Abstract
Lectins (carbohydrate-binding proteins) are able to distinguish different patterns of glycosylation on cell surfaces. This study investigated the effects of lectins from Alpinia purpurata inflorescence (ApuL) and Schinus terebinthifolia leaf (SteLL) on the viability of human leukemia cells (K562, chronic myeloid leukemia; JURKAT, acute lymphoblastic leukemia) and mesenchymal stem cells (MSCs) from human umbilical cords. In addition, possible immunomodulatory effects of ApuL and SteLL on MSCs were assessed by determining cytokine levels in cultures. ApuL reduced the viability of JURKAT cells (IC50: 12.5 μg/mL), inducing both apoptosis and necrosis. For K562 cells, ApuL at 50 µg/mL caused a decrease in viability, but of only 8.8%. Conversely, SteLL exerted a cytotoxic effect on K562 (IC50: 6.0 μg/mL), inducing apoptosis, while it was not cytotoxic to JURKAT. ApuL and SteLL (0.19–100 μg/mL) did not decrease MSCs viability. Treatment with ApuL strongly suppressed (99.5% reduction) the release of IL-6 by MSCs. SteLL also reduced the levels of this cytokine in culture supernatant. In conclusion, ApuL and SteLL showed potential to reduce the viability of leukemia cells, as well as immunomodulatory effect on MSCs without being toxic to them. These biological properties can be explored biomedically and biotechnologically in the future.
1. Introduction
Lectins are proteins capable of recognizing and binding to carbohydrates in a specific and reversible way, without causing structural modifications. Plant lectins are widely studied and have several biological activities, such as antimicrobial, antitumor, and immunomodulatory actions [1,2,3]. ApuL is a lectin found in the inflorescences of Alpinia purpurata that has immunomodulatory effect on human peripheral blood mononuclear cells [4] and antimicrobial effect on bacteria and fungi [5]. Agglutination inhibition assays indicated that the carbohydrate-recognizing domain (CRD) of ApuL can bind the glycan moieties of fetuin and ovalbumin; on the other hand, the monosaccharides glucose, galactose, N-acetylglucosamine, ribose, methyl-D-glucopyranoside, and mannose were not able to inhibit ApuL hemagglutinating activity [4]. The lectin isolated from leaves of Schinus terebinthifolia (SteLL) showed in vivo antitumor [6], antinociceptive [7], anti-depressive-like [8], and anxiolytic [9] activities in mice. An in vivo antiangiogenic property of SteLL was proved in the Coturnix japonica embryo model [10]. Furthermore, SteLL showed immunomodulatory effect on mouse splenocytes, stimulating the release of interleukins (IL-2, IL-4, and IL-17A), tumor necrosis factor alpha (TNF-α), interferon-gamma (IFN-γ), and reducing the secretion of nitric oxide (NO) [11]. Similar to ApuL, the hemagglutinating ability of SteLL was also not affected by monosaccharides (such as N-acetylglucosamine), but it was inhibited by the glycoproteins fetuin and ovalbumin [12]. The presence of these glycoproteins, as well as of azocasein, also prevented the labeling of Cryptococcus neoformans cells by SteLL conjugated to quantum dots [12].
Cancer is one of the leading causes of death worldwide, accounting for nearly 10 million deaths in 2020 alone [13]. Given the complexity of cancer, the treatments include chemotherapy agents, radiotherapy, surgical removal, among others, the choice being dependent on the location, risk factors, severity, and tumor type. However, these therapies are aggressive and often affect normal cells and cause serious side effects; in addition, it has been reported that there is resistance of some tumors to anticancer drugs [14,15]. Thus, the search for new and less aggressive molecules that can fight cancer and improve cell selectivity drives research around the world [15]. Some lectins have stood out for their in vitro cytotoxic effects against tumor cell lines, in vivo antitumor action, and helping roles in the diagnosis and monitoring of cancer progression and evolution [2,6,16,17]. Lectins can specifically recognize changes in glycosylation patterns in tumor cell membranes, triggering effective responses, such as activation of cell death and tumor growth inhibition mechanisms [2,18].
Leukemias are hematological neoplasms and can be manifested acutely or chronically. Hematopoietic progenitors are capable of self-renewal and differentiation into cell lines that make up the hematopoietic system. However, in leukemias, these cells stop responding to external factors within the bone marrow microenvironment and, consequently, disordered growth in the bone marrow contributes to immature cells being found in the peripheral blood [19]. Ahmed et al. [20] showed that the incidence and mortality of acute myeloid leukemia is increasing globally for males and females, from 1990 to 2019, and that disability-adjusted life years (DALYs) increased for males. Plant lectins have been demonstrated to be potential antitumor drugs, including against leukemia [21,22,23,24].
Stem cells are capable of self-renewal and differentiation into different cell types. In the field of cell therapy, these cells stand out for their promising results in the recovery and cure of various diseases [25,26]. For example, Xifro et al. [27] showed that the infusion of stem cells from bone marrow into the midbrain significantly improved the loss and functionality of dopaminergic neurons in a model of Parkinson’s disease in mice. Mesenchymal stem cells (MSCs) can be isolated from almost all tissues in the human body, including the umbilical cord, bone marrow, adipose tissue, and dental tissues. Considered multipotent, they can differentiate into different mesenchymal lines (osteogenic, chondrogenic, and adipogenic), in addition to presenting immunomodulatory properties useful for therapies of degenerative disease and cancer [28,29,30,31,32]. Preclinical studies showed that MSCs modulate innate and adaptive immune responses in various diseases, inhibiting pro-inflammatory responses and stimulating anti-inflammatory responses. In the cellular microenvironment, MSCs secrete a range of chemokines, cytokines, growth factors, as well as extracellular matrix molecules, which have useful therapeutic properties [33,34].
Research involving lectins and stem cells is stimulated by the potential of lectins to recognize carbohydrates present in cell membranes [35]. A high-density microarray, using lectins, proved to be useful for the recognition of different stem cells [36,37]. The Erythrina cristagalli lectin served as a support matrix for proliferation and increased the efficiency of plating pluripotent stem cells and human embryonic stem cells [35]. In addition, it is likely that the interaction of lectins with glycosylated receptors in these cells also leads to intracellular responses.
Reports of the immunomodulatory potential of ApuL and SteLL, as well antitumor effect of this last, stimulated the investigation of the cytotoxic activity of these lectins on leukemic tumor cell lines (K562, chronic myelogenous leukemia; JURKAT, acute lymphoblastic leukemia) and mesenchymal stem cells (MSCs) isolated from human umbilical cord, as well as the ability of these lectins to modulate cytokine production by MSCs.
