Abstract
The food industry produces an exorbitant amount of solid waste of petrochemical origin as a result of the increase in the development of new products. Natural polymers are an alternative to this theme; however, their development with adequate properties is a challenge. The union of different polymers in the synthesis of packaging is usually carried out to improve these properties. The combination of agar-agar and chitosan biopolymers show particular advantages through hydrogen bonds and electrostatic attraction between oppositely charged groups, presenting a promising source of studies for the synthesis of green packaging. When combined with natural extracts with active properties, these polymers allow an increase in the microbiological stability of foods associated with lower chemical preservative content and greater environmental sustainability.
1. Introduction
Many materials are used in the elaboration of different types of packaging, such as glass, metals, plastics, and wood, among others, in addition to combinations of more than one material, such as composites or blends [1,2]. When no longer used, they generate waste; while some are destined for recycling, the vast majority are destined for municipal landfills, generating environmental concern due to the time they take to decompose [3].
With growing population and development, the search for new products and advanced technology has generated the need for production of packaging [4]. Solid waste takes hundreds and thousands of years to decompose in the environment, causing an environmental crisis and incurring economic and social problems [5]. It is estimated that around 300 million tons of plastic packaging are deposited in landfills annually [6]. In addition to overcrowding, improper disposal causes the death of hundreds of animals who end up consuming packaging. The food industry is the sector that most contributes to the generation of waste, as it represents around 50% by weight of the total packaging sold [7].
An alternative for the reduction of synthetic materials harmful to the environment is the use of biopolymers obtained from natural sources, which can be extracted from agro-industrial residues, plants, and the biomass of microorganisms, among other sources [8]. However, the development of new materials that present adequate properties to make up alternatives to synthetic packaging is a challenge, mainly in relation to mechanical and physicochemical properties, as they commonly present inferior properties to synthetic polymers [9].
Agar-agar is a natural polymer extracted from red algae which has numerous applications in the food industry and is highly sensitive to water [10]. Chitosan is another natural polymer; it is obtained from the deacetylation of chitin, extracted mainly from shrimp shells, and is widely used in several areas. It has good chemical properties, and stands out for being hydrophobic [11]. The union of these polymers to compose a blend has been little explored in the current literature, and presents potential for the synthesis of food packaging.
The degradation rate of packaging developed with biodegradable materials is much higher compared to those made with conventional materials [12]. Along with the reduction in waste generation, an increase in the shelf life of food products can be achieved by the use of active biodegradable packaging [13] through the incorporation of antimicrobials. Natural antimicrobials have been widely highlighted, as their use can efficiently reduce and/or inhibit microbial development, a phenomenon that is linked to the synthesis of environmentally friendly food packaging [14,15].
According to a survey carried out by the International Food Information Council Foundation (IFICF), consumers are more aware of the environment and healthy eating; of those interviewed, 69% showed an interest in buying foods with only natural nutrients in their formulation and reported that sustainable production is one of the main factors in choosing a food product [16]. The present article aims to review the properties of the natural polymers agar-agar and chitosan, highlighting the contribution of the combination of their properties in the characteristics of a functional film with the potential for use as food packaging. In addition, we provide a brief presentation of natural extracts with active functionality.
2. Food Packaging
Food packaging is intended to allow transport, distribution, and handling, ensuring protection against shocks and compression. It works by minimizing product losses due to deterioration through the control of humidity, oxygen, light, and microbial development by acting as a barrier to the surrounding atmosphere [1].
Packaging must have performance compatible with its functionality, meeting the four basic functions of protection, communication, convenience, and containment while taking into account the characteristics of each product. It requires good mechanical strength, flexibility, and elasticity in order to avoid tears and perforations during all stages of production, storage, and marketing of the product [2].
Packaging is considered a vehicle for selling and promoting the brand, as it is the consumer’s first contact with the product, becoming one of the main characteristics for the decision at the time of purchase [17]. The packaging must be composed of inert material to ensure that there is no migration of its compounds to the food and that it does not pose a risk to the consumer’s health and/or change its sensory characteristics [15].
The vast majority of food packaging originates from polymers of petrochemical origin, which are popular due to their flexibility and lightness; however, petrochemical products represent a non-renewable resource. Thus, their use results in socioeconomic problems such as increased oil prices and the generation and accumulation of waste that can take tens or hundreds of years to decompose in nature [7].
The production of solid waste from food packaging has grown at a rate of 4.2% per year since 2010. It is estimated that this will continue until at least 2024 [18]. Considering all of the materials used in the development of these packaging, plastic corresponds to the largest share. Single-use packaging accounts for an important share of the millions of tons of plastics that end up in the oceans annually [19]. In 2018, 1.130 billion items of food and beverage packaging were sent to landfills in the European Union alone [20].
The consumption of plastic film has increased greatly in recent years; in combination with their long period of decomposition, phthalic acid esters (PAEs) are used as a plasticizer in the production of polyvinyl chloride (PVC) plastic films, and have a carcinogenic effect to living beings [21]. PVC plastic films are widely used on a daily basis, and in view of this problem, alternative means have been sought to reduce such impacts through the development of bioplastics and through the search for new technologies [8].
3. Biopolymers
Biopolymers are compounds of natural origin that are precursors in the synthesis of bioplastic materials. According to the European Bioplastic Association (EBA), bioplastics are defined as plastics that are biodegradable, based on renewable resources, or based on biological materials [22]. These materials decompose in the presence of carbon dioxide, methane, water, and biomass through the enzymatic action of microorganisms, being able to decompose at the same rate as other known compostable materials. The initial stage of composting takes place through an abiotic process, that is, based on thermal conditions, and the fragments from this stage of decomposition must be completely used by microorganisms [23].
The food packaging industry seeks biodegradable alternatives in order to improve its sustainability, and has been investing in these natural materials, where current studies have been mainly focused on techniques for synthesizing packaging from these materials. However, in the course of an entire investigation, many issues remain, such as large-scale adoption, and certain properties are still restricted [15].
A number of the limitations regarding the material properties of these materials are related to fragility, thermal instability, low impact resistance, and high permeability to water vapor and oxygen; when used in fresh foods they can be susceptible to moisture loss, which can change the sensory properties of the product [24]. It is in this context that studies are currently focused, largely based on investigating strategies for improving the material properties to ensure that these new materials can resist the possible treatments required in the food industry while maintaining the sensory properties of food for longer [15].
Among the various materials used in the synthesis of bioplastic films, starch, cellulose, gums, chitosan, and pectins are popular, among which each has specific properties related to the elaboration of bioplastic materials [25]. In general, materials based on polysaccharides such as starch, chitosan, and carrageenan have limitations in terms of their mechanical properties while having low permeability to gases [26], When the base is a protein, such as casein or collagen, the mechanical properties are acceptable, while the physical characteristics are lacking [27]. Lipid-based materials, on the other hand, have an excellent moisture barrier, but are sensitive to oxidation [9].
For this reason, mixtures or blends of these biopolymers have been researched in the elaboration of bioplastic films in order to find improvements in the final characteristics. Such blends are able to present a wide range of structures with different properties, allowing for their characteristics to be directed towards the desired application [6]. The incorporation of additives such as plasticizers helps to improve the final characteristics, as they are low molecular weight molecules that act to modify the three-dimensional structure, reducing intermolecular forces, increasing the mobility of polymer chains, and decreasing permeability to gases [28].
3.1. Agar-Agar
Agar-agar is a biopolymer belonging to the natural polysaccharides extracted from red algae of the Rhodophyta class, being the structural carbohydrate of the wall of these cells. It is composed of agarose, which has a straight chain, and agaropectin, which has a branched chain, linked together by bonds α- (1 → 3) e β- (1 → 4) [29]. It is an attractive biopolymer due to its chemical structure, resistance to acids, and ability to form a consistent gel even at low concentrations, favoring its application in several industrial areas [30].
