Fully Biodegradable Edible Packaging Foils on the Basis of Potato Starch–Lipid–Protein Ternary Complexes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physical Properties of Foils
2.1.1. Water Content, Solubility, and Swelling Degree of Foils
2.1.2. Color Parameters of Foils
2.1.3. Thickness and Mechanical Properties of Foils
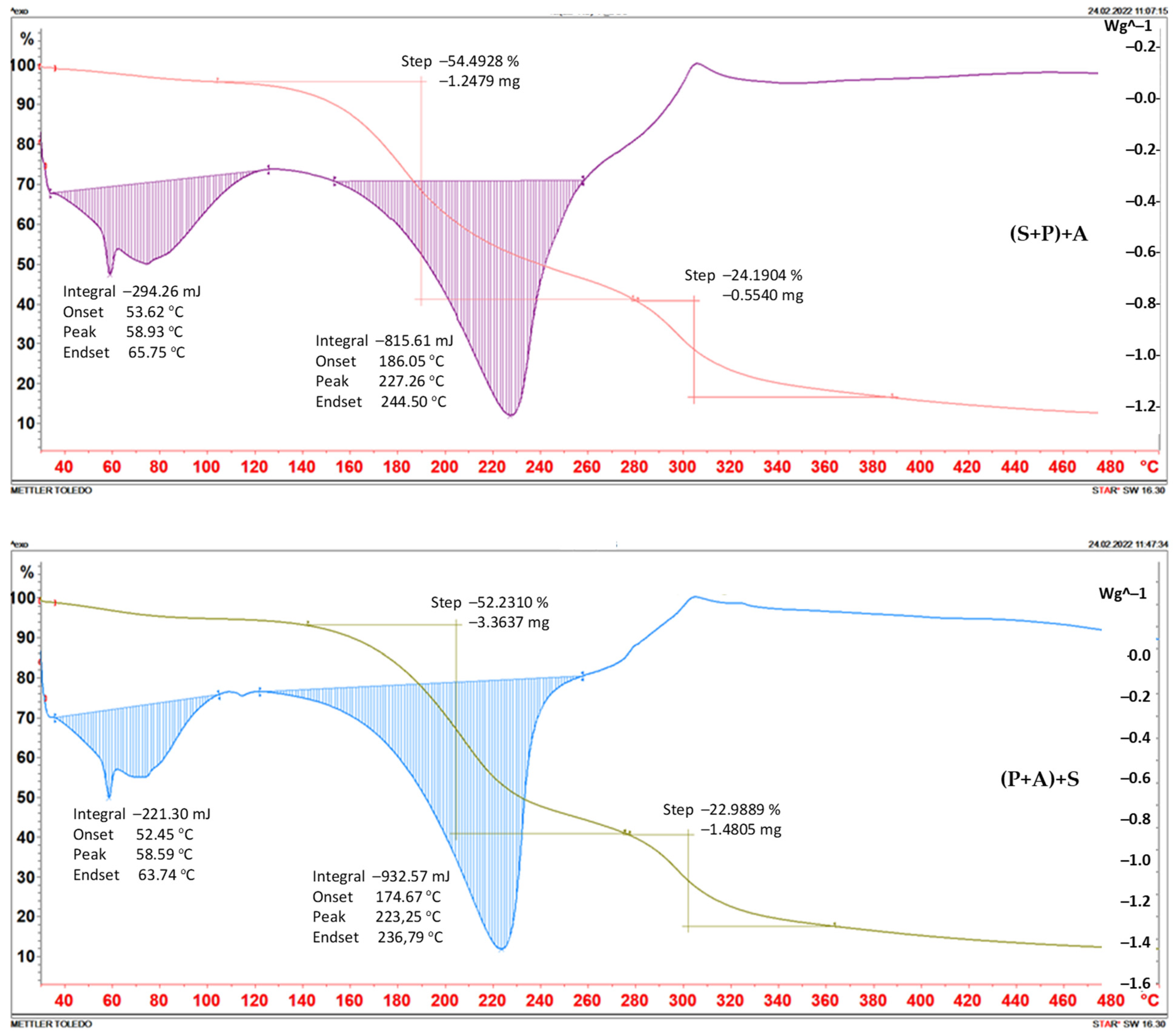
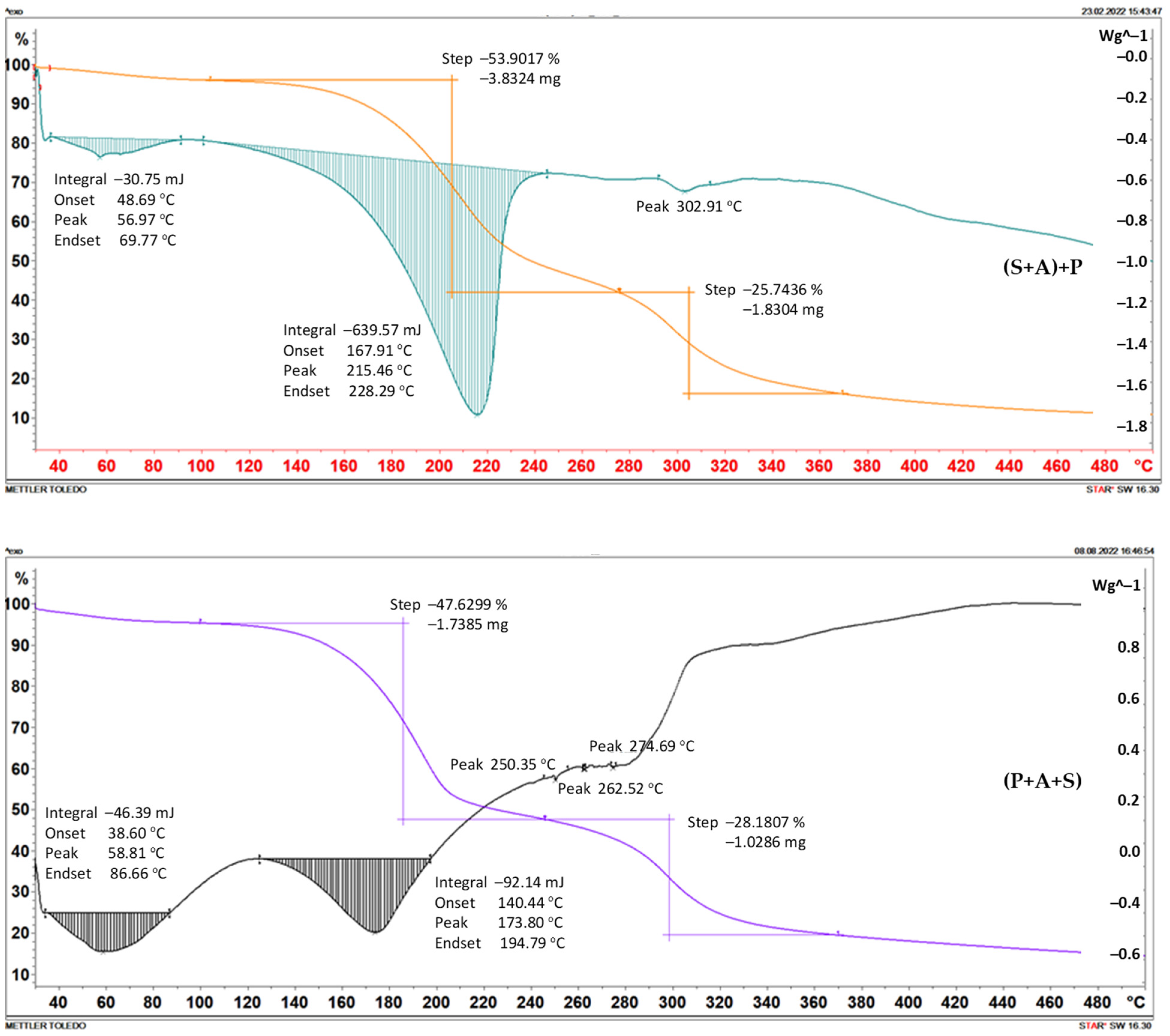
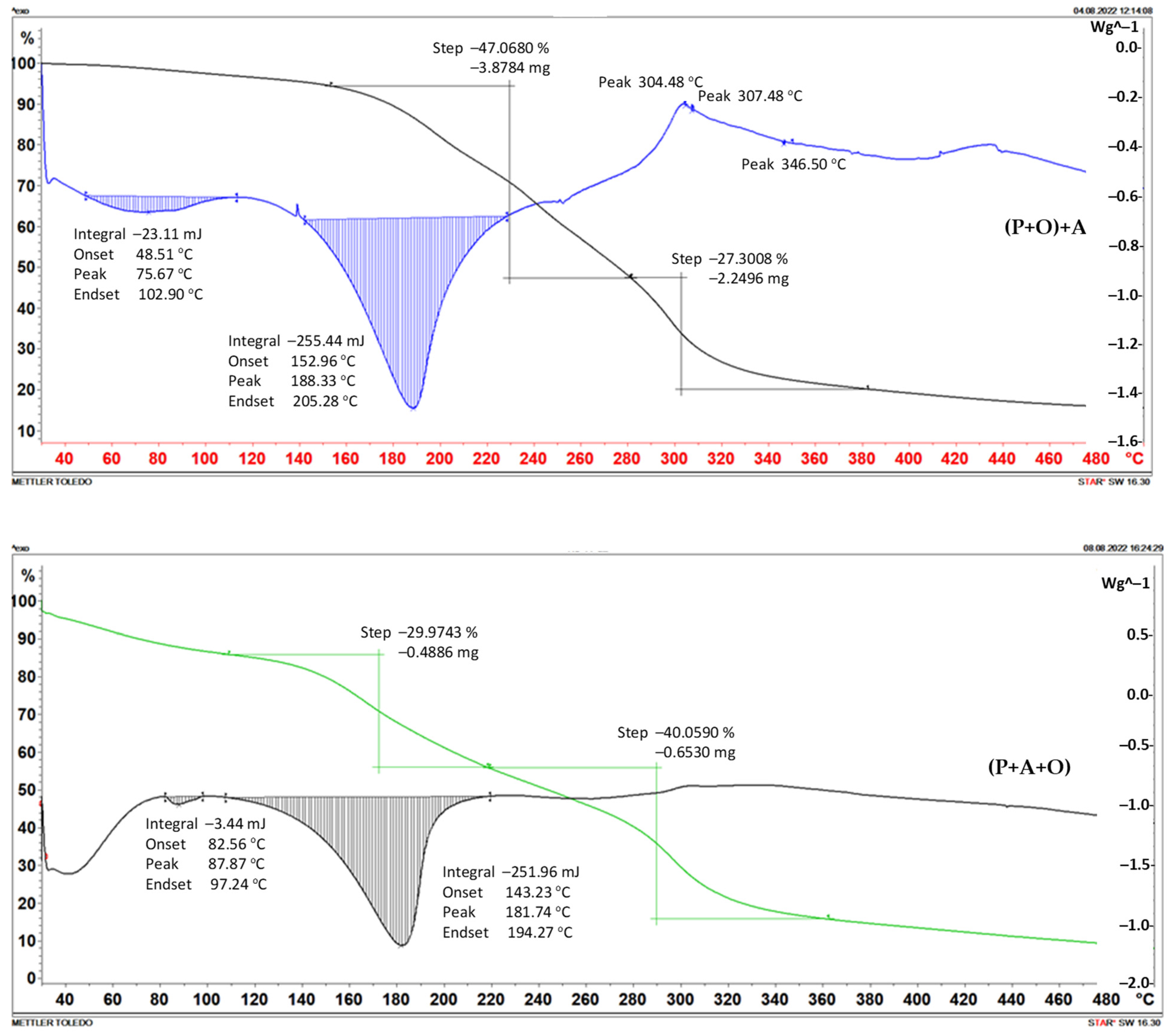
2.2. Thermogravimetry
