic industries as an emulsifier and viscosifier. The primary application of bacterial nanocellulose (BNC) remains its use in nata de coco, a popular culinary product primarily consumed in various Asian countries. However, the production costs associated with BNC are often considered high, which limits the ability to scale up production to industrial levels. Currently, the price of nata de coco is estimated to range from USD 50 to 80 per kilogram in dry weight. One significant challenge to large-scale BNC manufacturing is the cost of the fermentation medium [57]. A potential solution to this issue is the use of byproducts from the food industry as a more economical fermentation medium. This approach not only helps to reduce production costs but also addresses environmental concerns related to waste disposal. Using food industry byproducts may lead to more highly charged wastewater, which could complicate treatment and increase costs. Additionally, the nutrient impacts on production costs may not be as significant as previously thought. Another considerable hurdle in BNC production is the low yield [45,58]. Furthermore, it is important to note that a comprehensive assessment of BNC’s capacity for large-scale production has not yet been conducted. Consequently, its viability as a sustainable alternative in the textile industry remains to be fully evaluated.

Table 3.
Types of bacterial nanocellulose and their applications.
3. Plant-Based Biopolymers
3.1. Cellulose
The primary sources of cellulose are cotton and wood, which are both abundant and economically significant materials [59,60,61,62,63]. In addition to these plant sources, cellulose can also be derived from specific animal sources; however, this is less common in practice. Bacterial cellulose (BC), produced by certain bacteria such as Acetobacter xylinum, shares chemical similarities with plant-derived cellulose but possesses unique structural properties that make it distinct. Despite the differences, plant cellulose remains a crucial component of plant cell walls, providing essential support and structure [64,65,66]. Both plant and bacterial cellulose exhibit remarkable mechanical, physical, and biochemical properties, along with notable biological activity, good mechanical integrity, and biocompatibility, which enable their use in a variety of applications [67,68]. As a prevalent organic polymer, cellulose constitutes the primary structural element of numerous green plants, including cotton, bamboo, various crops, and even marine algae. Its molecular structure consists of long chains of β-1,4-linked D-glucose units, forming a linear polymer (Figure 3). The presence of numerous hydroxyl (-OH) active groups along the cellulose chain enhances its reactivity, contributing to the tendency of cellulose molecules to form strong hydrogen bonds. Each D-glucose molecule features six free hydroxyl groups that engage in hydrogen bonding, not only within the individual cellulose chains but also between chains, facilitating a robust three-dimensional network. Additionally, cellulose chains interact through van der Waals forces, which play a significant role in the cohesion of adjacent polymeric chains within plant structures [69,70]. Due to these intricate interactions, cellulose exhibits impressive mechanical properties including high axial stiffness, stability, and tensile strength, making it an invaluable material in various applications [71]. Moreover, the unique characteristics of cellulose and its derivatives make them among the most effective and widely utilized substrates for sensing devices. This is attributed to their abundant surface hydroxyl groups, which enhance connectivity and reactivity, as well as their large specific surface area, high aspect ratio, superior crystallinity, exceptional mechanical properties, and remarkable thermal resistance [72,73]. These features collectively position cellulose as a sustainable and versatile material for innovations in technology and materials science.
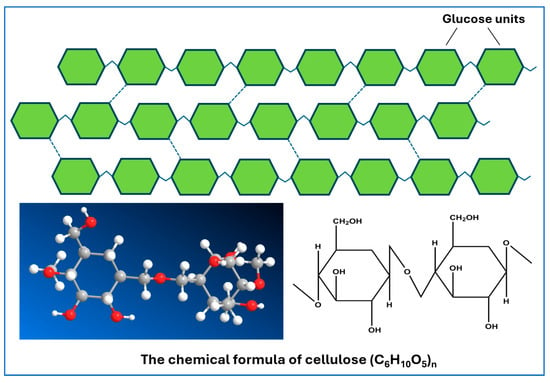
Figure 3.
Structure of cellulose.
Various techniques, including high-speed grinding and TEMPO-mediated oxidation, are utilized to produce cellulose nanofibers (CNFs), which are characterized by exceptional mechanical strength, and typically range from 5 to 100 nm in diameter and span several microns in length [74,75,76]. A primary source of cellulose is plant material, which consists of numerous cellulose molecule chains that interact and bundle together to form linear structures known as cellulose microfibrils. These microfibrils exhibit high tensile strength and are composed of hemicellulose, para-crystalline cellulose, and crystalline cellulose. The cellulose molecule itself consists of hydrated glucose units linked by beta 1–4 glycosidic bonds. To synthesize nanocellulose, strong acid hydrolysis is performed, and bacteria may be employed to produce cellulose nanofibers [77,78].
Cellulose stands as the most abundant renewable polymer in the biosphere, with an estimated annual production of 1 trillion tonnes via photosynthesis [79,80]. This linear polymer comprises D-glucose monomers linked through β (1,4) glycosidic bonds, with each monomer arranged in a 180-degree twist relative to its neighbors. The fundamental repeating unit of cellulose is cellobiose, a disaccharide [81]. Long, homopolymeric cellulose chains are formed by the polymerization of cellobiose units. During the biosynthesis process, van der Waals interactions and hydrogen bonding between adjacent chains facilitate the assembly of cellulose into elementary fibrils. These elementary fibrils aggregate to form microfibrils, which are further bundled into macrofibrils, or cellulosic fibers. Native cellulose fibers are characterized by both ordered (crystalline) and disordered (amorphous) regions, with crystallinity levels ranging from 40 to 70%, depending on the source and extraction method employed. The degree of polymerization for native cellulose can vary significantly, ranging from 1500 to 3500 [82]. The biosynthesis of natural cellulose occurs in plant cells through a membrane complex known as cellulose synthase, employing a bottom-up synthesis approach [83,84]. As cellulose chains are extruded from the cell wall, they do so in a coordinated manner, allowing for the simultaneous formation of interchain hydrogen bonds. The fibrous domains of cellulose are crystalline, and classified into allomorphs Iα and Iβ [85].
The structural integrity and rigidity of plant cell walls are primarily derived from the interaction of hydroxyl groups on cellulose chains, which form stable, rigid molec-ular assemblies. Cellulose’s insolubility in water arises from the extensive hydrogen bonding between its chains [86]. The aggregation of cellulose molecules leads to the formation of fibrils, which subsequently coalesce into larger bundles. These structures, along with lignin and hemicelluloses, are fundamental in constructing the cell wall [87,88,89]. The morphology of cellulose fibrils resembles a net-like architecture. Plant nanocellulose (NC) is typically classified into cellulose nanofibers (CNFs) and cellulose nanocrystals (CNCs) [90,91,92,93,94]. The ultimate chemical and physical characteristics of nanocellulose are closely tied to both the source material and the processing conditions employed in their production [95,96,97]. Notably, NC exhibits high strength and stiffness, low density, biodegradability, elevated surface area, and minimal thermal expansion, attributes that have garnered significant research interest and development over the past two decades. Various feedstocks suitable to produce nanocellulose (NC), including coconut husk fiber, mengkuang leaves (Pandanus tectorius), cotton, Agave tequilana, barley residues, tomato peel, garlic straw, forest byproducts, corncob residue, Gigantochloa scortechinni bamboo culms, industrial cotton waste, sugar palm fibers (Arenga pinnata), corn straw, and sago seed shells have been explored [98,99,100,101,102]. The initial step involves pretreatment processes such as milling, pulping, and bleaching to effectively remove lignin and hemicellulose. Cellulose nanocrystals (CNCs), also referred to as cellulose whiskers, nanowhiskers, or nanorods, are generated through the transverse cleavage of cellulose using strong acids like sulfuric and hydrochloric acid under controlled conditions of temperature, agitation, and time [103,104,105,106]. These cellulose nanocrystals are characterized by their nanoscale dimensions, with diameters ranging from 4 to 55 nm and lengths between 90 and 400 nm [107]. The production of CNCs involves subsequent washing, filtration or centrifugation, and dialysis to eliminate residual acid (Table 4).

Table 4.
Cellulose extraction methods and their applications.
The crystalline structure of CNCs is more rigid compared to CNFs, resulting in lower flexibility for CNCs [108,109]. Currently, CNCs are manufactured commercially in quantities ranging from 2 to 260 tons each year [62,110]. Meanwhile, cellulose nanofibers, also referred to as cellulose nanofibrils or nano-fibrillated cellulose, are produced through mechanical disintegration methods [44,111]. CNFs are characterized by their high aspect ratio and contain both crystalline and amorphous regions. The isolation of CNFs is typically achieved through mechanical destructuring techniques, including pressure, cavitation, shear, and impact forces. However, due to the high energy demands of these methods, integrating pretreatment processes has become increasingly important to enhance the efficiency of the subsequent fibrillation.
Cellulose, a prominent class of natural biopolymers, exhibits a remarkable combination of biocompatibility, biodegradability, and versatility in processing, making it suitable for a wide range of applications. Its sustainable production capabilities further enhance its appeal, alongside intrinsic properties such as shape anisotropy and tunable surface chemistry. Recent advancements have led to the innovative development of wearable and smart sensors leveraging cellulose’s unique characteristics. Significant progress in material fabrication techniques has enabled the engineering of cellulose into various dimensional architectures, including one-dimensional structures like nanofibers and yarns, two-dimensional forms such as films and fabrics, and three-dimensional configurations like hydrogels, aerogels, foams, and sponges. These cellulose-derived materials can be transformed into electrically conductive carbonaceous substrates with customizable structures and properties [112,113]. In the context of wearable technologies, cellulose-based materials have emerged as flexible biosupports, sensing layers, electrodes, and active components [114]. These applications capitalize on the distinct advantages offered by cellulose, making it an asset in the ongoing evolution of advanced sensor technologies [115,116].
3.2. Lignin
Lignocellulosic biomass (LCB) represents one of the most abundant and sustainable raw materials available globally, with an estimated production rate of 21,011 tons per year. This complex and heterogeneous mixture comprises key carbohydrate polymers, specifically cellulose (40–45% w/w), hemicellulose (25–35% w/w), and lignin (15–30% w/w). Sources of LCB include agricultural residues, dedicated energy crops such as temperate grasses, and various wood residues. Given its prevalence, LCB has the potential to address the challenges posed by depleting fossil resources, particularly if leveraged as a renewable feedstock for value-added materials. This biomass represents the largest share of terrestrial renewable materials available for sustainable energy and chemical production. The global demand for biofuels is projected to rise by 41 billion liters (approximately 28%) between 2021 and 2026. Second-generation biofuel facilities predominantly convert LCB into ethanol, diesel, methane, and other fuel types. Furthermore, per the Energy Independence and Security Act (EISA) 2007, the target for second-generation biofuel production is set at approximately 80 billion liters annually by 2022, which correlates to roughly 62 million tons of lignin as a byproduct [117]. Lignin is primarily synthesized from the monomers p-coumaroyl alcohol, coniferyl alcohol, and sinapyl alcohol through a series of enzyme-driven processes, including dehydrogenation, radical coupling, and dimerization [118]. This synthesis leads to the formation of an amorphous three-dimensional structure characterized by a complex network of both ether and carbon–carbon bonds [119]. Traditionally, lignin has been utilized as a renewable energy source by the pulp and paper sector, where lignin-rich black liquors from pulping processes are predominantly incinerated to recover inorganic substances and energy for the mills. Nevertheless, as lignin production surpasses the demand for its use as fuel and interest in renewable chemicals grow, there is a rising focus on the valorization of lignin into new chemicals and materials, as it is the most abundant natural resource of renewable aromatic compounds on the planet. Given the significance of aromatic functionalities in key chemical industries such as fragrances, flavors, polymers, coatings, and resins, these fields have all investigated the utilization of lignin. Due to its low toxicity, environmental compatibility, and excellent biocompatibility, lignin is being extensively researched for high-value applications instead of combustion [120,121]. Its aromatic characteristics also enable its use as a substitute for phenol in the production of phenolic resin adhesives [122].
There are several effective methods for converting biomass into biofuels, including enzymatic hydrolysis, soda, kraft, organosolv, and steam explosion. It is estimated that one ton of dry biomass can yield approximately 355 L of bioethanol, and annually, around 223 million tons of dry biomass are processed, resulting in the production of 62 million tons of lignin [123]. Lignin is an organic polymer with great potential for use as a raw material in both the chemical and biological sciences. As the second most abundant renewable biopolymer on Earth, lignin serves as a significant renewable source of carbon, and its heating value is comparable to that of carbon itself. Additionally, lignin is a substantial natural source of aromatic structures, which possess numerous unique chemical properties and significant biological effects. This opens exciting opportunities for innovation and application across various industries. Research into the energy dynamics of bioethanol production indicates that lignin possesses a higher energy yield than the amount needed for ethanol synthesis. This discrepancy ensures a consistent energy surplus, which can be harnessed for various applications beyond ethanol production, including utilization in external energy demands or as a feedstock for the synthesis of chemicals.
The primary monolignols—guaiacyl (G), syringyl (S), and p-hydroxyphenyl (H)—originate from the decomposition of lignin and are significantly influenced by the method of lignin deconstruction, the source material, and the extraction processes employed. These monolignols are often interconnected through various chemical linkages, including spirodienone and dibenzodioxocin configurations. Wood types can be categorized based on their lignin composition: softwoods predominantly feature guaiacyl lignin, hardwoods exhibit a balanced presence of guaiacyl and syringyl units with minimal inclusion of p-coumaryl alcohol or p-hydroxyphenyl units, while herbaceous biomass typically displays a higher ratio of guaiacyl units relative to p-hydroxyphenyl units. This compositional variability underscores the diverse structural characteristics of lignin in different plant sources.
