Sustainable Food Packaging with Chitosan Biofilm Reinforced with Nanocellulose and Essential Oils
Abstract
:1. Introduction
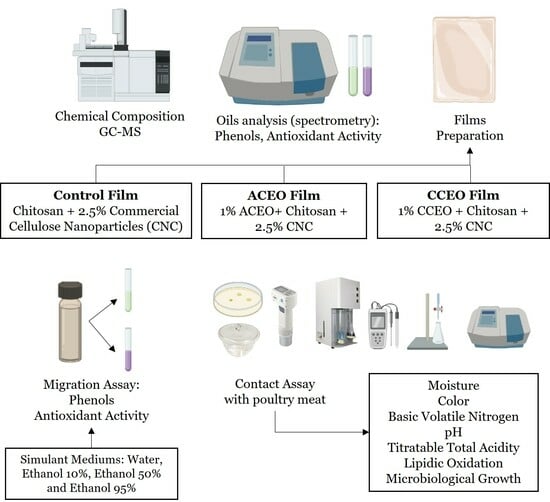
2. Materials and Methods
2.1. Materials and Reagents
2.2. Essential Oils Characterization
2.2.1. GC-MS Analysis
2.2.2. Total Phenolic Content
2.2.3. Antioxidant Activity
2.3. Bionanocomposites Preparation
2.4. In Vitro Bioactivity Study
2.5. Packaging of Fresh Poultry Meat
2.6. Poultry Meat Characterization
2.6.1. Moisture Content
2.6.2. pH and Titratable Acidity
2.6.3. Lipid Oxidation
2.6.4. Total Volatile Basic Nitrogen
2.6.5. Color
2.6.6. Microbiological Analysis
3. Results and Discussion