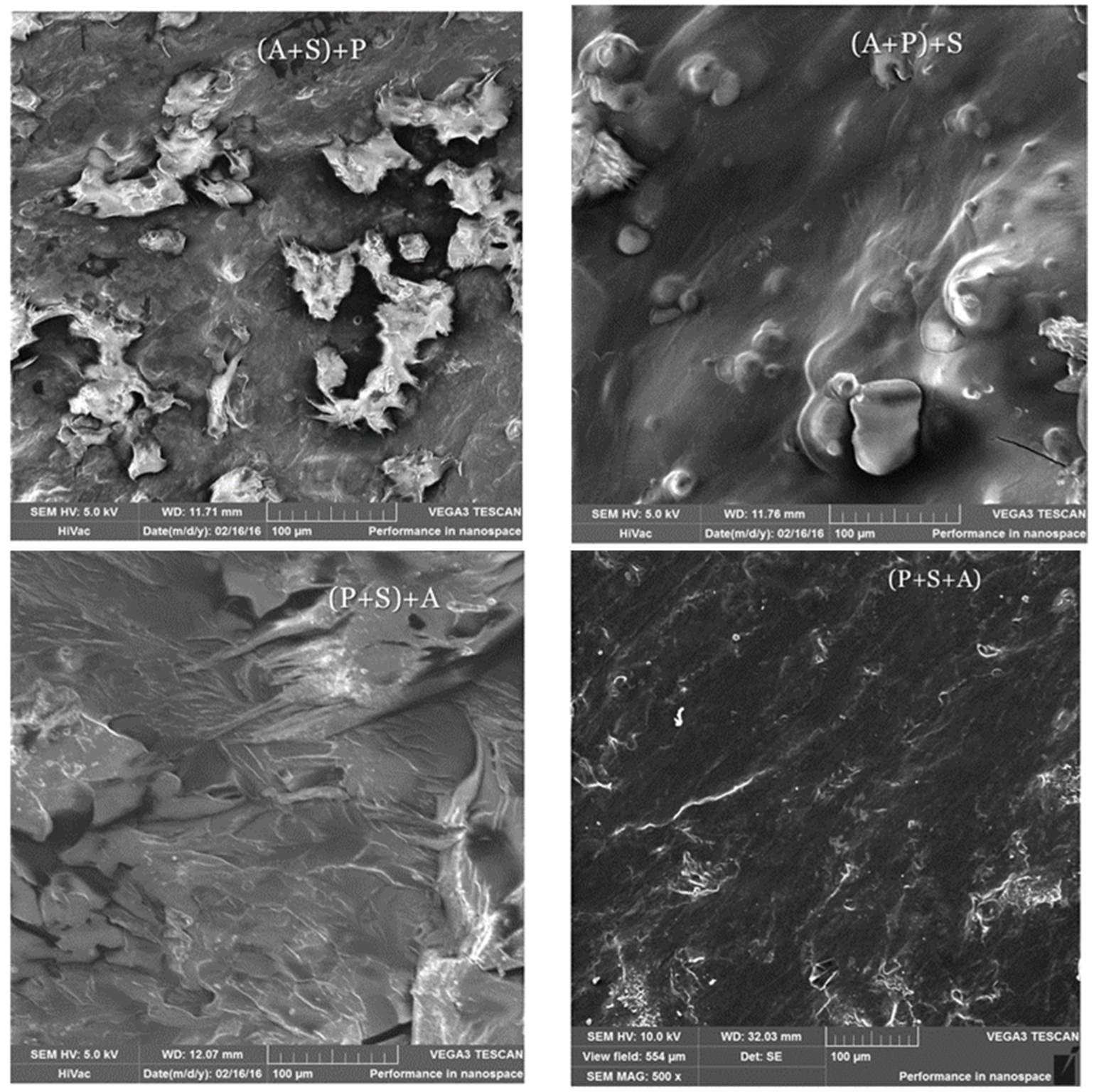
2.3. Scanning Electron Microscopy (SEM)
2.4. X-Ray Diffraction
2.5. Fourier Transformation Infrared Spectroscopy Attenuated Total Reflectance (FTIR-ATR)
2.6. Staling of Foils
3. Materials and Methods
3.1. Materials
3.2. Methods
Preparation of Foils
- Potato starch (P) (10 weight parts of 5% aq., gel), stearic acid (S) (5 weight parts of powder), and albumin (A) (1 weight part of liquid composed of 1 g albumin in 7 mL water).