2. Materials and Methods
2.1. Isolation of Lectins
Inflorescences of A. purpurata were collected at the campus of the Universidade Federal de Pernambuco (UFPE), with authorization (36301) from the Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio). The research was entered in the Sistema Nacional de Gestão do Patrimônio Genético e do Conhecimento Tradicional Associado (SisGen) (registration A83A2DE). A voucher specimen is deposited under number 53,376 at the herbarium UFP Geraldo Mariz from UFPE. The bracts were separated, washed with distilled water, and dried at 28 °C for 2 days. ApuL was then isolated according to Brito et al. [4]. The dry material was ground, and the resulting powder (10 g) was homogenized in 0.15 M NaCl (100 mL) for 16 h at 28 °C under constant stirring. The suspension was filtered through filter paper and then centrifuged (10 min, 3000× g) to obtain the extract. Ammonium sulfate (40% saturation) was added to the extract for protein fractionation [38]. After constant agitation for 4 h at 28 °C, the material was centrifuged (10 min, 3000× g), and the supernatant was dialyzed (6 h) against distilled water. The dialyzed supernatant was subjected to gel filtration chromatography on a column (30.0 × 1.5 cm) of Sephadex G-75 (GE Healthcare Life Sciences, Uppsala, Sweden), previously equilibrated with 0.15 M NaCl. The column was eluted with 0.15 M NaCl at a flow rate of 20 mL/h. Fractions of 5 mL were collected and monitored for absorbance at 280 nm. The pool of unadsorbed fractions corresponds to ApuL.
Leaves of S. terebinthifolia were also collected on the campus of UFPE, as authorized by the ICMBio. The record in the SisGen was made under the protocol AED6BF8. A voucher is archived (no. 73,431) at the Instituto Agronômico de Pernambuco (Recife, Brazil). The leaves were washed with distilled water, dried for 4 days at 28 °C, and then ground. To obtain the extract, the flour was suspended in the proportion of 10% (w/v) in 0.15 M NaCl. After homogenization for 16 h at 4 °C and centrifugation (15 min, 9000× g, 4 °C), the protein extract was obtained. SteLL was isolated according to the protocol described by Gomes et al. [39]. The extract was applied onto a column (7.5 × 1.5 cm) of chitin (Sigma-Aldrich, St. Louis, MO, USA), previously equilibrated with 0.15 M NaCl. Subsequently, the column was washed to remove unbound proteins, and then SteLL was eluted (20 mL/h) with 1.0 M acetic acid. The collected fractions were followed for absorbance at 280 nm. SteLL was dialyzed against distilled water for about 6 h, using a cellulose membrane (Sigma-Aldrich) to remove acetic acid.
2.2. Determination of Hemagglutinating Activity (HA) and Protein Concentration
The carbohydrate-binding capacity of the lectins was determined through the hemagglutinating activity (HA) assay, as described by Procopio et al. [16], using rabbit erythrocytes fixed with glutaraldehyde [40]. Protein concentration was determined by the method described by Lowry et al. [41] using a standard curve of bovine albumin (31.25–500 μg/mL).
2.3. Leukemic Tumor Lines
Human cell lines K562 (chronic myeloid leukemia) and JURKAT (acute lymphoblastic leukemia) were obtained from the Cell Bank of Rio de Janeiro, Brazil. Cells were cultured in complete RPMI medium containing 10% fetal bovine serum (FBS) and 1% antibiotic solution (100 µg/mL streptomycin and 100 U/mL penicillin).
2.4. Evaluation of Cytotoxicity to Leukemic Tumor Lines
K562 (105 cells/well) and JURKAT (105 cells/well) were cultured in 24-well microplates containing RPMI 1640 medium with HEPES supplemented with 10% fetal bovine serum (FBS, w/v). Next, K562 and JURKAT cells were treated with ApuL or SteLL (6.25–50 µg/mL). Wells containing only cells and culture medium were used as the negative control. Ethanol-treated wells were used as a positive death control. After a 24-h incubation period (37 °C, 5% CO2), the cells were evaluated for viability using the MTT test ([3-4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]) [42]. After each incubation period, MTT (20 μL; 5 mg/mL) was added to the wells and the plate incubated for 4 h. Then, the culture medium was removed, and dimethyl sulfoxide (DMSO, 100 μL) was added to solubilize the formed formazan crystals. The absorbance reading (590 nm) was performed in a microplate reader. Etoposide phosphate (0.625–10 μg/mL) was used as positive control. Each experiment was performed in triplicate.
2.5. Evaluation of Cell Death
The cells were cultured for 24 h in the absence and the presence of the lectins, as described above, and then they were washed with phosphate-buffered saline (PBS), collected by centrifugation (450× g, 5 min), and evaluated for occurrence of apoptosis and/or necrosis using the Annexin V-FITC/PI kit (Invitrogen, Waltham, MA, USA) following the manufacturer’s instructions. The analysis was performed on a BD Accuri C6 flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) with a collection of 10,000 events per sample. Double-labeled cells were in late apoptosis; cells labeled with AnnV-FITC alone were considered to be in an early stage of apoptosis; cells labeled only with PI were considered in the process of necrosis; and unlabeled cells were considered viable. Each experiment was performed in triplicate.
2.6. Human Umbilical Cords
Mesenchymal stem cells (MSCs) were isolated from human umbilical cords collected after cesarean deliveries at the Hospital D’Avila, in the city of Recife, Pernambuco, Brazil. Donor mothers signed a Free and Informed Consent Term. The procedures were approved by the Human Research Ethics Committee of the UFPE (protocol 90172918.3.0000.5208). The cords were transported to the laboratory in a sterile container, containing saline solution with EDTA (2 mM) and antibiotics—penicillin (150 U/mL), streptomycin (150 μg/mL) and amphotericin (5 μg/mL)—being processed in up to 6 h after delivery.
2.7. Isolation and Cultivation of MSCs
To isolate the cells, the cords were washed, and the veins were perfused with PBS. Then, the cords were cut into small pieces lengthwise (approximately 2 cm in length), thus removing veins and arteries, which were discarded. Tissue containing Wharton’s jelly was then minced and transferred to sterile culture bottles (75 cm2), containing DMEM-low glucose medium (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) supplemented with FBS (10% v/v), Ham’s F nutrient factor −12 (20% v/v; Gibco), and the antibiotics penicillin (100 U/mL) and streptomycin (100 μg/mL) were kept in an oven at 37 °C, 80% humidity, and 5% CO2. The MSCs isolation method was conducted without enzymatic treatment, based on the spontaneous migration of tissue cells and their adhesion to plastic with an average duration of 21 days. Identification was performed by known characteristics of MSCs, such as ability to adhere to plastic, morphology (fibroblastoid aspect), and by immunophenotyping [43].
2.8. Evaluation of Cytotoxicity of ApuL and SteLL to MSCs
MSCs (105 cells/well) were grown in 96-well plates in DMEM low glucose medium and incubated in an oven (37 °C, 5% CO2) for a period of 24 h for adherence to the plate. After this period, ApuL or SteLL were resuspended in the culture medium and added to the plates in serial dilution to reach final concentrations between 0.139 and 100 µg/mL in the wells. Wells containing only cells and culture medium were used as negative controls. Plates were incubated again for 24 and 48 h. After each incubation period, the MTT test was performed, as described in Section 2.4.
To investigate cell death, MSCs were cultivated in 6-well plates in DMEM low glucose medium and incubated in an oven (37 °C, 5% CO2) for a period of 24 h to adherence to the board. After this period, the cells were treated with ApuL or SteLL (50 µg/mL). Wells containing only cells and culture medium were used as negative controls. After treatment, the plates were incubated in an oven for 24 h. Then, cells were collected using trypsin (Gibco) and incubated again in an oven for 5 min to dissociate cells from the plate. Next, the MSCs were collected, washed in PBS, and centrifuged (3000 rpm, 10 min). The occurrence of apoptosis and/or necrosis was then assessed, as described in Section 2.5.