In view of its various applications, the food sector stands out, where it is used as a thickener and in food packaging [31]. In agriculture, it acts as a soil conditioner and water absorber, and is very efficient in places with little water availability [32], while in medicine it is used in the microencapsulation of medicines and bioactive compounds [33]. In addition to having a highly porous matrix, it is interesting for particle trapping [29]. Films based on this polymer, however, are brittle in nature, have poor mechanical properties, and are highly sensitive to water, as they are hydrophilic in nature, which limits their application with high-moisture products [10]. However, there are studies that have used blending or reinforcement of this polymer for a final result with different characteristics, whether chemical, physical, or mechanical. Jridi et al. [34] used a combination of gelatin and agar, resulting in mechanically stronger films. Wongphan and Harnkarnsujarit [35] obtained improvements in the solubility of the film when developed using mixtures of starch, agar-agar, and maltodextrin.
3.2. Chitosan
Chitosan is derived from chitin, and was discovered in 1859 when Rouget cooked chitin (itself discovered in 1811) in potassium hydroxide and found that it became soluble in organic acids [36]. Chitin is the second most abundant natural polymer in nature, and is obtained mainly from crab and shrimp shell residues, generally used in seafood industries. However it can be obtained from several other sources as well, such as mollusc shells, fungal cell walls and membranes, cell walls of algae, and the exoskeletons of insects and arachnids [36].
The deacetylation of chitin occurs by the transformation of acetamide (NHCO3) into amine (NH2) in a basic medium, being produced under different degrees of deacetylation and molecular weights that vary based on the alkaline concentration, time, and temperature used in the process [37].
Chitosan is the only polysaccharide of alkaline nature, the others being of acidic or neutral origin. It is a non-toxic, biocompatible, and biodegradable compound, and is absorbed by the body [26]. Chitosan’s properties are directly linked to its molecular weight, degree of deacetylation, and degree of crystallinity. Properties such as viscosity, solubility, tensile strength, and elongation are influenced by molecular weight, which corresponds to the number of sugar units per polymer molecule; the viscosity of chitosan solution is increased with increasing degree of deacetylation [37]. Chitosan polymers are aminopolysaccharides with unique structures, having several properties and high functionality, and can be applied to many diverse areas, both industrial and biomedical [38]. It is one of the most promising polymers of biological origin, and can be used as a food additive in the diet [39], in medicines, where it has great potential as an antacid and for protecting the stomach from other drugs in addition to acting as a transporter and drug releaser in the human body [40], and in cosmetics for the treatment of hair and skin, where it acts as a hydrating agent and has the ability to adhere to fragrance [41]. It has reported antiviral properties [42], in addition to acting as an antimicrobial agent. In this context, it acts on the external surface of bacteria, such as the cell walls of Gram-negative microorganisms (composed of lipopolysaccharides), on the peptidoglycan associated with teichoic acid, and on the cell membranes of Gram-positive bacteria [43].
However, chitosan has disadvantages for applications in bioplastic films when used as the sole source of polymer, as it has low solubility, which does not allow for interaction with other compounds often used to make films, such as plasticizers [21,40]. The union of chitosan with other polymers, however, results in a film with excellent characteristics [36]. Ghaderi et al. [44] obtained improvements in the barrier properties and solubility of films based on chitosan and vinyl alcohol when fish gelatin was added. Mendes et al. [45] produced films with better extensibility and thermal stability using a mixture of chitosan and corn starch. Li et al. [21] developed chitosan and sodium alginate films with good mechanical and hydrophobic properties as well as high light blocking capacity.
4. Impact of the Formulation of Bioplastics on Their Properties
The compatibility of the mixture of two or more materials is a great challenge; when this interaction is achieved, whether between polymers of petrochemical origin or of biological origin such as biopolymers, the resulting polymers have high potential for various applications. The interaction of these compounds enables a range of physicochemical, mechanical, and barrier characteristics [21]. When other compounds are used, such as extracts with active properties, the possibility of altering these characteristics is even greater due to the different bonds between the compounds and the polymer matrix and in the matrix itself [13].
Mir et al. [46] described a number of these changes, mainly with respect to the thickness, water vapor permeability, tensile strength, solubility, and barrier properties. Thickness is a parameter that directly influences the physical, mechanical, and barrier properties of a film; generally, the addition of different extracts provides a thicker film due to the increase in the solid content added, sometimes changing the crystallinity of the polymer structure [47].
Mechanical properties such as tensile strength and elongation at break are very important in film properties, as they allow the resulting film to have adequate strength for maintaining the integrity of the packaged products during transport, handling, and storage. Tensile strength is the maximum force that a film can resist before breaking, while elongation is the maximum flexibility of the film before breaking. The addition of natural extracts influences these properties due to their binding interactions; thus, the origin of the extract and the polymer matrix interfere, acquiring different combinations [13]. Tan et al. [48] obtained more amorphous films and with lower tensile strength when adding grapefruit seed extract, while Siripatrawan and Harte [49] obtained an increase in tensile strength and elongation when adding green tea to a chitosan matrix; however, these properties were reduced when using an agar-gelatin matrix.
Barrier properties are one of the most important properties for application in food, as they determines the shelf life of the product based on the permeability of the packaging to water vapor. This property depends on the morphological structure of the film. The more compact it is, the lower the permeability, the higher increase the barrier property; when added, extracts can significantly alter these properties [50]. Studies focusing on improving the properties of bioplastics through changes in the formulation (Table 1) have been developed.

Table 1.
Improvement of polymer matrices.
In general, the union of two or more biopolymers contributes positively to the properties of films. Nonetheless, in-depth investigation of the combination of the polymer matrix and the other components added to the film formulation is necessary, as each combination results in distinct properties [50] (Figure 1). The improvement of matrices through the combination of chitosan and agar-agar is promising, as both are edible natural polymers.
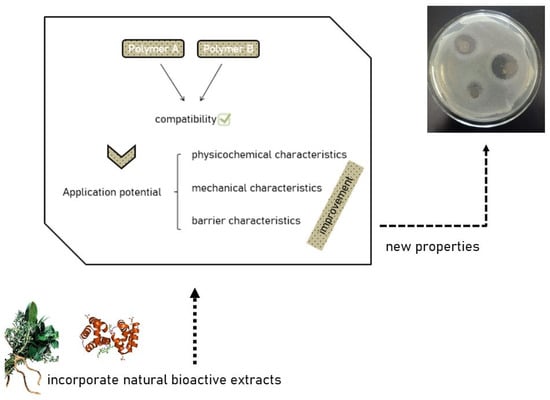
Figure 1.
The union of different polymers incorporated from natural extracts results in a polymer matrix with different properties.
5. Agar and Chitosan Blends
Agar is composed of a mixture of agarose and agaropectin, which correspond to the gelling and non-gelling fractions, respectively. In the agar-agar industrialization process, a large part of the agaropectin is removed, making a powder with greater gel strength [10]. The film formation process begins by (i) the formation of a viscous fluid through the dissolution of agar-agar powder, water, and gelation temperature (90–103 °C); (ii) cooling; (iii) thermoreversible gel formation; and (iv) evaporation. Gelation chemically occurs by the formation of hydrogen bonds between agarose molecules, forming a network of agarose double helices stabilized by water molecules [62]. In the drying process, the films have a high rate of retraction caused by the syneresis of the gel. The Agar-agar films allow easy interaction with mainly aqueous bioactive extracts. In film synthesis, when the solvent is replaced by an aqueous extract, it allows a homogeneous interaction with the agar-agar matrix [63]. Due to the high compaction of pure agar film, it becomes very brittle. A promising alternative to overcome this limitation is a combination with other biopolymeric substances.