- Potato starch (P) (10 weight parts of 5% aq., gel), oleic acid (O) (5 weight parts of liquid), and albumin (A) (1 weight part of liquid composed of 1 g aq. albumin in 7 mL water).
- Potato starch (P), lipid acid (S or O), and albumin (A) taken in the proportions above were blended simultaneously.
- (1)
- (polysaccharide+protein)+lipid in the ratio 10:5:1 (polysaccharide:protein:lipid)
- (2)
- (polysaccharide+lipid)+protein in the ratio 10:5:1 (polysaccharide:lipid:protein)
- (3)
- (lipid+protein)+polysaccharide in the ratio 10:5:1 (lipid:protein:polysaccharide)
3.3. Physical Properties of Foils
3.3.1. Water Content, Solubility, and Swelling Degree
3.3.2. Surface Color Measurements
3.3.3. Thickness Measurement
3.3.4. Mechanical Properties
3.4. Thermogravimetry
3.5. Scanning Electron Microscopy (SEM)
3.6. X-ray Diffraction
3.7. Fourier Transformation Infrared Spectroscopy Attenuated Total Reflectance (FTIR-ATR)
3.8. Staling of Foils
- Sample preparation: Biodegradable material samples were carefully prepared, placed on Teflon discs, and marked.
- Monitoring changes: After a specified period of time (3 months), changes in the materials were monitored by taking measurements of physical properties, such as thickness and mechanical, water content, solubility and swelling degree, and color parameters.
3.9. Statistics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Hassa, R.; Mrzigod, J.; Nowakowski, J. Podręczny Słownik Chemiczny; Videograf II: Katowice, Poland, 2004; p. 143. [Google Scholar]
- Vert, M.; Doi, Y.; Hellwich, K.-H.; Hess, M.; Hodge, P.; Kubisa, P.; Rinaudo, M.; Schue, F. Terminology for biorelated polymers and applications (IUPAC Recommendations 2012). Pure Appl. Chem. 2012, 84, 377–410. [Google Scholar] [CrossRef]
- De Graaf, R.A.; Karman, A.P.; Janssen, L.P.B.M. Material properties and glass transition temperatures of different thermoplastic starches after extrusion processing. Starke 2023, 55, 80–86. [Google Scholar] [CrossRef]
- Mościcki, L.; Janssen, L.P.B.M.; Mitrus, M.; Oniszczuk, T.; Rejak, A.; Juśko, S. Baro-thermal techniques in processing of thermoplastic starch. Acta Agroph. 2007, 9, 431–442. [Google Scholar]
- Park, H.M.; Lee, S.R.; Chowdhury, S.R.; Kang, T.K.; Kim, H.K.; Park, S.H.; Ha, C.S. Tensile properties, morphology and biodegradability of blends of starch with various thermoplastics. J. Appl. Polym. Sci. 2002, 86, 2907–2915. [Google Scholar] [CrossRef]
- Wollerdorfer, M.; Bader, H. Influence of natural fibres on the mechanical properties of biodegradable polymers. Ind. Crops Prod. 1998, 8, 105–112. [Google Scholar] [CrossRef]
- Serghat-Derradji, H.; Copinet, A.; Bureau, G.; Couturier, Y. Aerobic biodegradation of extruded polymer blends with native starch as major component. Starke 1999, 51, 369–375. [Google Scholar] [CrossRef]
- Gołębiewski, J.; Gibas, E.; Malinowski, R. Selected biodegradable polymers—Preparation. Properties and applications. Polimery 2008, 53, 799–809. [Google Scholar] [CrossRef]
- Malinowski, R. Biodegradable polymers. In Teka Kom. Budowy I Eksploat. Masz. Elektrotechechnicznych I Budownictwa—OL PAN; Polish Academy Of Sciences: Lublin, Poland, 2008; Volume 103, Available online: https://www.pan-ol.lublin.pl/archiwum/wydawnictwa/TBud2/spis.pdf (accessed on 17 April 2023).
- Badia, J.D.; Moriana, R.; Santonja-Blasco, L.; Ribes-Greus, A. A thermogravimetric approach to study the influence of a biodegradation in soil test to a poly(lactic acid). Macromol. Symp. 2008, 272, 93–99. [Google Scholar] [CrossRef]
- Fay, F.; Linossier, I.; Legendre, G.; Vallee-Rehel, K. Microencapsulation and antifouling coatings: Development of poly(lactic acid) microspheres containing bioactive molecules. Macromol. Symp. 2008, 272, 45–51. [Google Scholar] [CrossRef]
- Tomaszewski, W.; Duda, A.; Szadkowski, M.; Libiszowski, J.; Ciechańska, D. Poly(L-lactide) nano- and microfibers by electrospinning: Influence of poly(L-lactide) molecular weight. Macromol. Symp. 2008, 272, 70–74. [Google Scholar] [CrossRef]
- Kawalec, M.; Janeczek, H.; Adamus, G.; Kurcok, P.; Kowalczuk, M.; Scandola, M. The study of kinetics of poly[(R,S)-3-hydroxybutyrate] degradation induced by carboxylate. Macromol. Symp. 2007, 272, 63–69. [Google Scholar] [CrossRef]
- Koller, M.; Atlicc, A.; Gonazalez-Garcia, Y.; Kutschera, C.; Braunegg, G. Polyhydroxyalkanoate (PHA) biosynthesis from whey lactose. Macromol. Symp. 2007, 272, 87–92. [Google Scholar] [CrossRef]
- Chaudhuri, J.B.; Davidson, M.G.; Ellis, M.J.; Jones, M.D.; Wu, X. Fabrication of honeycomb-structured poly(DL-lactide) and poly[(DL-lactide)-coglycolide)] films and their use as scaffolds for osteoblast-like cell culture. Macromol. Symp. 2008, 272, 52–57. [Google Scholar] [CrossRef]
- Poljansek, I.; Gricar, M.; Zagar, E.; Zigon, M. Molar mass and structural characteristics of poly[lactide-co-(aspartic acid)] block copolymers. Macromol. Symp. 2007, 272, 75–80. [Google Scholar] [CrossRef]
- Labet, M.; Thielemans, W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484–38504. [Google Scholar] [CrossRef] [PubMed]
- Reimschuessel, N. Nylon 6: Chemistry and mechanisms. J. Polym. Sci. Macromol. Rev. 1977, 12, 65–139. [Google Scholar] [CrossRef]
- Independent. ‘Green’ Plastics May Be Worse for Environment. Available online: http://www.independent.co.uk/environment/climate-change/green-plastics-may-be-worse-for-environment-1920707 (accessed on 17 April 2023).
- Symphony Environmental Ltd. Available online: http://www.degradable.net/what-is-d2w/what-is-d2w (accessed on 17 April 2023).