2.9. Cytokine Dosage in MSCs Culture Supernatants
Supernatants from MSC cultures treated or not for 24 h with ApuL or SteLL (50 µg/mL) were collected for cytokine quantification using the Th1/Th2/Th17 human cytokine kit –CBA (BD Biosciences) to detect interleukins (IL-2, IL-4, IL-6, IL-10, IL17A), tumor necrosis factor alpha (TNF-α), and interferon gamma (IFN-γ). Assays were performed according to the manufacturer’s instructions, and data were acquired on the BD Accuri C6 cytometer.
2.10. Statistical Analysis
The results obtained were analyzed using the GraphPad Prism® software, version 8.0, expressed as mean ± standard deviation (SD). Statistically significant differences between groups were calculated by applying analysis of variance (ANOVA), followed by Tukey’s post test. The t-test was used to analyze the cytokine assay results. p values < 0.05 indicated significance when compared to the control. IC50 (the concentration required to reduce the cell viability by 50%) values were calculated by linear regression.
3. Results
ApuL and SteLL were isolated following the purification procedures previously established [4,39], and they showed specific HA of 180 and 1,024, respectively. This result shows that the carbohydrate-binding ability of the lectin molecules was preserved in the samples used in the present work.
ApuL caused a reduction in the viability of JURKAT tumor cells, with an IC50 of 12.5 ± 1.3 μg/mL. On the other hand, the viability of the JURKAT cells was not significantly reduced in the presence of SteLL. For K562 cells, ApuL caused a significant increase in the number of non-viable cells only at the concentration of 50 µg/mL and even when the inhibition percentage was low, ca. 8.8%. Conversely, SteLL significantly reduced the viability of K562 cells, with an IC50 of 6.0 ± 0.27 μg/mL. Etoposide phosphate (positive control) showed IC50 2.5 ± 0.2 and 2.9 ± 0.7 μg/mL on K562 and JURKAT, respectively.
When analyzing the type of cell death induced by the lectins, it was observed that ApuL predominantly induced apoptosis (26.6 ± 1.3% of cells) of JURKAT, but a high number of cells in late apoptosis or necrosis (20.4 ± 0.8%) was also detected (Figure 1a). In its turn, SteLL induced mainly apoptotic death of K562 cells (26.0 ± 0.7%) (Figure 1b).
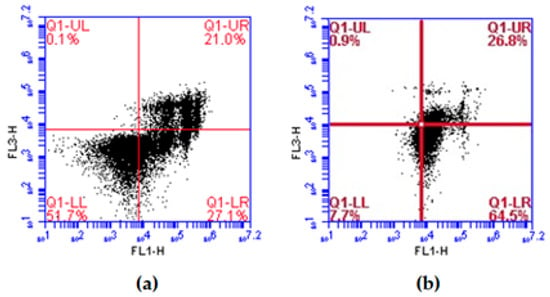
Figure 1.
Representative dot plots of the cell death evaluation of leukemic tumor lines. (a) JURKAT cells treated with ApuL. (b) K562 cells treated with SteLL. Lectins were used at their respective IC50. Quadrant LL: viable cells. UL quadrant: cells stained with propidium iodide. Quadrant LR: cells labeled with annexin V. Quadrant UR: cells labeled with annexin V and propidium iodide.
MSCs showed no significant reduction (p > 0.05) in viability after being treated with ApuL or SteLL for periods of 24 h (Figure 2a,b) and 48 h (Figure 2c,d). In the period of 24 h, it was observed that ApuL at 100 µg/mL caused higher cell viability (110.7%) than the control. After 48 h, ApuL at 50 and 100 µg/mL also promoted higher cell viability (123.9% and 111.5% respectively) when compared to the control.
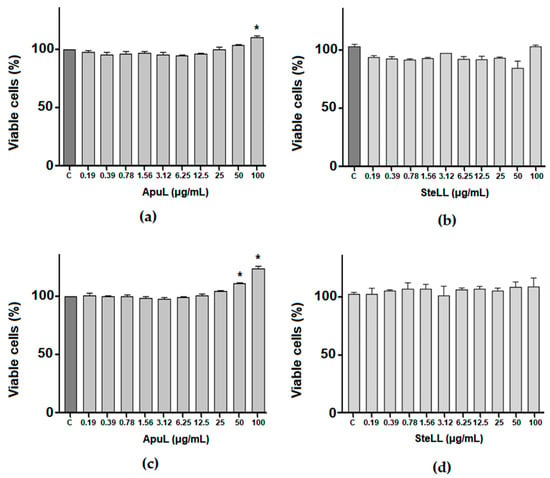
Figure 2.
Evaluation of the viability of MSCs treated with different concentrations (0.19–100 μg/mL) of ApuL (a,c) or SteLL (b,d) for 24 h (a,b) and 48 h (c,d), using the MTT assay. The bars represent the mean cell viability (%) ± SD for three independent experiments performed in triplicate, considering the control group as 100% viability. (*) p < 0.05 in comparison with control (C).
To confirm the absence of cytotoxicity and to verify if the lectins induced apoptosis or necrosis in the MSCs, we performed the cell death evaluation assay. As expected, no significant differences were detected in the number of cells undergoing necrosis, early apoptosis, or late apoptosis compared to the untreated control (Figure 3). The viability of MSCs treated with ApuL (50 μg/mL) was 96.9 ± 1.0%, and, with SteLL (50 μg/mL), it was 97.4 ± 0.9%.
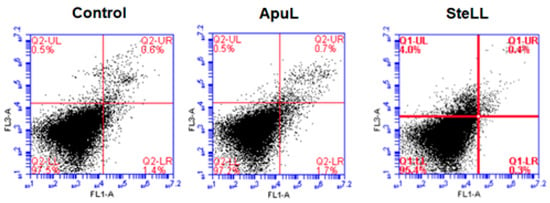
Figure 3.
Representative dot plots of the cell death evaluation of mesenchymal stem cells (MSCs) from control or treated with ApuL or SteLL at 50 µg/mL. Quadrant LL: viable cells. UL quadrant: cells stained with propidium iodide. Quadrant LR: cells labeled with annexin V. Quadrant UR: cells labeled with annexin V and propidium iodide.
MSCs produced pro-inflammatory (TNF-α and IFN-γ, IL-2, IL-6, IL-17) and anti-inflammatory (IL-4 and IL-10) cytokines, but the levels of most of these cytokines were not significantly changed after treatment with ApuL or SteLL (50 μg/mL) (Figure 4). However, the high concentration of IL-6 (23,431 pg/mL) released by MSCs was reduced in the presence of SteLL to 15,045 pg/mL, and it was strongly suppressed to 101.8 pg/mL (99.5% reduction) when MSCs were treated with ApuL (Figure 4).
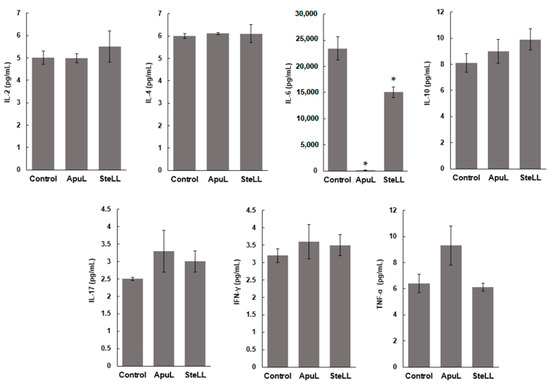
Figure 4.