Chitosan films are synthesized from the dissolution of chitosan in aqueous solutions of organic acids, and have gained much attention from researchers due to their biocompatibility. Chitosan is used in combination with natural polymers such as starch and gelatin in order to improve its properties. It easily allows hydrogen bonds with these polymers, which makes it biocompatible [31].
Films developed with pure biopolymers have insufficient mechanical properties for application as packaging compared to films that use a mixture of polymers. The electrostatic interactions between agar-agar and chitosan are compatible, allowing for the production of stable films with good properties. The electrostatic interaction is caused by the –NH3+ groups of chitosan and –COO− of the ester group of agar-agar [64] in addition to intermolecular interactions through hydrogen bonds between functional groups, such as –OH [65] (Figure 2). The bond between the polymers allows synthesis of a homogeneous film that combines the benefits of both, depending on the concentrations used and the presence or absence of extracts, resulting in materials with particular properties [63].
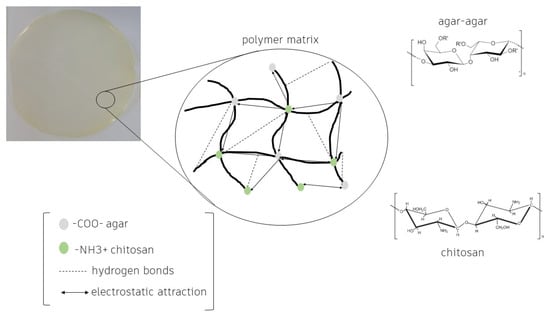
Figure 2.
Agar-agar and chitosan polymer matrix.
Cao et al. [66] used different concentrations of agar-agar and chitosan, and found that when the proportions of the polymers were equal the permeability to water vapor was reduced. This effect is caused by the total interaction between the hydroxyls (-OH) of agar and chitosan, leaving no free hydroxyls to interact with water. In agar-agar and chitosan blends, the lower the concentration of agar in the mixture, the greater the elongation of the material; the agar absorbs moisture from the environment, reducing the bond with chitosan; the resulting polymer allows greater mobility of the film, increasing its elongation to break [63].
The current literature presents few reports of chitosan and agar-agar blends in their ground state, other than in the form of nanocomposites or other complexes, and even these are limited [63,66,67,68]. The combination of these polymers without any treatment with solvents allows the synthesis of a totally green film without any impact on the environment. Their appropriate combination results in a material with good characteristics for food storage, and if bioactive natural extracts are added this can allows for the incorporation of antioxidants and/or antimicrobials as well [69].
6. Trends in Active Bioplastic Packaging
Active packaging aims to improve the characteristics of food beyond the passive protective role. They are capable of modifying the conditions of the product in order to prolong its shelf life while maintaining its sensory and safety properties (Figure 3). Additional functions are divided into compound absorption and compound release. Absorbent systems contribute to the removal of undesirable compounds responsible for accelerating food degradation, such as oxygen, excess water, ethylene, and carbon dioxide, among others. Emitting systems, on the other hand, have the function of releasing compounds which help to prolong shelf life, which can include carbon dioxide, ethanol, antioxidants, and antimicrobials [70].
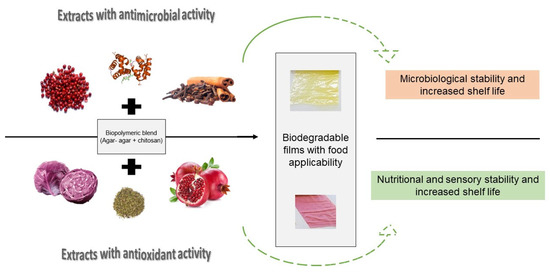
Figure 3.
Biopolymeric films of blends (i.e., chitosan and agar) with active extracts.
Active packaging incorporating antimicrobials have been highlighted, as deterioration reactions start on the surface of the food; thus the use of an active film is efficient in reducing and/or inhibiting microbial development [71]. When used as primary packaging directly in the product, the incorporation of these agents has the advantage of reducing the content of preservatives used in the food, serving consumers who seek foods with minimum levels of additives [72]. The commercialization of portioned and exhibited foods with the use of bioplastic films on supermarket shelves has grown, as they can provide greater consumer attraction [73]. The use of biodegradable films incorporating active extracts has potential for application in these cases. A number of studies have highlighted increases in the shelf life of foods packaged with active films, with examples including lamb [74] and fish fillets [75]. Other studies have reported the replacement of aluminum foil by active films in different processed cheeses [63,69,76], confirming an increase in the shelf life of these products.
The development of biodegradable films for application in food products incorporating extracts with antioxidant and antimicrobial properties has motivated several research groups (Table 2). Plant extracts have received great focus due to their high concentrations of phenolic compounds, which confer high antioxidant activity [77].

Table 2.
Bioplastic films incorporating different extracts.
The addition of extracts in films results in impacts on physicochemical, mechanical, barrier, antioxidant, and antimicrobial properties. Extracts with a wide variety of functions have been used, not only as antimicrobials or antioxidants, but also to modify the properties of the packaging and improve its application in general [13]. The use of natural extracts has as its main objective the addition of active compounds to the food product. However, numerous studies have used compounds of non-renewable origin, such as metal nanoparticles, in order to achieve efficient antimicrobial characteristics.
Xu et al. [88] incorporated silver nanoparticles into chitosan films to develop a packaging with antimicrobial activity. Peighmbardoust et al. [89] developed active starch-based films incorporating a combination of Ag, ZnO, and CuO nanoparticles for potential use as food packaging. Zhixiang et al. [90] developed an antimicrobial film based on curdlan (gum extracted from a bacterium of the human digestive system, used as a thickener in the food industry) and silver nanoparticles synthesized with Glycyrrhiz (a plant with medicinal properties). The use of nanoparticles sometimes restricts the application of the resulting polymers in food products due to their toxicity and alteration of sensory characteristics.
Studies aiming to investigate the improvement of matrices by the combination of unmodified natural polymers and natural extracts with active properties represent a promising approach to synthesizing a packaging with antimicrobial and/or antioxidant properties and with mechanical, physical, and chemical properties suitable for food packaging.
6.1. Packaging Providing Microbiological Stability of Food
Active antimicrobial compounds act by inactivating pathogenic microorganisms transmitted by food and/or deteriorating microorganisms. From this perspective, the use of potential bioactive agents in packaging is a promising strategy for extending the shelf life of food products [91]. Several natural compounds with antimicrobial capacity have been described in the literature, such as cinnamon essential oil [92], clove essential oil [93], grape pomace extract [94], prune peel [86], zein essential oil [95], and apple peel [85], among others.
For the same purpose, a more limited approach is the use of natural antimicrobials produced by microorganisms. Certain bacteriocins, for example, have been studied in combination with different polymer matrices, obtaining satisfactory results in extending the shelf life of minimally processed papaya [96], sliced ham [97], fresh pork [98], and different types of cheese [63,69]. Bacteriocins are compounds with antimicrobial activity produced by microorganisms considered safe or of qualitative presumption of safety, being generally digested by the human organism without intoxication and pathogenicity indexes [99].
For an efficient antimicrobial package, it is fundamental that it is in direct contact with the food to ensure that the compound migrates to the surface of the product. However, when using compounds with volatility properties, direct contact is not necessary [100]. Contessa et al. [63] studied the improvement of the polymeric matrix by the combination of chitosan and agar; when adding bacteriocin from Lactobacillus sakei as an active compound, the matrix showed bactericidal effects due to volatility. Fontes et al. [76] reported antimicrobial activity by volatility when applying pink pepper essential oil to simulated cream cheese packaging.
6.2. Packaging with Active Antioxidant Property
The literature includes several studies regarding the extraction of natural antioxidants, especially in the form of plant extracts and essential oils [101,102,103]. The use of these compounds is common due to their high safety and lack of toxicity [104]. These chemical compounds have biological activities beneficial to humans, including anti-inflammatory [105], anticancer [106], prevention and treatment of diabetes [107], and beneficial effects on the immune system [108]. Antioxidants act by inhibiting free radical reactions, suppressing oxidative processes, and avoiding consequent cell damage [109].
