Biodegradable Films from Kefiran-Based Cryogel Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Kefiran Extraction and Purification
2.2. Kefiran-Film Preparation and Experimental Design
2.3. Evaluation of the Films’ Physical Properties
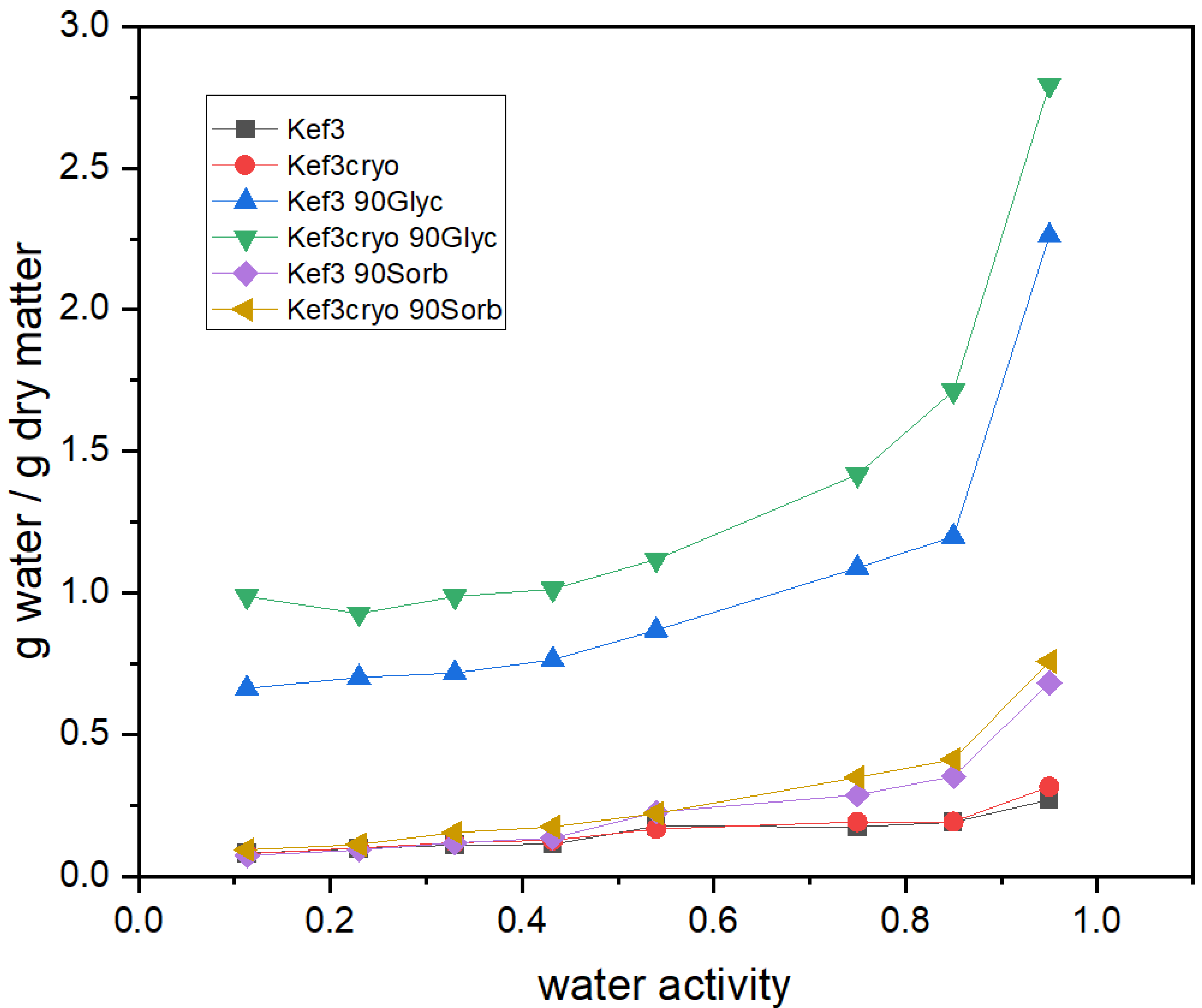
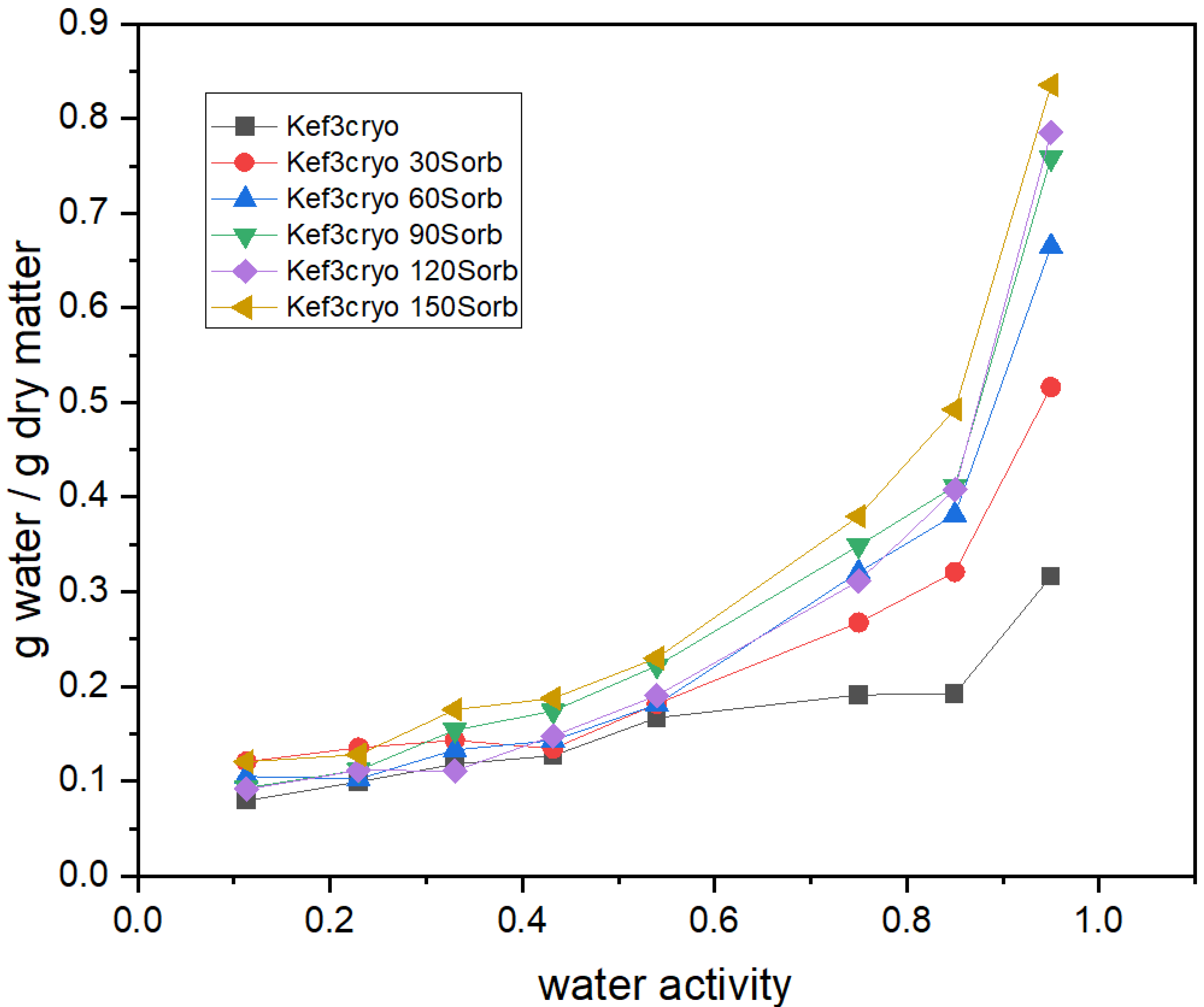
2.4. Kefiran-Film Vapor Adsorption Isotherms
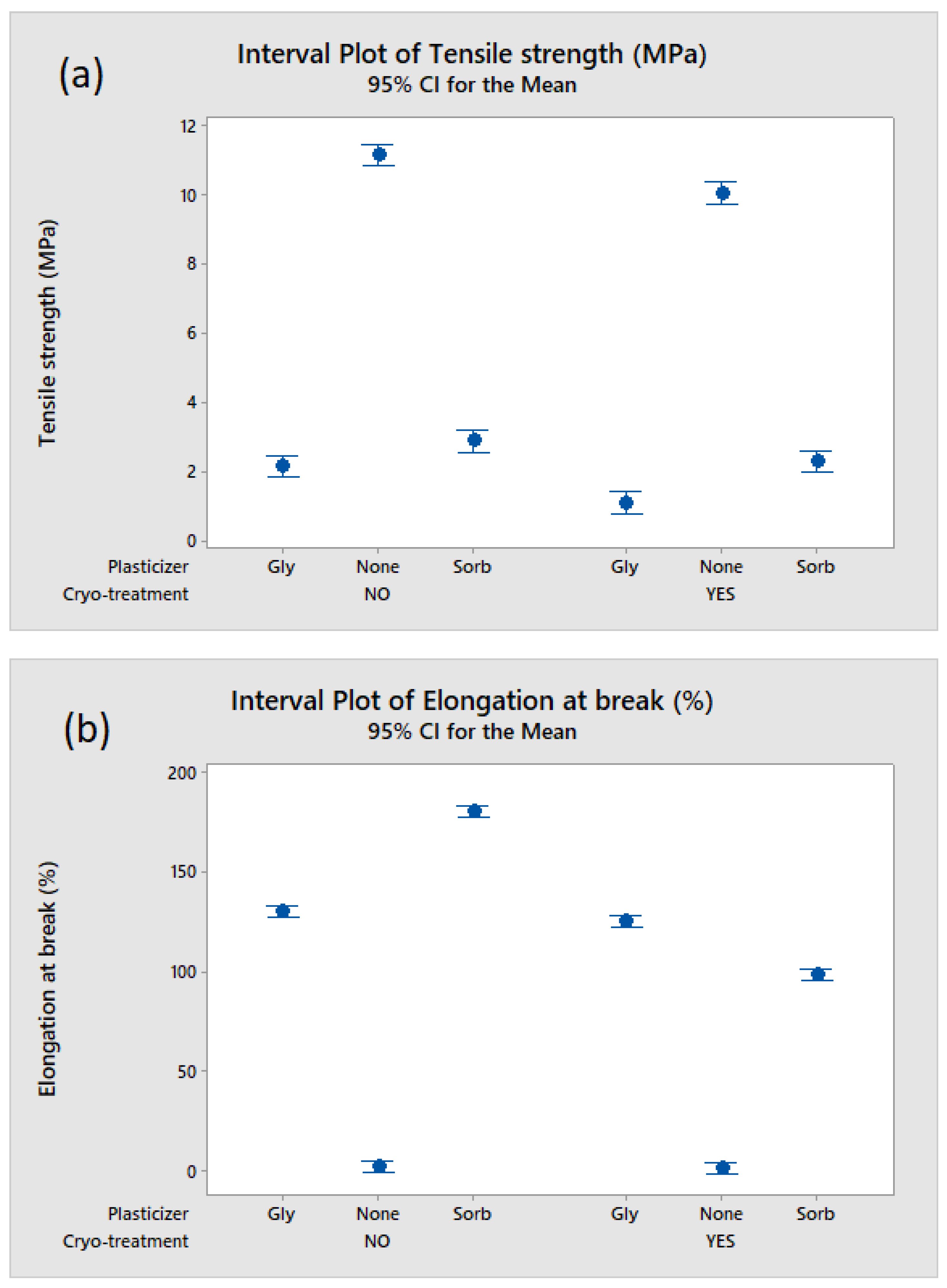
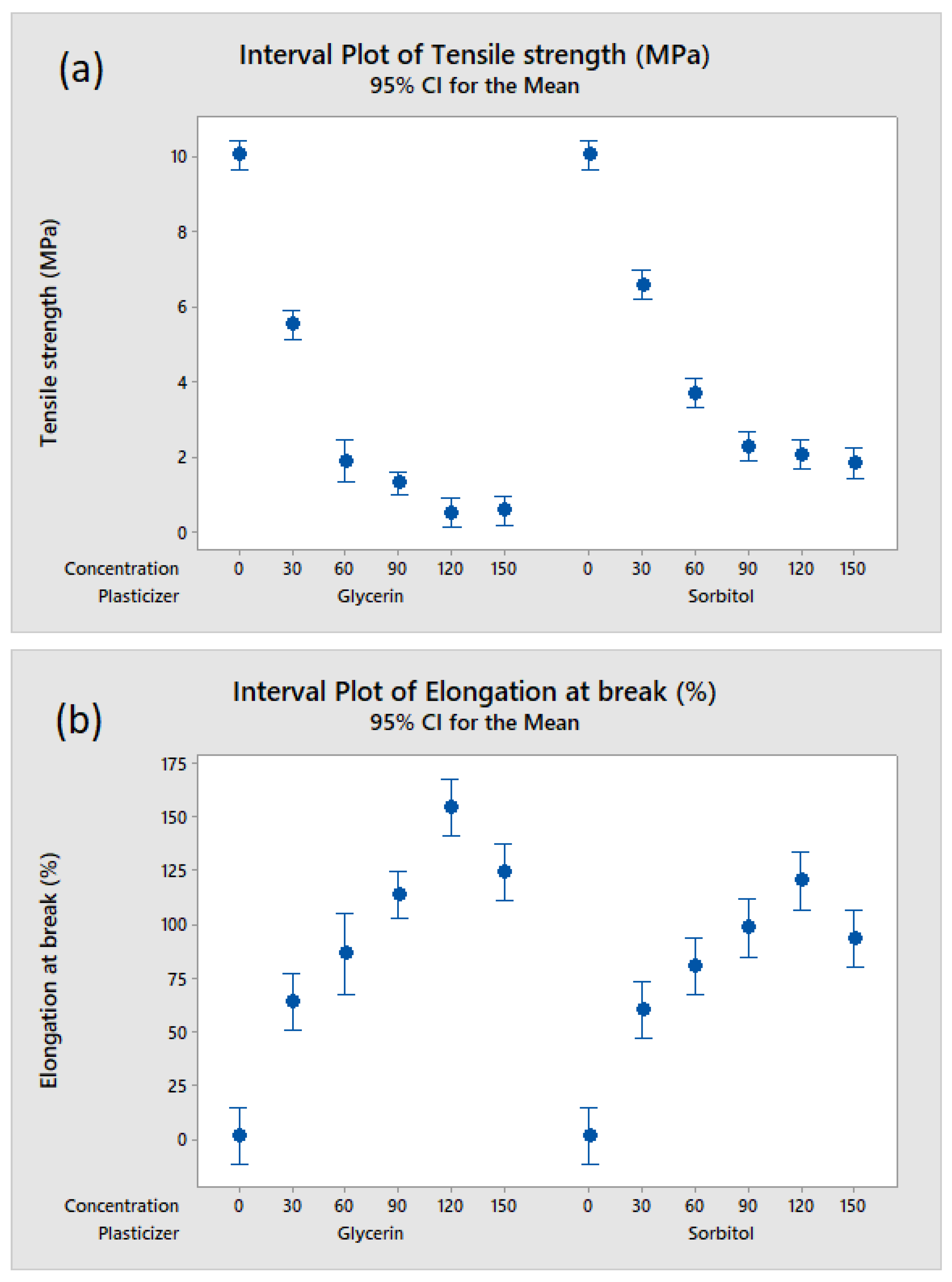
2.5. Evaluation of the Films’ Mechanical Properties
2.6. Film Thermal Analysis
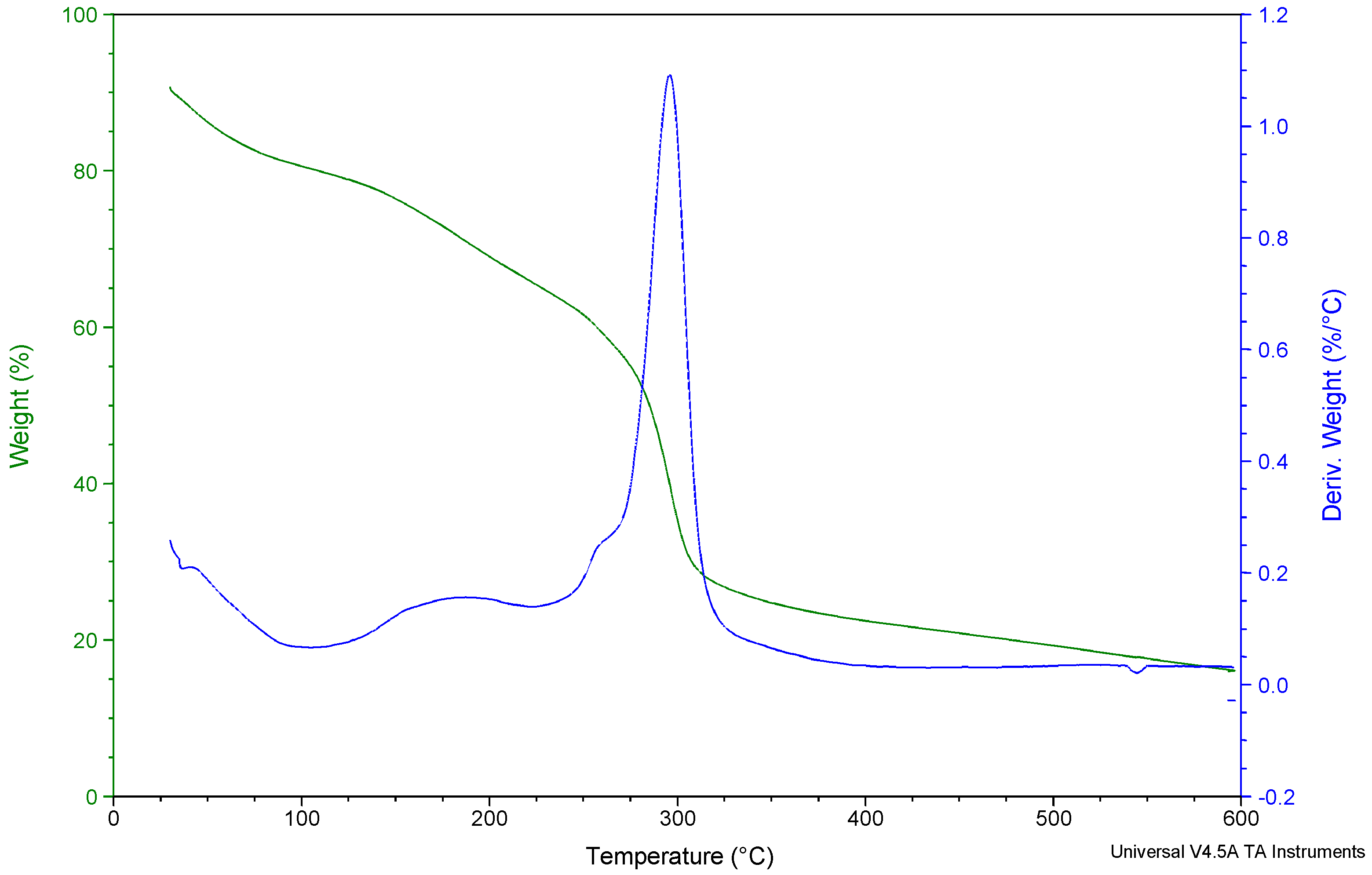
2.6.1. Thermogravimetric Analysis (TGA)
2.6.2. Differential Scanning Calorimetry (DSC)
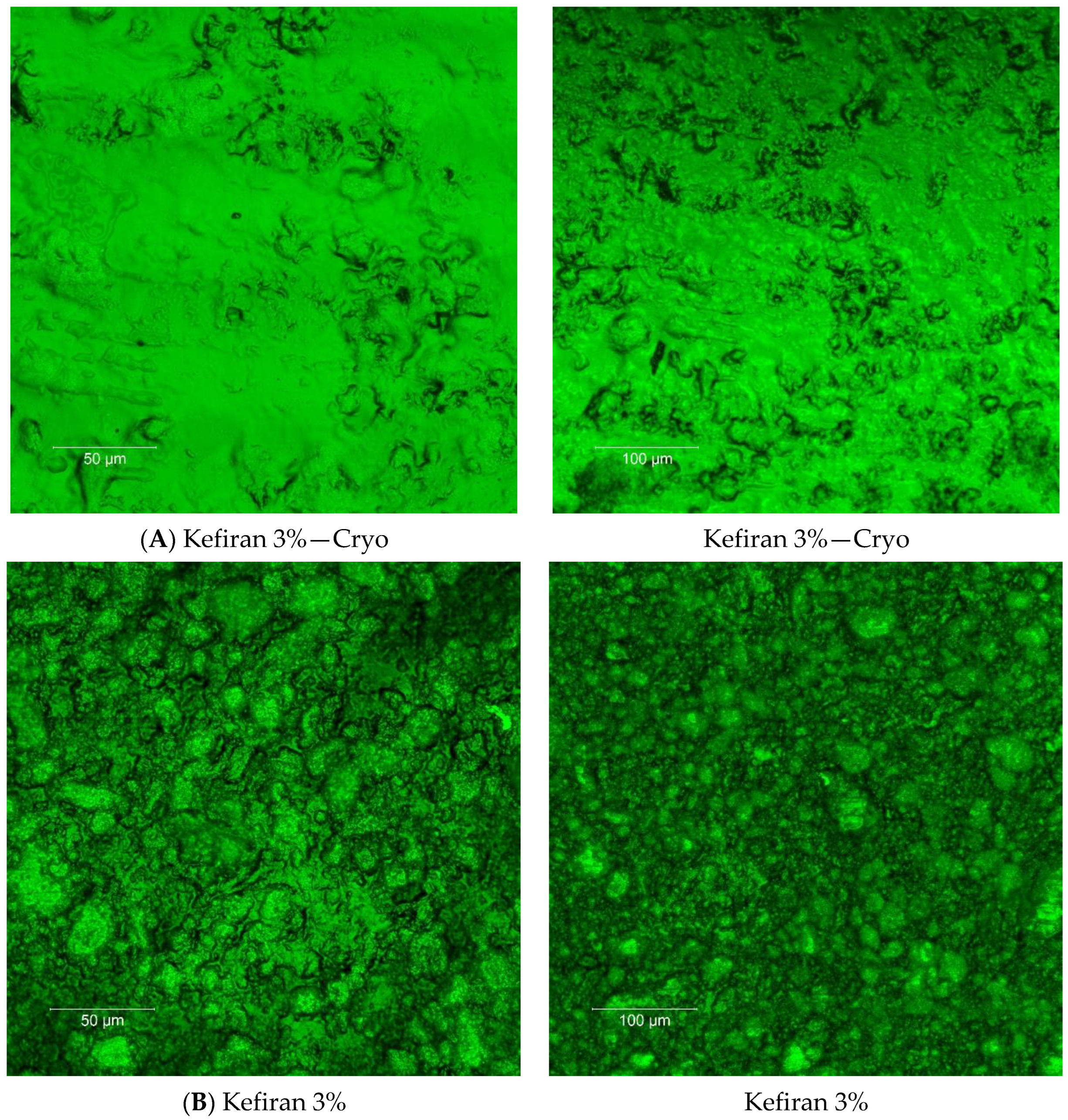
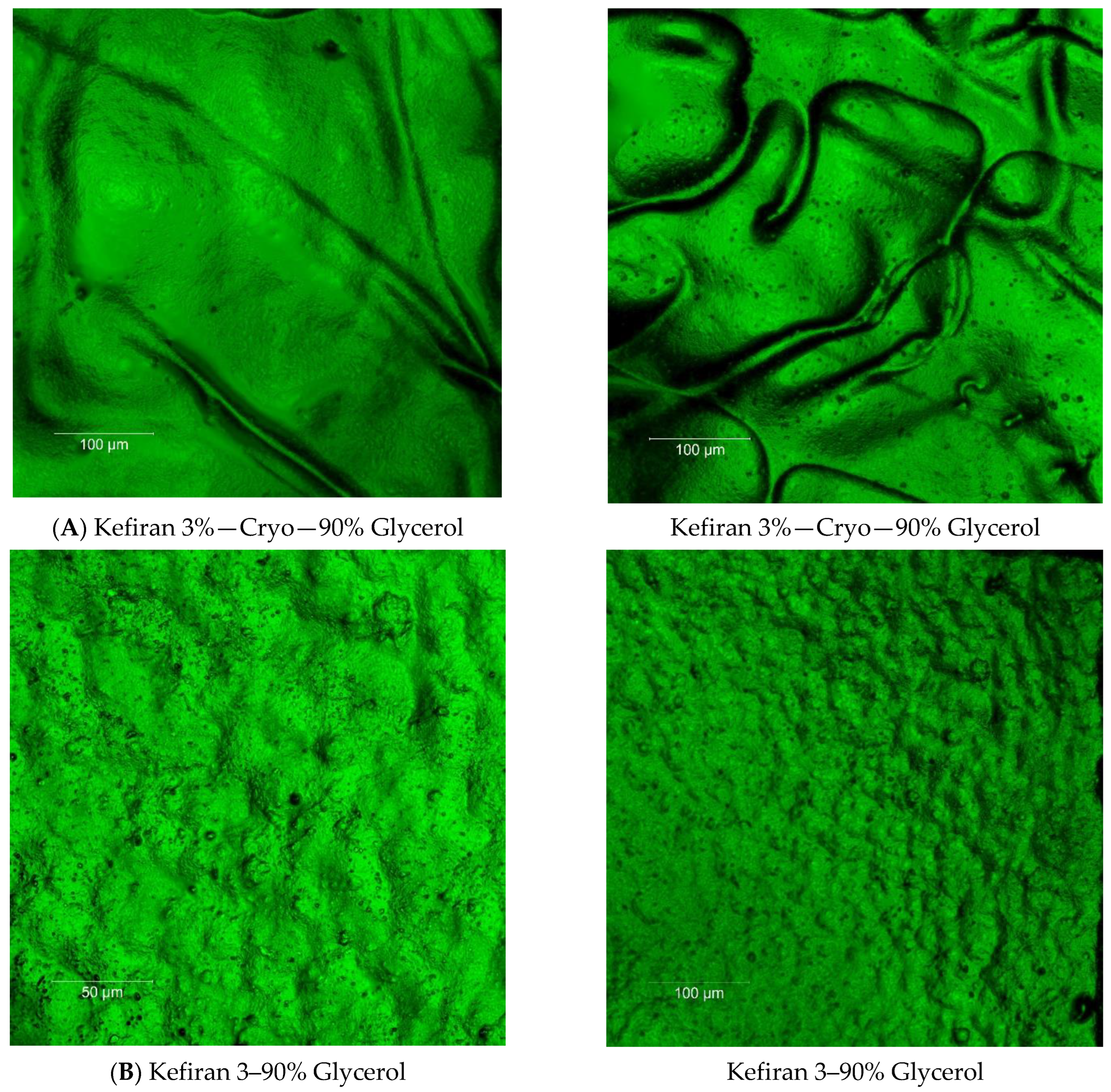
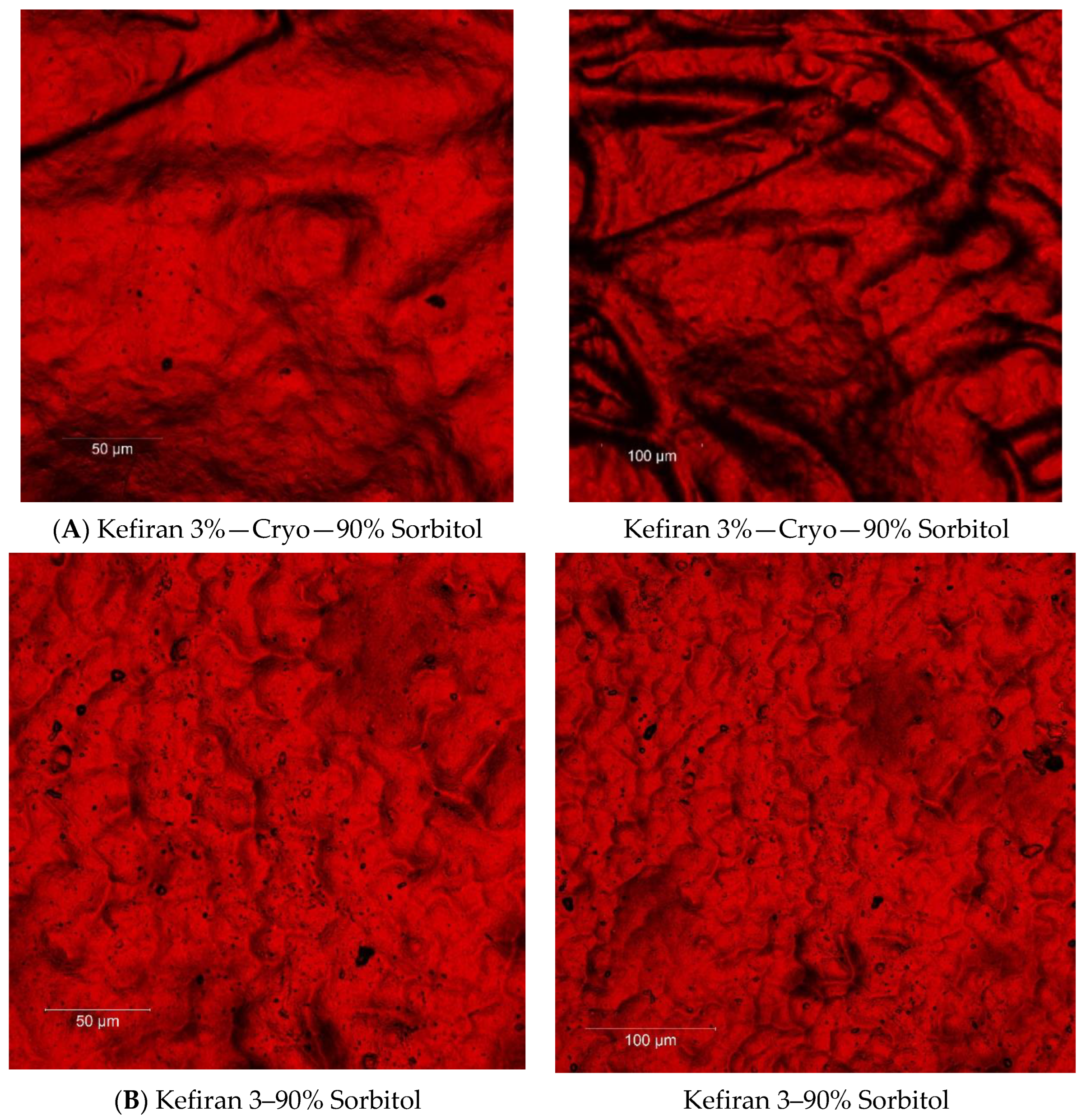
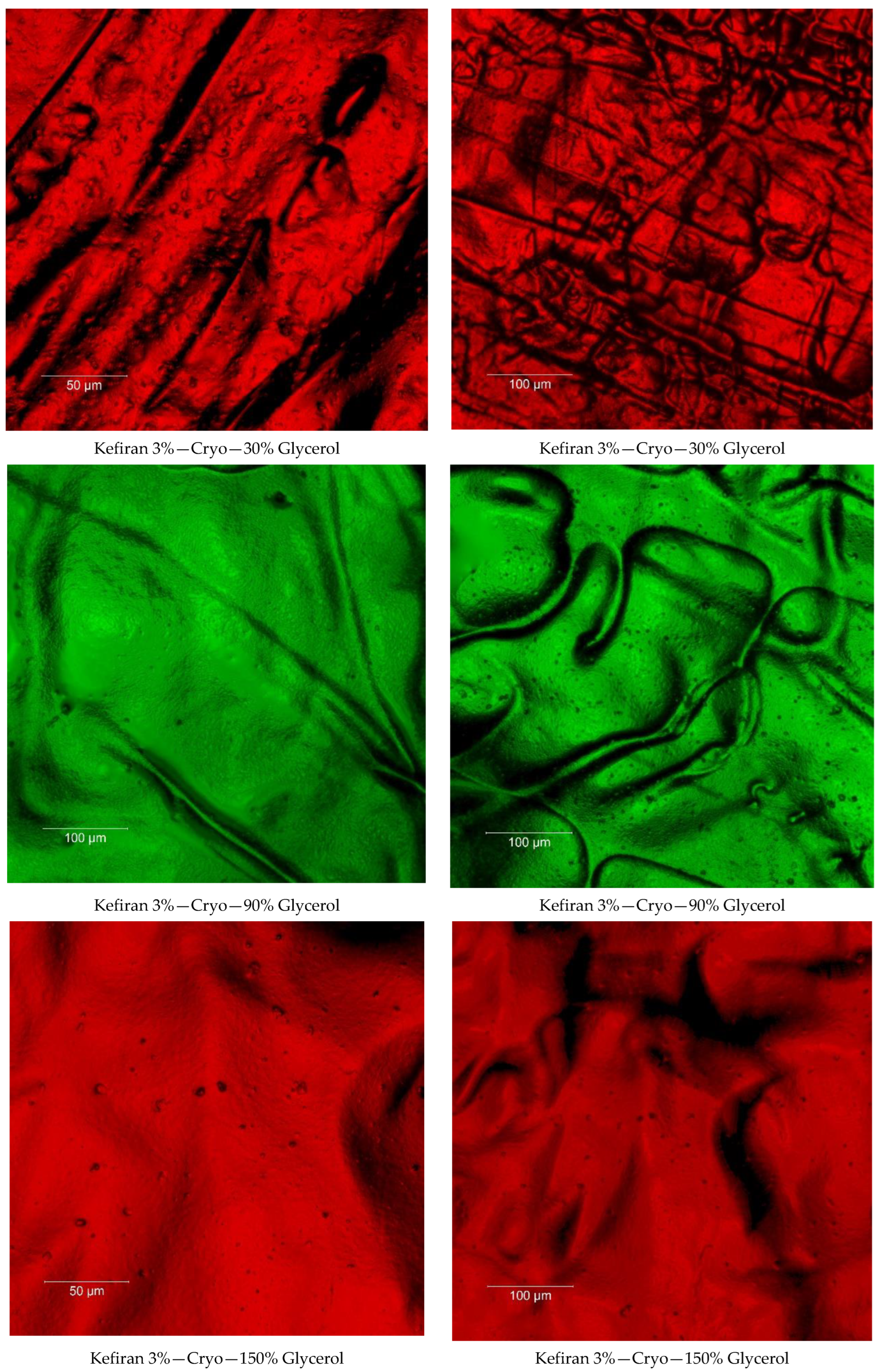
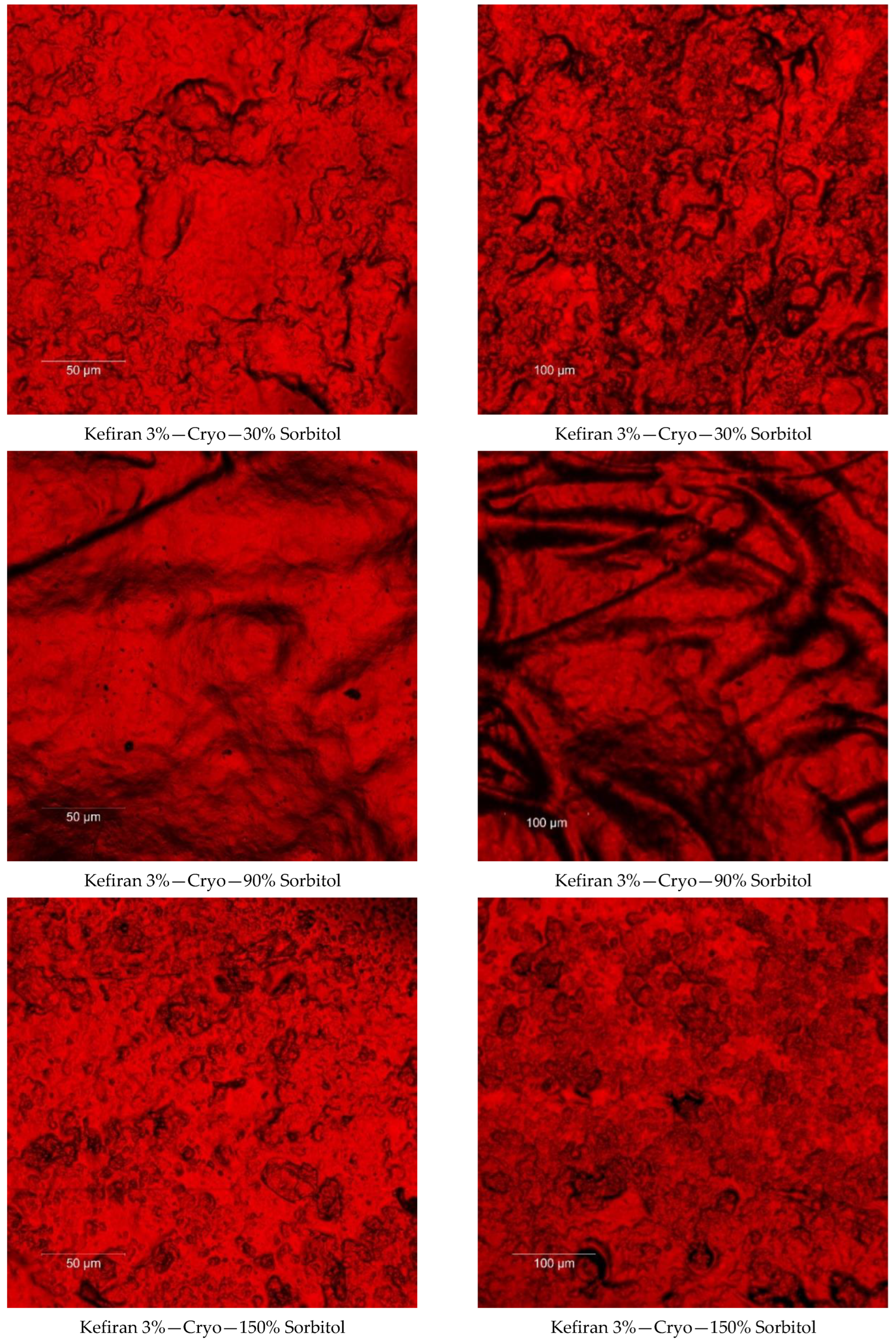
2.7. Surface Morphology of the Kefiran Films
2.8. Statistical Analysis
3. Results
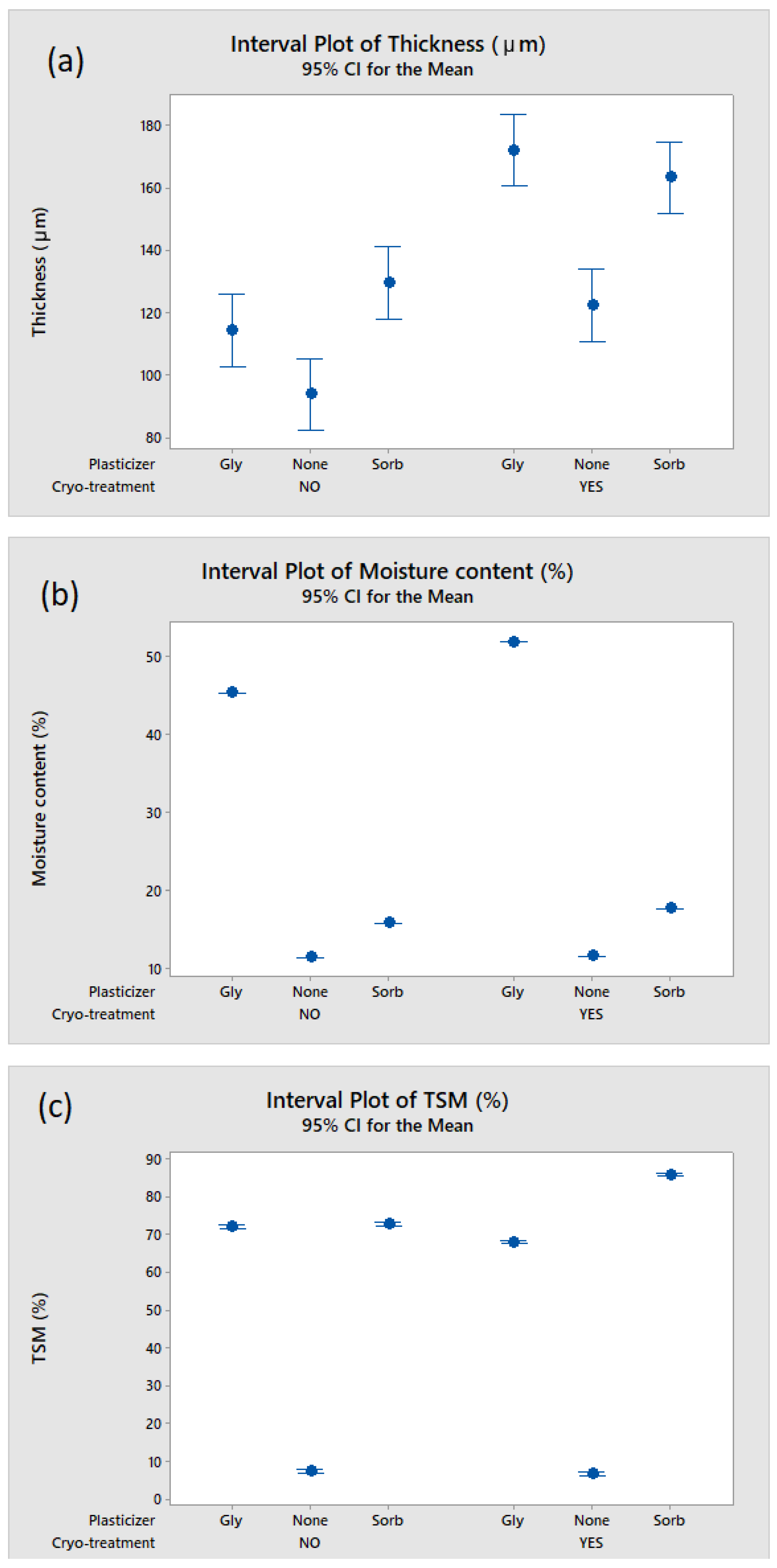
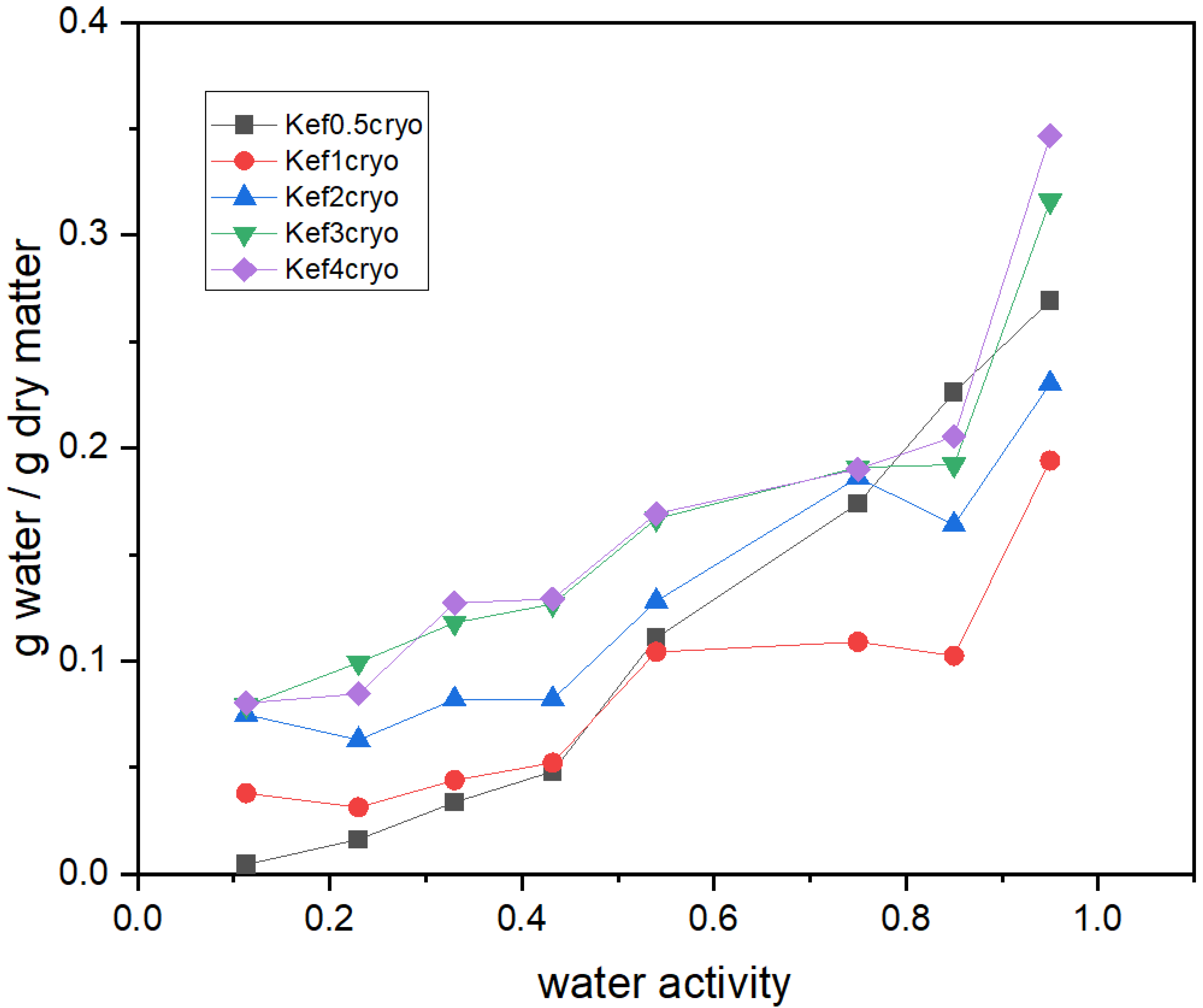
3.1. Effect of Cryo-Treatment—With or without Plasticizer Addition