Evaluation of pro-inflammatory (TNF-α, IFN-γ, IL-2, IL-6, IL-17) and anti-inflammatory (IL-4 and IL-10) cytokine levels in supernatants of cultures of human MSCs maintained in the absence (control) and in the presence of ApuL or SteLL (50 μg/mL) for 24 h. Bars represent mean cytokine concentration (pg/mL) ± SD for three independent experiments performed in triplicate. (*) p < 0.05 in comparison with control.
4. Discussion
Previous data on the carbohydrate-binding specificity of ApuL and SteLL, obtained through HA inhibition assay, showed that both lectins can recognize the oligosaccharide moieties of ovalbumin [4,12], which are rich in mannose and N-acetylglucosamine [44], but they did not have their agglutinating properties prevented by monosaccharides in the free form. Another similarity between them is that fetuin (containing sialic acid and galactose) inhibited their hemagglutinating activity. In other studies, the preference of some lectins to oligosaccharides has been associated with a better geometric fitting between the lectin CRD and oligoglycans in comparison with mono- or disaccharides [45,46].
Studies have reported the presence of glycans containing mannose, sialic acid, and galactose in K562 and JURKAT cells. An immunofluorescent assay using concanavalin A conjugated to quantum dots revealed the presence of mannosyl groups on the surface of K562 cells [47]. Nayak et al. [48] reported the presence of complex, hybrid, paucimannose, and oligomannose glycans in membrane fraction of K652 cells, being predominantly oligomannose- and complex-type glycans. Zhang et al. [49] also detected high mannoses in K562, and Lizzi et al. [50] reported a high expression of both mannose and galactose in this cell line. Leukosialin is a major sialoglycoprotein found on leukocytes that contains high-mannose type N-glycan chains and further elongation with O-glycans containing galactose and sialic acid [51].
Despite the similarities described above, ApuL and SteLL were cytotoxic against leukemia cells, but each lectin was highly active against one of the two cell lines evaluated: ApuL mainly reduced the viability of JURKAT, while SteLL showed effect only on K562 cells. Indeed, the biosynthesis of surface glycans in these cells occurs in a different way. It was demonstrated that O-glycan elongation of leukosialin occurs very rapidly in K562 cells, probably before the final processing of the N-glycans of high-mannose type, while, in JURKAT cells, there is a difference in enzymatic action that arrests O-glycan synthesis at the first step [51,52]. Thus, although the HA inhibition assay was not able to evidence differences in the carbohydrate-binding specificity of ApuL and SteLL, its CRDs possess binding abilities strong enough to distinguish the point of recognition of the differences between JURKAT and K562 cells. This evidence reinforces the complexity of the interactions between lectins and glycans, as well as points to the need of studying the fine glycan specificity of ApuL and SteLL in the future, which can be determined more precisely through glycan arrays, for example.
The interaction of lectins with glycoconjugates present on tumor cell surfaces can cause intracellular responses that lead to apoptosis and inhibition of tumor growth [3,53]. As described above, SteLL induced death of K562 by apoptosis. This result is similar to those found by Ramos et al. [6], who described cytotoxicity of SteLL against sarcoma 180 cells (IC50: 8.30 μg/mL) through induction of apoptosis. Other lectins are capable of inducing apoptosis in leukemic cells. Lectin isolated from the marine sponge Cliona varians (CvL; 1.0–150.0 μg/mL) inhibited the growth of K562 and JURKAT leukemic cells, with IC50 of 70 and 100 μg/mL, respectively. After a period of 72 h of incubation with the lectin, cell death of K562 was induced by apoptosis [54]. Lectin from Astragalus membranaceus roots (AML; 20 μg/mL) caused apoptosis in the K562 strain in a time-dependent manner, resulting in the detection of morphological changes, such as chromatin condensation, nuclear fragmentation, and apoptotic bodies [55]. Lectin from Calliandra surinamensis leaf pinnulae (CasuL) reduced the viability of K562 strains after 72 h of incubation, with an IC50 of 5.7 μg/mL [16].
MSCs are a type of multipotent adult stem cells, being considered promising cellular therapeutic agents, due to their self-renewal properties and differentiation into cell lineages [56,57]. Lectins have been applied in studies with stem cells due to their ability to recognize and bind to carbohydrates on the surface of these cells, being used mainly in the microarray technique in order to search for new biomarkers [58,59]. Thus, we proposed to evaluate the effects of ApuL and SteLL on cytokine production by MSCs.
Initially, we evaluated whether ApuL and SteLL would cause any cytotoxic effect in MSCs isolated from human umbilical cord. It was observed that they did not cause relevant cytotoxicity in MSCs and did not lead these cells to undergo necrosis or apoptosis. This result agrees with previous studies that showed that ApuL and SteLL do not have cytotoxic effects on healthy cells. Brito et al. [4] demonstrated the safety of ApuL with regards to human lymphocytes, while Santos et al. [11] showed that SteLL (3.12–50 μg/mL) is not cytotoxic for mouse splenocytes. Additionally, the viability of macrophages isolated from mice was not affected by SteLL (2.0–16.0 μg/mL) [60].
Evaluations of the cytotoxicity of other lectins to MSCs have been described. Microgramma vacciniifolia frond lectin (MvFL) did not show cytotoxicity for MSCs at concentrations of 0.1–50 μg/mL, but, at 100 μg/mL, it reduced their viability to 80% [61]. The lectin isolated from the bark of Crataeva tapia (CrataBL; 100 μM) also affected the viability of MSCs after 24 h of treatment; however, it did not induce apoptosis or necrosis [62]. Viscum album coloratum lectin (VCA; 1–10 pg/mL) showed no cytotoxic effect and increased MSC self-renewal and proliferation by interfering with the expression of factors involved in cell cycle control [63]. In another study, it was demonstrated that VCA (10 pg/mL) increased the viability of MSCs [64].
The immunomodulatory effect of MSCs is not fully understood, but there are data that indicate that MSCs can reduce local inflammation through the secretion of antiproliferative mediators, such as NO and, mainly, prostaglandin E2, and, systemically, they can shift the host response from Th1/Th17 to a Th2 immune profile [65]. Liu and Hwang [66] isolated MSCs from umbilical cord blood and found that this type of cell can produce several cytokines and growth factors. The results of this study strongly suggest that cytokine induction and signal transduction are important for the differentiation of umbilical cord blood MSCs. IL-6, IL-8, MCP-1, RANTES, GRO-a, IFN-γ, IL-1α, TGF-β, GM-CSF, angiogenin, and oncostatin M were constitutively expressed.