In foods, antioxidants act mainly on sensory quality, as lipid oxidation is the main alteration of food products, and causes nutritional loss [110]. Oxidative changes result in loss of color, changes in taste and odor, and additional production of substances with potential harmful effects on the consumer [111]. In this sense, active packaging/films incorporating natural antioxidants act on the sensory and nutritional quality of packaged food.
There are several natural sources for extracting antioxidants with potential application in active films, such as oregano extract [112], mango [113], cranberry extract [114], onion [115], pomegranate peel [116], red cabbage [117], rice straw extract [118], lemon essential oil [119], and tomato extract [120], among others. In addition to the antioxidant activity itself, these extracts have different properties from the other constituents of the polymeric matrix, potentially acting to improve material properties such as opacity and elasticity [121], mechanical properties and water resistance [122], and permeability to water vapor [123].
7. Challenges and Future Prospects
The food industry is responsible for much of the accumulation of solid waste due to high consumption of food and the need for packaging that acts as a barrier to the external environment. Many studies have focused on alternative approaches to this problem. The development of biodegradable packaging from natural polymers is a promising field; however, it presents challenges regarding the mechanical, physical, and chemical properties of these materials. The union of agar-agar and chitosan shows promise for the synthesis of food packaging, as they are both non-toxic and edible natural polymers. Unlike the union of other polymers, agar and chitosan present good interactions in terms of their electrostatic forces and hydrogen bonds, allowing for particular properties. With the addition of active natural extracts to the resulting biodegradable material, active packaging can be produced which acts to prolong the microbiological stability of food. The exploration of new combined packaging substances from the union of these polymers in combination with the addition of natural extracts with active properties is a promising field of research in keeping with the latest trends in food packaging.
Author Contributions
C.R.C.: Conceptualization, writing, original draft preparation, proofreading, and editing. G.S.d.R.: Project Management, supervision, formal review, and review. C.C.M.: Project management, supervision, writing, and editing. J.F.d.M.B.: Project management, supervision, writing, and editing. All authors have read and approved the final manuscript.
Funding
The authors are grateful for FAPERGS (Foundation Research Support in the State of Rio Grande do Sul), CNPq (National Council of Science and Technological Development), and the support of the Coordination of Improvement of Higher-Level Personnel—Brazil (CAPES) (Financing Code 001).
Data Availability Statement
All data generated or analysed during this study are included in the published article.
Conflicts of Interest
The authors confirm that this is an original research article, and that no conflicts of interests are associated with this publication.
References
- Jadhav, E.B.; Sankhla, M.S.; Bhat, R.A.; Bhagat, D.S. Microplastics from food packaging: An overview of human consumption, health threats, and alternative solutions. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100608. [Google Scholar] [CrossRef]
- Rydz, J.; Musiol, M.; Zawidlak-Wegrzynsk, B.; Sikorska, W. Chapter 14—Present and Future of Biodegradable Polymers for Food Packaging Applications. In Biopolymers for Food Design; Elsevier: Amsterdam, The Netherlands, 2018; pp. 431–467. [Google Scholar] [CrossRef]
- Jiménez-Rosado, M.; Zarate-Ramírez, L.; Romero, A.; Bengoechea, C.; Partal, P.; Guerrero, A. Bioplastics based on wheat gluten processed by extrusion. J. Clean. Prod. 2019, 239, 117994. [Google Scholar] [CrossRef]
- Waring, R.H.; Harris, R.M.; Mitchell, S.C. Plastic contamination of the food chain: A threat to human health? Maturitas 2018, 115, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Golwala, H.; Zhang, X.; Iskander, S.M.; Smit, A.L. Solid waste: An overlooked source of microplastics to the environment. Sci. Total Environ. 2021, 769, 144581. [Google Scholar] [CrossRef]
- Onu, Organização das Nações Unidas. 2019. Available online: https://nacoesunidas.org/onu-meio-ambiente-aponta-lacunas-na-reciclagem-global-de-plastico/ (accessed on 30 March 2023).
- Provin, A.P.; Dutra, A.R.A.; Gouveia, I.C.A.S.S.; Cubas, A.L.V. Circular economy for fashion industry: Use of waste from the food industry for the production of biotextiles. Technol. Forecast. Soc. Chang. 2021, 169, 120858. [Google Scholar] [CrossRef]
- Liu, Y.; Ahmed, S.; Sameen, D.E.; Lu, R.; Dai, J.; Li, S.; Qin, W. A review of cellulose and its derivatives in biopolymer-based for food packaging application. Trends Food Sci. Technol. 2021, 112, 532–546. [Google Scholar] [CrossRef]
- Moeini, A.; Germamm, N.; Maliconico, M.; Santagata, G. Formulation of secondary compounds as additives of biopolymer-based food packaging: A review. Trends Food Sci. Technol. 2021, 114, 342–354. [Google Scholar] [CrossRef]
- Mostafavi, F.S.; Zaeim, D. Agar-based edible films for food packaging applications—A review. Int. J. Biol. Macromol. 2020, 159, 1165–1176. [Google Scholar] [CrossRef]
- Moeini, A.; Pedram, P.; Makvandi, P.; Malinconico, M.; D’Ayla, G.G. Wound healing and antimicrobial effect of active secondary metabolites in chitosan-based wound dressings: A review. Carbohydr. Polym. 2020, 233, 115839. [Google Scholar] [CrossRef]
- Kabir, E.; Kaur, R.; Lee, J.; Kim, K.-H.; Kwon, E.E. Prospects of biopolymer technology as an alternative option for non-degradable plastics and sustainable management of plastic wastes. J. Clean. Prod. 2020, 258, 120536. [Google Scholar] [CrossRef]
- Cheikh, D.; Majdoub, H.; Darder, M. An overview of clay-polymer nanocomposites containing bioactive compounds for food packaging applications. Appl. Clay Sci. 2022, 216, 106335. [Google Scholar] [CrossRef]
- Latos-Brozio, M.; Mazek, A. The application of natural food colorants as indicator substances in intelligent biodegradable packaging materials. Food Chem. Toxicol. 2020, 135, 110975. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ji, K.; Kurt, K.; Cornish, K.; Vodovotz, Y. Optimal mechanical properties of biodegradable natural rubber-toughened PHBV bioplastics intended for food packaging applications. Food Packag. Shelf Life 2019, 21, 100348. [Google Scholar] [CrossRef]
- IFICF. International Food Information Council Foundation. Food & Health Survey “A Healthy Perspective: Understanding American Food Values”. 2018. Available online: https://www.foodinsight.org/2018-food-and-health-survey (accessed on 30 March 2023).
- Schifferstein, H.N.J.; Lemke, M.; Boer, A. An exploratory study using graphic design to communicate consumer benefits on food packaging. Food Qual. Prefer. 2022, 97, 104458. [Google Scholar] [CrossRef]
- All4pack. Packaging: Market and Challenges in 2016. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=2ahUKEwjT3sLFkdXiAhWO4IUKHcYsCb4QFjAAegQIARAC&url=https%3A%2F%2Fwww.all4pack.com%2FMedia%2FAll-4-Pack-Medias%2FFiles%2FFiches-marches%2FPackaging-market-and-challenges-in-2016&usg=AOvVaw1NRZNaZACeCTU88su2U2wZ (accessed on 30 March 2023).