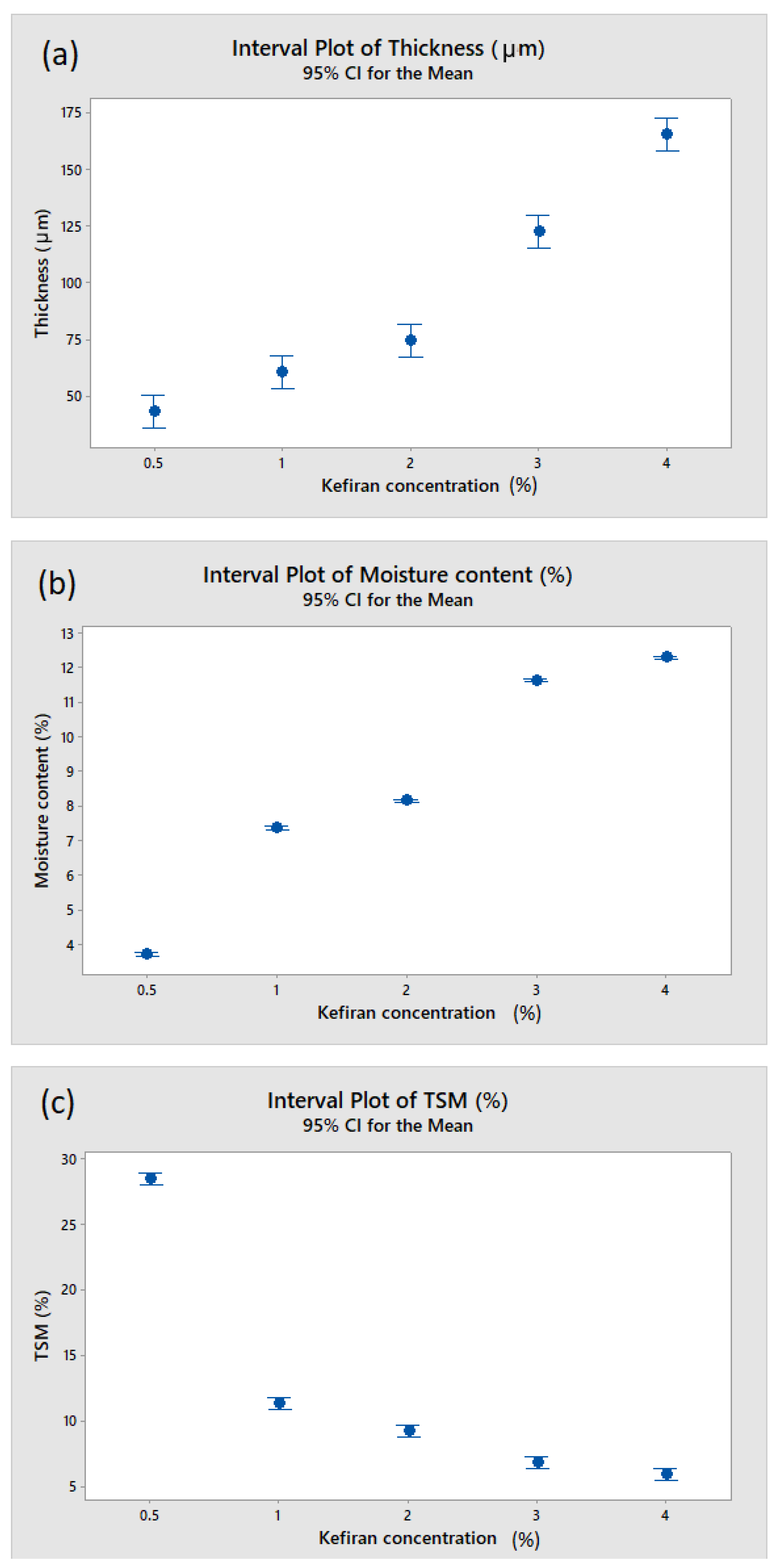
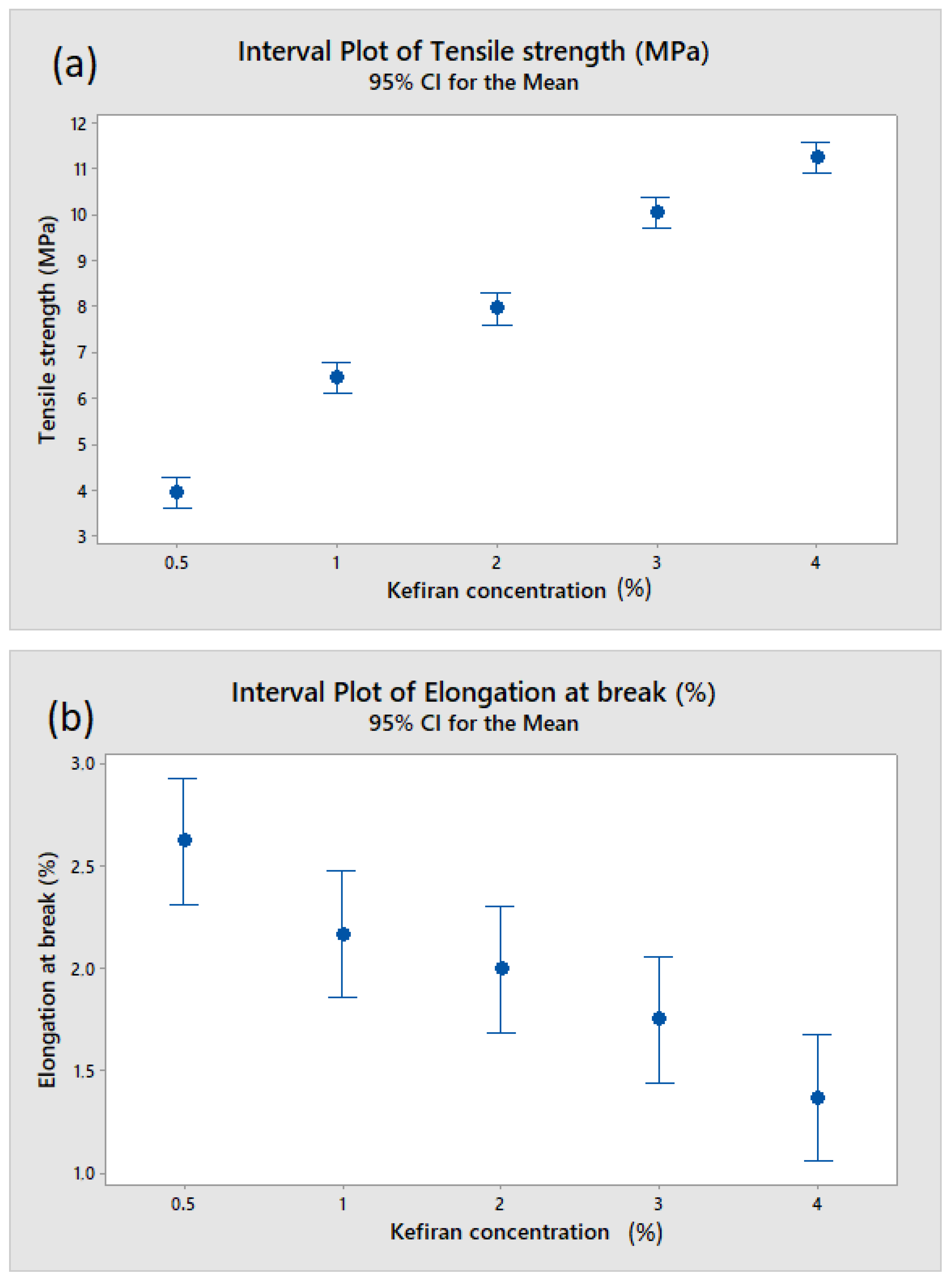
3.2. Effect of Kefiran Concentration
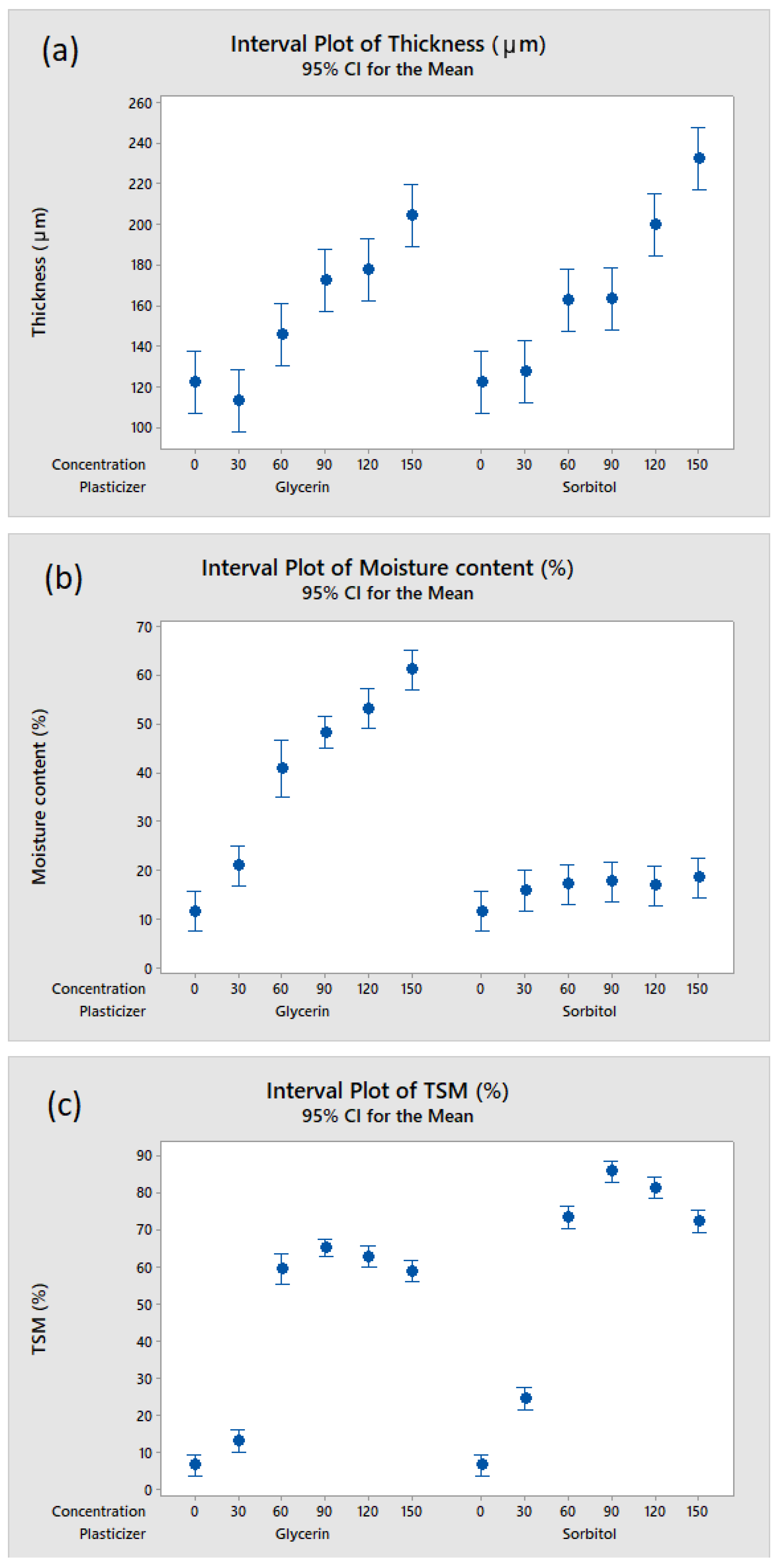
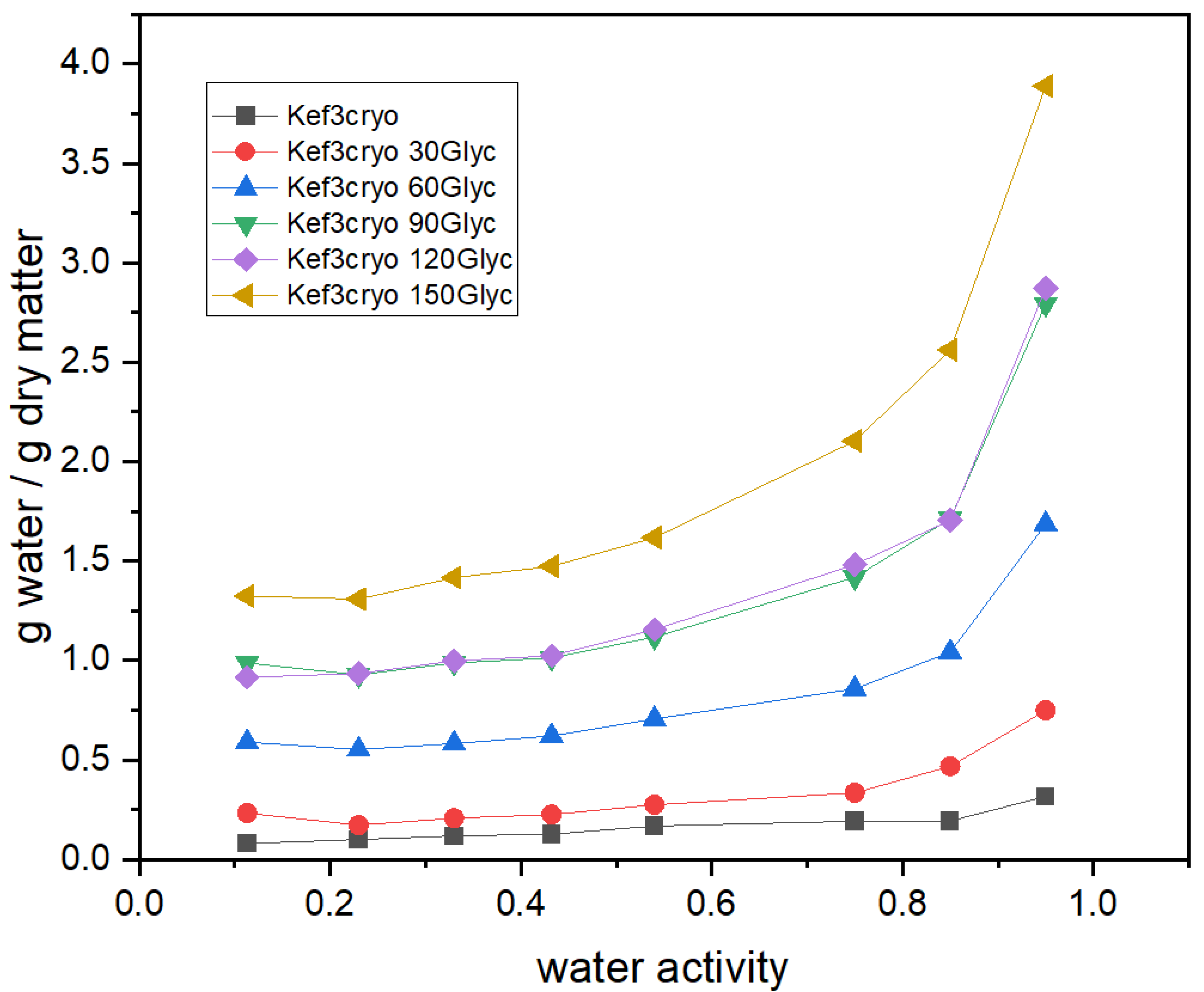
3.3. Effect of Plasticizer Addition
4. Conclusions
Author Contributions
Funding

Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sorrentino, A.; Gorrasi, G.; Vittoria, V. Potential perspectives of bio-nanocomposites for food packaging applications. Trends Food Sci. Technol. 2007, 18, 84–95. [Google Scholar] [CrossRef]
- Siracusa, V.; Rocculi, P.; Romani, S.; Dalla Rosa, M. Biodegradable polymers for food packaging: A review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Tan, K.-X.; Chamundeswar, V.N.; Loo, S.C.J. Prospects of kefiran as a food-derived biopolymer for agri-food and biomedical applications. RSC Adv. 2020, 10, 25339–25351. [Google Scholar] [CrossRef] [PubMed]
- Radhouani, H.; Correia, S.; Gonçalves, C.; Reis, R.L.; Oliveira, J.M. Synthesis and characterization of biocompatible methacrylated kefiran hydrogels: Towards tissue engineering applications. Polymers 2021, 13, 1342. [Google Scholar] [CrossRef] [PubMed]