In the present work, MSCs produced pro-inflammatory (TNF-α and IFN-γ, IL-2, IL-6, and IL-17) and anti-inflammatory (IL-4 and IL-10) cytokines, but the IL-6 levels were remarkably higher in comparison with the others. More interestingly, only the levels of IL-6 significantly changed after treatment with ApuL or SteLL. Accumulating evidence supports an essential role for IL-6 in the development, differentiation, and regeneration of stem cells [67]. IL-6 is a pleiotropic cytokine with a role in immune regulation, hematopoiesis, and tissue regeneration in vivo. Pricola et al. [68] demonstrated that MSCs isolated from bone marrow were able to secrete and respond to IL-6. One study showed that autocrine/paracrine IL-6 contributes to chondrogenic differentiation in MSCs isolated from bone marrow [69]. Xie et al. [70] presented evidence that the IL-6/IL-6 receptor complex promotes osteogenic differentiation of bone marrow MSCs. This cytokine, when produced by MSCs, was also able to act in regenerative processes in the central nervous system [71].
Other studies show that lectins can exert an immunomodulatory effect on MSC cell cultures. The VCA lectin (1 pg/mL) increased the expression of IL-6 [64]. On the other hand, CrataBL reduced the secretion of cytokines (IL-6, IL-8) at concentrations of 50 and 100 µM [62]. MSCs modulate the functionality of antigen-presenting cells, T cells, and NK cells (natural killer), mainly by immunosuppressive effects [72,73]. These cells can migrate to injured or inflamed sites in response to inflammatory mediators. They exert repairing effects through the transdifferentiating of tissue-specific cells or by secreting immunoregulatory factors that facilitate the reestablishment of tissue homeostasis [73,74]. A study conducted by Raffaghello et al. [75] demonstrated that MSCs isolated from bone marrow inhibit apoptosis of activated neutrophils, and the main soluble factor derived from MSCs was IL-6. In this case, this cell exerted an anti-apoptotic action through soluble factors, without the need for cell–cell contact.
In previous studies, SteLL (12.5 μg/mL) demonstrated the ability to stimulate the release of cytokines (IL-6, IL-10, IL-17A, TNF-α), NO, and superoxide anion in cultured macrophages isolated from mice. In addition, it stimulated the production of IL-17A, TNF-α, IFN-γ, IL-4, and IL-2 in cultured splenocytes isolated from mice [11,60]. It has already been shown that ApuL is capable of inducing lymphocytes isolated from humans to produce a Th17 response, producing IL-17 and IL-10, the latter with an immunoregulatory function [4]. Our results show that the immunomodulatory action of ApuL and SteLL is cell-type specific.
5. Conclusions
ApuL and SteLL were cytotoxic to leukemic tumor cells, and they did not exhibit toxic activity on MSCs isolated from the umbilical cord, and they functioned as immunomodulators of these cells, inhibiting the release of IL-6. Future studies can be conducted to unveil the glycan-binding differences between ApuL and SteLL that led them to exert distinct effects on the same leukemia cell line. In addition, our data open windows to investigate the effects of ApuL and SteLL on the differentiation of MSCs, as well as to evaluate possibilities of exploring their effects on stem cells biomedically and biotechnologically in the future (for example, as immunomodulators of these cells when used in disease therapies).
Author Contributions
Conceptualization, J.d.S.B., A.d.O.M., M.B.d.S. and T.H.N.; methodology, J.d.S.B., A.d.O.M., W.D.C.G., V.M.B.d.L., C.G.R., M.B.d.S. and T.H.N.; validation, J.d.S.B., A.d.O.M., L.L.d.S.P., W.D.C.G., D.J.L.T. and T.H.N.; formal analysis, J.d.S.B., A.d.O.M., L.L.d.S.P., W.D.C.G., D.J.L.T., P.M.G.P., V.M.B.d.L., C.G.R., M.B.d.S. and T.H.N.; investigation, J.d.S.B., A.d.O.M., L.L.d.S.P., W.D.C.G. and D.J.L.T.; resources, P.M.G.P., V.M.B.d.L., C.G.R., M.B.d.S. and T.H.N.; data curation, J.d.S.B., A.d.O.M., L.L.d.S.P., W.D.C.G., D.J.L.T. and T.H.N.; writing—original draft preparation, J.d.S.B., A.d.O.M. and L.L.d.S.P.; writing—review and editing, P.M.G.P. and T.H.N.; visualization, J.d.S.B., A.d.O.M., L.L.d.S.P. and T.H.N.; supervision, M.B.d.S. and T.H.N.; project administration, M.B.d.S. and T.H.N.; funding acquisition, P.M.G.P., V.M.B.d.L., C.G.R., M.B.d.S. and T.H.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Conselho Nacional de Desenvolvimento Científico e Tecnológico, grant number 407192/2018-2, Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco, grant number APQ-0108-2.08-14, and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Financial Code 001.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data are not publicly available due to privacy issues.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Coelho, L.C.B.B.; Silva, P.M.S.; Oliveira, W.F.; Moura, M.C.; Pontual, E.V.; Gomes, F.S.; Paiva, P.M.G.; Napoleão, T.H.; Correia, M.T.S. Lectins as antimicrobial agents. J. Appl. Microbiol. 2018, 125, 1238–1252. [Google Scholar] [CrossRef]
- Patriota, L.L.S.; Brito, J.S.; Ramos, D.B.M.; Procópio, T.F.; Paiva, P.M.G.; Pontual, E.V.; Melo, C.M.L.; Napoleão, T.H. Plant-derived lectins: A review of their status as alternatives to anticancer chemotherapy. In Horizons in Cancer Research; Watanabe, H.S., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2019; pp. 171–205. [Google Scholar]
- Patriota, L.L.S.; Brito, J.S.; Barboza, B.R.; Paiva, P.M.G.; Melo, C.M.L.; Napoleão, T.H. A review on the immunomodulatory effects of plant lectins. In Hemagglutinins: Structures, Functions and Mechanisms; Ng, T.B., Wong, J., Tse, R., Tse, T.F., Chan, H., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2019; pp. 53–82. [Google Scholar]