3.1. Essential Oils Characterization
3.1.1. Chemical Characterization by GC-MS
3.1.2. Total Phenolic Content and Antioxidant Activity
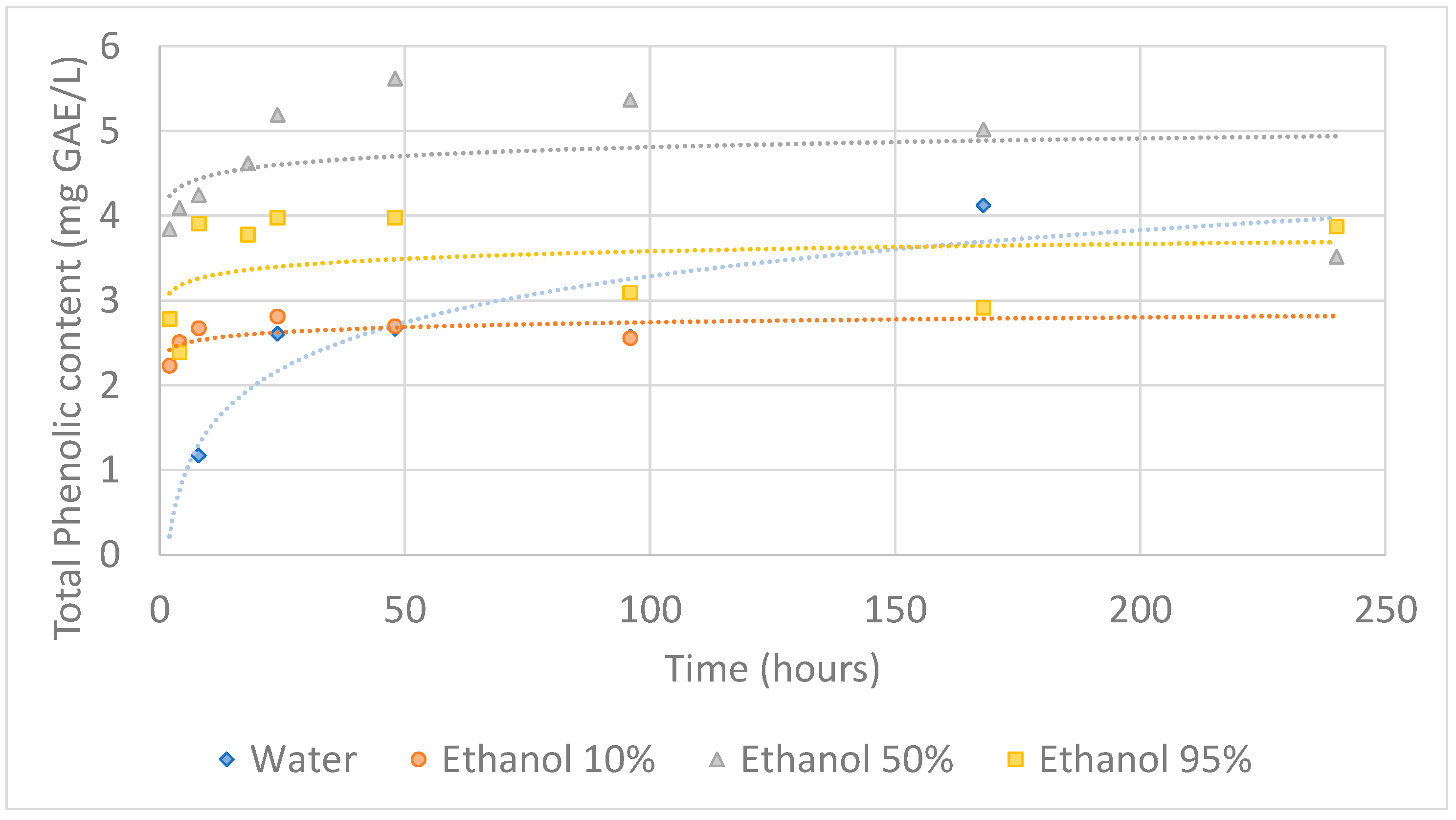
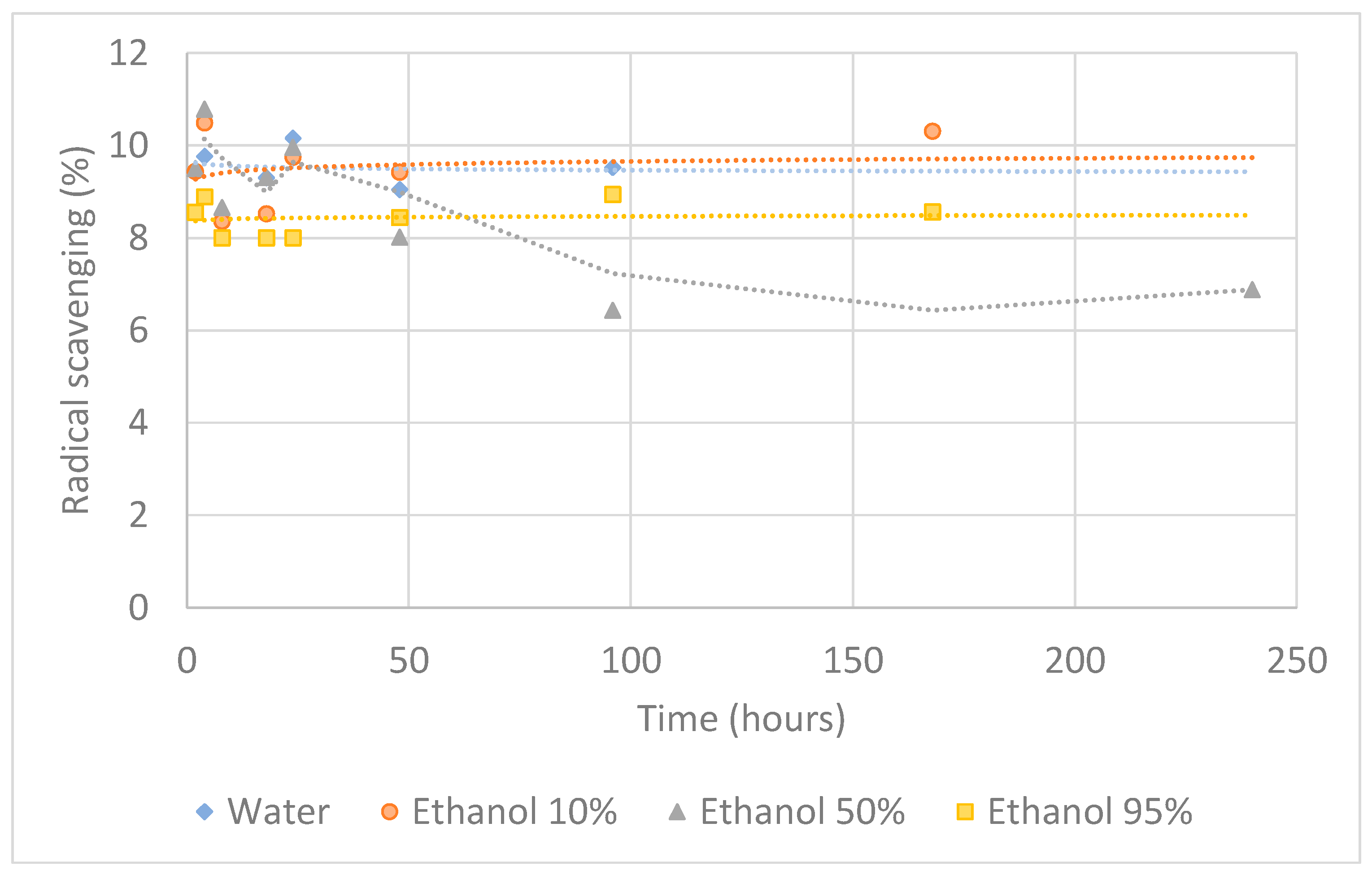
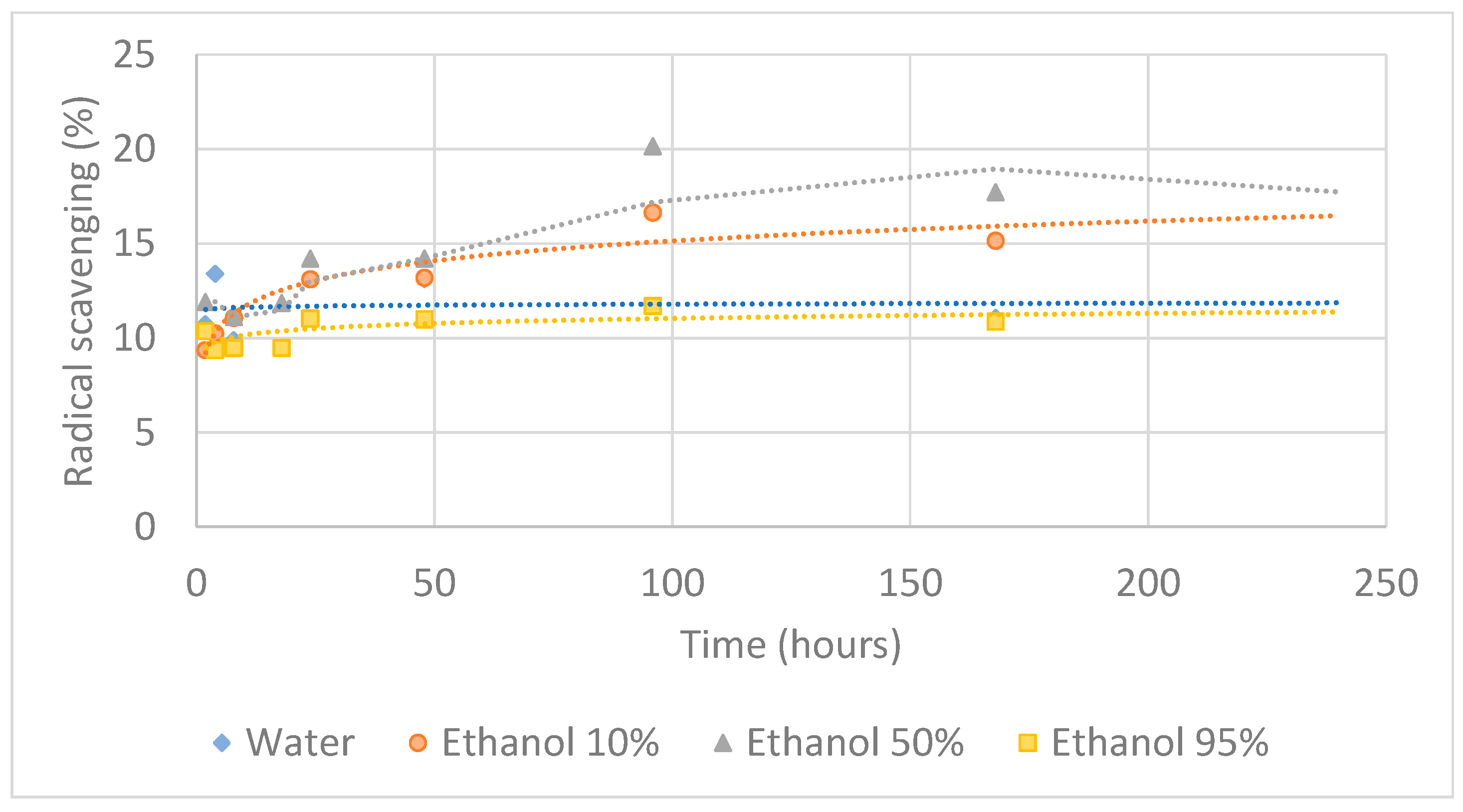
3.2. Migration Assay
3.2.1. Total Phenolic Content
3.2.2. Antioxidant Activity
3.3. Contact Assay
3.3.1. Moisture Content
3.3.2. pH and Titratable Acidity
3.3.3. Color Variation
3.3.4. Lipid Oxidation (TBARS Assays)
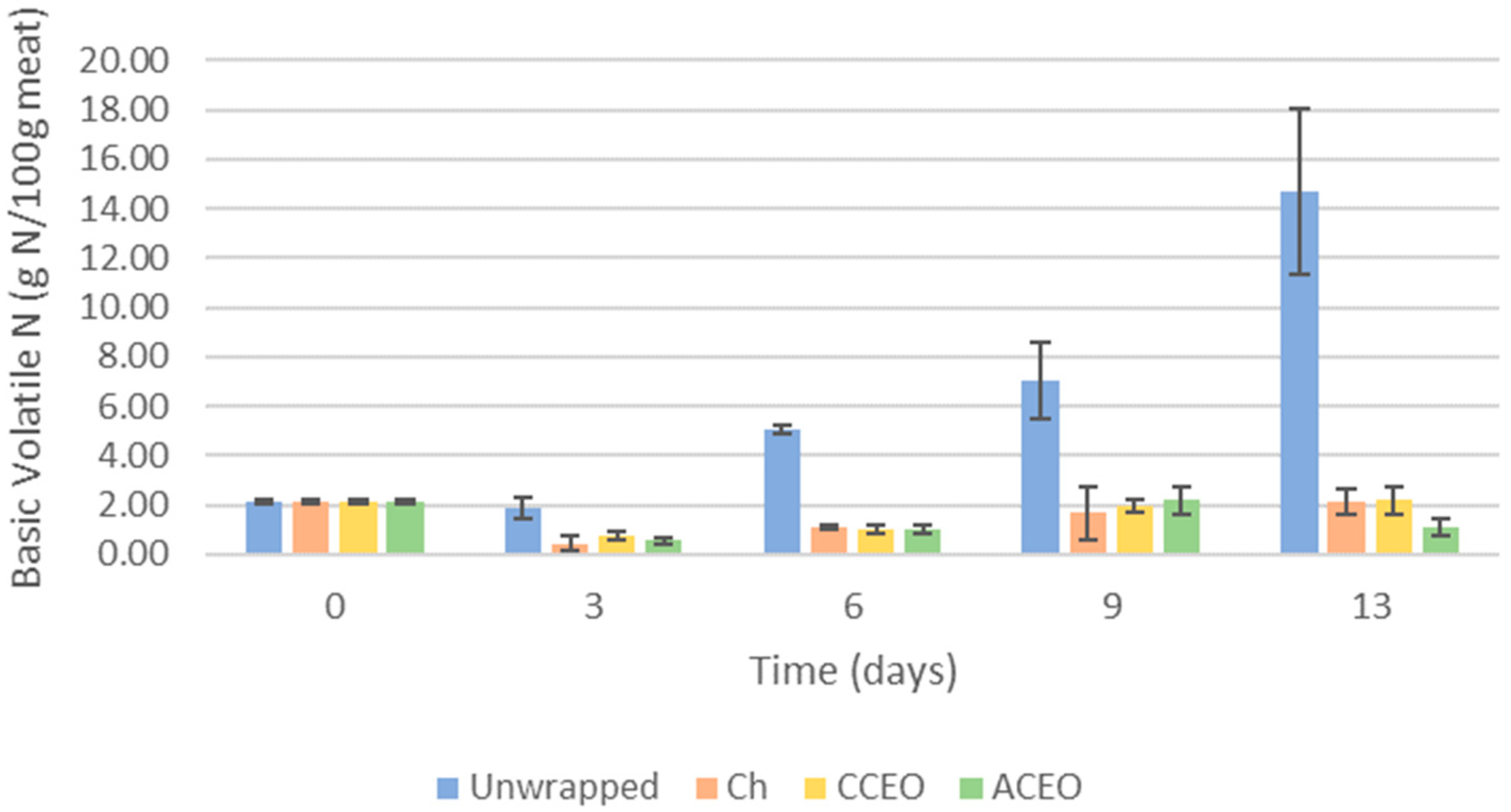
3.3.5. Basic Volatile Nitrogen
3.3.6. Microbial Growth
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019, 2, 49–55. [Google Scholar] [CrossRef]