- Rimada, P.S.; Abraham, A.G. Comparative study of different methodologies to determine the exopolysaccharide produced by kefir grains in milk and whey. Lait 2003, 83, 79–87. [Google Scholar] [CrossRef]
- Farnworth, E.R. Kefir—A complex probiotic. Food Sci. Technol. Bull. Funct. Foods 2005, 2, 1–17. [Google Scholar] [CrossRef]
- Dimitreli, G.; Exarhopoulos, S.; Goulas, A.; Antoniou, K.D.; Raphaelides, S.N. Effect of kefiran and milk proteins addition on the rheological behavior of glucono-δ-lactone induced milk gels. J. Food Res. 2015, 5, 121–128. [Google Scholar] [CrossRef][Green Version]
- Radhouani, H.; Goncalves, C.; Maia, F.R.; Oliveira, J.M.; Reis, R.L. Kefiran biopolymer: Evaluation of its physicochemical and biological properties. J. Bioact. Compat. Polym. 2018, 33, 461–478. [Google Scholar] [CrossRef]
- Piermaria, J.A.; de la Canal, M.L.; Abraham, A.G. Gelling properties of kefiran, a food-grade polysaccharide obtained from kefir grain. Food Hydrocoll. 2008, 22, 1520–1527. [Google Scholar] [CrossRef]