The unique thermoplastic properties of lignin at lower temperatures are attributed to its intra- and intermolecular hydrogen bonding, making it a valuable component for bio-based polymers and films. Conversely, its extensive crosslinking results in thermosetting behavior at elevated temperatures (T > 200 °C), with significant thermal degradation noted at these conditions [117,124]. Given its high carbon content, cost-effectiveness, and bio-renewable nature, lignin presents substantial potential for the development of a diverse array of products [125]. Major sources of technical lignin include pulp and paper mills, cellulosic biorefineries, and residues from forestry and agriculture. Industrial lignin is primarily derived as a byproduct from the pulp and paper industry as well as from emerging cellulosic biorefineries. A cellulosic ethanol facility has the potential to produce between 100,000 to 200,000 tons of lignin annually [126]. At present, only a small fraction of the lignin produced as a byproduct is effectively recovered; the majority is either incinerated for energy recovery or disposed of in landfills, resulting in substantial waste management challenges. The limited amount of lignin that is recovered (~2%) is utilized in various applications, including the production of vanillin, dispersants, animal nutrition, cement additives, carbon fibers, agglomerates, adhesives, and renewable materials [127]. Given its distinctive chemical properties, lignin and its derivatives have considerable potential in the fabrication of high-value materials. Moreover, due to its elevated carbon content, lignin serves as an exceptional precursor for various carbon-based materials.
Lignin-derived carbon products are extensively utilized in various applications such as catalyst supports, adsorbents, and energy storage systems. Recent research has focused on converting lignin into highly engineered materials due to its valuable properties. As an aromatic biopolymer, lignin boasts a high carbon content (exceeding 60 atomic percent), exceptional thermal stability, biodegradability, antioxidant characteristics, UV absorption capabilities, and antibacterial properties. The degradation of lignin into low molecular weight phenolic compounds involves the cleavage of inter-unit linkages, with aryl ether linkages (β-O-4′) representing over 50% of the bonds formed during the polymerization process. Other significant linkages present in lignin include resinol (β-β), phenyl coumaran (β-5′), biphenyl (5-5′), diphenyl ether (4-O-5′), and diphenyl methane (β-1). These additional linkages are notably more challenging to deconstruct, complicating the overall conversion process.
The chemical structure of lignin is intricately linked to its botanical source and the methods employed for its extraction. Recent advancements in analytical techniques have significantly streamlined the analysis of lignin’s chemical bonds. Thermochemical treatments facilitate the depolymerization of lignin extracted from cellulosic fibers, enhancing its solubility while simultaneously generating non-native “condensed” linkages at reactive sites. The predominant technical lignins pertinent to material applications include lignosulfonates (LSs), kraft lignin (KL), organosolv lignin (OSL), and soda lignin (SL). It is crucial to note that the choice of lignin type is closely aligned with its intended application. For instance, less purified lignin preparations may be readily utilized in the fabrication of lignin-derived plastics and composites, whereas highly purified or fractioned lignin is preferable for advanced applications, particularly in biomedical fields. This tailored approach ensures optimal performance and efficiency in each specific application. The chemical modification of lignin is increasingly recognized as a pivotal strategy to enhance its properties for various material production applications. These modification processes elevate the reactivity of lignin by specifically targeting its functional groups, which include hydroxyl, methoxyl, carbonyl, and carboxyl groups. Techniques such as hydroxyalkylation, esterification, and amination are employed to facilitate these transformations. Most notably, these chemical modifications aim to convert lignin’s complex macromolecules into more versatile macromonomers. This transformation allows for the subsequent grafting of traditional monomers or polymers, ultimately leading to the synthesis of innovative lignin-based functional polymers. Through these advanced processes, lignin, a natural polymer, is reimagined, opening up a wealth of possibilities for sustainable materials with enhanced performance characteristics.
Lignin possesses a complex aromatic structure characterized by multiple phenolic rings, presenting significant challenges in the synthesis of lignin nanoparticles (LNPs). Recent advancements have facilitated the production of various nanosized lignin forms, including irregular nanoparticles, hollow nanotubes, hollow nanospheres, and nanofibers. The synthesis of LNPs enables the transformation of heterogeneous and structurally intricate lignin into uniform nanoparticles with consistent size and morphology. These engineered nanoparticles exhibit advantageous properties such as tailored structural characteristics, enhanced compatibility with polymer matrices, and superior antioxidant activity, making them promising candidates for various applications in materials science and nanotechnology. LNPs can be synthesized through various methodologies, including self-assembly, solvent exchange, acid precipitation, polymerization, ultrasonication, crosslinking, electrospinning, and utilizing CO2 as a non-solvent. In the self-assembly approach, ordered morphologies emerge due to specific intermolecular non-covalent interactions—such as hydrophobic, electrostatic, hydrogen bonding, and Van der Waals forces—without any external intervention.
In the solvent exchange method, LNPs are generated by dissolving materials in solvents like tetrahydrofuran, dioxane, dimethyl sulfoxide, acetone, and ethanol, followed by controlled precipitation in the presence of water. Techniques such as ultrasonication and oil-in-water emulsions are employed for the formation of microcapsules, typically within a size range of 0.3 to 1.4 μm. Lignin micro- and nanocapsules can be produced via ultra-stirring or by electrospinning softwood organosolv with the inclusion of 2 wt% FeCl3. Notably, hollow lignin nanospheres with a diameter around 200 nm have been successfully synthesized through these processes. Tetrahydrofuran (THF) was employed as a solvent to initially dissolve lignin, followed by the controlled addition of water under specific conditions. The resulting LNPs, which encapsulated an anticancer drug, demonstrated successful release profiles under tightly regulated conditions. Lignin stands out among potential drug carriers due to its excellent biocompatibility and non-cytotoxic nature. Prior to the formation of nanospheres, cyclodextrin was conjugated to the lignin to facilitate the encapsulation of the anticancer agent. Various types of lignin can be utilized for LNP preparation, including hydrolysis lignin, kraft/alkali lignin, lignosulfonate, enzymatic hydrolysis lignin, and organosolv lignin. Notably, the cyclodextrin-modified lignin-based LNPs exhibited a significant sustained release (SR) capability for the antitumor agent hydroxycamptothecin (HCPT).
4. Physicochemical Properties
4.1. PHB
Polyhydroxybutyrate (PHB) exists in three distinct forms: high-molar mass PHB, low-molar mass D-PHB, and short-chain oligohydroxybutyrate (OHB). The repeat unit of PHB has a molecular weight (Mw) of 86.09 g mol−1, though Mw can vary significantly due to factors such as feedstock choice, cultivation conditions, and extraction methodologies [128]. PHB exhibits notable characteristics, including optical activity and piezoelectric properties, alongside effective barrier capabilities against hydrophobic substances and water vapor, which are influenced by its degree of crystallinity (Xc) [129]. Particularly, the exceptional oxygen barrier properties of PHB have attracted substantial attention for applications in food packaging. Polyhydroxyalkanoates (PHAs) and their composites, including PHB and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV), demonstrate superior gas barrier performance, rendering them suitable for use in films and coated paper. Moreover, their increased water vapor permeability makes them particularly advantageous for EMAP (Enhanced Modified Atmosphere Packaging) solutions in fresh food applications [130,131]. The degree of crystallinity in PHB affects its physical and mechanical properties, leading to limited ductility and processing capabilities. The intrinsic brittleness of pure PHB limits its applicability in practical applications. However, copolymerization of PHB with specific structural segments from natural polyhydroxyalkanoates (PHAs) can decrease its crystallinity and improve its mechanical performance. Despite these modifications, polyhydroxybutyrate-co-valerate (PHBV) retains the inherent brittleness associated with PHB [132]. Additionally, PHB is susceptible to thermal instability, which can result in a reduction in both molar mass and viscosity during thermal processing. The melting temperature (Tm) of PHB typically ranges from 173 °C to 180 °C, while the glass transition temperature (Tg) is around 5 °C. The primary thermal degradation mechanism involves chain cleavage at elevated temperatures, which leads to a notable decrease in molecular weight (Mw) [133].
Poly(3-hydroxybutyrate) (P3HB) is a crystalline biopolymer distinguished by its linear chain morphology, which encompasses both amorphous and crystalline domains. P3HB is synthesized through bacterial fermentation processes and offers several advantages for packaging applications, including superior barrier properties, rigidity, and biodegradability. Its performance under biologically active environments—such as soil, aquatic systems, and both aerobic and anaerobic composting—demonstrates notable resilience against degradation [134]. The polymer’s structure is characterized by a methyl functional group (CH3) coupled with an ester linkage (−COOR), which confers thermoplastic behavior, hydrophobicity, high crystallinity, and inherent brittleness. The thermal properties of PHB and its derivatives are defined by key thermal transition points: specifically, the glass transition temperature (Tg) indicative of the amorphous phase and the melting temperature (Tm) associated with the crystalline phase. The degradation temperature (Td) marks the onset of material degradation [135]. To accurately determine these temperatures, Differential Scanning Calorimetry (DSC) and X-Ray Diffraction (XRD) are routinely employed. Additionally, Fourier Transform Infrared Spectroscopy (FTIR) serves as a vital analytical tool for elucidating the molecular structure of these materials and evaluating their degree of purity. Furthermore, P3(HB) has been subjected to a comparative analysis of mechanical properties against polypropylene (PP), polyethylene terephthalate (PET), polyethylene (PE), and polylactic acid (PLA) [136].
Crystallinity plays a crucial role in determining the mechanical properties of polymer materials. For instance, P3(HB) exhibits a crystallinity value typically exceeding 50%, often falling within the range of 60% to 70%. Higher levels of crystallinity result in materials that are stiffer, stronger, and more brittle. Additionally, crystallinity influences various characteristics, including tactility, hardness, modulus, density, transparency, and behaviors during cold drawing or ductile flows [137]. However, P3(HB) materials face challenges due to a narrow processing window, particularly highlighted by the difference between their melting temperature (Tm) and degradation temperature (Td). This gap makes them susceptible to thermal degradation when exposed to temperatures near the melting point. Several approaches have been evaluated to improve processability, including adjusting the ratios of monomers used in the polymerization process. Notably, incorporating additional units of 3 HV can significantly enhance impact strength, although it may result in a decrease in tensile strength and modulus. P4(HB), a promising new material, can be synthesized either through the condensation of 4-hydroxybutyric acid (4HB) or via ring-opening polymerization (ROP) of γ-lactone. This material boasts several advantageous properties, including non-toxicity, biocompatibility, optical activity, and stability under UV radiation. Furthermore, it can be processed using various techniques such as extrusion, injection molding, blowing, and thermoforming. These qualities make P4(HB) an excellent candidate for applications in the biomedical field, particularly in the production of medical devices due to its safety and compatibility with biological systems [138].
4.2. Lignin
Lignin, a complex biopolymer, typically appears colorless or pale yellow but can change color upon treatment with acids or alkalis. The molecular weights of isolated lignin typically range from 1000 to 20,000 g/mol; however, estimating the degree of polymerization remains problematic due to fragmentation that occurs during the extraction process. Thermal treatment at approximately 150–160 °C can enhance the molecular weight of lignin. The total grain size (Tg) is a critical metric that assesses the extent of the rubbery region in materials and indirectly reflects the levels of crosslinking and crystallinity present [139]. Lignin demonstrates degradable properties, with hydrogenation and oxidation being the predominant degradation pathways. It also possesses significant attributes such as excellent thermal stability, antioxidant activity, biodegradability, antibacterial effects, adhesive properties, and capability for dust dispersion and blending. These properties position lignin as a versatile additive with applications in dust control and material blending [140].
4.3. Cellulose
Cellulose, the predominant constituent of plant cell walls, represents the most abundant natural polymer. Its structure as a (1-4) β-D glucan varies by plant species, comprising between 1000 and 8000 polymerized glucose units. Typically, cellulose fibers extracted from wood pulp exhibit a chain length ranging from 500 to 2000 glucose units [141]. The capacity of cellulose to undergo substitution with larger functional groups, such as nitrate or acetate, has been pivotal in the advancement of commercial plastic materials. This modification reduces intermolecular interactions and enhances the solubility of the resulting plastic. Additionally, cellulose can be processed through solubilization, involving derivatization followed by the removal of substituent groups. This method ultimately facilitates the production of rayon fibers and cellophane. Recently, the development of compostable polymers suitable for diverse molding applications in the plastics sector has been documented. These thermoplastics are synthesized from cellulose-based resins [142].
5. Methods of Synthesis/Extraction/Fabrication
5.1. PHB Extraction
Solvent extraction, chemical digestion with surfactants, and enzymatic digestion are methods employed to extract PHB from microorganisms for bioplastic production. Some processes for extracting PHB are effective but can be environmentally harmful when used on a large scale. Surfactants such as SDS involved in chemical digestion disrupt cell membranes, enabling PHB to be released into the lysis solution. Nonetheless, these approaches have drawbacks, including high costs and complex recovery processes [143]. Bioextraction offers groundbreaking methods for PHB extraction from producer cells that do not require harmful agents, minimize the use of hazardous chemicals, and lower expenses. A comprehensive understanding of bioextraction processes is essential for the strategic development of eco-friendly technology solutions aimed at extracting ultrapure PHB with sustainable properties. Bacteriophage-based systems, along with predatory organisms and mealworm digestive processes, are garnering interest for their capabilities in self-disruption and biotic extraction. The phage lysis mechanism, which represents an innovative approach for PHB extraction, involves the disintegration of bacterial cell walls via lytic-phage infection, offering an eco-friendly and cost-effective solution. Key components of the lytic system include holin, endolysin, late transcription factors, and their synergistic interactions within the tandem structure. The lytic action results in cell wall rupture, enabling the extraction of bacterial biomass following cell lysis. Martínez et al. (2011) developed KT2440, a stable strain of Pseudomonas putida, to regulate the expression of genes related to the extrinsic holin–endolysin system derived from the EJ-1 phage [144]. The plasmid pCNBHL, which is constructed from xylS/pm and ejh/ejl cassettes, is commonly utilized for the expression of genes and gene clusters, synthesis of recombinant proteins, and reporter gene expression in both Gram-negative and Gram-positive bacteria. The Pseudomonas putida BXHL, engineered with a tolB/pal mutation, generated inducible self-disruptive genes aimed at enhancing PHB production. This strain manages genetically engineered cell death and facilitates the release of PHB while presenting fewer environmental and economic challenges compared to the wild type. Hand et al. (2016) demonstrated the efficacy of utilizing lytic bacteriophage Ke14 for the bioextraction of PHB from Pseudomonas oleovorans [145]. Despite this success, the hydrophobic interactions between carbonosomes and cellular debris impede the complete release of PHB in aqueous environments. To secure PHB storage, it is vital to eliminate PHB depolymerase from the host cells. Notably, during the exponential growth phase of P. oleovorans, only a marginal increase of less than 20% in PHB accumulation has been observed.
