1. Introduction
With recent improvements in processing, equipment, and understanding of resulting properties, the commercialisation of wood-modification processes has increased significantly, particularly in Europe [
1]. This has been driven due a range of factors, including:
A change in wood properties as a result of changes in silvicultural practices and the way of using wood, e.g., in construction;
Awareness of the use of rare species with outstanding properties such as durability and appearance;
Awareness and restrictions by law of using environmental non-friendly chemicals for increased durability and reduced maintenance of wood products;
Increased interest from the industry to add value to sawn timber and by-products from the sawmill and refining processes;
(Specifically within Europe) Polices supporting the development of a sustainable society; and
The international dimension on climate change and related activities mainly organised within the frame of the United Nations (UN), such as the Paris Agreement under the UN Framework Convention on Climate Change [
2].
The most common form of wood modification is thermal modification. Controlling the conditions during treatment is of critical importance, given the fact that excess oxygen can lead to more conventional pyrolysis processes. Hill [
3] referred to thermal modification as “the application of heat to wood in order to bring about a desired improvement in the performance of the material”. Industrial thermal modification processes focus on improving the properties of less durable species, with improvements in biological durability and/or dimensional stability depending on the conditions employed. Among the pioneering work in this subject, Stamm et al. [
4] heated wood beneath molten metal, such that Sitka spruce (
Picea abies) was heated to between 140 and 320 °C, resulting in reduced swelling, improvements in the dimensional stability, and increased resistance to microbial attack. The success of the method was, however, limited, and the thermal modification was advanced through the use of gaseous atmospheres in works by Thunell and Elken [
5] and Buro [
6,
7]. However, the current commercialized methods tend to be derived from the pioneering work of Burmester [
8,
9,
10,
11]. Since these early studies, various timber species have been treated, and multiple commercialization processes have been marketed, with the resulting increase in durability as a result of thermal modification against fungi usually occurring after a process, resulting in a weight loss of at least 10% [
1].
A recent review [
12] considered the difference between wet and dry thermal modification processes, and more specifically, how moisture content during the modification process affects the degradation and repolymerization chemistry, and ultimately, the mass loss, hygroscopic behavior, and dimensional stability. As would be expected, dry systems are those where the wood has been dried to close to zero moisture content prior to undergoing thermal modification. Alternatively, wet systems can refer to two similar systems depending on the nature of the moisture: steam conditions are typically referred to as hygrothermal systems, whilst those involving immersion in liquid water are referred to as hydrothermal systems, with higher mass losses noted for the hydrothermal systems. This difference between wet and dry thermal treatment systems has been identified in terms of the water activity (a
w) against the treatment temperature [
13], whereby a
w refers to the ratio of the vapor pressure of water compared to the pressure of pure water at the same temperature. They identified that dry conditions lead to dry thermolysis. Wet systems were typically at lower temperatures, with pressure having significant impact on resulting mass loss.
Within wood, it is recognized that hemicelluloses are the first components to undergo chemical changes such as degradation. In hygrothermal and hydrothermal processes, the degradation of hemicelluloses is often referred to as autohydrolysis, yielding a range of oligomeric sugars, usually as a result of water reacting with the glycosidic bonds, whilst further degradation at higher temperatures can yield furfurals [
14]. The furfural has been shown to participate in cross-linking reaction between lignin moieties [
15].
In addition to thermal modification, there have been significant advances in chemical modification. Among the chemical-modification processes that have achieved commercialization is furfurylation. Furfurylation is based on the impregnation/polymerization of furfuryl alcohol (FFA, or its derivative/prepolymer), which is commonly obtained from agricultural wastes, such as sugar cane and corn cobs, in the presence of a mild acid catalyst. After impregnating, the polymerization stage occurs with a heat-curing step and drying, so as to recover and re-use as much remaining FFA as possible. The overall process results in a hard and resistant product. The resin contributes to the dark (brownish) color of the product, though it weathers to a grey color with direct solar radiation. Commercial furfurylation of wood is now undertaken in Norway and Belgium by Kebony, and in The Netherlands by Foreco Dalfsen, though this process uses prepolymerized FFA resin [
16]. The typical polymerization processes on a chemical level are summarized in
Figure 1, though long curing times (between 12–16 h) have been indicated [
17,
18], with typical reaction conditions reported shown in
Table 1 [
1], which often require the use of catalysts.