- Radhouani, H.; Bicho, D.; Gonçalves, C.; Maia, F.R.; Reis, R.L.; Oliveira, J.M. Kefiran cryogels as potential scaffolds for drug delivery and tissue engineering applications. Mater. Today Commun. 2019, 20, 100554. [Google Scholar] [CrossRef]
- Moradi, Ζ.; Kalanpour, Ν. Kefiran, a branched polysaccharide: Preparation, properties and applications: A review. Carbohydr. Polym. 2019, 223, 115100. [Google Scholar] [CrossRef] [PubMed]
- Marangoni Júnior, L.; Vieira, R.P.; Anjos, C.A.R. Kefiran-based films: Fundamental concepts, formulation strategies and properties. Carbohydr. Polym. 2020, 246, 116609. [Google Scholar] [CrossRef] [PubMed]
- Piermaria, J.; Bosch, A.; Pinotti, A.; Yantorno, O.; Garcia, M.A.; Abraham, A.G. Kefiran films plasticized with sugars and polyols: Water vapor barrier and mechanical properties in relation to their microstructure analyzed by ATR/FT-IR spectroscopy. Food Hydrocoll. 2011, 25, 1261–1269. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Khodaiyan, F.; Oromiehie, A. Rheological and structural characterisation of film-forming solutions and biodegradable edible film made from kefiran as affected by various plasticizer types. Int. J. Biol. Macromol. 2011, 49, 814–821. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Khodaiyan, F.; Oromiehie, A.; Yarmand, M.S. Development and characterisation of a new biodegradable edible film made from kefiran, an exopolysaccharide obtained from kefir grains. Food Chem. 2011, 127, 1496–1502. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Khodaiyan, F.; Oromiehie, A.; Yarmand, M.S. Characterization of edible emulsified films with low affinity to water based on kefiran and oleic acid. Int. J. Biol. Macromol. 2011, 49, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Lozinsky, V.I.; Galaev, I.Y.; Plieva, F.M.; Savina, I.N.; Jungvid, H.; Mattiasson, B. Polymeric cryogels as promising materials of biotechnological interest. Trends Biotechnol. 2003, 21, 445–451. [Google Scholar] [CrossRef]