- Obiro, W.C.; Sinha Ray, S.; Emmambux, M.N. V-amylose structural characteristics, methods of preparation, significance, and potential applications. Food Rev. Int. 2012, 28, 412–438. [Google Scholar] [CrossRef]
- Panyoo, A.E.; Emmambux, M.N. Amylose-lipid complex production and potential health benefits: A mini-review. Starke 2017, 69, 1600203. [Google Scholar] [CrossRef]
- Wang, S.; Copeland, L. Molecular disassembly of starch granules during gelatinization and its effect on starch digestibility: A review. Food Funct. 2013, 4, 1564–1580. [Google Scholar] [CrossRef]
- Marinopoulou, A.; Papastergiadis, E.; Raphaelides, S.N.; Kontominas, M.G. Morphological characteristics, oxidative stability and enzymic hydrolysis of amylose-fatty acid complexes. Carbohydr. Polym. 2016, 141, 106–115. [Google Scholar] [CrossRef]
- Chen, W.; Chao, C.; Yu, J.; Copeland, L.; Wang, S.; Wang, S.-J. Effect of protein-fatty acid interactions on the formation of starch-lipid-protein complexes. Food Chem. 2021, 364, 130390. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Zhuang, H.; Chen, F.; Campanella, O.; Bhopatkar, D.; Carignano, M.A.; Park, S.H. Starch-lipid and starch-protein complexes and their application. In Functional Starch and Applications in Food; Jin, Z., Ed.; Springer Nature: Singapore, 2018; pp. 177–226. [Google Scholar] [CrossRef]
- Wang, S.; Chao, C.; Cai, J.; Niu, B.; Copeland, L.; Wang, S. Starch-lipid and starch-lipid-protein complexes: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1056–1079. [Google Scholar] [CrossRef] [PubMed]
- Mazurkiewicz, J.; Tomasik, P. Intermolecular interactions in water-1,4-dioxane—Organic solvent ternary mixtures. Bull. Soc. Chim. Belg. 1993, 102, 441–446. [Google Scholar] [CrossRef]
- Baranowska, H.M.; Sikora, M.; Krystyjan, M.; Tomasik, P. Contribution to understanding gelatinization of granular starch in starch recent advances in biopolymer science and technology. In Polish Society of Food Technologists, Małopolska Branch; Fiedorowicz, M., Bertoft, E., Eds.; Polish Academy of Sciences: Krakow, Poland, 2010; pp. 13–28. [Google Scholar]
- Tomasik, P. Specific physical and chemical properties of potato starch. In Food 3 (Special Issue 1); Teixeira da Silva, J., Ed.; Global Science Books: New York, NY, USA, 2009; pp. 45–56. [Google Scholar]
- The European Parliament and the Council of the European Union. Directive (EU) 2018/2001 on Promotion of the Use of Energy from Renewable Sources. Off. J. Eur. Union 2018, L328/82–L328/209. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32018L2001 (accessed on 17 April 2023).
- Bruckner, M.; Häyhä, T.; Giljum, S.; Maus, V.; Fischer, G.; Tramberend, S.; Börner, J. Quantifying the global cropland footprint of the European Union’s non-food bioeconomy. Environ. Res. Lett. 2019, 14, 45011. [Google Scholar] [CrossRef]
- Klein, A. Albumins in Biological Encyclopedy; Otałęga, Z., Ed.; Agencja Publicystyczno-Wydawnicza: Krakow, Poland, 1998. [Google Scholar]
- McGee, H. On Food and Cooking: The Science and Lore of the Kitchen; Scribner Book Company: New York, NY, USA, 2004. [Google Scholar]
- National Libray of Medicine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Stearic-acid#section=Vapor-Pressure (accessed on 17 April 2023).
- National Libray of Medicine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Oleic-acid (accessed on 17 April 2023).