- Phelan, A.A.; Meissner, K.; Humphrey Ross, H. Plastic pollution and packaging: Corporate commitments and actions from the food and beverage sector. J. Clean. Prod. 2022, 331, 129827. [Google Scholar] [CrossRef]
- Ketelsen, M.; Janssen, M.; Hamm, U. Consumers’ response to environmentally-friendly food packaging—A systematic review. J. Clean. Prod. 2020, 254, 120123. [Google Scholar] [CrossRef]
- Li, Y.; Yan, H.; Li, X.; Ge, J.; Cheng, J.; Yu, X. Presence, distribution and risk assessment of phthalic acid esters (PAEs) in suburban plastic film pepper-growing greenhouses with different service life. Ecotoxicol. Environ. Saf. 2020, 196, 110551. [Google Scholar] [CrossRef]
- Santagata, G.; Valerio, F.; Cimmino, A.; Poggetto, G.D.; Masi, M.; Biase, M.D.; Malinconico, M.; Lavermicocca, P.; Evidente, A. Chemico-physical and antifungal properties of poly(butylene succinate)/cavoxin blend: Study of a novel bioactive polymeric based system. Eur. Polym. J. 2017, 94, 230–247. [Google Scholar] [CrossRef]
- Degli-Innocenti, F. Is composting of packaging real recycling? Waste Manag. 2021, 130, 61–64. [Google Scholar] [CrossRef]
- Khan, B.; Niazi, M.B.K.; Samin, G.; Jahan, Z. Thermoplastic Starch: A Possible Biodegradable Food Packaging Material—A Review. J. Food Process Eng. 2017, 40, e12447. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, S. Starch, cellulose, pectin, gum, alginate, chitin and chitosan derived (nano) materials for sustainable water treatment: A review. Carbohydr. Polym. 2021, 251, 116986. [Google Scholar] [CrossRef] [PubMed]
- Kaushalya, K.G.D.; Gunathilake, K.D.P.P. Encapsulation of phlorotannins from edible brown seaweed in chitosan: Effect of fortification on bioactivity and stability in functional foods. Food Chem. 2022, 377, 132012. [Google Scholar] [CrossRef] [PubMed]
- Sanjay, A.V.M.R.; Suchart, S.; Jyotishkumar, P. Renewable and sustainable biobased materials: An assessment on biofibers, biofilms, biopolymers and biocomposites. J. Clean. Prod. 2020, 258, 120978. [Google Scholar] [CrossRef]
- Alonso-González, M.; Felix, M.; Guerrero, A.; Romero, A. Rice bran-based bioplastics: Effects of the mixing temperature on starch plastification and final properties. Int. J. Biol. Macromol. 2021, 188, 932–940. [Google Scholar] [CrossRef]
- Pervez, S.; Nawaz, M.A.; Jamal, M.; Jan, T.; Maqbool, F.; Shah, I.; Aman, A.; Qader, S.A.U. Improvement of catalytic properties of starch hydrolyzing fungal amyloglucosidase: Utilization of agar-agar as an organic matrix for immobilization. Carbohydr. Res. 2019, 486, 107860. [Google Scholar] [CrossRef]
- Nie, Z.; Peng, K.; Lin, Z.; Yang, J.; Cheng, Z.; Gan, Q.; Chen, Y.; Feng, C. A conductive hydrogel based on nature polymer agar with self-healing ability and stretchability for flexible sensors. Chem. Eng. J. 2023, 454, 139843. [Google Scholar] [CrossRef]
- Huang, D.; Zhang, Z.; Zheng, Y.; Quan, Q.; Wang, W.; Wang, A. Synergistic effect of chitosan and halloysite nanotubes on improving agar film properties. Food Hydrocoll. 2020, 101, 105471. [Google Scholar] [CrossRef]
- Hasija, V.; Sharma, K.; Kumar, V.; Sharma, S.; Sharma, V. Green synthesis of agar/Gum Arabic based superabsorbent as an alternative for irrigation in agriculture. Vacuum 2018, 157, 458–464. [Google Scholar] [CrossRef]
- Kavoosi, G.; Derakhshan, M.; Salehi, M.; Rahmati, L. Microencapsulation of zataria essential oil in agar, alginate and carrageenan. Innov. Food Sci. Emerg. Technol. 2018, 45, 418–425. [Google Scholar] [CrossRef]
- Jridi, M.; Abdelhedi, O.; Zouari, N.; Fakhfakh, N.; Nasri, M. Development and characterization of grey triggerfish gelatin/agar bilayer and blend films containing vine leaves bioactive compounds. Food Hydrocoll. 2019, 89, 370–378. [Google Scholar] [CrossRef]
- Wongphan, P.; Harnkarnsujarit, N. Characterization of starch, agar and maltodextrin blends for controlled dissolution of edible films. Int. J. Biol. Macromol. 2020, 156, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N. Comparative study on antimicrobial activity and biocompatibility of N-selective chitosan derivatives. React. Funct. Polym. 2018, 124, 149–155. [Google Scholar] [CrossRef]
- Oladzadabbasabadi, N.; Mohammadi, A.; Nafchi, F.; Ariffin, M.M.; Wijekoon, J.O.; Al-Hassan, A.A.; Dheyab, M.A.; Guasemlou, M. Recent advances in extraction, modification, and application of chitosan in packaging industry. Carbohydr. Polym. 2022, 277, 118876. [Google Scholar] [CrossRef] [PubMed]
- Huq, T.; Khan, A.; Brown, D.; Dhayagude, N.; He, Z.; Ni, Y. Sources, production and commercial applications of fungal chitosan: A review. J. Bioresour. Bioprod. 2022, 23, 85–98. [Google Scholar] [CrossRef]
- Cheba, B.A. Chitosan: Properties, Modifications and Food Nanobiotechnology. Procedia Manuf. 2020, 46, 652–658. [Google Scholar] [CrossRef]
- Khan, A.; Wang, B.; Ni, Y. Chitosan-Nanocellulose Composites for Regenerative Medicine Applications. Curr. Med. Chem. 2020, 28, 4584–4592. [Google Scholar] [CrossRef]
- Tzaneva, D.; Simitchiev, A.; Petkova, N.; Nenov, V.; Stoyanova, A.; Denev, P. Synthesis of Carboxymethyl Chitosan and its Rheological Behaviour in Pharmaceutical and Cosmetic Emulsions. J. Appl. Pharm. Sci. 2017, 7, 70–78. [Google Scholar] [CrossRef]
- Bakashi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an environment friendly biomaterial—A review on recent modifications and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar] [CrossRef]
- Badawy, M.E.I. Structure and antimicrobial activity relationship of quaternary N-alkyl chitosan derivatives against some plant pathogens. J. Appl. Polym. Sci. 2010, 117, 960–969. [Google Scholar] [CrossRef]
- Ghaderi, J.; Hosseini, S.F.; Keyvani, N.; Gómez-Guillén, M.C. Polymer blending effects on the physicochemical and structural features of the chitosan/poly(vinyl alcohol)/fish gelatin ternary biodegradable films. Food Hydrocoll. 2019, 95, 122–132. [Google Scholar] [CrossRef]
- Mendes, J.F.; Paschoalin, R.; Carmona, V.; Neto, A.R.S.; Marques, A.; Marconcini, J.; Mattoso, L.; Medeiros, E.; Oliveira, J. Biodegradable polymer blends based on corn starch and thermoplastic chitosan processed by extrusion. Carbohydr. Polym. 2016, 137, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Mir, S.A.; Dar, B.N.; Wani, A.A.; Shah, M.A. Effect of plant extracts on the techno-functional properties of biodegradable packaging films. Trends Food Sci. Technol. 2018, 80, 141–154. [Google Scholar] [CrossRef]
- Delgado, J.F.; Peltzer, M.A.; Wagner, J.R.; Salvay, A.G. Hydration and water vapour transport properties in yeast biomass based films: A study of plasticizer content and thickness effects. Eur. Polym. J. 2018, 99, 9–17. [Google Scholar] [CrossRef]