- Cuicui, L.; Lixia, H. Review on Volatile Flavor Components of Roasted Oilseeds and Their Products. Grain Oil Sci. Technol. 2018, 1, 151–156. [Google Scholar] [CrossRef]
- Kamala Kumari, P.V.; Akhila, S.; Srinivasa Rao, Y.; Rama Devi, B. Alternative to Artificial Preservatives. Syst. Rev. Pharm. 2019, 10, 99–102. [Google Scholar]
- Pires, J.R.A.; Almeida, K.M.; Augusto, A.S.; Vieira, É.T.; Fernando, A.L.; Souza, V.G.L. Application of Biocomposite Films of Chitosan/Natural Active Compounds for Shelf Life Extension of Fresh Poultry Meat. J. Compos. Sci. 2022, 6, 342. [Google Scholar] [CrossRef]
- Rodrigues, C.; Paula, C.D.D.; Lahbouki, S.; Meddich, A.; Outzourhit, A.; Rashad, M.; Pari, L.; Coelhoso, I.; Fernando, A.L.; Souza, V.G.L. Opuntia spp.: An Overview of the Bioactive Profile and Food Applications of This Versatile Crop Adapted to Arid Lands. Foods 2023, 12, 1465. [Google Scholar] [CrossRef]
- Falleh, H.; Ben Jemaa, M.; Saada, M.; Ksouri, R. Essential oils: A promising eco-friendly food preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef]
- Mihai, A.L.; Popa, M.E. Essential oils utilization in food industry—A literature review. Sci. Bulletin. Ser. F Biotechnol. 2013, 17, 187–192. Available online: https://biotechnologyjournal.usamv.ro/pdf/vol.LVI/Art33.pdf (accessed on 1 July 2022).