Typically, furfurylated wood has an increased hardness, elasticity, and rupture modulus than untreated wood, though it is more brittle [
19]. Increasing weight gain leads to higher hardness, but lower impact strength [
17]. Moderate to high loading levels (weight gains above 30%) provide significant resistance against fungal decay [
20]. Aesthetically, commercially available furfurylated wood has an appealing color, even with weathering over whole sections [
1].
Whilst options exist for both thermal modification and the furfurylation of wood, a logical progression would be to combine the two processes, thereby generating the benefits exhibited by each system. Klaas [
21] outlined a method for modifying wood performed in one, simultaneous hybrid process, and does not involve any additional step with physical and/or chemical treatment, such as a drying step, between the impregnation step and the polymerization step, with elevated pressure and/or temperature.
Table 1.
Furfurylation process of wood species (adapted from [
1]).
Table 1.
Furfurylation process of wood species (adapted from [
1]).
| Species | Process Conditions | Ref. |
|---|
| European beech, European birch, Scots pine | FFA resin (BioRez)/vacuum for 45 min, pressure at 12 bar for 2 h. warming at 20–40 °C for 4 h, heating at 103 °C for 16 h. | [17] |
| Scots pine (only sapwood) | Vacuum, 7–12 bar, and drying at 130 °C for 16 h. | [18] |
| Rubberwood, kelempayan, sena (Pterocarpus indicus Willd.), European beech, Scots pine sapwood | FFA/maleic anhydride or FFA/citric acid. Vacuum, 12 bar, and drying at 130 °C for 16 h. | [22] |
| European beech, European ash, Radiata pine, Southern yellow pine, Scots pine | FFA/citric acid, cyclic anhydride, KebonyTM process for outdoor level. | [23] |
| Maritime pine boards | FFA/additives, vacuum/pressure stage, curing, vacuum drying. | [24] |
| European beech, European ash, Radiata pine, Southern yellow pine | FFA (30%), full-cell impregnation, vacuum drying, steam cure, drying. | [25] |
| Scots pine (sapwood) | FFA/citric acid, vacuum, pressure at 13 bar for 2 h, heating at 130 °C for 0.5–24 h. | [26] |
| Chinese white poplar, Cunninghamia, Swamp mahogany, Masson’s pine | FFA/additives vacuum 30 min, 12 h soaking, and 100 °C for 12 h. | [27] |
| Masson’s pine | FFA/citric acid/oxalic acid/sodium borate, vacuum, curing at <115 °C for up to 8 h, drying 60–103 °C. | [28] |
| Scots pine (sapwood) | 40% FFA (full-cell impregnation). | [29] |
| European beech | FFA/various catalysts, vacuum for 5 min, 12 bar for 5 min, drying for 10 h at 20 °C, heating < 120 °C for up to 24 h. | [30] |
| Poplar | 180 °C water, FFA/maleic anhydride, borate, vacuum for 1 h, heating at <103 °C for 3 h, then at 60–80 °C for 4 h, drying at 103 °C. | [31] |
| Radiata pine | FFA/additives, soaking for 15 days, heating at 120 °C for 16.5 h. | [32] |
| Chinese fir, Poplar | FFA, sodium borate (buffer), catalyst. Vacuum 30 min, atmospheric immersion 3–36 h, curing at 95–125 °C for 1.5–8 h, dry 2 h 60 °C, 2 h 80 °C, 103 °C until oven-dry. | [33] |
| Jabon, Sengon, Mangium, Merkus pine | FFA/catalyst. Vacuum 30 min, pressure 10 bar 30 min. Heat 100 °C 24 h, ambient conditions for 4 weeks. | [34] |
| Poplar, Bamboo | FFA, vacuum 30 min, atmospheric immersion 12 h. Heat 100 °C 10 h, dry at 100 °C. | [35] |
The fundamental concept of the process is to perform a chemical reaction/polymerization modification process step at elevated pressure, as opposed to atmospheric pressure in the majority of existing processes. This facilitates a second, simultaneous process of thermal hydrolysis modification, which improves desired wood properties beyond those only obtained by chemical reaction/polymerization modes of modification, in a novel simultaneous combined modification process, further creating a synergetic effect.