- Tan, Y.M.; Lim, S.; Tay, B.; Lee, M.; Thian, E. Functional chitosan-based grapefruit seed extract composite films for applications in food packaging technology. Mater. Res. Bull. 2015, 69, 142–146. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Harte, B.R. Physical properties and antioxidant activity of an active film from chitosan incorporated with green tea extract. Food Hydrocoll. 2010, 24, 770–775. [Google Scholar] [CrossRef]
- Aloui, H.; Deshmukh, A.R.; Khomlaem, C.; Kim, B.S. Novel composite films based on sodium alginate and gallnut extract with enhanced antioxidant, antimicrobial, barrier and mechanical properties. Food Hydrocoll. 2021, 113, 106508. [Google Scholar] [CrossRef]
- Ren, L.; Yan, X.; Zhou, J.; Tong, J.; Su, X. Influence of chitosan concentration on mechanical and barrier properties of corn starch/chitosan films. Int. J. Biol. Macromol. 2017, 105, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Ghavi, F.F. Preparation and functional properties of fish gelatin–chitosan blend edible films. Food Chem. 2013, 136, 1490–1495. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Ahmed, J.; Jacob, H. Preparation and characterization of agar-based nanocomposite films reinforced with bimetallic (Ag-Cu) alloy nanoparticles. Carbohydr. Polym. 2017, 155, 382–390. [Google Scholar] [CrossRef]
- Vejdan, A.; Ojagh, S.M.; Adeli, A.; Abdollahi, M. Effect of TiO2 nanoparticles on the physico-mechanical and ultraviolet light barrier properties of fish gelatin/agar bilayer film. LWT Food Sci. Technol. 2016, 71, 88–95. [Google Scholar] [CrossRef]
- Wang, X.; Guo, C.; Hao, W.; Ullah, N.; Chen, L.; Li, Z.; Feng, X. Development and characterization of agar-based edible films reinforced with nano-bacterial cellulose. Int. J. Biol. Macromol. 2018, 118, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Al-Naamani, L.; Dobretsov, S.; Dutta, J. Chitosan-zinc oxide nanoparticle composite coating for active food packaging applications. Innov. Food Sci. Emerg. Technol. 2016, 38, 231–237. [Google Scholar] [CrossRef]
- Kumar, S.; Shukla, A.; Baul, P.P.; Mitra, A.; Halder, D. Biodegradable hybrid nanocomposites of chitosan/gelatin and silver nanoparticles for active food packaging application. Food Packag. Shelf Life 2018, 16, 178–184. [Google Scholar] [CrossRef]
- Bigi, F.; Haghighi, H.; Fabio, H.W.S.; Pulvirenti, A. Characterization of chitosan-hydroxypropyl methylcellulose blend films enriched with nettle or sage leaf extract for active food packaging applications. Food Hydrocoll. 2021, 120, 106979. [Google Scholar] [CrossRef]
- Bhat, V.G.; Narasagoudr, S.S.; Masti, S.P.; Chougale, R.B.; Vantamuri, A.B.; Kasai, D. Development and evaluation of Moringa extract incorporated Chitosan/Guar gum/Poly (vinyl alcohol) active films for food packaging applications. Int. J. Biol. Macromol. 2022, 200, 50–60. [Google Scholar] [CrossRef]
- Rangaraj, V.M.; Devaraju, S.; Rambabu, K.; Banat, F.; Mittal, V. Silver-sepiolite (Ag-Sep) hybrid reinforced active gelatin/date waste extract (DSWE) blend composite films for food packaging application. Food Chem. 2022, 369, 130983. [Google Scholar] [CrossRef]
- Cui, H.; Surendhiran, D.; Li, C.; Lin, L. Biodegradable zein active film containing chitosan nanoparticle encapsulated with pomegranate peel extract for food packaging. Food Packag. Shelf Life 2020, 24, 100511. [Google Scholar] [CrossRef]
- Rocha, M.; Alemán, A.; Romani, V.P.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Montero, P.; Prentice, C. Effects of agar films incorporated with fish protein hydrolysate or clove essential oil on flounder (Paralichthys orbignyanus) fillets shelf-life. Food Hydrocoll. 2018, 81, 351–363. [Google Scholar] [CrossRef]
- Contessa, C.R.; Rosa, G.S.; Moraes, C.C. New Active Packaging Based on Biopolymeric Mixture Added with Bacteriocin as Active Compoun. Int. J. Mol. Sci. 2021, 22, 10628. [Google Scholar] [CrossRef]
- Li, K.; Zhu, J.; Guan, G.; Wu, H. Preparation of chitosan-sodium alginate films through layer-by-layer assembly and ferulic acid crosslinking: Film properties, characterization, and formation mechanism. Int. J. Biol. Macromol. 2019, 122, 485–492. [Google Scholar] [CrossRef]
- Fathiraja, P.; Gopalrajan, S.; Karunanithi, M.; Nagarajan, M.; Obaiah, M.C.; Durairajm, S.; Neethirajan, N.V. Development of a biodegradable composite film from chitosan, agar and glycerol based on optimization process by response surface methodology. Cellul. Chem. Technol. 2021, 55, 849–865. [Google Scholar] [CrossRef]
- Cao, Q.; Zhang, Y.; Chen, W.; Meng, X.; Liu, B. Hydrophobicity and physicochemical properties of agarose film as affected by chitosan addition. Int. J. Biol. Macromol. 2018, 106, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Nasef, M.M.; El-Hefian, E.; Saalah, S.; Yahaya, A.H. Preparation and Properties of Non-Crosslinked and Ionically Crosslinked Chitosan/Agar Blended Hydrogel Films. J. Chem. 2011, 8, 409–419. [Google Scholar] [CrossRef]
- El-Efiam, E.; Nasef, M.M.; Yahaya, A.H. Preparation and Characterization of Chitosan/Agar Blended Films: Part 1. Chemical Structure and Morphology. J. Chem. 2011, 9, 1431–1439. [Google Scholar] [CrossRef]
- Contessa, C.R.; Souza, N.B.; Gonçalo, G.B.; Moura, C.M.; Rosa, G.S.; Moraes, C.C. Development of Active Packaging Based on Agar-Agar Incorporated with Bacteriocin of Lactobacillus sakei. Biomolecules 2021, 11, 1869. [Google Scholar] [CrossRef]
- Flórez, M.; Guerra-Rodriguez, E.; Cazón, P.; Vázquez, M. Chitosan for food packaging: Recent advances in active and intelligent films. Food Hydrocoll. 2022, 124, 107328. [Google Scholar] [CrossRef]
- Bernardi, A.O.; Garcia, M.V.G.; Copetti, M.V. Food industry spoilage fungi control through facility sanitization. Curr. Opin. Food Sci. 2019, 29, 28–34. [Google Scholar] [CrossRef]
- Zanetti, M.; Carniel, T.K.; Dalcanton, F.; dos Anjos, R.S.; Riella, H.; de Araújo, P.H.; Oliveira, D.; Fiori, M.A. Use of encapsulated natural compounds as antimicrobial additives in food packaging: A brief review. Trends Food Sci. Technol. 2018, 81, 51–60. [Google Scholar] [CrossRef]
- Vittuari, M.; Masotti, M.; Iori, E.; Falasconi, L.; Toschi, T.G.; Segré, A. Does the COVID-19 external shock matter on household food waste? The impact of social distancing measures during the lockdown. Resour. Conserv. Recycl. 2021, 174, 105815. [Google Scholar] [CrossRef]