Employing a cost-effective self-disruption mechanism for the cell wall alongside inexpensive raw materials, such as crude glycerol, could substantially reduce production cost via bacteriophage-mediated lysis. Additionally, Pseudomonas putida not only generates protein-based hydrocarbons but also facilitates the internal or extracellular degradation of PHB depolymerase, thus providing carbon and energy substrates for its metabolic processes. Furthermore, Bdellovibrio bacteriovorus has been isolated and cultivated to obtain amino acids and other macromolecules essential for protein synthesis [146].
To enhance progeny and nutritional efficiency, Bdellovibrio bacteriovorus exhibits predatory behavior on Pseudomonas putida. Notably, PHB consumers harbor B. bacteriovorus, which contains the phaZBd gene encoding an extracellular PHA depolymerase, facilitating the degradation and release of PHAs. This results in accelerated growth, effective predation, and increased motility [147]. A newly isolated predatory strain demonstrates superior PHB extraction capabilities compared to previous variants, leading to high-purity biopolymer recovery through predator-mediated lysis. Furthermore, phaZ knockout mutants show substantial accumulation of PHA biopolymers due to significantly impaired PHB depolymerization. Employing metabolic engineering strategies in obligate predation systems can optimize biopolymer yield. The phaZ mutants also exhibit a low polydispersity index (PDI), indicating their potential applicability in industrial production and extraction processes [148]. The administration of lyophilized Cupriavidus necator H16 resulted in an increased recovery of PHB in rat models; however, a notable decline in growth rate was observed over a month, potentially indicating complications related to nephrolithiasis [149]. Identifying an optimal digestive host for PHB-producing microorganisms could facilitate the biological removal of fecal matter. Furthermore, mealworm larvae can be subjected to extraction processes yielding an impressive 89% purity through the use of sodium dodecyl sulfate (SDS). Given the associated environmental concerns, this methodology presents a promising alternative for low-chemical PHA production.
The enzyme digestion method for recovering PHB is designed to be ecologically sustainable, employing moderate operational conditions to enhance efficiency. Current research focuses on utilizing proteolytic enzymes, such as proteases and glycosidases, to achieve higher purity and cost-effectiveness while ensuring minimal environmental impact. Additionally, the application of lytic bacteriophages, predatory bacteria, and mealworms serves to induce cell lysis, representing a promising green biotechnology approach that effectively reduces both production costs and ecological footprints [150]. While economic and environmental considerations are critical, conventional methods still prevail in numerous applications. PHB is sourced from carbon contained within proteins essential for its synthesis and depolymerization. Advances in biotechnology have the potential to lower production costs and address environmental issues by optimizing the use of solvents and chemical additives. Enzymatic proteins derived from biotechnological processes effectively degrade cell walls, facilitating the extraction of PHB [151].
5.2. Cellulose Extraction
Cellulose extraction from wood is a highly effective process, as wood serves as one of the most established sources of cellulose. To achieve pure cellulose, it is essential to separate it from other wood components, specifically lignin and hemicellulose. This purification process involves several key steps. Initially, dried plant tissue is treated with a 4% sodium hydroxide solution at 80 °C for 4 h, effectively removing most of the lignin along with a significant portion of hemicellulose. Following this digestion step, the cellulose product undergoes a bleaching process using sodium chlorite and glacial acetic acid, which helps eliminate any remaining lignin and hemicellulose. The bleached cellulose fibers are then rigorously washed to ensure they achieve a neutral pH [152]. An alternative method of isolating cellulose is through ultrasonic treatment, which combines sequential application of water at 55 °C for 2 h with ultrasonic irradiation for 40 min. Afterward, the material is treated with 0.5 M NaOH and then subjected to varying concentrations of hydrogen peroxide (0.5%, 1.0%, 1.5%, 2.0%, and 3.0%) in conjunction with 0.5 M NaOH and 2 M NaOH at 55 °C for 2 h. The resulting insoluble residue is collected through filtration, rinsed with distilled water until the filtrate achieves a neutral pH, and finally dried at 60 °C. Once isolated, cellulose fibers can be transformed into nanofibers utilizing a range of mechanical, chemical, physical, or biological processing techniques (Figure 4). The choice of method will depend on the desired final dimensions of the nanofibers [76].

Figure 4.
Lignin and cellulose-based nanocomposite film preparation.
Enzymatic technology facilitates the selective hydrolysis of non-cellulosic components in plant fibers, specifically targeting hemicelluloses and lignin, thereby isolating the cellulose fraction effectively. This method integrates bioremediation, cryocrushing, and dilute acid pretreatment. Initially, air-dried plant material undergoes milling using a Wiley mill, and is then pre-soaked in a 1% dilute sulfuric acid solution at 5% dry solids concentration for four hours. Following this, the mixture is filtered and thoroughly rinsed with deionized water. The pretreated biomass is subsequently subjected to a 4560 mini-Parr 300 mL pressure reactor, where it is heated to 160 °C for a 30 min period, maintaining this temperature for predetermined residence times. The resultant slurry is filtered again, rinsed with deionized water, and air-dried overnight at ambient conditions. Reported biomass yields post-treatment range from 75% to 85% of the initial mass. Nonetheless, the removal of hemicellulose paired with hydrothermal treatment can result in cellulose annealing, which may increase crystallinity and compromise the overall effectiveness of this pretreatment strategy [153,154].
5.3. Lignin Extraction
Chemical treatment processes that decompose lignin–carbohydrate complexes allow for the industrial recovery of lignin from cellulose fibers (Table 5). During this procedure, partial depolymerization of the lignin macromolecules takes place, resulting in smaller fractions that enhance solubility [75]. Numerous reactive sites facilitate repolymerization (condensation) within the matrix, thereby reinforcing C–C linkages and modifying the original structure of the lignin used [152]. The variation in feedstock and extraction methods leads to isolated lignin with diverse structures and a range of physical properties. At present, biorefineries and the paper pulping industry contribute to the commercial production of technical lignin.

Table 5.
Types of polymers obtained before and after different treatments and their applications.
The pulp and paper industry generates approximately 50 million metric tons of lignin, with most of it utilized as fuel for electricity generation in paper mills [155]. Only 2% of the total lignin produced is utilized in the chemical industry. Numerous valuable evaluations include techniques for lignin extraction [74]. The radical polymerization of lignin can be complicated by covalently bonded reactive components from hemicelluloses, resulting in the formation of a lignin–carbohydrate complex (LCC) [156]. Therefore, lignin needs to be partially depolymerized to enhance its extractability, and LCC linkages must be disrupted to isolate it from carbohydrates. To promote biomass solubilization, depolymerization breaks lignin down into smaller fragments. Given that lignin and its various fractions are highly reactive, repolymerization (condensation) activities create new C-C bonds during extraction, leading to unique lignin intermediates with differing properties [71].
Biorefineries harness biomass as a renewable resource for the production of chemicals and fuels. To ensure the optimal utilization of all lignocellulosic components—cellulose, hemicellulose, and lignin—it is essential to effectively separate or isolate these components, given their distinct physical and chemical properties. The process of removing lignin, hemicellulose, and cellulose from biomass typically occurs during pretreatment or fractionation [72]. The biorefining process begins with the pretreatment of biomass, which is crucial for reducing recalcitrance and enhancing the valorization of these materials. Various biomass pretreatment methods have been developed, including solvolysis fractionation, reductive or oxidative catalytic fractionation, acid hydrolysis, and alkaline hydrolysis. It is important to note that the efficiency of delignification, the purity of isolated components, and the structural integrity can significantly differ even among the same type of biomass, depending on the chosen fractionation method and its intensity (Table 5) [73]. Emphasizing tailored approaches in biomass pretreatment can lead to more effective and sustainable biorefining outcomes.
6. Next-Generation Materials and Their Integration with Industry
Polyhydroxyalkanoates (PHAs) are gaining importance as biodegradable biopolymers with applications in packaging, agriculture, and biomedical fields. Their biocompatibility and biodegradability make them attractive for sustainable plastic alternatives, tissue engineering scaffolds, and controlled drug delivery systems. In agriculture, PHAs can be used for slow-release fertilizers and biodegradable mulch films. Furthermore, industries are exploring PHAs for making sustainable textiles, coatings, and 3D printing materials, positioning them as key contributors to reducing plastic waste and fostering a circular economy (Figure 5). Table 6 comprises a list of a few industries involved in different types of PHA production.
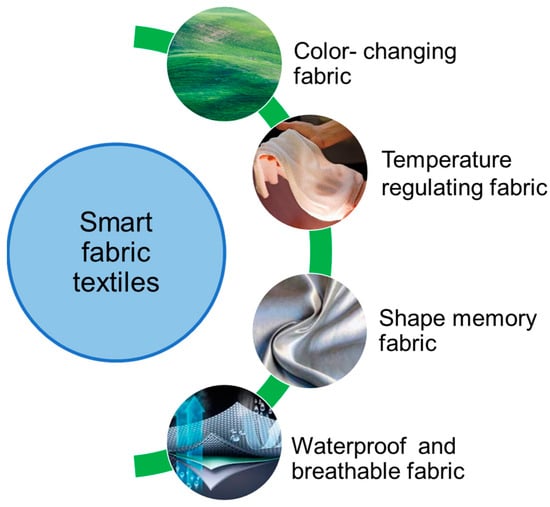
Figure 5.
Applications in smart fabric textiles.

Table 6.
List of companies with industrial-scale PHA production.
Cellulose exhibits a wide array of applications due to its regenerated forms like microcrystalline (MCC), nanocrystalline cellulose (NC), and CNFs, which serve as coatings for water and air filtration, electrical insulation, packaging, and components in construction and transportation sectors. The primary domain associated with cellulose’s technological relevance is the production of paper and various cellulose-derived products [157]. Considering emerging material constraints, both industrial and academic research initiatives are increasingly focused on the conversion of cellulose into biofuels or other high-value materials. As a widely recognized natural biopolymer, cellulose offers remarkable benefits such as excellent biocompatibility and biodegradability, versatility in processing into various material formats, the potential for sustainable large-scale production, and intrinsic characteristics like shape anisotropy, surface charge, and superior physical and mechanical properties. This makes cellulose a valuable resource for future material innovations.
In recent years, there has been significant innovation in wearable technology and smart sensors made from cellulose. This advancement can be attributed to the unique advantages that cellulose offers. As fabrication techniques for material processing have improved, researchers have been able to engineer cellulose into multidimensional structures. These cellulose-derived materials have emerged as versatile components in wearable sensors, serving as flexible biosupports, sensing layers, electrodes, and active elements. Their transformation into electrically conductive carbonaceous materials with customizable structures and properties enhances their effectiveness and functionality in various applications. Recent studies have explored various types of nanocellulose (NC) obtained from both plant and microbial sources as promising candidates for textile fiber production. One of the key factors driving the interest in nanocelluloses is their high crystallinity, which contributes to exceptional mechanical strength. It is important to note that nanocellulose encompasses a diverse range of materials, each exhibiting distinct properties, largely due to their different origins and synthesis methods rather than being a singular material type.
Nanocellulose represents a compelling alternative to conventional petroleum-based polymers, particularly in applications such as paper and textile coatings, due to its inherent biodegradability. This material exists in a nanometric fibrillar configuration derived from natural cellulose, which has garnered significant attention across various sectors, including industry, academia, and regulatory bodies. The growing interest is attributable to its remarkable characteristics, such as a high tensile strength, elastic modulus, extensive specific surface area, and a sustainable profile [8]. Furthermore, nanocellulose is often deemed non-toxic, biocompatible, and inherently biodegradable, reinforcing its potential as a viable substitute for traditional polymeric materials. Nanocellulose has significant potential in paper coatings due to its strong hydrogen bonding with paper fibers, enhancing mechanical, barrier, and hydrophobic properties [158,159]. Similar advantages are expected in textile coatings. Nanofiber extraction from cellulose can be achieved through mechanical, chemical, or combined methods.
Additionally, lignin-based hydrogels are promising stimuli-responsive polymers suitable for tissue engineering and drug delivery, as their porous structure mimics the biological extracellular matrix, promoting cell–matrix interactions. The swellability of hydrogels, which can be influenced by external stimuli like temperature, presents a significant opportunity for optimizing drug release profiles. The successful synthesis of thermo-responsive lignin-based hydrogels by incorporating thermo-responsive monomers into a lignin backbone, which undergoes phase transitions near physiological temperatures, makes them suitable for biomedical applications [160]. Furthermore, the presence of phenolic OH groups in the lignin structure plays a vital role, as these groups can interact effectively with COOH groups of the combined monomers, facilitating the production of these multifunctional hydrogels. Kraft lignin contains the highest concentration of phenolic OH groups, followed by soda lignin and organosolv [161,162]. Hydrogels made from soda lignin demonstrate superior mechanical properties due to their stronger crosslinking, resulting in greater elasticity. Hydrogels based on organosolv are a promising choice, while those derived from soda lignin are optimal for applications that require improved mechanical properties. Moreover, lignin is becoming recognized as a versatile strength enhancer, adhesive agent, and functional filler in various hydrogel formulations. Its potential uses extend across lignin fractionation, wearable electronics, UV protection, and the development of biomaterials, particularly in the formation of lignin-infused cellulose hydrogels for effective lignin separation [163,164]. The industrial potential and policy alignment strategies are listed in Table 7.

Table 7.
Industrial and policy integration strategies.
7. Applications in Next-Generation Materials
Cellulose is a multifunctional material that has emerged as a next-generation resource. In recent years, the focus on cellulose nanoparticles has intensified, particularly due to their potential applications across diverse fields such as sustainable ecology, bioremediation, and biomedical applications. Current research emphasizes the optimized utilization of cellulose, especially CNFs, which is characterized by its impressive mechanical strength, high hydrophilicity, and excellent biocompatibility. These attributes position CNFs as promising candidates for scaffolding in tissue engineering. Various fabrication techniques can be employed to create these scaffolds, including electrospinning, freeze-drying, 3D printing, and solvent casting, each offering unique advantages for controlled structural and functional properties.