- Galus, S.; Kadzińska, J. Whey protein edible films modified with almond and walnut oils. Food Hydrocoll. 2016, 52, 78–86. [Google Scholar] [CrossRef]
- Mayachiew, P.; Devahastin, S. Effects of drying methods and conditions on release characteristics of edible chitosan films enriched with Indian gooseberry extract. Food Chem. 2010, 118, 594–601. [Google Scholar] [CrossRef]
- Fakhouri, F.M.; Fontes, L.C.; Innocentini-Mei, L.H.; Collares-Queiroz, F.P. Effect of fatty acid addition on the properties of biopolymer films based on lipophilic maize starch and gelatin. Starke 2009, 61, 528–536. [Google Scholar] [CrossRef]
- Kester, J.; Fennema, O. Edible films and coatings: A review. Food Technol. 1986, 40, 47–59. [Google Scholar]
- Gontard, N.; Duchez, C.; Cuq, J.L.; Guilbert, S. Edible composite films of wheat gluten and lipids: Water vapor permeability and other physical properties. Int. J. Food Sci. Technol. 1994, 29, 39–50. [Google Scholar] [CrossRef]
- Krystyjan, M.; Khachatryan, G.; Grabacka, M.; Krzan, M.; Witczak, M.; Grzyb, J.; Woszczak, L. Physicochemical, Bacteriostatic, and Biological Properties of Starch/Chitosan Polymer Composites Modified by Graphene Oxide, Designed as New Bionanomaterials. Polymers 2021, 13, 2327. [Google Scholar] [CrossRef]
- Khachatryan, G.; Khachatryan, K.; Szczepankowska, J.; Krzan, M.; Krystyjan, M. Design of Carbon Nanocomposites Based on Sodium Alginate/Chitosan Reinforced with Graphene Oxide and Carbon Nanotubes. Polymers 2023, 15, 925. [Google Scholar] [CrossRef] [PubMed]
- Stanisławska, N.; Khachatryan, G.; Khachatryan, K.; Krystyjan, M.; Makarewicz, M.; Krzan, M. Formation and Investigation of Physicochemical and Microbiological Properties of Biocomposite Films Containing Turmeric Extract Nano/Microcapsules. Polymers 2023, 15, 919. [Google Scholar] [CrossRef] [PubMed]
- Janik, M.; Khachatryan, K.; Khachatryan, G.; Krystyjan, M.; Oszczęda, Z. Comparison of Physicochemical Properties of Silver and Gold Nanocomposites Based on Potato Starch in Distilled and Cold Plasma-Treated Water. Int. J. Mol. Sci. 2023, 24, 2200. [Google Scholar] [CrossRef] [PubMed]
- Dick, M.; Costa, T.M.H.; Gomaa, A.; Subirade, M.; de Oliveira Rios, A.; Flôres, S.H. Edible film production from chia seed mucilage: Effect of glycerol concentration on its physicochemical and mechanical properties. Carbohydr. Polym. 2015, 130, 198–205. [Google Scholar] [CrossRef]
- Tapia-Blácido, D.; Mauri, A.N.; Menegalli, F.C.; Sobral, P.J.; Añón, M.C. Contribution of the starch, protein, and lipid fractions to the physical, thermal, and structural properties of amaranth (Amaranthus caudatus) flour films. J. Food Sci. 2007, 72, E293–E300. [Google Scholar] [CrossRef]
- Grzebieniarz, W.; Nowak, N.; Khachatryan, G.; Krzan, M.; Krystyjan, M.; Kosiński, J.; Khachatryan, K. The Preparation and Characterization of Quantum Dots in Polysaccharide Carriers (Starch/Chitosan) as Elements of Smart Packaging and Their Impact on the Growth of Microorganisms in Food. Materials 2021, 14, 7732. [Google Scholar] [CrossRef]
- Basiak, E.; Galus, S.; Lenart, A. Characterisation of composite edible films based on wheat starch and whey-protein isolate. Int. J. Food Sci. Technol. 2015, 50, 372–380. [Google Scholar] [CrossRef]