- Martiny, T.R.; Raghavan, V.; Moraes, C.C.; Rosa, G.S.; Dotto, G.L. Bio-Based Active Packaging: Carrageenan Film with Olive Leaf Extract for Lamb Meat Preservation. Foods 2020, 9, 1759. [Google Scholar] [CrossRef]
- Brazeiro, F.S.G.; Contessa, C.R.; Almeida, L.S.; Moura, J.M.; Moraes, C.C.; Moura, C.M. Antimicrobial evaluation of gelatin–based films incorporated with chitosan in the conservation of fish fillets. Braz. J. Dev. 2020, 6, 87543–87556. [Google Scholar] [CrossRef]
- Fontes, M.R.V.; Contessa, C.R.; Moraes, C.C.; Zavareze, E.R.; Dias, A.R.G. Antimicrobial properties of PLA membranes loaded with pink pepper (Schinus terebinthifolius Raddi) essential oil applied in simulated cream cheese packaging. Food Biophys. 2022, 18, 107–119. [Google Scholar] [CrossRef]
- Cirak, C.; Radusiene, J.; Raudone, L.; Vilkickyte, G.; Seyis, F.; Marksa, M.; Ivanauskas, L.; Yayla, F. Phenolic compounds and antioxidant activity of Achillea arabica populations. S. Afr. J. Bot. 2022, 147, 425–433. [Google Scholar] [CrossRef]
- Young, H.; Wang, X.; Bai, R.; Miao, Z.; Zhang, X.; Liu, J. Development of antioxidant and intelligent pH-sensing packaging films by incorporating purple-fleshed sweet potato extract into chitosan matrix. Food Hydrocoll. 2019, 90, 216–224. [Google Scholar] [CrossRef]
- Medina-Jaramillo, C.; Ochoa-Yepes, O.; Bernal, C.; Famá, L. Active and smart biodegradable packaging based on starch and natural extracts. Carbohydr. Polym. 2017, 176, 187–194. [Google Scholar] [CrossRef]
- Wang, X.; Yong, H.; Gao, L.; Li, L.; Jin, M.; Liu, J. Preparation and characterization of antioxidant and pH-sensitive films based on chitosan and black soybean seed coat extract. Food Hydrocoll. 2019, 89, 56–66. [Google Scholar] [CrossRef]
- Wang, K.; Lin, P.N.; Tong, S.Y.; Thian, E.S. Development of grapefruit seed extract-loaded poly(ε-caprolactone)/chitosan films for antimicrobial food packaging. Food Packag. Shelf Life 2019, 22, 100396. [Google Scholar] [CrossRef]
- Giménez, B.; Lacey, A.L.; Pérez-Santín, E.; López-Caballero, M.E.; Montero, P. Release of active compounds from agar and agar–gelatin films with green tea extract. Food Hydrocoll. 2013, 30, 264–271. [Google Scholar] [CrossRef]
- Kurek, M.; Garofulic, I.E.; Bakic, M.T.; Scetar, M.; Uzelac, V.D.; Galic, K. Development and evaluation of a novel antioxidant and pH indicator film based on chitosan and food waste sources of antioxidants. Food Hydrocoll. 2018, 84, 238–246. [Google Scholar] [CrossRef]
- Meira, S.M.M.; Zehetmeyer, G.; Werner, J.O.; Brandelli, A. A novel active packaging material based on starch-halloysite nanocomposites incorporating antimicrobial peptides. Food Hydrocoll. 2017, 63, 561–570. [Google Scholar] [CrossRef]
- Riaz, A.; Lei, S.; Akhtar, H.M.S.; Wan, P.; Chen, D.; Jabbar, S.; Abid, M.; Hashim, M.M.; Zheng, X. Preparation and characterization of chitosan-based antimicrobial active food packaging film incorporated with apple peel polyphenols. Int. J. Biol. Macromol. 2018, 114, 547–555. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Yong, H.; Qin, Y.; Liu, J.; Liu, J. Development of multifunctional food packaging films based on chitosan, TiO2 nanoparticles and anthocyanin-rich black plum peel extract. Food Hydrocoll. 2019, 94, 80–92. [Google Scholar] [CrossRef]
- Annu, A.A.; Ahmed, S. Eco-friendly natural extract loaded antioxidative chitosan/polyvinyl alcohol based active films for food packaging. Heliyon 2021, 7, e06550. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Huang, X.; Li, Y.-C.; Xiao, H.; Wang, X. Novel chitosan films with laponite immobilized Ag nanoparticles for active food packaging. Carbohydr. Polym. 2018, 199, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Peighambardoust, S.J.; Peighambardoust, S.H.; Pournasir, N.; Pakdel, P.M. Properties of active starch-based films incorporating a combination of Ag, ZnO and CuO nanoparticles for potential use in food packaging applications. Food Packag. Shelf Life 2019, 22, 100420. [Google Scholar] [CrossRef]
- Cai, Z.; Dai, Q.; Guo, Y.; Wei, Y.; Wu, M.; Zhang, H. Glycyrrhiza polysaccharide-mediated synthesis of silver nanoparticles and their use for the preparation of nanocomposite curdlan antibacterial film. Int. J. Biol. Macromol. 2019, 141, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, S.; Jafari, S.M.; Salehabadi, A.; Nafchi, A.M.; Kumar, U.S.U.; Khalil, H.P.S.A. Biodegradable green packaging with antimicrobial functions based on the bioactive compounds from tropical plants and their by-products. Trends Food Sci. Technol. 2020, 100, 262–277. [Google Scholar] [CrossRef]
- Lucas-Gonzalez, R.; Yilmaz, B.; Khaneghah, A.M.; Hano, C.; Shariati, M.A.; Bangar, S.P.; Goksen, G.; Dhama, K.; Lorenzo, J.M. Cinnamon: An antimicrobial ingredient for active packaging. Food Packag. Shelf Life 2023, 35, 101026. [Google Scholar] [CrossRef]
- Zhu, W.; Chen, J.; Dong, Q.; Luan, D.; Tao, N.; Deng, S.; Wang, L.; Hao, Y.; Li, L. Development of organic-inorganic hybrid antimicrobial materials by mechanical force and application for active packaging. Food Packag. Shelf Life 2023, 37, 101060. [Google Scholar] [CrossRef]
- Silva, D.J.; Oliveira, M.M.; Wang, S.H.; Carastan, D.J.; Rosa, D.S. Designing antimicrobial polypropylene films with grape pomace extract for food packaging. Food Packag. Shelf Life 2022, 34, 100929. [Google Scholar] [CrossRef]
- Ge, X.; Huang, X.; Zhou, L.; Wang, Y. Essential oil-loaded antimicrobial and antioxidant zein/poly (lactic acid) film as active food packaging. Food Packag. Shelf Life 2022, 34, 100977. [Google Scholar] [CrossRef]
- Narsaiah, K.; Wilson, R.A.; Gokul, K.; Mandge, H.M.; Jha, S.N.; Bhadwal., S.; Anurag, R.K.; Malik, R.K.; Vij, S. Effect of bacteriocin-incorporated alginate coating on shelf-life of minimally processed papaya (Carica papaya L.). Postharvest Biol. Technol. 2015, 100, 212–218. [Google Scholar] [CrossRef]
- Santiago-Silva, P.; Soares, N.F.; Nóbrega, J.E.; Júnior, M.A.; Barbosa, K.B.; Volp, A.C.P.; Zerdas, E.R.; Würlitzer, N.J. Antimicrobial efficiency of film incorporated with pediocin (ALTA® 2351) on preservation of sliced ham. Food Control 2009, 20, 85–89. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, M.; Warner, R.D.; Fang, Z. Incorporating nisin and grape seed extract in chitosan-gelatine edible coating and its effect on cold storage of fresh pork. Food Control 2020, 110, 107018. [Google Scholar] [CrossRef]
- Peng, S.; Song, J.; Zeng, W.; Wang, H.; Zhang, Y.; Xin, J.; Suo, H. A broad-spectrum novel bacteriocin produced by Lactobacillus plantarum SHY 21–2 from yak yogurt: Purification, antimicrobial characteristics and antibacterial mechanism. LWT Food Sci. Technol. 2021, 142, 110955. [Google Scholar] [CrossRef]