- Macwan, S.R.; Dabhi, B.K.; Aparnathi, K.D.; Prajapati, J.B. Essential Oils of Herbs and Spices: Their Antimicrobial Activity and Application in Preservation of Food. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 885–901. [Google Scholar] [CrossRef]
- Ramos, M.; Beltrán, A.; Valdes, A.; Peltzer, M.A.; Jiménez, A.; Garrigós, M.C.; Zaikov, G.E. Carvacrol and Thymol for Fresh Food Packaging. J. Bioequiv. Bioavailab. 2013, 5, 154–160. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Rodrigues, C.; Ferreira, L.; Pires, J.R.A.; Duarte, M.P.; Coelhoso, I.; Fernando, A.L. In vitro bioactivity of novel chitosan bionanocomposites incorporated with different essential oils. Ind. Crops Prod. 2019, 140, 111563. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential oils as additives in active food packaging. Food Chem. 2021, 343, 128403. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.A.; Vieira, É.T.; Coelhoso, I.M.; Duarte, M.P.; Fernando, A.L. Activity of chitosan-montmorillonite bionanocomposites incorporated with rosemary essential oil: From in vitro assays to application in fresh poultry meat. Food Hydrocoll. 2019, 89, 241–252. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Rodrigues, P.F.; Duarte, M.P.; Fernando, A.L. Antioxidant migration studies in chitosan films incorporated with plant extracts. J. Renew. Mater. 2018, 6, 548–558. [Google Scholar] [CrossRef]
- Xavier, L.O.; Sganzerla, W.G.; Rosa, G.B.; da Rosa, C.G.; Agostinetto, L.; de Lima Veeck, A.P.; Bertoldi, F.C. Chitosan packaging functionalized with Cinnamodendron dinisii essential oil loaded zein: A proposal for meat conservation. Int. J. Biol. Macromol. 2021, 169, 183–193. [Google Scholar] [CrossRef]
- Pires, J.R.A.; Rodrigues, C.; Coelhoso, I.; Fernando, A.L.; Souza, V.G.L. Current Applications of Bionanocomposites in FoodProcessing and Packaging. Polymers 2023, 15, 2336. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.A.; Rodrigues, P.F.; Lopes, A.A.S.; Fernandes, F.M.B.; Duarte, M.P.; Coelhoso, I.; Fernando, A.L. Bionanocomposites of chitosan/montmorillonite incorporated with Rosmarinus officinalis essential oil: Development and physical characterization. Food Packag. Shelf Life 2018, 16, 148–156. [Google Scholar] [CrossRef]
- Oliveira, L.I.G.; Oliveira, K.A.R.; Medeiros, E.S.; Batista, A.U.D.; Madruga, M.S.; Lima, M.S.; Souza, E.L.; Magnani, M. Characterization and efficacy of a composite coating containing chitosan and lemongrass essential oil on postharvest quality of guava. Innov. Food Sci. Emerg. Technol. 2020, 66, 102506. [Google Scholar] [CrossRef]
- Pires, J.; Paula, C.D.D.; Souza, V.G.L.; Fernando, A.L.; Coelhoso, I. Understanding the Barrier and Mechanical Behavior of Different Nanofillers in Chitosan Films for Food Packaging. Polymers 2021, 13, 721. [Google Scholar] [CrossRef] [PubMed]
- Pires, J.R.A.; Souza, V.G.L.; Gomes, L.A.; Coelhoso, I.M.; Godinho, M.H.; Fernando, A.L. Micro and nanocellulose extracted from energy crops as reinforcement agents in chitosan films. Ind. Crops Prod. 2022, 186, 115247. [Google Scholar] [CrossRef]
- Farahmandfar, R.; Asnaashari, M.; Pourshayegan, M.; Maghsoudi, S.; Moniri, H. Evaluation of antioxidant properties of lemon verbena (Lippia citriodora) essential oil and its capacity in sunflower oil stabilization during storage time. Food Sci. Nutr. 2018, 6, 983–990. [Google Scholar] [CrossRef]
- Elchaghaby, M.A.; El-Kader, S.F.A.; Aly, M.M. Bioactive composition and antibacterial activity of three herbal extracts (lemongrass, sage, and guava leaf) against oral bacteria: An in vitro study. J. Oral. Biosci. 2022, 64, 114–119. [Google Scholar] [CrossRef]
- Rezaeifar, M.; Mehdizadeh, T.; Mojaddar Langroodi, A.; Rezaei, F. Effect of chitosan edible coating enriched with lemon verbena extract and essential oil on the shelf life of vacuum rainbow trout (Oncorhynchus mykiss). J. Food Saf. 2020, 40, e12781. [Google Scholar] [CrossRef]
- United Nations. Sustainable Development Goals. 2023. Available online: https://sdgs.un.org/ (accessed on 1 April 2023).