Nanocellulose has several applications in tissue engineering, particularly for the repair of cutaneous, vascular, neurological, muscular, hepatic, and ophthalmological tissues [165,166,167]. When integrated with materials such as polyvinyl alcohol (PVA), GelMa, collagen, gelatin, and chitosan, nanocellulose facilitates the formation of scaffolds that provide an optimal microenvironment for cellular adhesion, proliferation, and metabolic activity. The incorporation of CNCs and CNFs in fibrous forms significantly enhances the tensile strength, elastic modulus, indentation modulus, and biocompatibility of scaffolds intended for vascular tissue engineering applications. Nanocellulose scaffolds play a crucial role in neural tissue engineering by supporting the development of neural cells, thereby maintaining axonal pathways and enhancing neural stimulation and activity [168,169]. Its elevated surface-to-volume ratio facilitates essential cellular processes such as growth, migration, and adhesion, making it an attractive candidate for advanced wound dressings. The increased density of hydroxyl (-OH) functional groups on the nanocellulose surface forms a hydrophilic layer over wounds, which helps to determine microbial biofilm formation, facilitate the cleansing of damaged tissues, alleviate pain, and mitigate odor. Furthermore, innovative bioactive wound dressings that inhibit bacterial colonization have been engineered by integrating nanocellulose with antibiotic therapies [170,171].
The straightforward conversion of CNFs into suspensions that act as carriers for pharmaceutical medications is achievable. CNFs have been utilized as a film to facilitate the rapid release of drugs that are poorly soluble and as a matrix for ongoing drug delivery [172,173]. Additional research into the efficacy of drug delivery has explored the use of BNC–acrylic acid (AA) hydrogels for this purpose. In terms of drug delivery, cellulose nanofibers (CNFs) have been studied; some investigations indicate slow-release rates over time, while others reveal rapid release rates within the CNF network. Made from nanocellulose, cellulose derivatives, and cellulose-based composites, cellulose-based nanogenerators show potential in enhancing the performance of cellulose and addressing environmental pollution issues associated with the non-biodegradability of traditional polymers. From an ecological perspective, the recycling and detection of heavy metals in water are critically important [67].
The development of cellulose coated with gold Qatarized capillaries (Au-QC) presents a significant advancement in high-resolution protein separation through electrophoresis. This innovative Au-QC coating has demonstrated its effectiveness in separating glycoproteins and successfully detecting lysozyme in both milk and tear samples. As a result, it offers a promising new approach for the detection of lysozyme from diverse sources [136]. Additionally, Turyanska et al. have successfully regenerated cellulose in aqueous dispersions of gold nanoparticles (AuNPs), leading to the formation of AuNP networks on cellulose sheets [174]. This advancement further highlights the potential of integrating nanotechnology with biomaterials for enhanced analytical applications. The resistivity characteristics of composite films play a critical role in dictating the mechanisms of electrical conduction. Notably, under low-temperature conditions and in the presence of high magnetic fields, these materials exhibit negative magneto-resistance. Gold nanoparticles (AuNPs) have been effectively incorporated into hybrid films, enhancing the functionality of both technological devices and biological sensors. A significant advancement in this area is the development of SiO2–cellulose paper with gold anodes, which demonstrates brightness three times that of standard bacterial cellulose sheets. This innovation presents a promising green substrate for flexible top-emission organic light-emitting diodes (TE-OLEDs) [175].
In addition to having antibacterial capabilities and being non-cytotoxic in nature, hydrogels that are produced from lignin exhibit increased mechanical strength and stiffness. There has been research conducted on the potential of lignin–poly lactic acid (PLA) composites as wound dressings. The results of this research have shown that these composites have enhanced wettability and lower fracture resistance. Hydrogels have been synthesized based on lignin with concentrations ranging from 24 to 40%, and these hydrogels have demonstrated water absorption capabilities of up to 500 percent. Because of its hydrophobic qualities, lignin could assist the incorporation of a hydrophobic drug model (curcumin), and it also gave a large amount of resistance to cell adhesion by bacteria [176]. Lignin was mixed with MCC and examined as an excipient in immediately compressed tetracycline tablets. This demonstrated the potential of lignin as an excipient in the pharmaceutical industry [177]. Applications for the biodegradable polymer lignin, which is derived from wood, have been discovered in the field of radiative cooling technologies.
Extraction of lignin from the residual biomass of sugarcane bagasse was carried out. Subsequently, chemical changes were made in order to produce a cationic surfactant. The inclusion of nano-clay into a polystyrene co-butyl acrylate latex matrix was proven to be facilitated by this surfactant, which indicated its potential to facilitate the process. The addition of lignin into this nanocomposite resulted in a significant improvement in its resistance to increasing amounts of ultraviolet light [178]. A melt extrusion technique known as fused deposition modeling (FDM), more generally known as 3D printing, is a technique that is utilized to produce three-dimensional structures. This technique involves the utilization of a nozzle that methodically deposits layers of thermoplastic polymer, adhering to a particular design. Acrylonitrile butadiene styrene, also known as ABS, is the thermoplastic that is most commonly used in three-dimensional printing. ABS is constructed from petroleum-based materials. An amorphous polymer was produced due to the replacement of forty percent of the ABS with organosolv lignin. Despite this, the incorporation of 10% acrylonitrile butadiene rubber mitigated the adverse effects of lignin, resulting in a polymer that exhibited a fivefold increase in tensile strength and was appropriate for 3D printing [179,180].
8. Challenges, Limitations, and Solutions
Widespread adoption of biomass-derived lignin and cellulose biopolymers is hindered by common factors like the intricate structure of ligno-cellulosic biomass, which makes it challenging to separate pure lignin and cellulose; the requirement for effective and sustainable pretreatment techniques to degrade the lignocellulose matrix; high production costs as a result of energy-intensive extraction procedures; and the variability in quality based on the biomass feedstock (Table 8). Biomass requires pretreatment procedures, such as chemical or enzymatic operations, to break down lignin and hemicellulose in order to yield cellulose. These procedures can be energy-intensive and produce potentially hazardous byproducts. Because the cellulose concentration and composition of various biomass sources fluctuate, it might be challenging to optimize extraction procedures for constant quality. Pretreatment of biomass, lignin and cellulose extraction, and purification are multi-step processes that can be costly. While cellulose is highly crystalline, insoluble in water, and non-reactive by its structural and molecular nature, lignin is highly reactive, toxic to the environment and complex in structure, and closely bound with cellulose and hemicellulose. These challenges can be widely addressed using organic chemistry interventions like natural deep eutectic solvents (NADESs) but have been limited to the research phase.

Table 8.
Challenges, limitations, and potential solutions of plant/bacterial biopolymers.
Solutions for ligno-cellulosic biopolymers can be developed by creating more sustainable and effective pretreatment techniques: studies on biological, ionic, and mild chemical treatments that enhance cellulose extraction while reducing environmental impact. Modifying enzyme mixtures to efficiently break down cellulose derived from various biomass sources. Biorefinery-based integrated processes can increase product yield, reduce energy use, and produce more value from byproduct streams.
The production of PHAs remains cost-prohibitive compared to traditional plastics like polyethylene and polypropylene. This is largely attributed to the energy-intensive fermentation processes necessary for their biosynthesis, coupled with the substantial expenses associated with downstream processing, including separation and purification methodologies. To compete with petroleum-based polymers, PHA production needs to become more cost-effective. While PHAs are biodegradable, the properties of the final polymer can vary significantly based on the type of PHA produced (e.g., PHA copolymers versus homopolymers). Some forms of PHA, like polyhydroxybutyrate (PHB), face limitations due to their brittleness and inferior mechanical properties. One of the key challenges is the development of PHA variants with enhanced performance characteristics, including strength, flexibility, and thermal stability. These issues within the biopolymer sector open up broader opportunities for the advancement of bio-based innovations at the convergence of science, chemistry, biotechnology, and engineering to facilitate commercial implementation (Table 9).

Table 9.
Comparison of biopolymers with conventional plastics.
9. Conclusions
Bio-based polymers hold incredible promise for the future, yet they face a few significant hurdles. Competing with the entrenched plastics made from fossil fuels that dominate the global market is no small feat. For bio-based polymers to shine, they must tackle challenges related to both cost and performance. While these sustainable alternatives are undeniably friendlier to our planet compared to their petroleum-based counterparts, it is crucial to take a holistic view of their environmental impact. We need to carefully assess the resources used, the energy consumed, and the land transitions required for feedstock production in order to avoid any unintended consequences. The pursuit of a more sustainable future for plastics is undoubtedly a commendable endeavor. Developing infrastructure for proper disposal and recycling, along with boosting consumer awareness and acceptance, is vital for bio-based polymers to truly replace traditional plastics. As they scale up, adapt, and become more economically viable, bio-based polymers hold immense promise for the future. With advancements in production technology, the adoption of sustainable feedstocks, and growing public demand for eco-friendly options, bio-based polymers can significantly contribute to reducing plastic pollution and facilitating the shift toward a sustainable, circular economy. However, to fully unlock their power and promise, we need continuous innovation and collaboration across various sectors.
10. Future Perspectives
In industrial biomanufacturing processes for biopolymers, microorganisms like bacteria, yeast, and fungi are engineered to produce polymers through fermentation. Plant-based biopolymers involve leveraging agricultural outputs, such as starch and cellulose, which are extracted and refined. Fermentation tanks help cultivate strains that metabolize sugars into polymer precursors. These precursors undergo polymerization to form materials like polylactic acid, PHAs, etc. The scalability of these processes is enhanced by optimizing microbial strains for higher yield and efficiency, ultimately enabling sustainable alternatives to petrochemical-derived plastics. Unlike traditional polymers derived from fossil fuels, biopolymers utilize renewable resources, such as plant material and microorganisms, which absorb carbon dioxide during their growth, resulting in significant environmental benefits, particularly in terms of reduced carbon footprint. This process offsets emissions, contributing to a lower overall carbon footprint. Moreover, biopolymer production often requires less energy, further minimizing greenhouse gas emissions. As these materials are biodegradable, they reduce long-term environmental impact, enhancing sustainability in material science. They provide versatile applications, from packaging to biomedical devices, due to their unique properties such as flexibility, strength, and biocompatibility. As awareness of ecological issues grows, the development of these biopolymers is gaining momentum, paving the way for innovative solutions that bridge technology and nature responsibly.
Bioengineering and synthetic biology will play pivotal roles in developing functional biopolymers by enabling precise manipulation of organisms at the molecular level. These disciplines facilitate the design of novel pathways and the optimization of microbial and plant cell factories to produce biopolymers with enhanced properties. Through techniques such as CRISPR-Cas9 and metabolic engineering, scientists can tailor the functionality, biodegradability, and mechanical properties of these materials. This targeted approach accelerates the development of sustainable alternatives to conventional plastics, fostering advancement in environmentally friendly materials. Harnessing biomimetics involves drawing inspiration from nature to enhance material performance by mimicking biological processes and structures. This approach in material science allows researchers to create innovative biopolymers that emulate the resilient and adaptable qualities found in plants and microorganisms. By studying the ways in which nature efficiently uses resources and energy, scientists engineer materials that are sustainable and highly functional. These bio-inspired designs lead to the development of new materials with enhanced strength, flexibility, and environmental compatibility, driving forward the capabilities and applications of biopolymers.
Green chemistry and biomanufacturing will play central roles in pioneering sustainable biopolymer production methods. NADESs and ionic/green solvents will change the way biomass will be processed for the extraction of useful compounds and metabolites with high recycling efficiency. This shift not only reduces carbon footprints but also fosters biodegradability, leading to materials that harmonize with natural cycles, thereby promoting a more sustainable future for material science. These materials decompose naturally, minimizing environmental impact and reducing reliance on fossil fuels. The integration of natural fibers not only enhances the mechanical properties of biopolymers but also promotes a circular economy, opening new pathways for eco-friendly production and waste management practices that align with global sustainable development goals (SDGs).
The rapid advancement of cellulosic nanomaterials, ranging from conducting polymers to carbon nanotubes (CNTs), and 2D materials such as graphene, MXenes, and Transition Metal Dichalcogenides (TMDs), has paralleled developments in cellulose nanotechnology. This has opened new opportunities by combining the mechanical strength of cellulose nanomaterials with the electronic conductivity of advanced nanomaterials like MXenes with tremendous applications in energy storage systems, supercapacitors, and bio-batteries. Another approach is to use cellulose nanomaterials, such as nanopapers, as a substrate for electronics integration in a monolithic design or as cellulose mixed water dispersions to generate electrical composites. These materials are closer to industrialization, due to their huge potential to be used as novel electronic substrates, thus making conventional and highly recalcitrant printed circuit board (PCB) market obsolete.
Cellulose-based materials are emerging as game-changers in sustainable technologies, combining environmental friendliness with remarkable functional potential. Future cellulose materials with unique self-assembly and nanostructuring capabilities will self-assemble from water into well-defined 3D nanostructures. This precision at the nanometer scale allows functions to be embedded deep within the material’s bulk, enabling sophisticated applications. These materials could power brain-like or neuromorphic computing systems, where 3D electrochemical junctions form dense arrays of memory and computational elements. This development could lead to water-based neuromorphic computers mimicking human brain functionality for evolving high-performance computing. Combining cellulose nanocomposites with tissue engineering holds promise for creating electronic biohybrid materials. Such materials could revolutionize biomedical devices, wearable technologies, and bio-interfacing systems leading to bio-inspired electronics, and living neural and brain–machine interfaces (BMIs). The next generation of cellulose nanocomposites could integrate computing, energy storage, sensors, and actuators into monolithic smart systems [182,183,184]. These systems could respond to their environment, process data, and adapt in real time. Advanced cellulose-based materials may be utilized in the construction industry for building massive structures like skyscrapers, offering strong, renewable alternatives to conventional materials. Additionally, they could serve as green electronic substrates, helping the electronics industry transition to more sustainable practices. Cellulose-based next-generation materials offer immense promises across computing, healthcare, construction, and sustainable electronics. They represent a key step toward environmentally responsible innovation without compromising on advanced functionality.
The rise of microbial and plant-based biopolymers presents significant future implications for both industry and the environment, thus truly living up to the title of revolutionary future materials or next-generation materials. These sustainable materials offer a pathway to reduce dependency on fossil fuels, diminish carbon footprints, and promote circular economies. Industries such as construction, electronics, packaging, textiles, and automotive can transform their traditional processes to incorporate biopolymers, enhancing biodegradability and reducing waste. Environmentally, widespread adoption of these biopolymers could lead to healthier ecosystems, minimize pollution, and address climate change challenges, paving the way for a more sustainable planet.