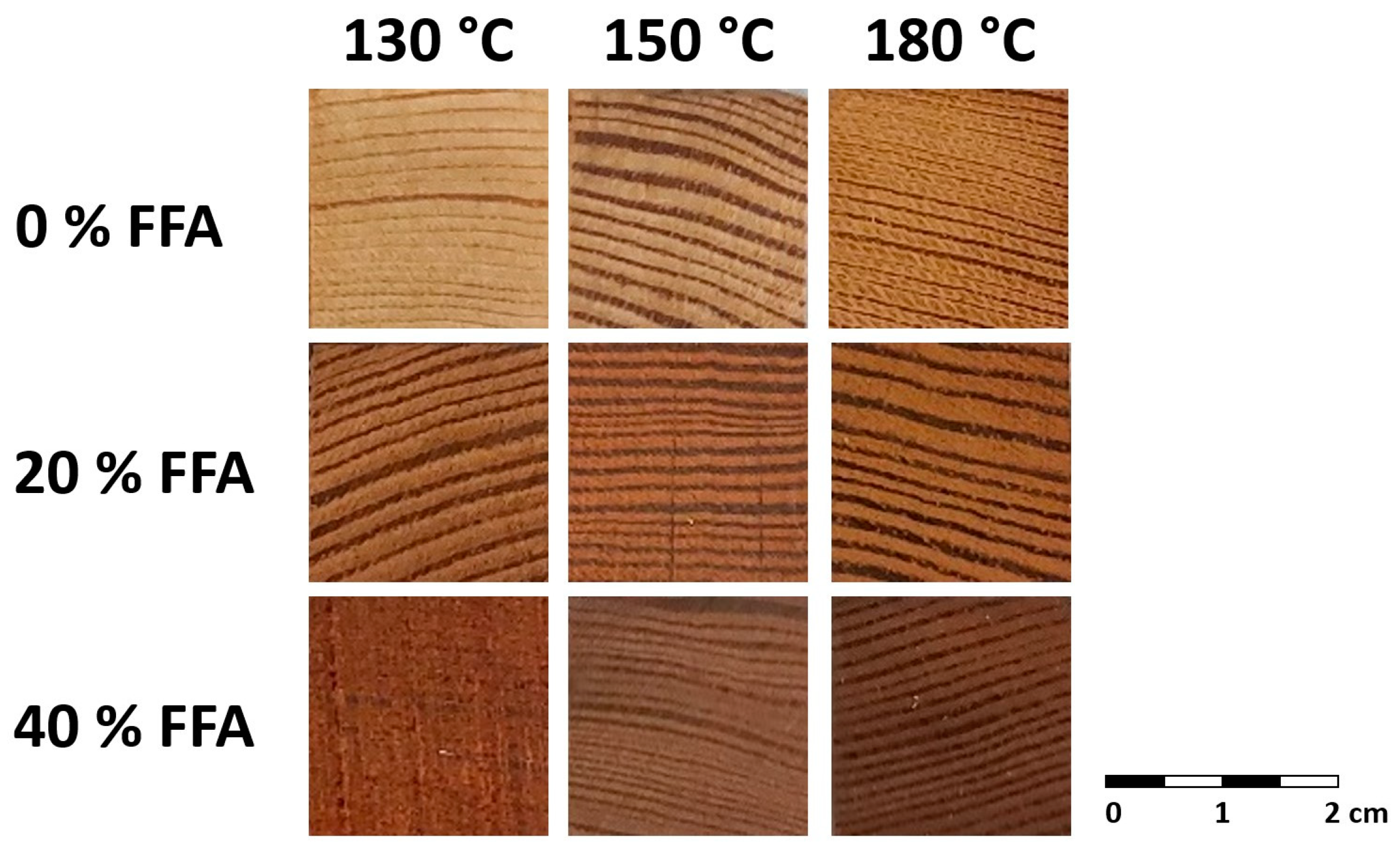
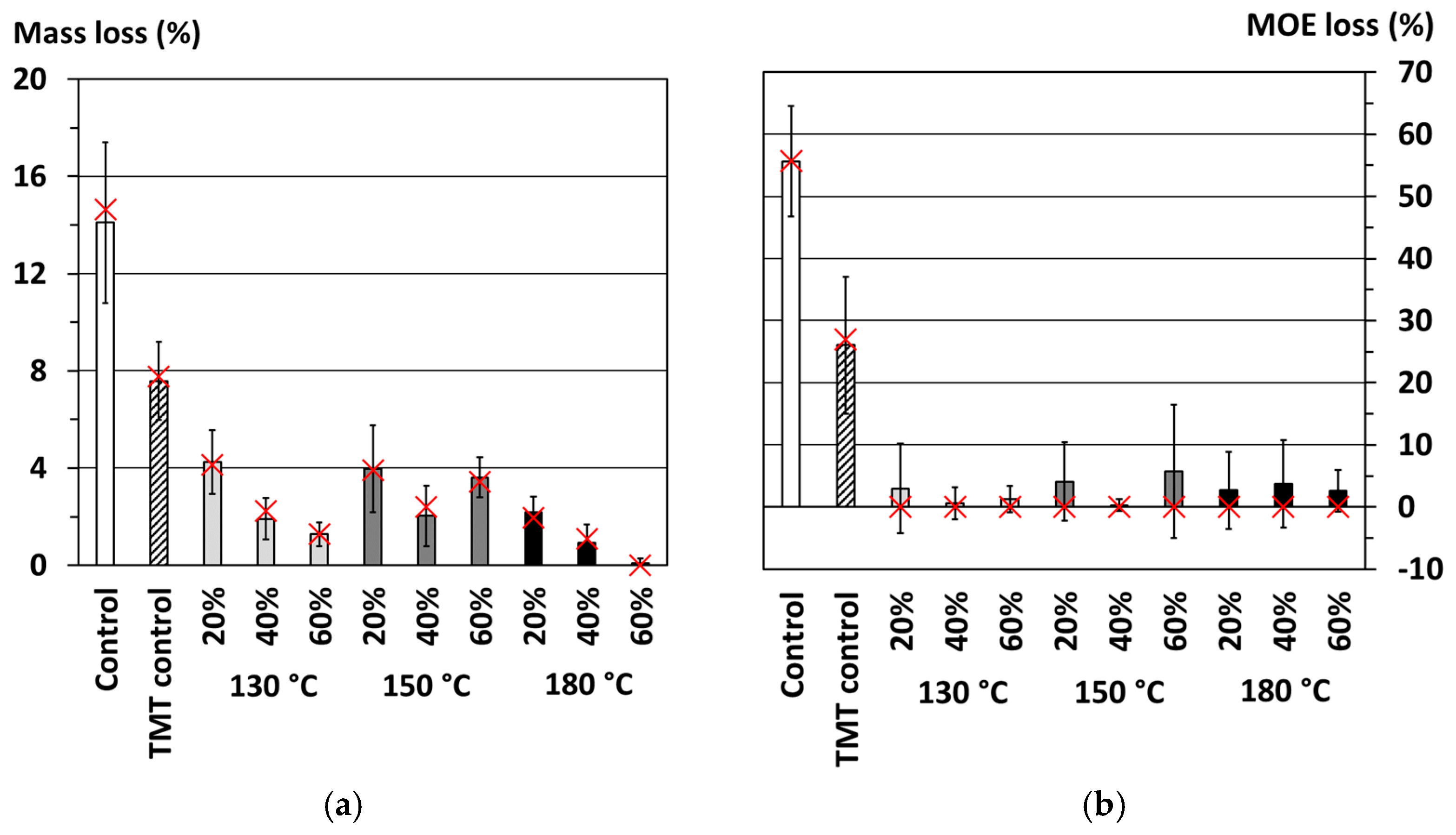
This paper considers the potential of a novel wood-modification process, combining furfurylation and thermal modification of wood under pressure, in a synergistic way in the absence of catalysts, and thereby outlining the effectiveness of the treatment (in terms of weight gain). The effectiveness of the combined treatment, where mass losses as a result of the process has been minimized, is hereby evaluated, particularly in terms of the resulting durability classes according to losses in the modulus of elasticity (MOE) and potential resistance against termites.