- Gun’ko, V.M.; Savina, I.N.; Mikhalovsky, S.V. Cryogels: Morphological, structural and adsorption characterisation. Adv. Colloid. Interface Sci. 2013, 187, 1–46. [Google Scholar] [CrossRef]
- Exarhopoulos, S.; Raphaelides, S.N.; Kontominas, M.G. Flow behavior studies of kefiran systems. Food Hydrocoll. 2018, 79, 282–290. [Google Scholar] [CrossRef]
- Exarhopoulos, S.; Raphaelides, S.N.; Kontominas, M.G. Conformational studies and molecular characterization of the polysaccharide kefiran. Food Hydrocoll. 2018, 77, 347–356. [Google Scholar] [CrossRef]
- Ruas-Madiedo, P.; de los Reyes-Gavilan, C.G. Methods for the screening, isolation and characterization of exopolysaccharides produced by lactic acid bacteria. J. Dairy Sci. 2005, 88, 843–856. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar]
- Maeda, H.; Zhu, X.; Suzuki, S.; Suzuki, K.; Kitamura, S. Structural characterization and biological activities of an exopolysaccharide kefiran produced by Lactobacillus kefiranofaciens WT-2. J. Agric. Food Chem. 2004, 52, 5533–5538. [Google Scholar] [CrossRef] [PubMed]