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Harte, B.R. Physical properties and antioxidant activity of an active film from chitosan incorporated with green tea extract. Food Hydrocoll. 2010, 24, 770–775. [Google Scholar] [CrossRef]
- Dias, M.V.; Machado Azevedo, V.; Borges, S.V.; Soares, N.F.; de Barros Fernandes, R.V.; Marques, J.J.; Medeiros, E.A. Development of chitosan/montmorillonite nanocomposites with encapsulated α-tocopherol. Food Chem. 2014, 165, 323–329. [Google Scholar] [CrossRef]
- Pires, J.R.A.; de Souza, V.G.L.; Fernando, A.L. Chitosan/montmorillonite bionanocomposites incorporated with rosemary and ginger essential oil as packaging for fresh poultry meat. Food Packag. Shelf Life 2018, 17, 142–149. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.A.; Vieira, É.T.; Coelhoso, I.M.; Duarte, M.P.; Fernando, A.L. Shelf life assessment of fresh poultry meat packaged in novel bionanocomposite of chitosan/montmorillonite incorporated with ginger essential oil. Coatings 2018, 8, 177. [Google Scholar] [CrossRef]
- European Commission. Regulation (EU) No 10/2011 of 14 January 2011 on Plastic Materials and Articles Intended to Come into Contact with Food; European Commission: Brussels, Belgium, 2011. [Google Scholar]
- Association of the Official Analytical Chemists (AOAC). Official Methods of Analysis of the Association of Official Analytical Chemists, 20th ed.; AOAC: Gaithersburg, MD, USA, 2016. [Google Scholar]
- Rosmini, M.R.; Perlo, F.; Pérez-Alvarez, J.A.; Pagán-Moreno, M.J.; Gago-Gago, A.; López-Santoveña, F.; Aranda-Catalá, V. TBA test by an extractive method applied to ‘paté’. Meat Sci. 1996, 42, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Malle, P.; Poumeyrol, M. A New Chemical Criterion for the Quality Control of Fish: Trimethylamine/Total Volatile Basic Nitrogen (%). J. Food Prot. 1989, 52, 419–423. [Google Scholar] [CrossRef] [PubMed]
- ISO 4833-1:2013; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 Degrees C by the Pour Plate Technique. ISO: Geneva, Switzerland, 2013.
- ISO 17410:2019; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Psychrotrophic Microorganisms. ISO: Geneva, Switzerland, 2001.
- ISO 21528-2:2017; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Enterobacteriaceae—Part 2: Colony-Count Technique. ISO: Geneva, Switzerland, 2017.
- Golmakani, M.-T.; Farahmand, M.; Ghassemi, A.; Eskandari, M.H.; Niakousari, M. Enrichment of citral isomers in different microwave-assisted extraction of essential oil from fresh and dried lemon verbena (Aloysia citridora) leaves. J. Food Process Preserv. 2017, 41, e13215. [Google Scholar] [CrossRef]
- Hadjilouka, A.; Mavrogiannis, G.; Mallouchos, A.; Paramithiotis, S.; Mataragas, M.; Drosinos, E.H. Effect of lemon grass essential oil on Listeria monocytogenes gene expression. LWT-Food Sci. Technol. 2017, 77, 510–516. [Google Scholar] [CrossRef]
- Matasyoh, J.C.; Wagara, I.N.; Nakavuma, J.L.; Kiburai, A.M. Chemical composition of Cymbopogon citratus essential oil and its effect on mycotoxigenic Aspergillus species. Afr. J. Food Sci. 2011, 5, 138–142. Available online: http://www.academicjournals.org/ajfs (accessed on 1 April 2023).
- Lohvina, H.; Sándor, M.; Wink, M. Effect of Ethanol Solvents on Total Phenolic Content and Antioxidant Properties of Seed Extracts of Fenugreek (Trigonella foenum-graecum L.) Varieties and Determination of Phenolic Composition by HPLC-ESI-MS. Diversity 2022, 14, 7. [Google Scholar] [CrossRef]
- Onawunmi, G.O.; Yisak, W.A.; Ogunlana, E.O. Anti bacterial constituents in the essential oil of Cymbopogon citratus (D.C) Stapf. J. Ethnopharmacol. 1984, 12, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Naik, M.I.; Fomda, B.A.; Jaykumar, E.; Bhat, J.A. Antibacterial activity of lemongrass (Cymbopogon citratus) oil against some selected pathogenic bacterias. Asian Pac. J. Trop. Med. 2010, 3, 535–538. [Google Scholar] [CrossRef]