Author Contributions
Conceptualization, Y.-C.C., M.V.R. (M. Venkateswar Reddy) and M.V.R. (M. V. Rohit); methodology, writing—original draft preparation, M.V.R. (M. V. Rohit), P.K.D. and I.; writing—review and editing, Y.-C.C., M.V.R. (M. Venkateswar Reddy), M.V.R. (M. V. Rohit), O.S., G.M., I. and A.P.; supervision, Y.-C.C. and M.V.R. (M. Venkateswar Reddy); project administration, funding acquisition, Y.-C.C. and M.V.R. (M. V. Rohit). All authors have read and agreed to the published version of the manuscript.
Funding
PKD and MVR acknowledge the financial support in the form of the Lignin–Cellulose-based Nanocomposites project sponsored by Gujarat State Biotechnology Mission GSBTM/JD(R&D)/618/21-22/3674 and the Indian Council for Agricultural Research—All India Coordinated Research Project (ICAR-AICRP) on Energy in Agricultural and Agro-based Industries (EAAI). YC also acknowledges that this study was funded by JSPS KAKENHI (Grant Number 24K11471). Additionally, this research was supported by the Ogasawara Foundation for the Promotion of Science and Engineering (Japan) and the Iwatani Foundation for the Promotion of Science and Engineering (Japan).
Acknowledgments
P.K. Drishya, Aesha Patel and M.V. Rohit acknowledge the Director of SPRERI for support and encouragement in carrying out this work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ma, Z.; Zhang, Z.; Wang, C.; Cao, J.; Liu, Y.; Yan, H.; Zhou, X.; Feng, X.; Chen, D. Boosting Engineering Strategies for Plastic Hydrocracking Applications: A Machine Learning-Based Multi-Objective Optimization Framework. Green Chem. 2025, 27, 1169–1182. [Google Scholar] [CrossRef]
- Patria, R.D.; Rehman, S.; Yuen, C.-B.; Lee, D.-J.; Vuppaladadiyam, A.K.; Leu, S.-Y. Energy-Environment-Economic (3E) Hub for Sustainable Plastic Management–Upgraded Recycling, Chemical Valorization, and Bioplastics. Appl. Energy 2024, 357, 122543. [Google Scholar] [CrossRef]
- Anitha, K. Emerging Trends in Sustainability: A Conceptual Exploration. In Global Sustainability; World Sustainability Series; Springer: Cham, Switzerland, 2024; pp. 19–35. [Google Scholar]
- Chang, Y.-C.; Reddy, M.V.; Mawatari, Y.; Sarkar, O. Enhanced Polyhydroxyalkanoate Biosynthesis by Cupriavidus sp. CY-1 Utilizing CO2 under Controlled Non-Explosive Conditions. Chemosphere 2025, 373, 144181. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Mondal, P.; Jadhav, S.M.; Moholkar, V.S. Application of Enzymatic Technique for the Synthesis of Biodegradable Polymers from Different Agrobiomasses. In Agricultural Biomass for the Synthesis of Value-Added Materials; CRC Press: Boca Raton, FL, USA, 2024; pp. 273–299. [Google Scholar]
- Chang, Y.-C.; Reddy, M.V.; Suzuki, H.; Terayama, T.; Mawatari, Y.; Seki, C.; Sarkar, O. Characterization of Ralstonia Insidiosa C1 Isolated from Alpine Regions: Capability in Polyhydroxyalkanoates Degradation and Production. J. Hazard. Mater. 2024, 471, 134348. [Google Scholar] [CrossRef]
- Kothawade, S.N.; Pande, V.V.; Suryawanshi, J.; Kumar, A.; Kumar, P.; Kumar, A. Biodegradable and Non-Biodegradable Sustainable Biomaterials. In Advances in Sustainable Biomaterials; CRC Press: Boca Raton, FL, USA, 2024; pp. 92–116. [Google Scholar]
- Bugarčić, M.; Jovanović, A.; Petrović, J.; Mišić, M.; Sokić, M.; Milivojević, M. Advances in Biopolymer Production and Applications: A Comprehensive Review of Key Biomaterials. Metall. Mater. Data 2024, 2, 81–98. [Google Scholar] [CrossRef]
- Tagliaferri, S.; Gaspard, L.; Au, H.; Mattevi, C.; Titirici, M.-M.; Ribadeneyra, M. Nature-Inspired Batteries: From Biomaterials to Biomimetic Design Strategies. Green Chem. 2024, 26, 6944–6958. [Google Scholar] [CrossRef]
- Kalpana, M.R.; Sharma, N.; Kumar, V.; Jayaraj, I.; Vimal, S.; Umesh, M. A Comprehensive Review on Natural Macromolecular Biopolymers for Biomedical Applications: Recent Advancements, Current Challenges, and Future Outlooks. Carbohydr. Polym. Technol. Appl. 2024, 8, 100536. [Google Scholar] [CrossRef]
- Heidari, M.; Al Hasani, S.; Thangavel, S.; Kumar, A. Challenges and Perspectives of Polymeric Nanocomposites for Biomedical Applications. In Nanomanufacturing Techniques in Sustainable Healthcare Applications; CRC Press: Boca Raton, FL, USA, 2024; pp. 317–340. [Google Scholar]
- Cháirez-Ramírez, M.H.; Díaz-Rivas, J.O.; Contreras-Ramírez, J.I.; González-Laredo, R.F.; Gallegos-Infante, J.A. Green Polymer-based Materials as Promising Therapeutical Agents for Non-communicable Diseases. Polym. Int. 2024. [Google Scholar] [CrossRef]
- Ali, G.; Sharma, M.; Salama, E.-S.; Ling, Z.; Li, X. Applications of Chitin and Chitosan as Natural Biopolymer: Potential Sources, Pretreatments, and Degradation Pathways. Biomass Convers. Biorefin. 2024, 14, 4567–4581. [Google Scholar] [CrossRef]
- Lalarukh; Hussain, S.M.; Ali, S.; Zahoor, A.F.; Azmat, H.; Nazish, N.; Alshehri, M.A.; Riaz, D.; Naeem, E.; Mahrukh. Innovation of Advanced Polymers from Seafood Waste: Applications of Chitin and Chitosan. Polym. Adv. Technol. 2024, 35, e6471. [Google Scholar] [CrossRef]
- Chudasama, N.A.; Sequeira, R.A.; Moradiya, K.; Prasad, K. Seaweed Polysaccharide Based Products and Materials: An Assessment on Their Production from a Sustainability Point of View. Molecules 2021, 26, 2608. [Google Scholar] [CrossRef] [PubMed]
- Anjana, K.; Arunkumar, K. Brown Algae Biomass for Fucoxanthin, Fucoidan and Alginate; Update Review on Structure, Biosynthesis, Biological Activities and Extraction Valorisation. Int. J. Biol. Macromol. 2024, 280, 135632. [Google Scholar] [CrossRef] [PubMed]
- Benalaya, I.; Alves, G.; Lopes, J.; Silva, L.R. A Review of Natural Polysaccharides: Sources, Characteristics, Properties, Food, and Pharmaceutical Applications. Int. J. Mol. Sci. 2024, 25, 1322. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, B.; Li, C.; Xu, Y.; Luo, Y.; Liang, D.; Huang, C. Comprehensive Review of Polysaccharide-Based Materials in Edible Packaging: A Sustainable Approach. Foods 2021, 10, 1845. [Google Scholar] [CrossRef]
- Arif, Z.U. The Role of Polysaccharide-Based Biodegradable Soft Polymers in the Healthcare Sector. Adv. Ind. Eng. Polym. Res. 2025, 8, 132–156. [Google Scholar] [CrossRef]
- Kramar, A.; González-Benito, F.J. Cellulose-Based Nanofibers Processing Techniques and Methods Based on Bottom-up Approach—A Review. Polymers 2022, 14, 286. [Google Scholar] [CrossRef]
- Obruca, S.; Sedlacek, P.; Slaninova, E.; Fritz, I.; Daffert, C.; Meixner, K.; Sedrlova, Z.; Koller, M. Novel unexpected functions of PHA granules. Appl. Microbiol. Biotechnol. 2020, 104, 4795–4810. [Google Scholar] [CrossRef]
- Obruca, S.; Sedlacek, P.; Koller, M. The underexplored role of diverse stress factors in microbial biopolymer synthesis. Bioresour. Technol. 2021, 326, 124767. [Google Scholar] [CrossRef]
- Alcantara, J.M.; Distante, F.; Storti, G.; Moscatelli, D.; Morbidelli, M.; Sponchioni, M. Current trends in the production of biodegradable bioplastics: The case of polyhydroxyalkanoates. Biotechnol. Adv. 2020, 42, 107582. [Google Scholar]
- Yao, F.; Yuan, K.; Zhou, W.; Tang, W.; Tang, T.; Yang, X.; Liu, H.; Li, F.; Xu, Q.; Peng, C. Unlocking growth potential in Halomonas bluephagenesis for enhanced PHA production with sulfate ions. J. Ind. Microbiol. Biotechnol. 2024, 51, kuad006. [Google Scholar] [CrossRef]
- Saito, S.; Imai, R.; Miyahara, Y.; Nakagawa, M.; Orita, I.; Tsuge, T.; Fukui, T. Biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from glucose by Escherichia coli through butyryl-CoA formation driven by CCR-EMD combination. Front. Bioeng. Biotechnol. 2022, 10, 860177. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cui, J.; Bilal, M.; Hu, H.; Wang, W.; Zhang, X. Pseudomonas spp. as microbial cell factories for value-added products: From rational design to industrial applications. Crit. Rev. Biotechnol. 2020, 40, 1232–1249. [Google Scholar] [CrossRef] [PubMed]
- Favaro, L.; Basaglia, M.; Casella, S. Improving polyhydroxyalkanoate production from inexpensive carbon sources by genetic approaches: A review. Biofuels Bioprod. Biorefin. 2019, 13, 208–227. [Google Scholar] [CrossRef]
- Sindhu, R.; Madhavan, A.; Arun, K.B.; Pugazhendhi, A.; Reshmy, R.; Awasthi, M.K.; Sirohi, R.; Tarafdar, A.; Pandey, A.; Binod, P. Metabolic circuits and gene regulators in polyhydroxyalkanoate producing organisms: Intervention strategies for enhanced production. Bioresour. Technol. 2021, 327, 124791. [Google Scholar] [CrossRef]
- Adeleye, A.T.; Odoh, C.K.; Enudi, O.C.; Banjoko, O.O.; Osiboye, O.O.; Odediran, E.T.; Louis, H. Sustainable synthesis and applications of polyhydroxyalkanoates (PHAs) from biomass. Process Biochem. 2020, 96, 174–193. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, Y.; Xu, P.; Yang, W.; Chen, M.; Dong, W.; Ma, P. Crystallization of microbial polyhydroxyalkanoates: A review. Int. J. Biol. Macromol. 2022, 209, 330–343. [Google Scholar] [CrossRef]
- Song, H.M.; Joo, J.C.; Lim, S.H.; Lim, H.J.; Lee, S.; Park, S.J. Production of polyhydroxyalkanoates containing monomers conferring amorphous and elastomeric properties from renewable resources: Current status and future perspectives. Bioresour. Technol. 2022, 366, 128114. [Google Scholar]
- Mai, J.; Kockler, K.; Parisi, E.; Chan, C.M.; Pratt, S.; Laycock, B. Synthesis and Physical Properties of Polyhydroxyalkanoate (PHA)-Based Block Copolymers: A Review. Int. J. Biol. Macromol. 2024, 263, 130204. [Google Scholar] [CrossRef]
- Kadier, A.; Ilyas, R.A.; Huzaifah, M.R.M.; Harihastuti, N.; Sapuan, S.M.; Harussani, M.M.; Azlin, M.N.M.; Yuliasni, R.; Ibrahim, R.; Atikah, M.S.N. Use of Industrial Wastes as Sustainable Nutrient Sources for Bacterial Cellulose (BC) Production: Mechanism, Advances, and Future Perspectives. Polymers 2021, 13, 3365. [Google Scholar] [CrossRef]
- Augimeri, R.V.; Varley, A.J.; Strap, J.L. Establishing a Role for Bacterial Cellulose in Environmental Interactions: Lessons Learned from Diverse Biofilm-Producing Proteobacteria. Front. Microbiol. 2015, 6, 1282. [Google Scholar] [CrossRef]
- Stanisławska, A.; Staroszczyk, H.; Szkodo, M. The Effect of Dehydration/Rehydration of Bacterial Nanocellulose on Its Tensile Strength and Physicochemical Properties. Carbohydr. Polym. 2020, 236, 116023. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ling, C.; Chen, Y.; Jiang, X.; Chen, G.-Q. Microbial Engineering for Easy Downstream Processing. Biotechnol. Adv. 2019, 37, 107365. [Google Scholar] [CrossRef] [PubMed]
- Kale, İ.; Kırdök, O.; Bilgi, E.; Akyol-Altun, T.D.; Tokuç, A.; Köktürk, G.; Özkaban, F.; Şendemir, A.; Andiç-Çakir, Ö.; Hameş, E.E. Potential of Bacterial Cellulose for Sustainable Cities: A Review and Bibliometric Analysis on Bacterial Cellulose. In A Sustainable Green Future: Perspectives on Energy, Economy, Industry, Cities and Environment; Springer: Berlin/Heidelberg, Germany, 2023; pp. 329–357. [Google Scholar]
- Choi, S.M.; Rao, K.M.; Zo, S.M.; Shin, E.J.; Han, S.S. Bacterial Cellulose and Its Applications. Polymers 2022, 14, 1080. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J. XIX.—The Chemical Action of Pure Cultivations of Bacterium Aceti. J. Chem. Soc. Trans. 1886, 49, 172–187. [Google Scholar] [CrossRef]