- Brito, J.S.; Ferreira, G.R.S.; Klimczak, E.; Gryshuk, L.; Santos, N.D.L.; Patriota, L.L.S.; Moreira, L.R.; Sores, A.K.A.; Barboza, B.R.; Paiva, P.M.G.; et al. Lectin from inflorescences of ornamental crop Alpinia purpurata acts on immune cells to promote Th1 and Th17 responses, nitric oxide release, and lymphocyte activation. Biomed. Pharmacother. 2017, 94, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.R.S.; Brito, J.S.; Procópio, T.F.; Santos, N.D.L.; Lima, B.J.R.C.; Coelho, L.C.B.B.; Navarro, D.M.A.F.; Paiva, P.M.G.; Soares, T.S.; Moura, M.C.; et al. Antimicrobial potential of Alpinia purpurata lectin (ApuL): Growth inhibitory action, synergistic effects in combination with antibiotics, and antibiofilm activity. Microb. Pathogen. 2018, 124, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Ramos, D.B.M.; Araújo, M.T.M.; Araújo, T.C.L.; Neto, O.G.S.; Silva, M.G.; Silva, Y.A.; Torres, D.J.L.; Patriota, L.L.S.; Melo, C.M.L.; Lorena, V.M.B.; et al. Evaluation of antitumor activity and toxicity of Schinus terebinthifolia leaf extract and lectin (SteLL) in sarcoma 180-bearing mice. J. Ethnopharmacol. 2019, 233, 148–157. [Google Scholar] [CrossRef]
- Marinho, A.O.; Brito, J.S.; Costa, J.A.; Silva, A.B.; Silva, S.P.; Amorim, L.C.; Correia, M.T.S.; Paiva, P.M.G.; Oliveira, A.M.; Patriota, L.L.S.; et al. Schinus terebinthifolia leaf lectin has central and peripheral antinociceptive action mediated by its carbohydrate-recognition domain and delta-opioid receptors. J. Ethnopharmacol. 2023, 301, 115817. [Google Scholar] [CrossRef]
- Lima, B.R.F.; Patriota, L.L.S.; Marinho, A.O.; Costa, J.A.; Napoleão, T.H.; Rosa, M.M.; Paiva, P.M.G. The lectin from Schinus terebinthifolia leaf (SteLL) reduces immobility of mice on the tail suspension test dependent on the monoaminergic and nitric oxide signaling. Neurosci. Lett. 2023, 801, 137092. [Google Scholar] [CrossRef]
- Lima, B.R.F.; Patriota, L.L.S.; Marinho, A.O.; Costa, J.A.; Napoleão, T.H.; Rosa, M.M.; Paiva, P.M.G. The anxiolytic activity of Schinus terebinthifolia leaf lectin (SteLL) is dependent on monoaminergic signaling although independent of the carbohydrate-binding domain of the lectin. Pharmaceuticals 2022, 15, 136. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.J.C.A.; Araujo, V.F.S.; França, R.P.M.; Silva, E.L.A.; Almeida, W.A.; Procópio, T.F.; Paiva, P.M.G.; Napoleão, T.H.; Costa, E.V.L.; Nogueira, R.A.; et al. Schinus terebinthifolia Raddi leaf lectin is an antiangiogenic agent for Coturnix japonica embryos. An. Acad. Bras. Ciênc. 2022, 94, e20211297. [Google Scholar] [CrossRef]
- Santos, A.J.C.A.; Barros, B.R.S.; Aguiar, L.M.S.; Patriota, L.L.S.; Lima, T.A.; Zingali, R.B.; Paiva, P.M.G.; Napoleão, T.H.; Melo, C.M.L.; Pontual, E.V. Schinus terebinthifolia leaf lectin (SteLL) is an immunomodulatory agent by altering cytokine release by mice splenocytes. 3 Biotech 2020, 10, 144. [Google Scholar] [CrossRef]
- Silva, A.R.; Oliveira, W.F.; Silva, P.M.; Patriota, L.L.S.; Alves, R.R.V.; Oliveira, A.P.S.; Correia, M.T.S.; Paiva, P.M.G.; Vainstein, M.H.; Filho, P.E.C.; et al. Quantum dots conjugated to lectins from Schinus terebinthifolia leaves (SteLL) and Punica granatum sarcotesta (PgTeL) as potential fluorescent nanotools for investigating Cryptococcus neoformans. Int. J. Biol. Macromol. 2021, 192, 232–240. [Google Scholar] [CrossRef]
- World Health Organization. Cancer Fact Sheet; OMS: Genebra, Switzerland, 2022; Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 15 March 2023).
- Tessmann, J.W.; Buss, J.; Begnini, K.R.; Berneira, L.M.; Paula, F.R.; Pereira, C.M.P.; Collares, T.; Seixas, F.K. Antitumor potential of 1-thiocarbamoyl-3,5-diaryl-4,5-dihydro-1H- pyrazoles in human bladder cancer cells. Biomed. Pharmacother. 2017, 94, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Shivamadhu, M.C.; Srinivas, B.K.; Jayarama, S.; Chandrashekaraiah, S.A. Anti-cancer and anti-angiogenic effects of partially purified lectin from Praecitrullus fistulosus fruit on in vitro and in vivo model. Biomed. Pharmacother. 2017, 96, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Procópio, T.F.; Patriota, L.L.S.; Moura, M.C.; Silva, P.M.; Oliveira, A.P.S.; Carvalho, L.V.N.; Lima, T.A.; Soares, T.; Silva, T.D.; Coelho, L.C.B.B.; et al. CasuL: A new lectin isolated from Calliandra surinamensis leaf pinnulae with cytotoxicity to cancer cells, antimicrobial activity and antibiofilm effect. Int. J. Biol. Macromol. 2017, 98, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Poiroux, G.; Barre, A.; Van Damme, E.; Benoist, H.; Rougé, P. Plant lectins targeting O-glycans at the cell surface as tools for cancer diagnosis, prognosis and therapy. Int. J. Mol. Sci. 2017, 18, 1232. [Google Scholar] [CrossRef] [PubMed]
- Patriota, L.L.S.; Ramos, D.B.M.; Silva, Y.A.; Santos, A.C.L.A.; Araújo, M.T.M.F.; Brito, J.S.; Torres, D.J.L.; Oliveira, A.M.; Silva, D.C.N.; Lorena, V.M.B.; et al. Microgramma vacciniifolia frond lectin (MvFL) exhibits antitumor activity against sarcoma 180 in mice. Phytomed. Plus 2021, 1, 100013. [Google Scholar] [CrossRef]
- Wong, S.W.; Lenzini, S.; Shin, J.W. Perspective: Biophysical regulation of cancerous and normal blood cell lineages in hematopoietic malignancies. APL Bioeng. 2018, 2, 031802. [Google Scholar] [CrossRef]
- Ahmed, A.; Jani, C.; Bhatt, P.; Singh, H.; Jani, R.; Shalhoub, J.; Marshall, D.; Lam, P.; Salciccioli, J. EPR22-104: A comparison of the burden of leukemia amongst various regions of the world, 1990–2019. J. Natl. Compr. Cancer Netw. 2022, 20, EPR22–EPR104. [Google Scholar] [CrossRef]
- Faheina-Martins, G.V.; Silveira, A.L.; Cavalcanti, B.C.; Ramos, M.V.; Moraes, M.O.; Pessoa, C.; Araújo, D.A.M. Antiproliferative effects of lectins from Canavalia ensiformis and Canavalia brasiliensis in human leukemia cell lines. Toxicol. Vitr. 2012, 26, 1161–1169. [Google Scholar] [CrossRef]
- Yau, T.; Dan, X.; Ng, C.C.; Ng, T.B. Lectins with potential for anti-cancer therapy. Molecules 2015, 20, 3791–3810. [Google Scholar] [CrossRef]
- Catanzaro, E.; Calcabrini, C.; Bishayee, A.; Fimognari, C. Antitumor potential of marine and freshwater lectins. Mar. Drugs 2019, 18, 11. [Google Scholar] [CrossRef]
- Konozy, E.H.E.; Osman, M.E.M. Plant lectin: A promising future anti-tumor drug. Biochimie 2022, 202, 136–145. [Google Scholar] [CrossRef]