- Falcao, M.A.; Fianco, A.L.B.; Lucas, A.M.; Pereira, M.A.A.; Torres, F.C.; Vargas, R.M.F.; Cassel, F. Determination of antibacterial activity of vacuum distillation fractions of lemongrass essential oil. Phytochem. Rev. 2012, 11, 405–412. [Google Scholar] [CrossRef]
- Guimarães de Lima, L.G.; Cardoso das Graças, M.; de Sousa, P.E.; de Andrade, J.; Vieira, S.S. Antioxidant and fungitoxic activities of the lemongrass essential oil and citral. Rev. Ciência Agronômica 2011, 42, 464–472. [Google Scholar] [CrossRef]
- Baschieri, A.; Ajvazi, M.D.; Tonfack, J.L.F.; Valgimigli, L.; Amorati, R. Explaining the antioxidant activity of some common non-phenolic components of essential oils. Food Chem. 2017, 232, 656–663. [Google Scholar] [CrossRef]
- López-De-Dicastillo, C.; Gómez-Estaca, J.; Catalá, R.; Gavara, R.; Hernández-Muñoz, P. Active antioxidant packaging films: Development and effect on lipid stability of brined sardines. Food Chem. 2012, 131, 1376–1384. [Google Scholar] [CrossRef]
- Souza, V.G.L. Desenvolvimento de Bio-Nanocompósitos de Quitosano/Montmorilonite Incorporados com Extratos Naturais como Embalagens Ativas para Alimentos; Faculdade de Ciências e Tecnologia, Universidade Nova de Lisboa: Lisbon, Portugal, 2018. [Google Scholar]
- Majewska, E.; Kozlowska, M.; Gruczynska-Sekowska, E.; Kowalska, D.; Tarnowska, K. Lemongrass (Cymbopogon citratus) essential oil: Extraction, composition, bioactivity and uses for food preservation—A review. Polish J. Food Nutr. Sci. 2019, 69, 327–341. [Google Scholar] [CrossRef]
- Pastor, C.; Sánchez-González, L.; Chiralt, A.; Cháfer, M.; González-Martínez, C. Physical and antioxidant properties of chitosan and methylcellulose based films containing resveratrol. Food Hydrocoll. 2013, 30, 272–280. [Google Scholar] [CrossRef]
- Osman, Z.; Arof, A.K. Chitosan and Phthaloylated Chitosan in Electrochemical Devices. In Biological Activities and Application of Marine Polysaccharides; Shalaby, E.A., Ed.; InTech.: Cairo, Egypt, 2017; Available online: https://www.intechopen.com/chapters/52586 (accessed on 1 July 2022). [CrossRef]
- Barbut, S. Pale, soft, and exudative poultry meat—Reviewing ways to manage at the processing plant. Poult. Sci. 2009, 88, 1506–1512. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Jamshidi, A.; Raeisi, M.; Azizzadeh, M. Effect of sodium alginate coating containing clove (Syzygium aromaticum) and lemon verbena (Aloysia citriodora) essential oils and different packaging treatments on shelf life extension of refrigerated chicken breast. J. Food Process. Preserv. 2021, 45, e14946. [Google Scholar] [CrossRef]
- Hosseinzadeh, M.H.; Ebrahimzadeh, M.A. Protective effects of ethanolic extract of Lemon Beebrush (Aloysia citrodora) leaf against hypoxia-induced lethality in mice. Tabari Biomed. Stud. Res. J. 2020, 1, 1–7. [Google Scholar] [CrossRef]
- Tyl, C.; Sadler, G.D. pH and Titratable Acidity. In Food Analysis; Food Science Text Series; Nielsen, S.S., Ed.; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Hunt, M.C.; King, A.; Barburt, S.; Clause, J.; Cornforth, D.; Hanson, D. AMSA Meat Color Measurement Guidelines; American Meat Science Association: Savoy, IL, USA, 2012; Volume 61820. [Google Scholar]
- Carvalho, R.; Shimokomaki, M.; Estévez, M. Poultry Meat Color and Oxidation. In Poultry Quality Evaluation Quality Attributes and Consumer Values; Petracci, M., Berri, C., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 133–157. [Google Scholar] [CrossRef]
- Olorunsanya, A.O.; Olorunsanya, E.O.; Bolu, S.A.O.; Adejumobi, C.T.; Kayode, R.M.O. Effect of Graded Levels of Lemongrass (Cymbopogon citratus) on Oxidative Stability of Raw or Cooked Pork Patties. Pak. J. Nutr. 2010, 9, 1680–5194. [Google Scholar] [CrossRef]