- Rovera, C.; Carullo, D.; Bellesia, T.; Büyüktaş, D.; Ghaani, M.; Caneva, E.; Farris, S. Extraction of High-Quality Grade Cellulose and Cellulose Nanocrystals from Different Lignocellulosic Agri-Food Wastes. Front. Sustain. Food Syst. 2023, 6, 1087867. [Google Scholar] [CrossRef]
- Sarkar, M.; Upadhyay, A.; Pandey, D.; Sarkar, C.; Saha, S. Cellulose-Based Biodegradable Polymers: Synthesis, Properties, and Their Applications. In Biodegradable Polymers and Their Emerging Applications; Springer: Berlin/Heidelberg, Germany, 2023; pp. 89–114. [Google Scholar]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and Its Derivatives: Towards Biomedical Applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Potočnik, V.; Gorgieva, S.; Trček, J. From Nature to Lab: Sustainable Bacterial Cellulose Production and Modification with Synthetic Biology. Polymers 2023, 15, 3466. [Google Scholar] [CrossRef]
- De Amorim, J.D.P.; de Souza, K.C.; Duarte, C.R.; da Silva Duarte, I.; de Assis Sales Ribeiro, F.; Silva, G.S.; de Farias, P.M.A.; Stingl, A.; Costa, A.F.S.; Vinhas, G.M. Plant and Bacterial Nanocellulose: Production, Properties and Applications in Medicine, Food, Cosmetics, Electronics and Engineering. A Review. Environ. Chem. Lett. 2020, 18, 851–869. [Google Scholar] [CrossRef]
- Jozala, A.F.; de Lencastre-Novaes, L.C.; Lopes, A.M.; de Carvalho Santos-Ebinuma, V.; Mazzola, P.G.; Pessoa-Jr, A.; Grotto, D.; Gerenutti, M.; Chaud, M.V. Bacterial Nanocellulose Production and Application: A 10-Year Overview. Appl. Microbiol. Biotechnol. 2016, 100, 2063–2072. [Google Scholar] [CrossRef]
- Chandana, A.; Mallick, S.P.; Dikshit, P.K.; Singh, B.N.; Sahi, A.K. Recent Developments in Bacterial Nanocellulose Production and Its Biomedical Applications. J. Polym. Environ. 2022, 30, 4040–4067. [Google Scholar] [CrossRef]
- Ansari, M.M.; Heo, Y.; Do, K.; Ghosh, M.; Son, Y.-O. Nanocellulose Derived from Agricultural Biowaste By-Products–Sustainable Synthesis, Biocompatibility, Biomedical Applications, and Future Perspectives: A Review. Carbohydr. Polym. Technol. Appl. 2024, 8, 100529. [Google Scholar] [CrossRef]
- Ullah, M.W.; Alabbosh, K.F.; Fatima, A.; Islam, S.U.; Manan, S.; Ul-Islam, M.; Yang, G. Advanced Biotechnological Applications of Bacterial Nanocellulose-Based Biopolymer Nanohybrids: A Review. Adv. Ind. Eng. Polym. Res. 2024, 7, 100–121. [Google Scholar] [CrossRef]
- Heise, K.; Kontturi, E.; Allahverdiyeva, Y.; Tammelin, T.; Linder, M.B.; Nonappa; Ikkala, O. Nanocellulose: Recent Fundamental Advances and Emerging Biological and Biomimicking Applications. Adv. Mater. 2021, 33, 2004349. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Chen, S.; Chen, Y.; Wang, B.; Pei, Q.; Wang, H. Macrofibers with High Mechanical Performance Based on Aligned Bacterial Cellulose Nanofibers. ACS Appl. Mater. Interfaces 2017, 9, 20330–20339. [Google Scholar] [CrossRef]
- Ullah, M.W.; Manan, S.; Ul-Islam, M.; Revin, V.V.; Thomas, S.; Yang, G. Introduction to Nanocellulose. In Nanocellulose: Synthesis, Structure, Properties and Applications; World Scientific: Singapore, 2021; pp. 1–50. [Google Scholar]
- Pogorelova, N.; Rogachev, E.; Digel, I.; Chernigova, S.; Nardin, D. Bacterial Cellulose Nanocomposites: Morphology and Mechanical Properties. Materials 2020, 13, 2849. [Google Scholar] [CrossRef]
- Suryanto, H.; Yanuhar, U.; Mansingh, B.B.; Binoj, J.S. Bacterial Nanocellulose from Agro-Industrial Wastes. In Handbook of Biopolymers; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–39. [Google Scholar]
- Sapuan, S.M.; Norrrahim, M.N.F.; Ilyas, R.A. Industrial Applications of Nanocellulose and Its Nanocomposites; Woodhead Publishing: Cambridge, UK, 2022; ISBN 032389917X. [Google Scholar]
- Girard, V.; Chaussé, J.; Vermette, P. Bacterial Cellulose: A Comprehensive Review. J. Appl. Polym. Sci. 2024, 141, e55163. [Google Scholar] [CrossRef]
- Lunardi, V.B.; Soetaredjo, F.E.; Putro, J.N.; Santoso, S.P.; Yuliana, M.; Sunarso, J.; Ju, Y.-H.; Ismadji, S. Nanocelluloses: Sources, Pretreatment, Isolations, Modification, and Its Application as the Drug Carriers. Polymers 2021, 13, 2052. [Google Scholar] [CrossRef]
- Martínez, E.; Posada, L.; Botero, J.C.; Rios-Arango, J.A.; Zapata-Benabithe, Z.; López, S.; Molina-Ramírez, C.; Osorio, M.A.; Castro, C.I. Nata de Fique: A Cost-Effective Alternative for the Large-Scale Production of Bacterial Nanocellulose. Ind. Crops Prod. 2023, 192, 116015. [Google Scholar] [CrossRef]
- Zhong, C. Industrial-Scale Production and Applications of Bacterial Cellulose. Front. Bioeng. Biotechnol. 2020, 8, 605374. [Google Scholar] [CrossRef]
- Keijsers, E.R.P.; Yılmaz, G.; van Dam, J.E.G. The Cellulose Resource Matrix. Carbohydr. Polym. 2013, 93, 9–21. [Google Scholar] [CrossRef]
- Lavanya, D.; Kulkarni, P.K.; Dixit, M.; Raavi, P.K.; Krishna, L.N.V. Sources of Cellulose and Their Applications—A Review. Int. J. Drug Formul. Res. 2011, 2, 19–38. [Google Scholar]
- Sundarraj, A.A.; Ranganathan, T.V. A Review on Cellulose and Its Utilization from Agro-Industrial Waste. Drug Invent. Today 2018, 10, 89–94. [Google Scholar]
- Felgueiras, C.; Azoia, N.G.; Gonçalves, C.; Gama, M.; Dourado, F. Trends on the Cellulose-Based Textiles: Raw Materials and Technologies. Front. Bioeng. Biotechnol. 2021, 9, 608826. [Google Scholar] [CrossRef] [PubMed]
- French, A.D.; Pérez, S.; Bulone, V.; Rosenau, T.; Gray, D. Cellulose. In Encyclopedia of Polymer Science and Technology, 4th ed.; John Wiley: Hoboken, NJ, USA, 2002; Volume 1838, pp. 1–69. [Google Scholar]
- Delmer, D.; Dixon, R.A.; Keegstra, K.; Mohnen, D. The Plant Cell Wall—Dynamic, Strong, and Adaptable—Is a Natural Shapeshifter. Plant Cell 2024, 36, 1257–1311. [Google Scholar] [CrossRef]
- Doblin, M.S.; Ma, Y.; Novaković, L.; Bacic, A.; Johnson, K.L. Plant Cell Walls–Past, Present, and Future. In Plant Cell Walls; CRC Press: Boca Raton, FL, USA, 2024; pp. 1–28. [Google Scholar]
- Beck, C.B. An Introduction to Plant Structure and Development: Plant Anatomy for the Twenty-First Century; Cambridge University Press: Cambridge, UK, 2010; ISBN 1139486365. [Google Scholar]
- Singh, G.; Gauba, P.; Mathur, G. Bacterial Cellulose-Based Composites: Recent Trends in Production Methods and Applications. Cellul. Chem. Technol. 2024, 58, 799–818. [Google Scholar] [CrossRef]
- Naomi, R.; Bt Hj Idrus, R.; Fauzi, M.B. Plant-vs. Bacterial-Derived Cellulose for Wound Healing: A Review. Int. J. Environ. Res. Public Health 2020, 17, 6803. [Google Scholar] [CrossRef]
- Etale, A.; Onyianta, A.J.; Turner, S.R.; Eichhorn, S.J. Cellulose: A Review of Water Interactions, Applications in Composites, and Water Treatment. Chem. Rev. 2023, 123, 2016–2048. [Google Scholar] [CrossRef]
- Nishiyama, Y. Molecular Interactions in Nanocellulose Assembly. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2018, 376, 20170047. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; Van den Bosch, S.; Koelewijn, S.-F.; Beckham, G.T.; Sels, B.F. Chemicals from Lignin: An Interplay of Lignocellulose Fractionation, Depolymerisation, and Upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef]
- Galletti, A.M.R.; Antonetti, C. Biomass Pretreatment: Separation of Cellulose, Hemicellulose, and Lignin-Existing Technologies and Perspectives. In Biorefinery: From Biomass to Chemicals and Fuels; De Gruyter: Berlin, Germany, 2012; pp. 101–121. [Google Scholar]
- Smit, A.T.; van Zomeren, A.; Dussan, K.; Riddell, L.A.; Huijgen, W.J.J.; Dijkstra, J.W.; Bruijnincx, P.C.A. Biomass Pre-Extraction as a Versatile Strategy to Improve Biorefinery Feedstock Flexibility, Sugar Yields, and Lignin Purity. ACS Sustain. Chem. Eng. 2022, 10, 6012–6022. [Google Scholar] [CrossRef]
- Haile, A.; Gelebo, G.G.; Tesfaye, T.; Mengie, W.; Mebrate, M.A.; Abuhay, A.; Limeneh, D.Y. Pulp and Paper Mill Wastes: Utilizations and Prospects for High Value-Added Biomaterials. Bioresour. Bioprocess 2021, 8, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.; Adhikari, S.; Cross, P.; Jahromi, H. Progress in the Solvent Depolymerization of Lignin. Renew. Sustain. Energy Rev. 2020, 133, 110359. [Google Scholar] [CrossRef]
- Sun, J.X.; Sun, X.F.; Sun, R.C.; Su, Y.Q. Fractional Extraction and Structural Characterization of Sugarcane Bagasse Hemicelluloses. Carbohydr. Polym. 2004, 56, 195–204. [Google Scholar] [CrossRef]
- Moohan, J.; Stewart, S.A.; Espinosa, E.; Rosal, A.; Rodríguez, A.; Larrañeta, E.; Donnelly, R.F.; Domínguez-Robles, J. Cellulose Nanofibers and Other Biopolymers for Biomedical Applications. A Review. Appl. Sci. 2019, 10, 65. [Google Scholar] [CrossRef]
- Thomas, B.; Raj, M.C.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a Versatile Green Platform: From Biosources to Materials and Their Applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [CrossRef]
- Kamarudin, S.H.; Mohd Basri, M.S.; Rayung, M.; Abu, F.; Ahmad, S.; Norizan, M.N.; Osman, S.; Sarifuddin, N.; Desa, M.S.Z.M.; Abdullah, U.H. A Review on Natural Fiber Reinforced Polymer Composites (NFRPC) for Sustainable Industrial Applications. Polymers 2022, 14, 3698. [Google Scholar] [CrossRef]
- Sundarraj, A.A.; Ranganathan, T.V. Comprehensive Review on Cellulose and Microcrystalline Cellulose from Agro-Industrial Wastes. Drug Invent. Today 2018, 10, 2783–2788. [Google Scholar]
- Gardner, K.H.; Blackwell, J. The Structure of Native Cellulose. Biopolym. Orig. Res. Biomol. 1974, 13, 1975–2001. [Google Scholar] [CrossRef]
- Hallac, B.B.; Ragauskas, A.J. Analyzing Cellulose Degree of Polymerization and Its Relevancy to Cellulosic Ethanol. Biofuels Bioprod. Biorefining 2011, 5, 215–225. [Google Scholar] [CrossRef]
- Doblin, M.S.; Kurek, I.; Jacob-Wilk, D.; Delmer, D.P. Cellulose Biosynthesis in Plants: From Genes to Rosettes. Plant Cell Physiol. 2002, 43, 1407–1420. [Google Scholar] [CrossRef]
- Brown, R.M.; Saxena, I.M.; Kudlicka, K. Cellulose Biosynthesis in Higher Plants. Trends Plant Sci. 1996, 1, 149–156. [Google Scholar]
- Zhou, J.; Wang, H.; Du, C.; Zhang, D.; Lin, H.; Chen, Y.; Xiong, J. Cellulose for Sustainable Triboelectric Nanogenerators. Adv. Energy Sustain. Res. 2022, 3, 2100161. [Google Scholar] [CrossRef]
- Bergenstråhle, M.; Wohlert, J.; Himmel, M.E.; Brady, J.W. Simulation Studies of the Insolubility of Cellulose. Carbohydr. Res. 2010, 345, 2060–2066. [Google Scholar] [CrossRef] [PubMed]
- Berglund, J.; Mikkelsen, D.; Flanagan, B.M.; Dhital, S.; Gaunitz, S.; Henriksson, G.; Lindström, M.E.; Yakubov, G.E.; Gidley, M.J.; Vilaplana, F. Wood Hemicelluloses Exert Distinct Biomechanical Contributions to Cellulose Fibrillar Networks. Nat. Commun. 2020, 11, 4692. [Google Scholar] [CrossRef]
- Sarkar, P.; Bosneaga, E.; Auer, M. Plant Cell Walls throughout Evolution: Towards a Molecular Understanding of Their Design Principles. J. Exp. Bot. 2009, 60, 3615–3635. [Google Scholar] [CrossRef]
- Chen, H.; Chen, H. Chemical Composition and Structure of Natural Lignocellulose. Theory and practice. In Biotechnology of Lignocellulos; Springer: Dordrecht, The Netherlands, 2014; pp. 25–71. [Google Scholar]