- Standard D882; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. Annual Book of ASTM. American Society for Testing and Materials: Philadelphia, PA, USA, 2001.
- Zhang, H.; Zhang, F.; Wu, J. Physically crosslinked hydrogels from polysaccharides prepared by freeze–thaw technique. React. Funct. Polym. 2013, 73, 923–928. [Google Scholar] [CrossRef]
- Montoille, L.; Vicencio, C.M.; Fontalba, D.; Ortiz, J.A.; Moreno-Serna, V.; Peponi, L.; Matiacevich, S.; Zapata, P.A. Study of the effect of the addition of plasticizers on the physical properties of biodegradable films based on kefiran for potential application as food packaging. Food Chem. 2021, 360, 129966. [Google Scholar] [CrossRef] [PubMed]

| Sample | Tg (°C) | Tm (°C) |
|---|---|---|
| Kefiran 3% | −4.23 | 92.67 |
| Kefiran 3%—Cryo | −3.90 | 92.31 |
| Kefiran 3%–90% Glycerol | −7.43 | 84.02 |
| Kefiran 3%–90% Sorbitol | −8.20 | 83.96 |
| Kefiran 3%—Cryo—30% Glycerol | −5.94 | 89.50 |
| Kefiran 3%—Cryo—60% Glycerol | −6.54 | 86.83 |
| Kefiran 3%—Cryo—90% Glycerol | −7.08 | 83.97 |
| Kefiran 3%—Cryo—120% Glycerol | −7.42 | 81.93 |