4. Conclusions
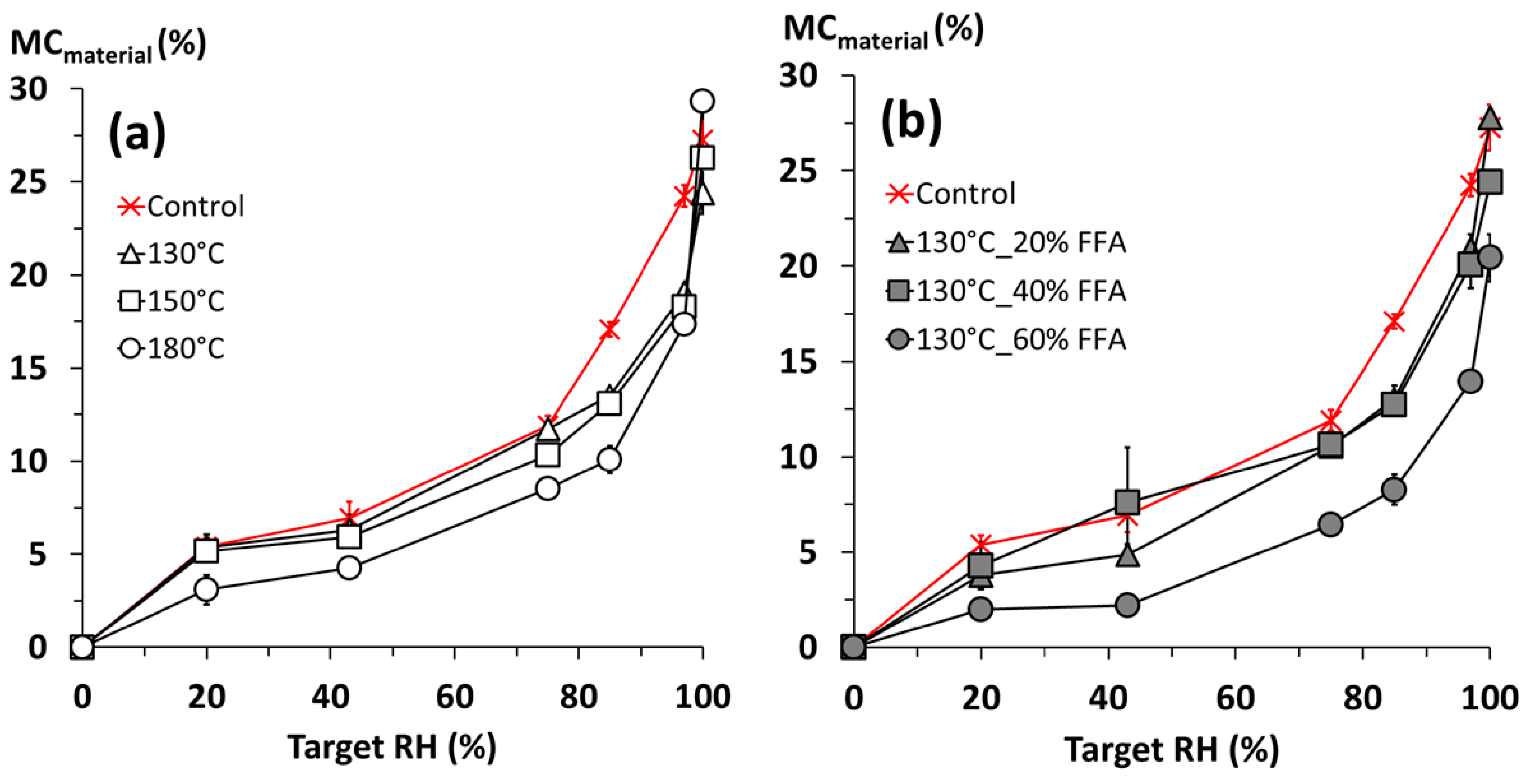
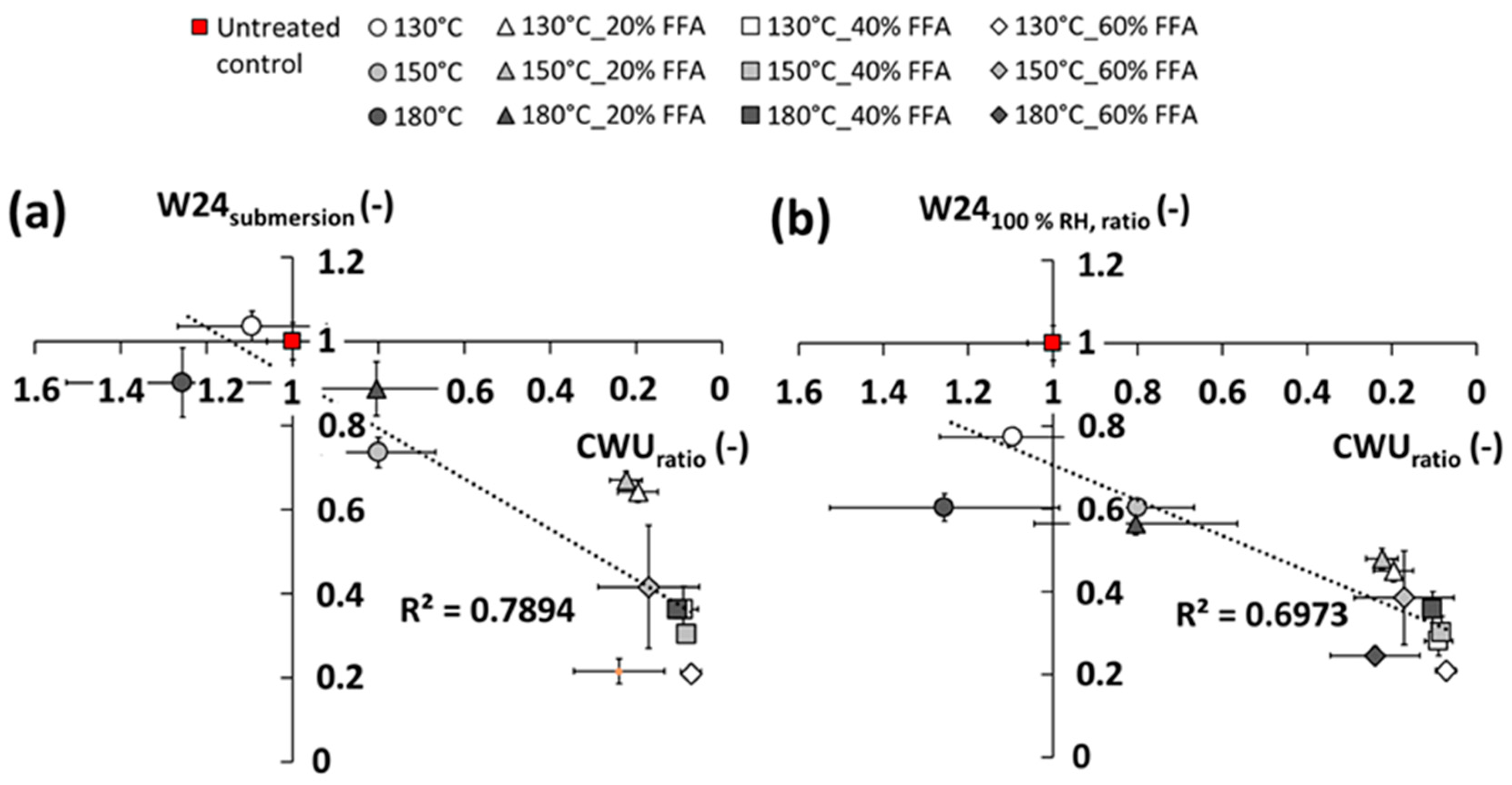
The potential of furfurylation and thermal modification of wood are well-established commercial processes. Despite their commercial status, there is scope for further improvement. This study shows the potential benefits of a novel pressurized process combining the FFA-impregnation process with thermal modification, in the absence of catalysts. Results according to CEN/TS 15083-2 show durability class 1 (very durable) treatments of Scots pine can be achieved, all showing a trend in reduced EMC depending on concentration of FFA and thermal modification applied, respectively.
Indeed, it would appear that there is little difference in properties of treated specimens across all the FFA concentrations and temperatures tested, suggesting results from milder conditions were very similar to those from more aggressive conditions. The only improvement from the more aggressive conditions would be the slight reduction in equilibrium moisture content, which would imply a more dimensionally stable material when treated at higher temperatures.
Further studies are necessary to optimize the reaction parameters using this hybrid system, as well as wider evaluations of resulting properties, including field trials. Despite this, these preliminary results would appear to suggest a means of combining the benefits from both the furfurylation and thermal modification processes into a combined treatment system, without the need for excessive curing times nor catalysts. As such, this new combined process appears to represent a considerable advance in achieving very durable modified wood.