- Kim, H.; Dutta, S.D.; Randhawa, A.; Patil, T.V.; Ganguly, K.; Acharya, R.; Lee, J.; Park, H.; Lim, K.-T. Recent Advances and Biomedical Application of 3D Printed Nanocellulose-Based Adhesive Hydrogels: A Review. Int. J. Biol. Macromol. 2024, 264, 130732. [Google Scholar] [CrossRef]
- Quirós, J.; Boltes, K.; Rosal, R. Bioactive Applications for Electrospun Fibers. Polym. Rev. 2016, 56, 631–667. [Google Scholar] [CrossRef]
- Fraser, S.A.; van Zyl, W.E. In Situ Polymerization and Electrical Conductivity of Polypyrrole/Cellulose Nanocomposites Using Schweizer’s Reagent. RSC Adv. 2022, 12, 22031–22043. [Google Scholar] [CrossRef]
- Ee, L.Y.; Li, S.F.Y. Recent Advances in 3D Printing of Nanocellulose: Structure, Preparation, and Application Prospects. Nanoscale Adv. 2021, 3, 1167–1208. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose Processing Properties and Potential Applications. Curr. For. Rep. 2019, 5, 76–89. [Google Scholar] [CrossRef]
- Dhali, K.; Ghasemlou, M.; Daver, F.; Cass, P.; Adhikari, B. A Review of Nanocellulose as a New Material towards Environmental Sustainability. Sci. Total Environ. 2021, 775, 145871. [Google Scholar] [CrossRef]
- Rajinipriya, M.; Nagalakshmaiah, M.; Robert, M.; Elkoun, S. Importance of Agricultural and Industrial Waste in the Field of Nanocellulose and Recent Industrial Developments of Wood Based Nanocellulose: A Review. ACS Sustain. Chem. Eng. 2018, 6, 2807–2828. [Google Scholar] [CrossRef]
- Selianitis, D.; Efthymiou, M.-N.; Tsouko, E.; Papagiannopoulos, A.; Koutinas, A.; Pispas, S. Nanocellulose Production from Different Sources and Their Self-Assembly in Composite Materials. In Handbook of Nanocelluloses: Classification, Properties, Fabrication, and Emerging Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–32. [Google Scholar]
- Zanchetta, E.; Damergi, E.; Patel, B.; Borgmeyer, T.; Pick, H.; Pulgarin, A.; Ludwig, C. Algal Cellulose, Production and Potential Use in Plastics: Challenges and Opportunities. Algal Res. 2021, 56, 102288. [Google Scholar] [CrossRef]
- Ratna, A.S.; Ghosh, A.; Mukhopadhyay, S. Advances and Prospects of Corn Husk as a Sustainable Material in Composites and Other Technical Applications. J. Clean. Prod. 2022, 371, 133563. [Google Scholar] [CrossRef]
- Rajanna, M.; Shivashankar, L.M.; Shivamurthy, O.H.; Ramachandrappa, S.U.; Betageri, V.S.; Shivamallu, C.; Hallur Lakshmana Shetty, R.; Kumar, S.; Amachawadi, R.G.; Kollur, S.P. A Facile Synthesis of Cellulose Nanofibers from Corn Cob and Rice Straw by Acid Hydrolysis Method. Polymers 2022, 14, 4383. [Google Scholar] [CrossRef] [PubMed]
- Nargotra, P.; Sharma, V.; Tsai, M.-L.; Hsieh, S.-L.; Dong, C.-D.; Wang, H.-M.D.; Kuo, C.-H. Recent Advancements in the Valorization of Agro-Industrial Food Waste for the Production of Nanocellulose. Appl. Sci. 2023, 13, 6159. [Google Scholar] [CrossRef]
- Padzil, F.N.M.; Lee, S.H.; Ainun, Z.M.A.; Lee, C.H.; Abdullah, L.C. Potential of Oil Palm Empty Fruit Bunch Resources in Nanocellulose Hydrogel Production for Versatile Applications: A Review. Materials 2020, 13, 1245. [Google Scholar] [CrossRef]
- Trache, D.; Hussin, M.H.; Haafiz, M.K.M.; Thakur, V.K. Recent Progress in Cellulose Nanocrystals: Sources and Production. Nanoscale 2017, 9, 1763–1786. [Google Scholar] [CrossRef]
- Elanthikkal, S.; Francis, T.; Sangeetha, C.; Unnikrishnan, G. Cellulose Whisker-Based Green Polymer Composites. In Handbook of Composites from Renewable Materials; Thakur, V.K., Thakur, M.K., Kessler, M.R., Eds.; Wiley: Hoboken, NJ, USA, 2017; pp. 461–494. [Google Scholar]
- Mounika, M.; Ravindra, K. Characterization of Nanocomposites Reinforced with Cellulose Whiskers: A Review. Mater. Today Proc. 2015, 2, 3610–3618. [Google Scholar] [CrossRef]
- Foster, E.J.; Moon, R.J.; Agarwal, U.P.; Bortner, M.J.; Bras, J.; Camarero-Espinosa, S.; Chan, K.J.; Clift, M.J.D.; Cranston, E.D.; Eichhorn, S.J. Current Characterization Methods for Cellulose Nanomaterials. Chem. Soc. Rev. 2018, 47, 2609–2679. [Google Scholar] [CrossRef]
- Nagarajan, K.J.; Ramanujam, N.R.; Sanjay, M.R.; Siengchin, S.; Surya Rajan, B.; Sathick Basha, K.; Madhu, P.; Raghav, G.R. A Comprehensive Review on Cellulose Nanocrystals and Cellulose Nanofibers: Pretreatment, Preparation, and Characterization. Polym. Compos. 2021, 42, 1588–1630. [Google Scholar] [CrossRef]
- Zinge, C.; Kandasubramanian, B. Nanocellulose Based Biodegradable Polymers. Eur. Polym. J. 2020, 133, 109758. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Z.-L.; Song, F.; Wang, X.-L.; Wang, Y.-Z. Rapid Synthesis of Polymer-Grafted Cellulose Nanofiber Nanocomposite via Surface-Initiated Cu (0)-Mediated Reversible Deactivation Radical Polymerization. Macromolecules 2021, 54, 7409–7420. [Google Scholar] [CrossRef]
- Cruz, M.A.; Flor-Unda, O.; Avila, A.; Garcia, M.D.; Cerda-Mejía, L. Advances in Bacterial Cellulose Production: A Scoping Review. Coatings 2024, 14, 1401. [Google Scholar] [CrossRef]
- Ye, Y.; Yu, L.; Lizundia, E.; Zhu, Y.; Chen, C.; Jiang, F. Cellulose-Based Ionic Conductor: An Emerging Material toward Sustainable Devices. Chem. Rev. 2023, 123, 9204–9264. [Google Scholar] [CrossRef]
- Wang, D.-C.; Lei, S.-N.; Zhong, S.; Xiao, X.; Guo, Q.-H. Cellulose-Based Conductive Materials for Energy and Sensing Applications. Polymers 2023, 15, 4159. [Google Scholar] [CrossRef]
- Zhao, D.; Zhu, Y.; Cheng, W.; Chen, W.; Wu, Y.; Yu, H. Cellulose-based Flexible Functional Materials for Emerging Intelligent Electronics. Adv. Mater. 2021, 33, 2000619. [Google Scholar] [CrossRef]
- Poonguzhali, R.; Basha, S.K.; Kumari, V.S. Synthesis and characterization of chitosan-PVP-nanocellulose composites for in-vitro wound dressing application. Int. J. Biol. Macromol. 2017, 105, 111–120. [Google Scholar] [CrossRef]
- Fu, Q.; Cui, C.; Meng, L.; Hao, S.; Dai, R.; Yang, J. Emerging Cellulose-Derived Materials: A Promising Platform for the Design of Flexible Wearable Sensors toward Health and Environment Monitoring. Mater. Chem. Front. 2021, 5, 2051–2091. [Google Scholar] [CrossRef]
- Du, X.; Zhang, K. Recent Progress in Fibrous High-Entropy Energy Harvesting Devices for Wearable Applications. Nano Energy 2022, 101, 107600. [Google Scholar] [CrossRef]
- Vasile, C.; Baican, M. Lignins as Promising Renewable Biopolymers and Bioactive Compounds for High-Performance Materials. Polymers 2023, 15, 3177. [Google Scholar] [CrossRef]
- An, M.; Wang, X.; Chang, D.; Wang, S.; Hong, D.; Fan, H.; Wang, K. Application of Compound Material Alleviates Saline and Alkaline Stress in Cotton Leaves through Regulation of the Transcriptome. BMC Plant Biol. 2020, 20, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.; Sipponen, M.H. Lignin-Based Smart Materials: A Roadmap to Processing and Synthesis for Current and Future Applications. Mater. Horiz. 2020, 7, 2237–2257. [Google Scholar] [CrossRef]
- Mariana, M.; Alfatah, T.; H.P.S., A.K.; Yahya, E.B.; Olaiya, N.G.; Nuryawan, A.; Mistar, E.M.; Abdullah, C.K.; Abdulmadjid, S.N.; Ismail, H. A Current Advancement on the Role of Lignin as Sustainable Reinforcement Material in Biopolymeric Blends. J. Mater. Res. Technol. 2021, 15, 2287–2316. [Google Scholar] [CrossRef]
- Yu, X.; Yang, B.; Zhu, W.; Deng, T.; Pu, Y.; Ragauskas, A.; Wang, H. Towards Functionalized Lignin and Its Derivatives for High-Value Material Applications. Ind. Crops Prod. 2023, 200, 116824. [Google Scholar] [CrossRef]
- Bertella, S.; Luterbacher, J.S. Lignin Functionalization for the Production of Novel Materials. Trends Chem. 2020, 2, 440–453. [Google Scholar] [CrossRef]
- Chauhan, P.S. Lignin Nanoparticles: Eco-Friendly and Versatile Tool for New Era. Bioresour. Technol. Rep. 2020, 9, 100374. [Google Scholar] [CrossRef]
- Li, W.; Sun, N.; Stoner, B.; Jiang, X.; Lu, X.; Rogers, R.D. Rapid dissolution of lignocellulosic biomass in ionic liquids using temperatures above the glass transition of lignin. Green Chem. 2011, 13, 2038–2047. [Google Scholar] [CrossRef]
- Nimz, H. Beech lignin proposal of a constitutional scheme. Angew. Chem. Int. Ed. Engl. 1974, 13, 313–321. [Google Scholar] [CrossRef]
- Wyman, C.E. Potential synergies and challenges in refining cellulosic biomass to fuels, chemicals, and power. Biotechnol. Prog. 2003, 19, 254–262. [Google Scholar] [CrossRef]
- Wang, T.; Li, H.; Diao, X.; Lu, X.; Ma, D.; Ji, N. Lignin to Dispersants, Adsorbents, Flocculants and Adhesives: A Critical Review on Industrial Applications of Lignin. Ind. Crops Prod. 2023, 199, 116715. [Google Scholar] [CrossRef]
- Briassoulis, D.; Tserotas, P.; Athanasoulia, I.-G. Alternative Optimization Routes for Improving the Performance of Poly (3-Hydroxybutyrate)(PHB) Based Plastics. J. Clean. Prod. 2021, 318, 128555. [Google Scholar] [CrossRef]
- Etxabide, A.; Kilmartin, P.A.; Guerrero, P.; de la Caba, K.; Hooks, D.O.; West, M.; Singh, T. Polyhydroxybutyrate (PHB) Produced from Red Grape Pomace: Effect of Purification Processes on Structural, Thermal and Antioxidant Properties. Int. J. Biol. Macromol. 2022, 217, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Keskin, G.; Kızıl, G.; Bechelany, M.; Pochat-Bohatier, C.; Öner, M. Potential of Polyhydroxyalkanoate (PHA) Polymers Family as Substitutes of Petroleum Based Polymers for Packaging Applications and Solutions Brought by Their Composites to Form Barrier Materials. Pure Appl. Chem. 2017, 89, 1841–1848. [Google Scholar] [CrossRef]
- Panda, P.K.; Dash, P. Preparation, Characterization, and Evaluation of Antibacterial Properties of Poly(3-Hydroxybutarate-Co-3-Hydroxyvalerate) (PHBV)-Based Films and Coatings. In Biopolymer-Based Films and Coatings; CRC Press: Boca Raton, FL, USA, 2023; pp. 291–308. [Google Scholar]
- Yeo, J.C.C.; Muiruri, J.K.; Thitsartarn, W.; Li, Z.; He, C. Recent Advances in the Development of Biodegradable PHB-Based Toughening Materials: Approaches, Advantages and Applications. Mater. Sci. Eng. C 2018, 92, 1092–1116. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, M.A.; Flynn, A.; Chiou, B.-S.; Imam, S.; Orts, W.; Chiellini, E. Thermal, Mechanical and Morphological Characterization of Plasticized PLA–PHB Blends. Polym. Degrad. Stab. 2012, 97, 1822–1828. [Google Scholar] [CrossRef]
- Alves, M.I.; Macagnan, K.L.; Rodrigues, A.A.; de Assis, D.A.; Torres, M.M.; de Oliveira, P.D.; Furlan, L.; Vendruscolo, C.T.; Moreira, A.d.S. Poly (3-Hydroxybutyrate)-P (3HB): Review of Production Process Technology. Ind. Biotechnol. 2017, 13, 192–208. [Google Scholar] [CrossRef]
- Djouonkep, L.D.W.; Tchameni, A.P.; Selabi, N.B.S.; Tamo, A.K.; Doench, I.; Cheng, Z.; Gauthier, M.; Xie, B.; Osorio-Madrazo, A. Bio-Based Degradable Poly (Ether-Ester) s from Melt-Polymerization of Aromatic Ester and Ether Diols. Int. J. Mol. Sci. 2022, 23, 8967. [Google Scholar] [CrossRef]
- Cavalcante, M.P.; Toledo, A.L.M.M.; Rodrigues, E.J.R.; Neto, R.P.C.; Tavares, M.I.B. Correlation between Traditional Techniques and TD-NMR to Determine the Morphology of PHB/PCL Blends. Polym Test 2017, 58, 159–165. [Google Scholar] [CrossRef]
- Fernández-Ronco, M.P.; Gradzik, B.; Gooneie, A.; Hufenus, R.; El Fray, M. Tuning Poly (3-Hydroxybutyrate)(P3HB) Properties by Tailored Segmented Biocopolymers. ACS Sustain. Chem. Eng. 2017, 5, 11060–11068. [Google Scholar] [CrossRef]