| Kefiran 3%—Cryo—150% Glycerol | −8.05 | 80.09 |
| Kefiran 3%—Cryo—30% Sorbitol | −6.19 | 89.25 |
| Kefiran 3%—Cryo—60% Sorbitol | −6.53 | 86.92 |
| Kefiran 3%—Cryo—90% Sorbitol | −6.44 | 84.01 |
| Kefiran 3%—Cryo—120% Sorbitol | −7.44 | 82.02 |
| Kefiran 3%—Cryo—150% Sorbitol | −7.63 | 79.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Exarhopoulos, S.; Goulas, A.; Dimitreli, G. Biodegradable Films from Kefiran-Based Cryogel Systems. Macromol 2022, 2, 324-345. https://doi.org/10.3390/macromol2030021
Exarhopoulos S, Goulas A, Dimitreli G. Biodegradable Films from Kefiran-Based Cryogel Systems. Macromol. 2022; 2(3):324-345. https://doi.org/10.3390/macromol2030021
Chicago/Turabian StyleExarhopoulos, Stylianos, Athanasios Goulas, and Georgia Dimitreli. 2022. "Biodegradable Films from Kefiran-Based Cryogel Systems" Macromol 2, no. 3: 324-345. https://doi.org/10.3390/macromol2030021
APA StyleExarhopoulos, S., Goulas, A., & Dimitreli, G. (2022). Biodegradable Films from Kefiran-Based Cryogel Systems. Macromol, 2(3), 324-345. https://doi.org/10.3390/macromol2030021