- Dannenberg, G.S.; Funck, G.D.; Cruxen, C.E.S.; Marques, J.L.; Silva, W.P.; Fiorentini, A.M. Essential oil from pink pepper as an antimicrobial component in cellulose acetate film: Potential for application as active packaging for sliced cheese. LWT Food Sci. Technol. 2017, 81, 314–318. [Google Scholar] [CrossRef]
- Lv, Q.-Z.; Long, J.-T.; Gong, Z.-F.; Nong, K.-Y.; Liang, X.-M.; Qin, T.; Huang, W.; Yang, L. Current State of Knowledge on the Antioxidant Effects and Mechanisms of Action of Polyphenolic Compounds. Nat. Prod. Commun. 2021, 16, 1934578X211027745. [Google Scholar] [CrossRef]
- Noronha, C.M.; Carvalho, S.M.; Lino, R.C.; Barreto, P.L.M. Characterization of antioxidant methylcellulose film incorporated with α-tocopherol nanocapsules. Food Chem. 2014, 159, 529–535. [Google Scholar] [CrossRef]
- Martiny, T.R.; Raghavan, V.; Moraes, C.C.; Rosa, G.S.; Dotto, G.L. Optimization of green extraction for the recovery of bioactive compounds from Brazilian olive crops and evaluation of its potential as a natural preservative. J. Environ. Chem. Eng. 2021, 9, 105130. [Google Scholar] [CrossRef]
- Wang, Q.; Song, Y.; Sun, J.; Jiang, G. A novel functionalized food packaging film with microwave-modified konjac glucomannan/chitosan/citric acid incorporated with antioxidant of bamboo leaves. LWT 2022, 166, 113780. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, P.; Xue, M.; Zhang, M.; Xiao, Z.; Xu, C.; Fan, Y.; Liu, W.; Wu, Y.; Wu, M.; et al. Anti-inflammatory and antioxidant properties of rice bran oil extract in copper sulfate-induced inflammation in zebrafish (Danio rerio). Fish Shellfish Immunol. 2023, 136, 108740. [Google Scholar] [CrossRef] [PubMed]
- Li, P.-H.; Shih, Y.-J.; Lu, W.-C.; Huang, P.-H.; Wang, C.-C. Antioxidant, antibacterial, anti-inflammatory, and anticancer properties of Cinnamomum kanehirae Hayata leaves extracts. Arab. J. Chem. 2023, 16, 104873. [Google Scholar] [CrossRef]
- Sun, H.; Chen, M.; He, X.; Sun, Y.; Feng, J.; Guo, X.; Li, L.; Zhu, J.; Xia, G.; Zang, H. Phytochemical analysis and in vitro and in vivo antioxidant properties of Plagiorhegma dubia Maxim as a medicinal crop for diabetes treatment. Arab. J. Chem. 2023, 16, 104788. [Google Scholar] [CrossRef]
- Wang, F.; Gao, Y.; Guan, C.; Jia, Y. Growth performance, hematology, antioxidant capacity, immunity, and intestinal microbiota of spotted knifejaw (Oplegnathus punctatus) reared in recirculating aquaculture system and offshore aquaculture net pen. Aquaculture 2023, 562, 738816. [Google Scholar] [CrossRef]
- Saparbekova, A.A.; Kantureyeva, G.O.; Kudasova, D.E.; Konarbayeva, Z.K.; Lafit, A.S. Potential of phenolic compounds from pomegranate (Punica granatum L.) by-product with significant antioxidant and therapeutic effects: A narrative review. Saudi J. Biol. Sci. 2023, 30, 103553. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Kim, N.-A.; Kim, H.-J.; Kerr, W.L.; Choi, S.-G. Effect of oil pressing and packaging under oxygen-free conditions on yield, oxidative stability, antioxidant activity, and physicochemical characteristics of perilla oil. LWT 2023, 179, 114647. [Google Scholar] [CrossRef]
- Galano, J.-M.; Lee, Y.Y.; Durand, T.; Lee, J.C.Y. Special Issue on “Analytical Methods for Oxidized Biomolecules and Antioxidants”—The use of isoprostanoids as biomarkers of oxidative damage, and their role in human dietary intervention studies. Free Radic. Res. 2015, 49, 583–598. [Google Scholar] [CrossRef]
- Parra, C.; Munoz, P.; Bustus, L.; Parra, F.; Simirgiots, M.J.; Escobar, H. UHPLC-DAD Characterization of Origanum vulgare L. from Atacama Desert Andean Region and Antioxidant, Antibacterial and Enzyme Inhibition Activities. Molecules 2021, 26, 2100. [Google Scholar] [CrossRef]
- Bhatt, A.; Patel, V. A Comparative Study Between Antioxidant Potential of Ripe and Pre-ripe Mango Using Conventional and Physiological Extraction. Int. J. Fruit Sci. 2016, 16, 57–68. [Google Scholar] [CrossRef]
- Anhê, F.F.; Pilon, G.; Roy, D.; Desjardins, Y.; Levy, E.; Marette, A. Triggering Akkermansia with dietary polyphenols: A new weapon to combat the metabolic syndrome? Gut Microbes 2016, 7, 146–153. [Google Scholar] [CrossRef]
- Ouyang, Y.; Hou, K.; Peng, W.; Liu, Z.; Deng, H. Antioxidant and xanthine oxidase inhibitory activities of total polyphenols from onion. Saudi J. Biol. Sci. 2018, 25, 1509–1513. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic compounds as beneficial phytochemicals in pomegranate (Punica granatum L.) peel: A review. Food Chem. 2018, 261, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Ji, Y.; Yang, X.; Pang, L.; Cheng, J.; Lu, X.; Zheng, J.; Yin, L.; Hu, W. Antioxidant activity and microbial safety of fresh-cut red cabbage stored in different packaging films. LWT 2023, 175, 114478. [Google Scholar] [CrossRef]
- Freitas, P.A.V.; Gil, N.J.B.; Gonzalez-Martinez, C.; Chiralt, A. Antioxidant poly (lactic acid) films with rice straw extract for food packaging applications. Food Packag. Shelf Life 2022, 34, 101003. [Google Scholar] [CrossRef]
- López-Gómez, A.; Navarro-Martinez, A.; Martinez-Hernandez, G.B. Effects of essential oils released from active packaging on the antioxidant system and quality of lemons during cold storage and commercialization. Sci. Hortic. 2023, 312, 111855. [Google Scholar] [CrossRef]
- Marino-Cortegoso, S.; Stanzione, M.; Andrade, M.A.; Restuccia, C.; Quiros, A.R.-B.; Buonocore, G.G.; Barbosa, C.H.; Vilarinho, F.; Silva, A.S.; Ramos, F.; et al. Development of active films utilizing antioxidant compounds obtained from tomato and lemon by-products for use in food packaging. Food Control 2022, 140, 109128. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, B.; Zhang, X.; Ma, Z.; Feng, X. Incorporating Portulaca oleracea extract endows the chitosan-starch film with antioxidant capacity for chilled meat preservation. Food Chem. 2023, 18, 100662. [Google Scholar] [CrossRef]
- Lei, Y.; Wu, H.; Jiao, C.; Jiang, Y.; Liu, R.; Xiao, D.; Lu, J.; Zhang, Z.; Shen, G.; Li, S. Investigation of the structural and physical properties, antioxidant and antimicrobial activity of pectin-konjac glucomannan composite edible films incorporated with tea polyphenol. Food Hydrocoll. 2019, 94, 128–135. [Google Scholar] [CrossRef]
- Yu, Z.; Sun, L.; Wang, W.; Zeng, W.; Mustapha, A.; Lin, M. Soy protein-based films incorporated with cellulose nanocrystals and pine needle extract for active packaging. Ind. Crops Prod. 2018, 112, 412–419. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).