- Zwetsloot, P.P.; Végh, A.M.; Jansen, S.J.L.; Hout, G.P.V.; Currie, G.L.; Sena, E.S.; Gremmels, H.; Buikema, J.W.; Goumans, M.J.; Macleod, M.R.; et al. Cardiac stem cell treatment in myocardial infarction: A systematic review and meta-analysis of preclinical studies. Circ. Res. 2016, 118, 1223–1232. [Google Scholar] [CrossRef]
- Mochida, T.; Ueno, H.; Yamazoe, N.T.; Hiyoshi, H.; Ito, R.; Matsumoto, H.; Toyoda, T. Insulin-deficient diabetic condition upregulates the insulin-secreting capacity of human induced pluripotent stem cell–derived pancreatic endocrine progenitor cells after implantation in mice. Diabetes 2020, 69, 634–646. [Google Scholar] [CrossRef]
- Xifro, W.A.; Vicino, U.; Martin, M.I.M.; Bortolozzi, A.; Bové, J.; Vila, M.; Cosma, M.P. Functional rescue of dopaminergic neuron loss in Parkinson’s disease mice after transplantation of hematopoietic stem and progenitor cells. EBioMedicine 2016, 8, 83–95. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Martin, B.J. Mesenchymal stem cells and their potential as cardiac therapeutics. Circulat. Res. 2004, 95, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Chao, K.C.; Yang, H.T.; Chen, M.W. Human umbilical cord mesenchymal stem cells suppress breast cancer tumourigenesis through direct cell-cell contact and internalization. J. Cell. Mol. Med. 2012, 16, 1803–1815. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Chang, Y.; Shyu, W.; Lin, S. Human umbilical cord mesenchymal stem cells: A new era for stem cell therapy. Cell Transpl. 2015, 24, 339–347. [Google Scholar] [CrossRef]
- Chen, X.; Wang, S.; Cao, W. Mesenchymal stem cell-mediated immunomodulation in cell therapy of neurodegenerative diseases. Cell. Immunol. 2018, 326, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Huang, L.; Li, Y.; Fang, B.; Li, G.; Chen, L.; Xu, L. Mesenchymal stem cells and cancer: Clinical challenges and opportunities. BioMed Res. Int. 2019, 2019, 2820853. [Google Scholar] [CrossRef]
- English, K. Mechanisms of mesenchymal stromal cell immunomodulation. Immunol. Cell Biol. 2013, 91, 19–26. [Google Scholar] [CrossRef]
- Dabrowska, S.; Andrzejewska, A.; Janowski, M.; Lukomska, B. Immunomodulatory and regenerative effects of mesenchymal stem cells and extracellular vesicles: Therapeutic outlook for inflammatory and degenerative diseases. Front. Immunol. 2021, 11, 591065. [Google Scholar] [CrossRef] [PubMed]
- Mikkola, M.; Toivonen, S.; Tamminen, K.; Alfthan, K.; Tuuri, T.; Satomaa, T.; Natunen, J.; Saarinen, J.; Tiittanen, M.; Lampinen, M.; et al. Lectin from Erythrina cristagalli supports undifferentiated growth and differentiation of human pluripotent stem cells. Stem Cells Dev. 2012, 22, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Tateno, H.; Toyota, M.; Saito, S.; Onuma, Y.; Ito, Y.; Hiemori, K.; Asashima, M. Glycome diagnosis of human induced pluripotent stem cells using lectin microarray. J. Biol. Chem. 2011, 286, 20345–20353. [Google Scholar] [CrossRef] [PubMed]
- Hasehira, K.; Hirabayashi, J.; Tateno, H. Structural and quantitative evidence of α2–6-sialylated N-glycans as markers of the differentiation potential of human mesenchymal stem cells. Glycoconj. J. 2017, 34, 797–806. [Google Scholar] [CrossRef]
- Green, A.A.; Hughes, L. Protein fractionation on the basis of solubility in aqueous solution of salts and organic solvents. In Methods in Enzymology; Colowick, S., Kaplan, N., Eds.; Academic Press: New York, NY, USA, 1955; pp. 67–90. [Google Scholar]
- Gomes, F.S.; Procópio, T.F.; Napoleão, T.H.; Coelho, L.C.B.B.; Paiva, P.M.G. Antimicrobial lectin from Schinus terebinthifolius leaf. J. Appl. Microbiol. 2013, 114, 672–679. [Google Scholar] [CrossRef]
- Bing, D.H.; Weyand, J.G.; Stavinsky, A.B. Hemagglutination with aldehyde-fixed erythrocytes for assay of antigens and antibodies. Proc. Soc. Exp. Biol. Med. 1967, 124, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Präbst, K.; Engelhardt, H.; Ringgeler, S.; Hübner, H. Basic Colorimetric Proliferation Assays: MTT, WST, and Resazurin. In Cell Viability Assays; Springer: New York, NY, USA, 2017; Volume 1601, pp. 1–17. [Google Scholar]
- Miranda, J.M.; Arruda, J.A.A.; Moreno, L.M.M.; Gaião, W.D.C.; Nascimento, S.V.B.; Silva, E.V.S.; Silva, M.B.; Rodrigues, C.G.; Albuquerque, D.S.; Braz, R.; et al. Photobiomodulation therapy in the proliferation and differentiation of human umbilical cord mesenchymal stem cells: An in vitro study. J. Laser Med. Sci. 2020, 11, 469–474. [Google Scholar] [CrossRef]
- Tai, T.; Yamashita, K.; Ito, S.; Kobata, A. Structures of the carbohydrate moiety of ovalbumin glycopeptide III and the difference in specificity of endo-beta-N-acetylglucosaminidases CII and H. J. Biol. Chem. 1977, 252, 6687–6694. [Google Scholar] [CrossRef]
- Singh, T.; Wu, J.H.; Peumans, W.J.; Rougé, P.; Van Damme, E.J.M.; Wu, A.M. Recognition profile of Morus nigra agglutinin (Morniga G) expressed by monomeric ligands, simple clusters and mammalian polyvalent glycotopes. Mol. Immunol. 2007, 44, 451–462. [Google Scholar] [CrossRef]
- Weis, W.I.; Drickamer, K. Structural basis of lectin-carbohydrate recognition. Ann. Rev. Biochem. 1996, 65, 441–473. [Google Scholar] [CrossRef]
- Cao, J.-T.; Chen, Z.-X.; Hao, X.-Y.; Zhang, P.-H.; Zhu, J.-J. Quantum dots-based immunofluorescent microfluidic chip for the analysis of glycan expression at single-cells. Anal. Chem. 2012, 84, 10097–10104. [Google Scholar] [CrossRef]
- Nayak, S.; Zhao, Y.; Mao, Y.; Li, N. System-wide quantitative N-glycoproteomic analysis from K562 cells and mouse liver tissues. J. Proteome Res. 2021, 20, 5196–5202. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Y.; Jiang, L.; Miao, X.; Zhou, H.; Ji, L. Glycomic alterations are associated with multidrug resistance in human leukemia. Int. J. Biochem. Cell Biol. 2012, 44, 1244–1253. [Google Scholar] [CrossRef] [PubMed]
- Lizzi, A.R.; D’alessandro, A.M.; Bozzi, A.; Cinque, B.; Oratore, A.; D’andrea, G. Pattern expression of glycan residues in AZT-treated K562 cells analyzed by lectin cytochemistry. Mol. Cell. Biochem. 2007, 300, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Piller, V.; Piller, F.; Klier, G.; Fukuda, M. O-Glycosylation of leukosialin in K562 cells. Eur. J. Biochem. 1989, 183, 123–135. [Google Scholar] [CrossRef]