- Bekhit, A.E.D.A.; Holman, B.W.B.; Giteru, S.G.; Hopkins, D.L. Total volatile basic nitrogen (TVB-N) and its role in meat spoilage: A review. Trends Food Sci. Technol. 2021, 109, 280–302. [Google Scholar] [CrossRef]
- Ercolini, D.; Russo, F.; Nasi, A.; Ferranti, P.; Villani, F. Mesophilic and psychrotrophic bacteria from meat and their spoilage potential in vitro and in beef. Appl. Environ. Microbiol. 2009, 75, 1990–2001. [Google Scholar] [CrossRef]





| Sample | Water | Ethanol 10% | Ethanol 50% | Ethanol 95% | |
|---|---|---|---|---|---|
| Total phenolic content (mg/L) | ACEO | 216.0 ± 5.0 | 255.0 ± 34.0 | 288 ± 59.0 | - |
| CCEO | 109.0 ± 18.0 | 110.0 ± 6.0 | 133.0 ± 5.0 | 268.0 ± 11.0 | |
| Antioxidant activity (% radical scavenging) | ACEO | 77.0 ± 12.0 | 78.0 ± 1.0 | 77.0 ± 0.4 | 60.0 ± 3.0 |
| CCEO | 83.0 ± 2.0 | 85.0 ± 15.0 | 87.0 ± 7.0 | 89.0 ± 5.0 |
| Time (Days) | Unwrapped | Chitosan | CCEO | ACEO |
|---|---|---|---|---|
| 0 | 73.9 ± 0.1 | 73.9 ± 0.1 | 73.9 ± 0.1 | 73.9 ± 0.1 |
| 3 | 75.3 ± 0.8 | 71.0 ± 0.2 | 72.6 ± 0.6 | 70.0 ± 3.6 |
| 6 | 75.2 ± 0.7 | 71.3 ± 0.6 | 71.0 ± 0.3 | 69.7 ± 0.1 |
| 9 | 75.9 ± 0.9 | 68.9 ± 0.7 | 69.4 ± 0.9 | 67.1 ± 3.8 |
| 13 | 76.6 ± 0.9 | 67.7 ± 0.7 | 70.4 ± 0.7 | 67.1 ± 0.7 |
| Time (Days) | Unwrapped | Chitosan | CCEO | ACEO | |
|---|---|---|---|---|---|
| pH | 0 | 5.95 ± 0.04 | 5.95 ± 0.04 | 5.95 ± 0.04 | 5.95 ± 0.04 |
| 3 | 6.30 ± 0.06 | 6.26 ± 0.01 | 6.40 ± 0.06 | 6.33 ± 0.02 | |
| 6 | 6.78 ± 0.19 | 5.63 ± 0.01 | 5.78 ± 0.01 | 5.51 ± 0.07 | |
| 9 | 6.73 ± 0.35 | 5.49 ± 0.04 | 5.77 ± 0.01 | 5.44 ± 0.03 | |
| 13 | 7.51 ± 0.16 | 5.58 ± 0.04 | 5.72 ± 0.01 | 5.39 ± 0.01 | |
| Total Titratable Acidity (g oleic acid/100 g meat) | 0 | 1.06 ± 0.07 | 1.06 ± 0.07 | 1.06 ± 0.07 | 1.06 ± 0.07 |
| 3 | 1.58 ± 0.16 | 0.98 ± 0.10 | 0.84 ± 0.17 | 0.89 ± 0.23 | |
| 6 | 0.62 ± 0.01 | 0.83 ± 0.15 | 0.91 ± 0.16 | 0.87 ± 0.06 | |
| 9 | 0.82 ± 0.59 | 0.90 ± 0.16 | 1.05 ± 0.21 | 1.11 ± 0.12 | |
| 13 | 0.38 ± 0.07 | 1.40 ± 0.07 | 1.13 ± 0.25 | 1.39 ± 0.08 |
| Time (Days) | Unwrapped | Chitosan | CCEO | ACEO |
|---|---|---|---|---|
| 0 | 40 ± 5 | 40 ± 5 | 40 ± 5 | 40 ± 5 |
| 3 | 46 ± 3 | 46 ± 1 | 46 ± 4 | 48 ± 1 |
| 6 | 41 ± 6 | 46 ± 1 | 49 ± 2 | 52 ± 0 |
| 9 | 47 ± 3 | 49 ± 2 | 52 ± 1 | 61 ± 1 |
| 13 | 48 ± 2 | 50 ± 1 | 53 ± 6 | 61 ± 0 |
| Time (Days) | Unwrapped | Chitosan | CCEO | ACEO |
|---|---|---|---|---|
| 0 | 2.10 ± 1.49 | 7.54 ± 1.68 | 8.55 ± 2.25 | 5.58 ± 0.15 |
| 3 | 5.42 ± 2.27 | 7.74 ± 3.04 | 9.19 ± 1.86 | 6.47 ± 0.13 |
| 6 | 4.52 ± 0.54 | 7.92 ± 0.77 | 9.53 ± 1.19 | 5.84 ± 0.63 |
| 9 | 4.77 ± 0.83 | 7.87 ± 2.02 | 8.92 ± 1.05 | 6.18 ± 1.23 |
| 13 | 2.10 ± 1.49 | 7.54 ± 1.68 | 8.55 ± 2.25 | 5.58 ± 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, S.J.; Samba, N.; Mendes, J.; Pires, J.R.A.; Rodrigues, C.; Curto, J.; Gomes, A.; Fernando, A.L.; Silva, L. Sustainable Food Packaging with Chitosan Biofilm Reinforced with Nanocellulose and Essential Oils. Macromol 2023, 3, 704-722. https://doi.org/10.3390/macromol3040040
Silva SJ, Samba N, Mendes J, Pires JRA, Rodrigues C, Curto J, Gomes A, Fernando AL, Silva L. Sustainable Food Packaging with Chitosan Biofilm Reinforced with Nanocellulose and Essential Oils. Macromol. 2023; 3(4):704-722. https://doi.org/10.3390/macromol3040040
Chicago/Turabian StyleSilva, Sofia J., Nsevolo Samba, José Mendes, João R. A. Pires, Carolina Rodrigues, Joana Curto, Arlindo Gomes, Ana Luísa Fernando, and Lúcia Silva. 2023. "Sustainable Food Packaging with Chitosan Biofilm Reinforced with Nanocellulose and Essential Oils" Macromol 3, no. 4: 704-722. https://doi.org/10.3390/macromol3040040
APA StyleSilva, S. J., Samba, N., Mendes, J., Pires, J. R. A., Rodrigues, C., Curto, J., Gomes, A., Fernando, A. L., & Silva, L. (2023). Sustainable Food Packaging with Chitosan Biofilm Reinforced with Nanocellulose and Essential Oils. Macromol, 3(4), 704-722. https://doi.org/10.3390/macromol3040040