- Macdougall, D. Colour measurement of food: Principles and practice. Colour Meas. 2010, 50, 312–342. [Google Scholar] [CrossRef]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.J. Colour Measurement and Analysis in Fresh and Processed Foods: A Review. Food Bioprocess Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Shiku, Y.; Hamaguchi, P.Y.; Benjakul, S.; Visessanguan, W.; Tanaka, M. Effect of surimi quality on properties of edible films based on Alaska pollack. Food Chem. 2004, 86, 493–499. [Google Scholar] [CrossRef]
- Piermaria, J.; Bosch, A.; Pinotti, A.; Yantorno, O.; Garcia, M.A.; Abraham, A.G. Kefiran films plasticized with sugars and polyols: Water vapor barrier and mechanical properties in relation to their microstructure analyzed by ATR/FT-IR spectroscopy. Food Hydrocoll. 2011, 25, 1261–1269. [Google Scholar] [CrossRef]
- Chao, C.; Yu, J.; Wang, S.; Copeland, L.; Wang, S. Mechanisms underlying the formation of complexes between maize starch and lipids. J. Agric. Food Chem. 2018, 66, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Yang, H.; Chi, C.; Ma, X. Effect of protein types on structure and digestibility of starch-protein-lipids complexes. LWT 2020, 134, 110175. [Google Scholar] [CrossRef]
- Singh, V.; Ali, S.Z.; Somashekar, R.; Mukherjee, P.S. Nature of crystallinity in native and acid modified starches. Int. J. Food Prop. 2006, 9, 845–854. [Google Scholar] [CrossRef]
- Jivan, M.J.; Yarmand, M.; Madadlou, A. Preparation of cold water-soluble potato starch and its characterization. J. Food Sci. Technol. 2014, 51, 601–605. [Google Scholar] [CrossRef]
- Kowsik, P.V.; Mazumder, N. Structural and chemical characterization of rice and potato starch granules using microscopy and spectroscopy. Microsc. Res. Tech. 2018, 81, 1533–1540. [Google Scholar] [CrossRef]
- Rodriguez-Pineda, L.M.; Munoz-Prieto, E.; Rius-Alonso, C.A.; Palacios-Alquista, J. Preparation and characterization of potato starch microparticles with acrylamide by micro wave radiation. Cienc. Desarro. 2018, 9, 149–159. [Google Scholar]
- Singh, V.; Tiwari, A.; Pandey, S.; Singh, S.K. Microwave-accelerated synthesis and characterization of potato starch-g-poly(acrylamide). Starke 2006, 58, 536–543. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Fernando, A.L.; Pires, J.R.A.; Rodrigues, P.F.; Lopes, A.A.S.; Fernandes, F.M.B. Physical properties of chitosan films incorporated with natural antioxidants. Ind. Crops Prod. 2017, 107, 565–572. [Google Scholar] [CrossRef]
- Sant’Anna, V.; Gurak, P.D.; Marczak, L.D.F.; Tessaro, I.C. Tracking bioactive compounds with colour changes in foods–A review. Dyes Pigm. 2013, 98, 601–608. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Britton, G.; Vicario, I.M.; Heredia, F.J. Colour and carotenoid profile of Spanish Valencia late ultrafrozen orange juices. Food Res. Int. 2005, 38, 931–936. [Google Scholar] [CrossRef]
- ISO 527-1:2019; Determination of Tensile Properties–Part 1: General Principles. ISO: Geneva, Switzerland, 2019. Available online: https://www.iso.org/standard/75824.html (accessed on 18 April 2023).
- Hulleman, S.H.D.; Kalisvaart, M.G.; Janssen, F.H.P.; Feil, H.; Vliegenthart, J.F.G. Origins of B-type crystallinity in glycerol-plasticised, compression-moulded potato starches. Carbohydr. Polym. 1999, 39, 351–360. [Google Scholar] [CrossRef]
- Kliem, S.; Kreutzbruck, M.; Bonten, C. Review on the Biological Degradation of Polymers in Various Environments. Materials 2020, 13, 4586. [Google Scholar] [CrossRef] [PubMed]