- McAdam, B.; Brennan Fournet, M.; McDonald, P.; Mojicevic, M. Production of Polyhydroxybutyrate (PHB) and Factors Impacting Its Chemical and Mechanical Characteristics. Polymers 2020, 12, 2908. [Google Scholar] [CrossRef]
- Andrianova, A.A.; Yeudakimenka, N.A.; Lilak, S.L.; Kozliak, E.I.; Ugrinov, A.; Sibi, M.P.; Kubátová, A. Size Exclusion Chromatography of Lignin: The Mechanistic Aspects and Elimination of Undesired Secondary Interactions. J. Chromatogr. A 2018, 1534, 101–110. [Google Scholar] [CrossRef]
- Lizundia, E.; Sipponen, M.H.; Greca, L.G.; Balakshin, M.; Tardy, B.L.; Rojas, O.J.; Puglia, D. Multifunctional Lignin-Based Nanocomposites and Nanohybrids. Green Chem. 2021, 23, 6698–6760. [Google Scholar] [CrossRef] [PubMed]
- Hallac, B.B. Fundamental Understanding of the Biochemical Conversion of Buddleja Davidii to Fermentable Sugars; Georgia Institute of Technology: Atlanta, GA, USA, 2011; ISBN 1124759301. [Google Scholar]
- Vasile, C.; Cazacu, G. Biocomposites and Nanocomposites Containing Lignin. In Biopolymer Nanocomposites: Processing, Properties, and Applications; Wiley: Hoboken, NJ, USA, 2013; pp. 565–598. [Google Scholar]
- Raza, Z.A.; Abid, S.; Banat, I.M. Polyhydroxyalkanoates: Characteristics, Production, Recent Developments and Applications. Int. Biodeterior. Biodegrad. 2018, 126, 45–56. [Google Scholar] [CrossRef]
- Martínez, V.; García, P.; García, J.L.; Prieto, M.A. Controlled Autolysis Facilitates the Polyhydroxyalkanoate Recovery in Pseudomonas Putida KT2440. Microb. Biotechnol. 2011, 4, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Hand, S.; Gill, J.; Chu, K.-H. Phage-Based Extraction of Polyhydroxybutyrate (PHB) Produced from Synthetic Crude Glycerol. Sci. Total Environ. 2016, 557, 317–321. [Google Scholar] [CrossRef]
- Martínez, V.; Jurkevitch, E.; García, J.L.; Prieto, M.A. Reward for BDellovibrio Bacteriovorus for Preying on a Polyhydroxyalkanoate Producer. Environ. Microbiol. 2013, 15, 1204–1215. [Google Scholar] [CrossRef]
- Kaplan, M.; Chang, Y.-W.; Oikonomou, C.M.; Nicolas, W.J.; Jewett, A.I.; Kreida, S.; Dutka, P.; Rettberg, L.A.; Maggi, S.; Jensen, G.J. Dynamic Structural Adaptations Enable the Endobiotic Predation of Bdellovibrio Bacteriovorus. bioRxiv 2022, 2022–2026. [Google Scholar]
- Cai, L.; Yuan, M.-Q.; Liu, F.; Jian, J.; Chen, G.-Q. Enhanced Production of Medium-Chain-Length Polyhydroxyalkanoates (PHA) by PHA Depolymerase Knockout Mutant of Pseudomonas Putida KT2442. Bioresour. Technol. 2009, 100, 2265–2270. [Google Scholar] [CrossRef]
- Kunasundari, B.; Murugaiyah, V.; Kaur, G.; Maurer, F.H.J.; Sudesh, K. Revisiting the Single Cell Protein Application of Cupriavidus Necator H16 and Recovering Bioplastic Granules Simultaneously. PLoS ONE 2013, 8, e78528. [Google Scholar] [CrossRef]
- Zhang, G.; Zheng, W.; Bai, X.; Xu, L.; Li, K.; Zhang, M.; Huang, Y. Polyhydroxyalkanoates (PHAs) Biological Recovery Approaches and Protein-Mediated Secretion Model Hypothesis. J. Clean. Prod. 2024, 440, 140851. [Google Scholar] [CrossRef]
- Bhakyaraj, R.; Arunkumar, D.; Anitha, A. Synthetic Microbial Research-Challenges and Prospects; Darshan Publishers: Tamil Nadu, India, 2022. [Google Scholar]
- Radotić, K.; Mićić, M. Methods for Extraction and Purification of Lignin and Cellulose from Plant Tissues. In Sample Preparation Techniques for Soil, Plant, and Animal Samples; Springer: Berlin/Heidelberg, Germany, 2016; pp. 365–376. [Google Scholar]
- Sun, Q.; Foston, M.; Meng, X.; Sawada, D.; Pingali, S.V.; O’Neill, H.M.; Kumar, R. Effect of Lignin Content on Changes Occurring in Poplar Cellulose Ultrastructure during Dilute Acid Pretreatment. Biotechnol. Biofuels 2014, 7, 150. [Google Scholar] [CrossRef]
- Bhutto, A.W.; Qureshi, K.; Harijan, K.; Abro, R.; Abbas, T.; Bazmi, A.A.; Karim, S.; Yu, G. Insight into Progress in Pre-Treatment of Lignocellulosic Biomass. Energy 2017, 122, 724–745. [Google Scholar] [CrossRef]
- Ma, C.; Kim, T.-H.; Liu, K.; Ma, M.-G.; Choi, S.-E.; Si, C. Multifunctional Lignin-Based Composite Materials for Emerging Applications. Front. Bioeng. Biotechnol. 2021, 9, 708976. [Google Scholar] [CrossRef] [PubMed]
- Balakshin, M.; Capanema, E.; Berlin, A. Isolation and Analysis of Lignin–Carbohydrate Complexes Preparations with Traditional and Advanced Methods: A Review. Stud. Nat. Prod. Chem. 2014, 42, 83–115. [Google Scholar]
- Aziz, T.; Farid, A.; Haq, F.; Kiran, M.; Ullah, A.; Zhang, K.; Li, C.; Ghazanfar, S.; Sun, H.; Ullah, R. A Review on the Modification of Cellulose and Its Applications. Polymers 2022, 14, 3206. [Google Scholar] [CrossRef]
- Wang, H.; Qiu, X.; Liu, W.; Fu, F.; Yang, D. A Novel Lignin/ZnO Hybrid Nanocomposite with Excellent UV-Absorption Ability and Its Application in Transparent Polyurethane Coating. Ind. Eng. Chem. Res. 2017, 56, 11133–11141. [Google Scholar] [CrossRef]
- Kaur, P.; Sharma, N.; Munagala, M.; Rajkhowa, R.; Aallardyce, B.; Shastri, Y.; Agrawal, R. Nanocellulose: Resources, Physio-Chemical Properties, Current Uses and Future Applications. Front. Nanotechnol. 2021, 3, 747329. [Google Scholar] [CrossRef]
- Long, H.; Hu, L.; Yang, F.; Cai, Q.; Zhong, Z.; Zhang, S.; Guan, L.; Xiao, D.; Zheng, W.; Zhou, W. Enhancing the Performance of Polylactic Acid Composites through Self-Assembly Lignin Nanospheres for Fused Deposition Modeling. Compos. B Eng. 2022, 239, 109968. [Google Scholar] [CrossRef]
- Kim, G.-H.; Um, B.-H. Fractionation and Characterization of Lignins from Miscanthus via Organosolv and Soda Pulping for Biorefinery Applications. Int. J. Biol. Macromol. 2020, 158, 443–451. [Google Scholar] [CrossRef]
- Martín-Sampedro, R.; Santos, J.I.; Fillat, Ú.; Wicklein, B.; Eugenio, M.E.; Ibarra, D. Characterization of Lignins from Populus alba L. Generated as by-Products in Different Transformation Processes: Kraft Pulping, Organosolv and Acid Hydrolysis. Int. J. Biol. Macromol. 2019, 126, 18–29. [Google Scholar] [CrossRef]
- Hachimi Alaoui, C.; Réthoré, G.; Weiss, P.; Fatimi, A. Sustainable Biomass Lignin-Based Hydrogels: A Review on Properties, Formulation, and Biomedical Applications. Int. J. Mol. Sci. 2023, 24, 13493. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Zhuang, J.; Xiang, Z.; Jiang, W.; He, S.; Xiao, H. Conductive Hydrogels Based on Industrial Lignin: Opportunities and Challenges. Polymers 2022, 14, 3739. [Google Scholar] [CrossRef] [PubMed]
- Lamm, M.E.; Li, K.; Ker, D.; Zhao, X.; Hinton, H.E.; Copenhaver, K.; Tekinalp, H.; Ozcan, S. Exploiting Chitosan to Improve the Interface of Nanocellulose Reinforced Polymer Composites. Cellulose 2022, 29, 3859–3870. [Google Scholar] [CrossRef]
- Khalil, H.P.S.A.; Jummaat, F.; Yahya, E.B.; Olaiya, N.G.; Adnan, A.S.; Abdat, M.; NAM, N.; Halim, A.S.; Kumar, U.S.U.; Bairwan, R. A Review on Micro-to Nanocellulose Biopolymer Scaffold Forming for Tissue Engineering Applications. Polymers 2020, 12, 2043. [Google Scholar] [CrossRef] [PubMed]
- Bacakova, L.; Pajorova, J.; Bacakova, M.; Skogberg, A.; Kallio, P.; Kolarova, K.; Svorcik, V. Versatile Application of Nanocellulose: From Industry to Skin Tissue Engineering and Wound Healing. Nanomaterials 2019, 9, 164. [Google Scholar] [CrossRef]
- Tamo, A.K. Nanocellulose-Based Hydrogels as Versatile Materials with Interesting Functional Properties for Tissue Engineering Applications. J. Mater. Chem. B 2024, 12, 7692–7759. [Google Scholar] [CrossRef]
- Kandhola, G.; Park, S.; Lim, J.-W.; Chivers, C.; Song, Y.H.; Chung, J.H.; Kim, J.; Kim, J.-W. Nanomaterial-Based Scaffolds for Tissue Engineering Applications: A Review on Graphene, Carbon Nanotubes and Nanocellulose. Tissue Eng. Regen. Med. 2023, 20, 411–433. [Google Scholar] [CrossRef]
- Kuzmenko, V.; Kalogeropoulos, T.; Thunberg, J.; Johannesson, S.; Hägg, D.; Enoksson, P.; Gatenholm, P. Enhanced Growth of Neural Networks on Conductive Cellulose-Derived Nanofibrous Scaffolds. Mater. Sci. Eng. C 2016, 58, 14–23. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, S.; Yang, J.; Schneider, K.H.; Xie, M.; Chen, Y.; Zheng, Z.; Wang, X.; Zhao, Z.; Yu, J. Recent Advances in Harnessing Biological Macromolecules for Wound Management: A Review. Int. J. Biol. Macromol. 2024, 266, 130989. [Google Scholar] [CrossRef]
- Oprică, G.M.; Panaitescu, D.M.; Lixandru, B.E.; Uşurelu, C.D.; Gabor, A.R.; Nicolae, C.-A.; Fierascu, R.C.; Frone, A.N. Plant-Derived Nanocellulose with Antibacterial Activity for Wound Healing Dressing. Pharmaceutics 2023, 15, 2672. [Google Scholar] [CrossRef]
- Ong, X.-R.; Chen, A.X.; Li, N.; Yang, Y.Y.; Luo, H.-K. Nanocellulose: Recent Advances toward Biomedical Applications. Small Sci. 2023, 3, 2200076. [Google Scholar] [CrossRef]
- Turyanska, L.; Makarovsky, O.; Patane, A.; Kozlova, N.V.; Liu, Z.; Li, M.; Mann, S. High Magnetic Field Quantum Transport in Au Nanoparticle–Cellulose Films. Nanotechnology 2012, 23, 045702. [Google Scholar] [CrossRef] [PubMed]
- Min, S.H.; Kim, C.K.; Moon, D.-G. Flexible Top Emission Organic Light Emitting Diodes with Ni and Au Anodes Deposited on a Cellulose Paper Substrate. Mol. Cryst. Liq. Cryst. 2013, 584, 27–36. [Google Scholar] [CrossRef]
- Lin, D.; Xiao, L.; Qin, W.; Loy, D.A.; Wu, Z.; Chen, H.; Zhang, Q. Preparation, Characterization and Antioxidant Properties of Curcumin Encapsulated Chitosan/Lignosulfonate Micelles. Carbohydr. Polym. 2022, 281, 119080. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Stewart, S.A.; Rendl, A.; González, Z.; Donnelly, R.F.; Larrañeta, E. Lignin and Cellulose Blends as Pharmaceutical Excipient for Tablet Manufacturing via Direct Compression. Biomolecules 2019, 9, 423. [Google Scholar] [CrossRef] [PubMed]
- Seyed Shahabadi, S.I.; Kong, J.; Lu, X. Aqueous-Only, Green Route to Self-Healable, UV-Resistant, and Electrically Conductive Polyurethane/Graphene/Lignin Nanocomposite Coatings. ACS Sustain. Chem. Eng. 2017, 5, 3148–3157. [Google Scholar] [CrossRef]
- Rahim, T.N.A.T.; Abdullah, A.M.; Md Akil, H. Recent Developments in Fused Deposition Modeling-Based 3D Printing of Polymers and Their Composites. Polym. Rev. 2019, 59, 589–624. [Google Scholar] [CrossRef]
- Rajan, K.; Samykano, M.; Kadirgama, K.; Harun, W.S.W.; Rahman, M.M. Fused Deposition Modeling: Process, Materials, Parameters, Properties, and Applications. Int. J. Adv. Manuf. Technol. 2022, 120, 1531–1570. [Google Scholar] [CrossRef]
- Plastic Market Size, Share & Trends Analysis Report. Grandview Research. 2024 Plastic Prices Database. Online Resource. Available online: https://www.grandviewresearch.com/industry-analysis/global-plastics-market (accessed on 12 March 2025).
- Koller, M.; Mukherjee, A. A New Wave of Industrialization of PHA Biopolyesters. Bioengineering 2022, 9, 205. [Google Scholar] [CrossRef]
- Winnacker, M. Selected Recent Advances in the Utilization of Biopolymers for Nano- and Microfiber Materials and Nonwovens Production. Macromol. Mater. Eng. 2022, 307, 2200270. [Google Scholar] [CrossRef]
- Hamedi, M.M.; Sandberg, M.; Olsson, R.T.; Pedersen, J.; Benselfelt, T.; Wohlert, J. Wood and Cellulose: The Most Sustainable Advanced Materials for Past, Present, and Future Civilizations. Adv. Mater. 2025, 35, 2207925. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).