- Piller, V.; Piller, F.; Fukuda, M. Biosynthesis of truncated O-glycans in the T cell line Jurkat. Localization of O-glycan initiation. J. Biol. Chem. 1990, 265, 9264–9271. [Google Scholar] [CrossRef] [PubMed]
- Mazalovska, M.; Kouokam, J.C. Transiently expressed mistletoe lectin II in Nicotiana benthamiana demonstrates anticancer activity in vitro. Molecules 2020, 25, 2562. [Google Scholar] [CrossRef]
- Queiroz, A.F.S.; Silva, R.A.; Moura, R.M.; Dreyfuss, J.L.; Gamero, E.J.P.; Souza, A.C.S.; Tersariol, I.L.S.; Santos, E.A.; Nader, H.B.; Justo, G.Z.; et al. Growth inhibitory activity of a novel lectin from Cliona varians against K562 human erythroleukemia cells. Cancer Chemother. Pharmacol. 2009, 63, 1023–1033. [Google Scholar] [CrossRef]
- Huang, L.H.; Yan, Q.J.; Kopparapu, N.K.; Jiang, Z.Q.; Sun, Y. Astragalus membranaceus lectin (AML) induces caspase-dependent apoptosis in human leucemia cells. Cell. Prolif. 2012, 45, 15–21. [Google Scholar] [CrossRef]
- Gao, F.; Chiu, S.; Motan, D.; Zhang, Z.; Chen, L.; Ji, H.-L.; Tse, H.-F.; Fu, Q.-L.; Lian, Q. Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death Dis. 2016, 7, e2062. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Sun, Y.; Shi, J.; Li, C.-F.; Fang, S.-B.; Wang, D.; Deng, X.-Q.; Wen, W.; Fu, Q.-L. Effects of mesenchymal stem cells from human induced pluripotent stem cells on differentiation, maturation, and function of dendritic cells. Stem Cell Res. Ther. 2017, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Nand, A.; Singh, V.; Wang, P.; Na, J.; Zhu, J. Glycoprotein profiling of stem cells using lectin microarray based on surface plasmon resonance imaging. Anal. Biochem. 2014, 465C, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Dang, K.; Zhang, W.; Jiang, S.; Lin, X.; Qian, A. Application of lectin microarrays for biomarker discovery. Chem. Open 2020, 9, 285–300. [Google Scholar] [CrossRef]
- Lima, I.M.S.F.; Zagmignan, A.; Santos, D.M.; Maia, H.S.; Silva, L.S.; Cutrim, B.S.; Vieira, S.L.; Filho, C.M.B.; Sousa, E.M.; Napoleão, T.H.; et al. Schinus terebinthifolia leaf lectin (SteLL) has anti-infective action and modulates the response of Staphylococcus aureus-infected macrophages. Sci. Rep. 2019, 9, 18159. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.R.; Patriota, L.L.S.; Brito, J.S.; Gaião, W.D.C.; Torres, D.J.L.; Lorena, V.M.B.; Silva, M.B.; Napoleão, T.H. Evaluation of the cytotoxicity of lectin from Microgramma vacciniifolia fronds (MvFL) to human mesenchymal stem cells and leukemia cell lines (K562 and JUKART). Biofarm 2020, 16, 44. [Google Scholar]
- Bonturi, C.R.; Silva, M.C.C.; Motaln, H.; Salu, B.R.; Ferreira, R.S.; Batista, F.P.; Correia, M.T.S.; Paiva, P.M.G.; Turnšek, T.L.; Oliva, M.L.V. A bifunctional molecule with lectin and protease inhibitor activities isolated from Crataeva tapia bark significantly affects cocultures of mesenchymal stem cells and glioblastoma cells. Molecules 2019, 24, 2109. [Google Scholar] [CrossRef]
- Choi, J.H.; Lyu, S.Y.; Lee, H.J.; Jung, J.; Park, W.B.; Kim, G.J. Korean mistletoe lectin regulates self-renewal of placenta-derived mesenchymal stem cells via autophagic mechanisms. Cell Prolif. 2012, 45, 420–429. [Google Scholar] [CrossRef]
- Kim, G.D.; Choi, J.H.; Lim, S.M.; Jun, J.H.; Moon, J.W.; Kim, G.J. Alterations in IL-6/STAT3 signaling by korean mistletoe lectin regulate the self-renewal activity of placenta-derived mesenchymal stem cells. Nutrients 2019, 11, 2604. [Google Scholar] [CrossRef]
- Bouffi, C.; Bony, C.; Courties, G.; Jorgensen, C.; Noël, D. IL-6-Dependent PGE2 secretion by mesenchymal stem cells inhibits local inflammation in experimental arthritis. PLoS ONE 2010, 5, e14247. [Google Scholar] [CrossRef]
- Liu, C.; Hwang, S. Cytokine interactions in mesenchymal stem cells from cord blood. Cytokine 2005, 32, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Klassen, H.J.; Imfeld, K.L.; Kirov, I.I.; Tai, L.; Gage, F.H.; Young, M.J.; Berman, M.A. Expression of cytokines by multipotent neural progenitor cells. Cytokine 2003, 22, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Pricola, K.L.; Kuhn, N.Z.; Haleem-Smith, H.; Song, Y.; Tuan, R.S. Interleukin-6 maintains bone marrow-derived mesenchymal stem cell stemness by an ERK1/2-dependent mechanism. J. Cell. Biochem. 2009, 108, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Kondo, M.; Yamaoka, K.; Sakata, K.; Sonomoto, K.; Lin, L.; Nakano, K.; Tanaka, Y. Contribution of the interleukin-6/STAT-3 signaling pathway to chondrogenic differentiation of human mesenchymal stem cells. Arthritis Rheumatol. 2015, 67, 1250–1260. [Google Scholar] [CrossRef]
- Xie, Z.; Tang, S.; Ye, G.; Wang, P.; Li, J.; Liu, W.; Li, M.; Wang, S.; Wu, X.; Cen, S.; et al. Interleukin-6/interleukin-6 receptor complex promotes osteogenic differentiation of bone marrow-derived mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 13. [Google Scholar] [CrossRef]
- He, M.; Shi, X.; Yang, M.; Yang, T.; Li, T.; Chen, J. Mesenchymal stem cells-derived IL-6 activates AMPK/mTOR signaling to inhibit the proliferation of reactive astrocytes induced by hypoxic-ischemic brain damage. Exp. Neurol. 2018, 311, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Rasmusson, I. Immune modulation by mesenchymal stem cells. Exp. Cell Res. 2006, 312, 2169–2179. [Google Scholar] [CrossRef]
- Engela, A.U.; Baan, C.C.; Dor, F.J.M.F.; Weimar, W.; Hoogduijn, M.J. On the interactions between mesenchymal stem cells and regulatory T cells for immunomodulation in transplantation. Front. Immunol. 2012, 3, 126. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Cao, W.; Shi, Y. Plasticity of mesenchymal stem cells in immunomodulation: Pathological and therapeutic implications. Nat. Immunol. 2014, 15, 1009–1016. [Google Scholar] [CrossRef]
- Raffaghello, L.; Bianchi, G.; Bartolotto, M.; Montecucco, F.; Busca, A.; Dallegri, F.; Ottonello, L.; Pistoia, V. Human mesenchymal stem cells inhibit neutrophil apoptosis: A model for neutrophil preservation in the bone marrow niche. Stem Cells 2008, 26, 151–162. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).