- Gaňa, D.; Markovičová, L.; Liptáková, T. Evaluation of the protective PE foils properties after exposure in various environments. IOP Conf. Ser. Mater. Sci. Eng. 2020, 776, 012088. [Google Scholar] [CrossRef]

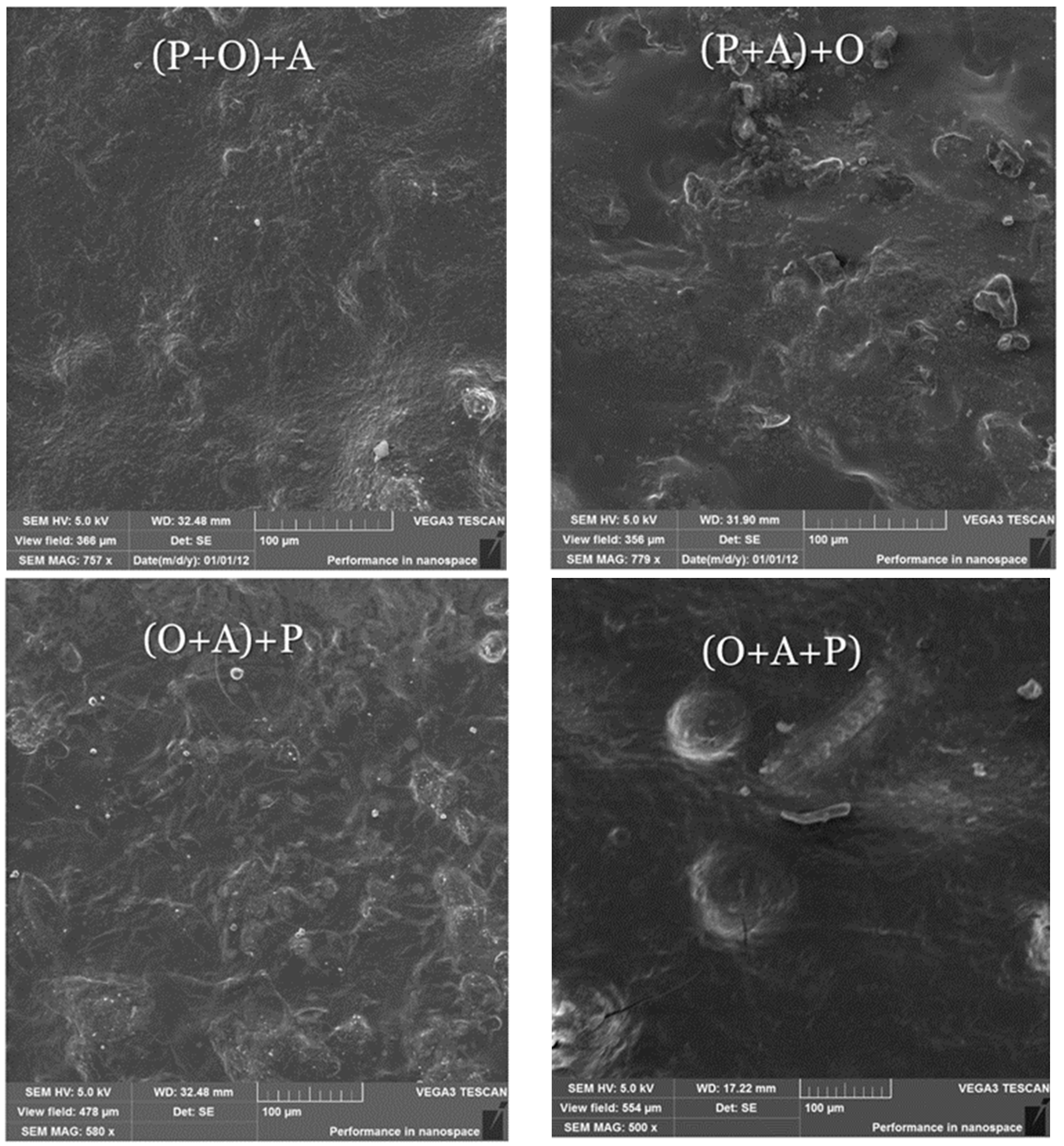

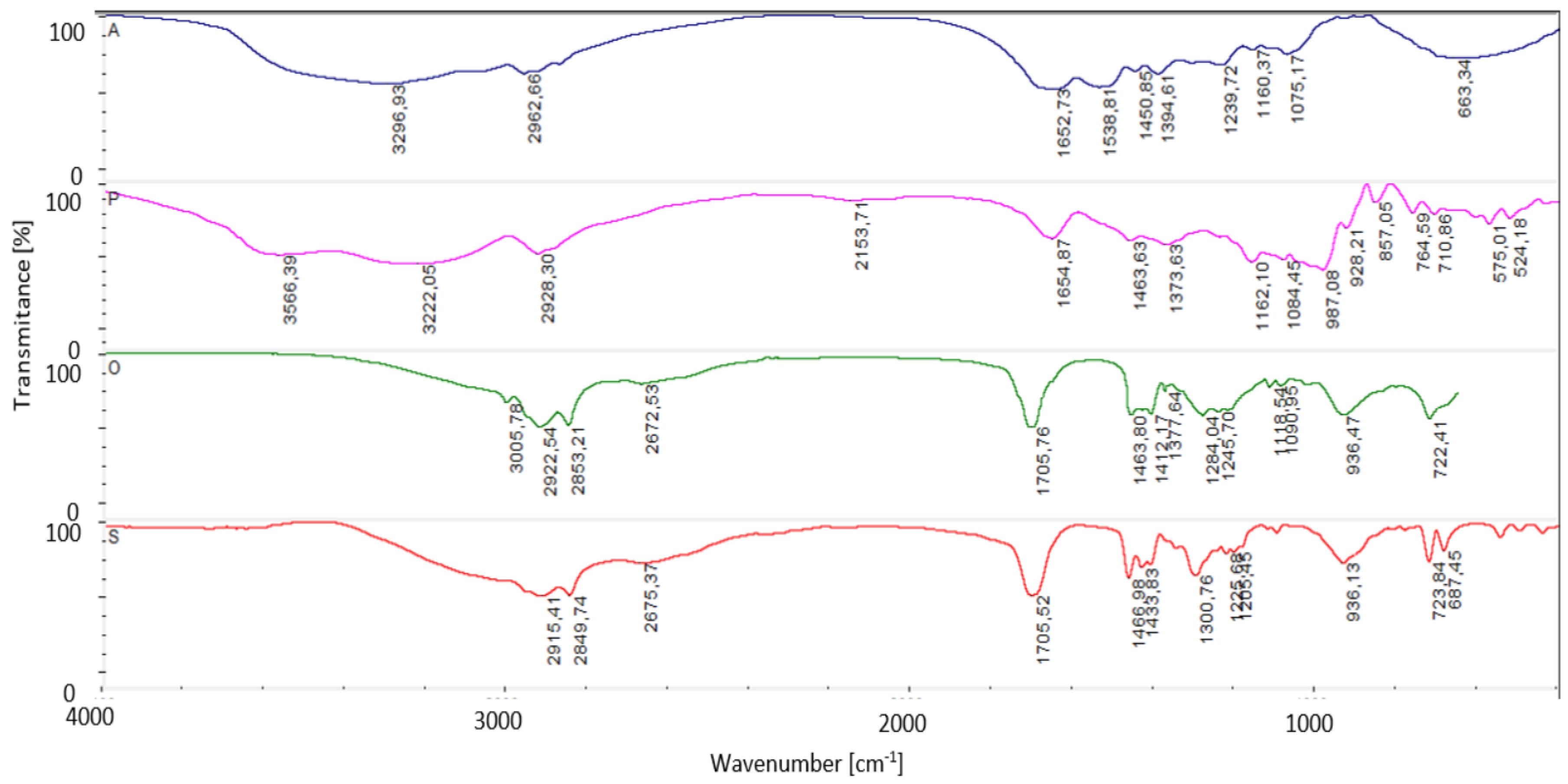
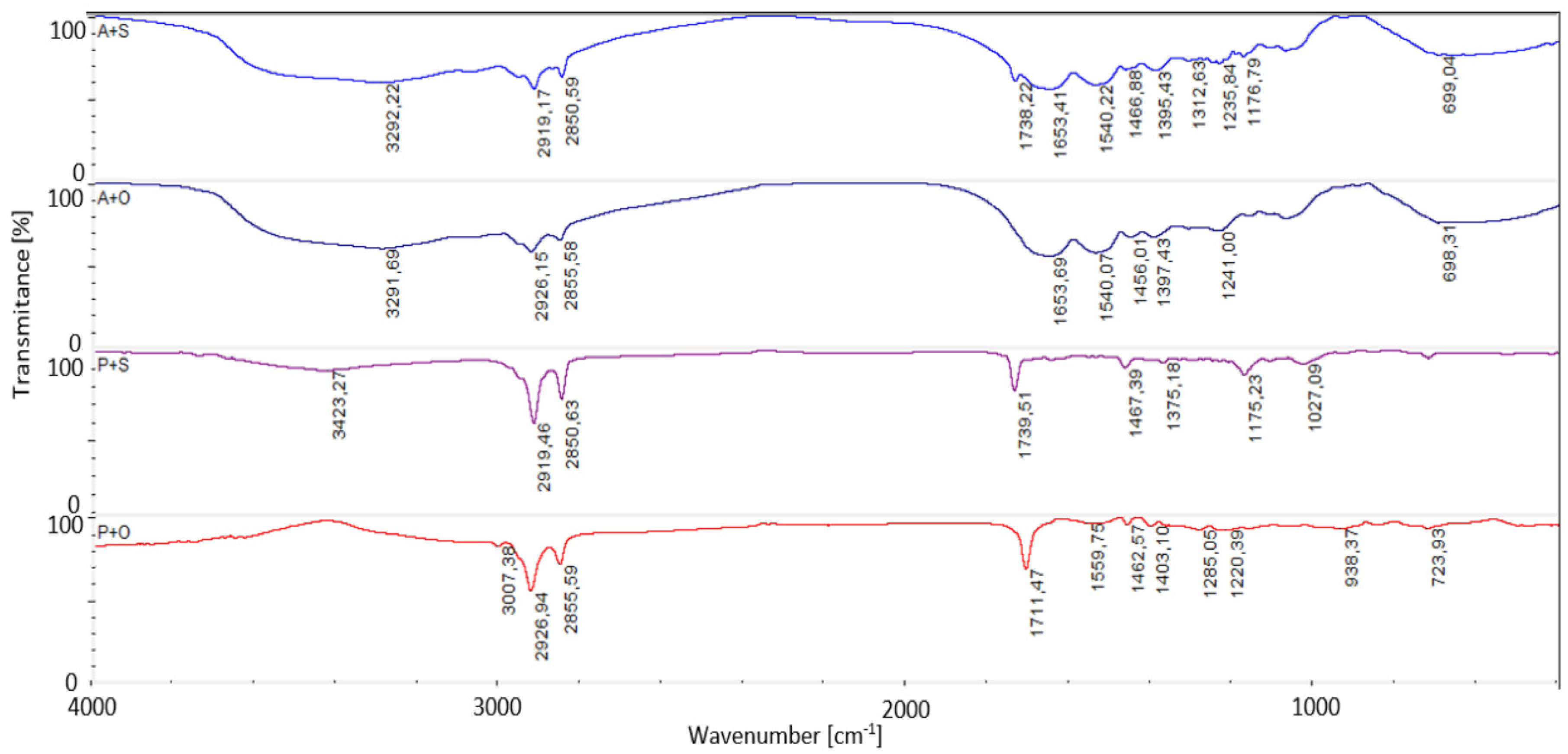
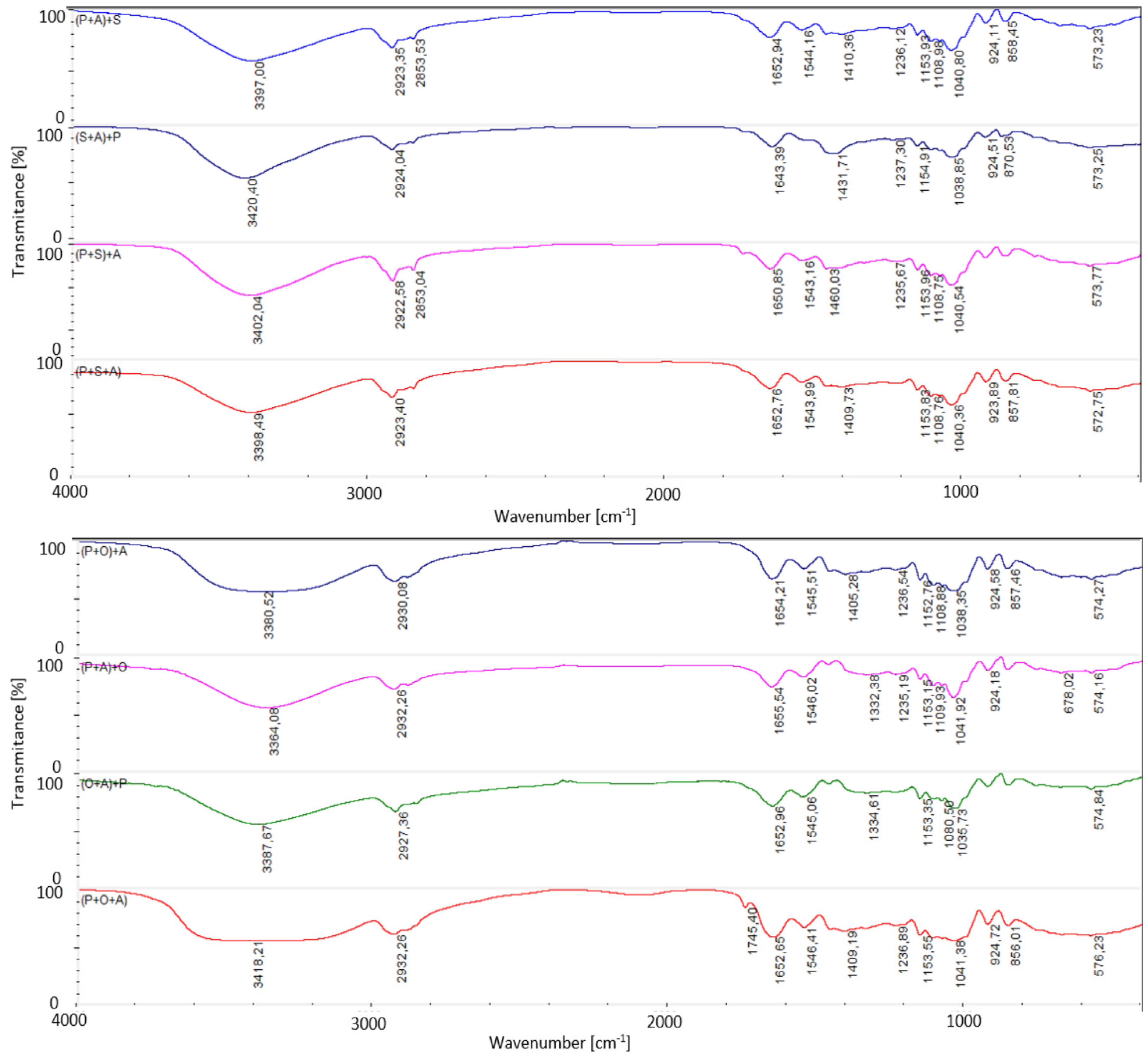
| Foil * | Water Content [%] | Solubility [%] | Swelling Degree [%] |
|---|---|---|---|
| (P+A+S) | 8.75 ± 0.06 c | 53.26 ± 0.09 d | 179.25 ± 0.62 b |
| (P+A)+S | 9.19 ± 0.03 a | 54.28 ± 0.11 c | 119.99 ± 0.98 d |
| (S+A)+P | 9.07 ± 0.05 b | 61.59 ± 0.12 a | 188.97 ± 0.75 a |
| (S+P)+A | 8.53 ± 0.07 d | 57.06 ± 0.17 b | 160.81 ± 1.12 c |
| (P+A+O) | 8.62 ± 0.06 d | 41.26 ± 0.08 g | 81.15 ± 0.68 h |
| (O+A)+P | 8.78 ± 0.03 c | 54.03 ± 0.16 c | 88.85 ± 1.17 g |
| (P+A)+O | 9.27 ± 0.07 a | 47.06 ± 0.13 e | 102.22 ± 1.06 e |
| (O +P)+A | 9.06 ± 0.03 b | 45.02 ± 0.14 f | 98.01 ± 0.85 f |
| Foil | L*(D65) | a*(D65) | b*(D65) | C* | h* |
|---|---|---|---|---|---|
| (P+A+S) | 86.12 ± 0.23 d | −0.61 ± 0.04 a | 7.75 ± 0.43 d | 7.77 ± 0.43 d | 94.52 ± 0.32 d |
| (P+A)+S | 89.19 ± 0.67 a | −0.70 ± 0.04 b | 8.95 ± 0.43 b | 8.97 ± 0.44 b | 94.50 ± 0.08 d |
| (S+A)+P | 87.12 ± 0.16 c | −0.64 ± 0.05 a | 8.19 ± 0.43 cd | 8.22 ± 0.43 cd | 94.50 ± 0.16 d |
| (S+P)+A | 87.93 ± 0.23 b | −0.93 ± 0.05 c | 10.21 ± 0.42 a | 10.25 ± 0.42 a | 95.23 ± 0.23 c |
| (P+A+O) | 83.12 ± 0.21 f | −0.88 ± 0.02 c | 8.72 ± 0.18 b | 8.76 ± 0.18 b | 95.76 ± 0.21 b |
| (O+A)+P | 86.89 ± 0.26 c | −0.73 ± 0.02 b | 8.64 ± 0.33 bc | 8.67 ± 0.32 bc | 94.86 ± 0.27 c,d |
| (P+A)+O | 88.94 ± 0.19 a | −1.00 ± 0.04 d | 8.80 ± 0.36 b | 8.86 ± 0.36 b | 96.51 ± 0.32 a |
| (O+P)+A | 84.63 ± 0.42 e | −0.60 ± 0.10 a | 7.73 ± 0.32 d | 7.76 ± 0.31 d | 94.49 ± 0.88 d |
| Foil * | Thickness [mm] | Tensile Strength [MPa] | Relative Elongation [%] |
|---|---|---|---|
| (P+A+S) | 0.438 ± 0.003 e | 28.43 ± 0.05 c | 13.14 ± 0.01 e |
| (P+A)+S | 0.483 ± 0.001d | 16.80 ± 0.52 e | 13.86 ± 0.05 d |
| (P+S)+A | 0.575 ± 0.001 b | 12.61 ± 0.13 f | 8.29 ± 0.02 g |
| (A+S)+P | 0.643 ± 0.003 a | 10.25 ± 0.10 g | 5.93 ± 0.02 h |
| (P+A+O) | 0.408 ± 0.004 f | 6.84 ± 0.04 h | 8.73 ± 0.01 f |
| (P+O)+A | 0.520 ± 0.003 c | 29.61 ± 0.12 b | 30.67 ± 0.02 b |
| (P+A)+O | 0.570 ± 0.020 b | 25.58 ± 0.37 d | 15.61 ± 0.04 c |
| (A+O)+P | 0.533 ± 0.050 c | 31.70 ± 0.10 a | 39.86 ± 0.02 a |
| Foil | Weight Loss | |||||
|---|---|---|---|---|---|---|
| Step I | Step II | |||||
| Temp. of Beginning [°C] | [mg] | [%] | Temp. of Beginning [°C] | [mg] | [%] | |
| (P+A)+S | 145 | 3.3697 | 52.2310 | 277 | 1.4805 | 22.9899 |
| (P+S)+A | 108 | 1.2479 | 54.4928 | 280 | 0.5540 | 24.1908 |
| (S+A)+P | 104 | 3.8324 | 53.9017 | 276 | 1.8304 | 25.7436 |
| (P+A+S) | 100 | 1.7385 | 47.6299 | 245 | 1.0286 | 28.1807 |
| (P+A)+O | 120 | 1.7509 | 47.9691 | 258 | 0.9526 | 26.0982 |
| (P+O)+A | 165 | 3.8784 | 47.0680 | 281 | 2.2496 | 27.3008 |
| (O+A)+P | 115 | 2.7618 | 38.5148 | 236 | 2.5306 | 35.2943 |
| (P+O+A) | 110 | 0.4886 | 29.9743 | 220 | 0.6530 | 40.0590 |
| Foil ** | Thickness [mm] | Tensile Strength [MPa] | Relative Elongation [%] |
|---|---|---|---|
| (P+A+S) | 0.407 ± 0.002 (−9.3%) f | 27.23 ± 0.04 (−9.2%) c | 11.20 ± 0.01 (−8.7) d |
| (P+A)+S | 0.452 ± 0.001 (−9.35%) e | 15.43± 0.13 (−9.2%) e | 10.74 ± 0.05 (−7.7%) e |
| (P+S)+A | 0.531 ± 0.001 (−9.2%) b | 11.13 ± 0.11 (−8.6%) f | 6.94 ± 0.02 (−8.4%) f |
| (A+S)+P | 0.587 ± 0.001 (−9.1%) a | 9.59 ± 0.08 (−9.4%) g | 4.63 ± 0.02 (- 7.8%) h |
| (P+A+O) | 0.369 ± 0.002 (−9.0%) g | 6.42 ± 0.03 (−9.3%) h | 6.23 ± 0.01 (−7.1%) g |
| (P+O)+A | 0.492 ± 0.004 (−9.5%) d | 28.69 ± 0.05 (−9.7%) b | 24.74 ± 0.02 (- 8.1%) b |
| (P+A)+O | 0.528 ± 0.007 (−9.2%) b | 24.82 ± 0.05 (−9.8) d | 12.32 ± 0.04 (−7.9%) c |
| (A+O)+P | 0.518 ± 0.009 (−9.7%) c | 30.82 ± 0.06 (−9.7) a | 33.42 ± 0.02 (−8.4%) a |
| Foil | Water Content [%] | Solubility [%] | Swelling Degree [%] |
|---|---|---|---|
| (P+A+S) | 8.32 ± 0.03 (−9.5%) c,d | 49.52 ± 0.06 (−9.3%) e | 173.11 ± 0.32 (−9.7%) b |
| (P+A)+S | 8.54 ± 0.05 (−9.3%) b | 51.11 ± 0.09 (−9.4%) d | 117.45 ± 0.68 (−9.8%) d |
| (S+A)+P | 8.31 ± 0.03 (−9.2%) c,d | 58.94 ± 0.05 (−9.6%) a | 183.36 ± 0.37 (−9.7%) a |
| (S+P)+A | 8.25 ± 0.02 (−9.6%) c,d,e | 53.24 ± 0.08 (−9.3%) b | 154.52 ± 0.65 (−9.6%) c |
| (P+A+O) | 8.19 ± 0.04 (−9.6%) e | 38.28 ± 0.09 (−9.3%) h | 82.62 ± 0.33 (−10.1%) h |
| (O+A)+P | 8.22 ± 0.06 (−9.4%) d,e | 52.18 ± 0.08 (−9.5%) c | 84.35 ± 0.26 (−9.5%) g |
| (P+A)+O | 8,73 ± 0.05 (−9.4%) a | 46.23 ± 0.06 (−9.8%) f | 96.11 ± 0.66 (−9.4%) e |
| (O+P)+A | 8.35 ± 0.08 (−9.2%) c | 44.22 ± 0.09 (−9.8%) g | 92.21 ± 0.49 (−9.4%) f |
| Foil | L*(D65) | a*(D65) | b*(D65) | C* | Hue Angle |
|---|---|---|---|---|---|
| (P+A+S) | 87.02 ± 0.21 c | −0.56 ± 0.03 a | 8.12 ± 0.32 e | 8.14 ± 0.32 e | 93.91 ± 0.06 e |
| (P+A)+S | 89.23 ± 0.45 a | −0.63 ± 0.02 c,d | 9.23 ± 0.25 b | 9.25 ± 0.25 b | 93.92 ± 0.11 e |
| (S+A)+P | 87.18 ± 0.11 c | −0.61 ± 0.05 b,c | 8.34 ± 0.36 d,e | 8.36 ± 0.36 d,e | 94.20 ± 0.18 d,e |
| (S+P)+A | 88.03 ± 0.21 b | −0.87 ± 0.04 f | 10.68 ± 0.48 a | 10.71 ± 0.48 a | 94.74 ± 0.32 c |
| (P+A+O) | 83.22 ± 0.20 e | −0.81 ± 0.05 e | 8.61 ± 0.26 c,d | 8.64 ± 0.26 c,d | 95.35 ± 0.17 b |
| (O+A)+P | 87.09 ± 0.21 c | −0.68 ± 0.03 d | 8.84 ± 0.28 c | 8.87 ± 0.28 c | 94.38 ± 0.31 d |
| (P+A)+O | 89.04 ± 0.15 a | −0.97 ± 0.02 g | 9.23 ± 0.18 b | 9.28 ± 0.17 b | 95.99 ± 0.06 a |
| (O+P)+A | 85.02 ± 0.36 d | −0.57 ± 0.05 a,b | 7.96 ± 0.11 e | 7.98 ± 0.11 e | 94.13 ± 0.36 de |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Folentarska, A.; Kulakowska, A.; Pavlyuk, V.; Krystyjan, M.; Tomasik, P.; Ciesielski, W. Fully Biodegradable Edible Packaging Foils on the Basis of Potato Starch–Lipid–Protein Ternary Complexes. Macromol 2023, 3, 723-741. https://doi.org/10.3390/macromol3040041
Folentarska A, Kulakowska A, Pavlyuk V, Krystyjan M, Tomasik P, Ciesielski W. Fully Biodegradable Edible Packaging Foils on the Basis of Potato Starch–Lipid–Protein Ternary Complexes. Macromol. 2023; 3(4):723-741. https://doi.org/10.3390/macromol3040041
Chicago/Turabian StyleFolentarska, Agnieszka, Anna Kulakowska, Volodymyr Pavlyuk, Magdalena Krystyjan, Piotr Tomasik, and Wojciech Ciesielski. 2023. "Fully Biodegradable Edible Packaging Foils on the Basis of Potato Starch–Lipid–Protein Ternary Complexes" Macromol 3, no. 4: 723-741. https://doi.org/10.3390/macromol3040041
APA StyleFolentarska, A., Kulakowska, A., Pavlyuk, V., Krystyjan, M., Tomasik, P., & Ciesielski, W. (2023). Fully Biodegradable Edible Packaging Foils on the Basis of Potato Starch–Lipid–Protein Ternary Complexes. Macromol, 3(4), 723-741. https://doi.org/10.3390/macromol3040041








